Abstract
Purpose
To examine the associations between myopic refractive error (MRE), corneal power (CP), axial length (AL), and the prevalence of open angle glaucoma (OAG) in an adult Latino population.
Design
Population-based cross-sectional epidemiologic study.
Participants
5927 Latinos aged 40 years and older in the Los Angeles Latino Eye Study (LALES).
Methods
MRE was defined as a non-cycloplegic sphero-cylindrical refractive error of ≤ −1 diopter (D). AL was measured by A-scan ultrasound. CP was measured using a Humphrey auto-refractor. OAG was diagnosed by a combination of optic nerve and visual field changes. Pseudophakic and aphakic eyes were excluded from the analysis. The associations between MRE, AL, CP, and the prevalence of OAG were calculated using a logistic regression model, adjusting for age, gender, intraocular pressure, central corneal thickness, diabetes mellitus, family history of glaucoma, and lens nuclear opacification (NO).
Main Outcome Measures
Odds ratios (OR) for the prevalence of OAG.
Results
After adjusting for covariates persons with greater MRE (OR 1.82, 1.20–2.77; P=0.005), longer AL (OR: 1.25, 1.03–1.50; P=0.02) and flatter corneas (OR 1.21, 1.08–1.35; P=0.0007) were associated with a higher prevalence of OAG when compared to those with emmetropic refractive error, shorter axial length and steeper corneas respectively.
Conclusions
Persons with a myopic refractive error, flatter corneas, and longer axial lengths should be considered to be at higher risk of having open-angle glaucoma.
Myopia, as defined by refractive error, has long been associated with primary open angle glaucoma (OAG).1 2 Large cross-sectional epidemiologic studies in the United States3, Australia4, India5, Japan6, China7, the Caribbean8–9, and Europe10 have found that myopes have a higher prevalence of open-angle glaucoma (OAG) than do non-myopes. Several studies have found that persons with higher myopic refractive error (MRE) have a greater prevalence of OAG than those with a lower MRE.4–6, 7 However, only one of these studies has taken into consideration the effect of nuclear opacification (NO) on MRE9, which could be a confounding factor when evaluating the independent relationship between myopia and prevalence of OAG. Furthermore, no studies to date have comprehensively evaluated the independent relationship between various biometric ocular characteristics and the prevalence of OAG particularly those that may be directly related with refractive error (axial length and corneal power). In this study we propose to examine the association between MRE, AL, CP, and prevalence of OAG.
The Los Angeles Latino Eye Study (LALES) is a population-based ophthalmologic survey of adult Latinos in Los Angeles County. Understanding eye disease among Latinos, an ethnic group projected to constitute a quarter of the U.S. population by 205011, has important public health implications. Previous studies of this population have shown that Latinos have a higher prevalence of OAG12 than non-Hispanic whites and a greater burden of myopia in older Latinos than either African-American or non-Hispanic whites.13 Previously, we have also shown that persons with thinner central corneas are more likely to be associated with OAG than those with thicker central corneas. However, that study did not assess the relationship between other biometric parameters and the prevalence of OAG.14 Previously, we have shown that one explanation for having a myopic refractive error is NO.15 However, since there can be other explanations for myopic refractive error, it would be important to consider utilizing a biometric surrogate (such as axial length) for myopic refractive error (particularly axial myopia) that is not confounded by lens opalescence may yield further insight into the relationship between myopia and OAG.
Recently there has been significant interest in the relationship between corneal characteristics and the measurement of intraocular pressure (IOP), as well as an IOP-independent relationship between central corneal thickness and the risk of developing OAG.16 17 Corneal power is a biometric characteristic related to refractive error and corneal hysteresis18, a parameter that has been associated with increased risk for OAG.16 Therefore, in the present study, we also examined the relationship between CP and OAG.
Participants and Methods
Study Population
The LALES population consists of self-identified Latino residents ≥ 40 years old in six census tracts in La Puente, California, a city with a socioeconomic demographic profile similar to Latinos in the US.19 The Institutional Review Board at the University of Southern California approved the study protocol. The study adhered to the tenets of the Declaration of Helsinki.
Data Collection
Details of the study design, sampling plan, and baseline data have been previously reported.19 Briefly, informed consent was obtained, and a detailed in-home interview was conducted documenting demographic information (age, gender, ocular and medical histories (having diabetes, family history of glaucoma), access to medical care, and level of acculturation. Subsequently, participants received a comprehensive standardized eye examination, including measurement of visual acuity, IOP, visual fields, manifest refractive error, ocular biometry and a slit-lamp and dilated fundus examination.
Definition of OAG
Open-angle glaucoma (OAG) was defined by the presence of an open angle and at least one of the following criteria: 1) congruent, characteristic, or compatible glaucomatous visual field abnormality and/or 2) evidence of characteristic or compatible glaucomatous optic disc damage in at least one eye, as determined by two independent glaucoma specialist graders who reviewed all cases. Visual field defects characteristic of glaucoma were defined as defects corresponding to the nerve fiber bundle pattern, which included nasal steps (either superior or inferior, but not both), paracentral defect, arcuate defect, central island, temporal island, and absolute defect. The optic nerve appearance was classified as characteristic/compatible if it met one and characteristic if it met two or more of the following criteria: horizontal or vertical cup/disc ratio ≥ 0.8, notching of the neural rim, localized or diffuse loss of neural rim with maximum remaining neural rim of <0.1, disc or peripapillary nerve fiber layer hemorrhage, or nerve fiber layer defect in the arcuate bundles. Intraocular pressure was not considered in the definition of OAG. Ocular hypertension was defined as IOP > 21mm Hg in either eye.
Biometry, and Lens Opacification Assessment
Visual acuity was measured for each eye at 4 meters using ETDRS protocols with a modified ETDRS distance chart transilluminated with a chart illuminator (Precision Vision, La Salle, IL).20 A participant able to read 55 letters or better without correction was considered emmetropic in the tested eye. If the participant read fewer than 55 letters at 4 m in either eye, an automated non-cycloplegic refraction and corneal power measurement were performed (Humphrey Autorefractor Model 599; Carl Zeiss Meditec, Dublin, CA). The automated refraction was refined by a standardized subjective refraction protocol to determine the spherical and cylindrical refractive error to the nearest 0.25 diopter (D). The spherical equivalent (sphere plus half cylinder) was calculated for each eye. Myopia at the individual level was defined as a non-cycloplegic spherocylindrical refractive error of −1 diopter or less in the worse eye (phakic eye with larger absolute sphero-cylindrical refractive error). The term myopic refractive error was used to quantify the degree of myopia in diopters. Low myopia was defined as MRE ≤ −1 D and > −3 D, and moderate to high myopia was defined as MRE ≤ −3 D. Corneal power was measured three times in each of two perpendicular axes, and these measurements were averaged to create a mean CP. Axial length (AL) measurements were obtained using an A-scan ultrasound (A-Scan pachymeter, Ultrasonic, Exton, PA). Three measurements were obtained and averaged for each eye of each participant. Lens nuclear opacification was graded for each eye of each participant at the slit lamp using the Lens Opacities Classification System (LOCS II)21 to categorize opacities into five nuclear grades of increasing severity (N0, NI, NII, NIII, NIV). A participant was classified as having nuclear opacity if he/she had a LOCS grading score of NII or greater in either eye.
Data Analysis
Pseudophakic and aphakic eyes were excluded from these analyses. First, Frequency procedures were used to look at the distribution of the risk factors with prevalence of open-angle glaucoma. Chi-square procedures were used to test the associations of categorical variables (such as age, gender, diabetes, history of glaucoma, refractive error groups, and nuclear opacity) with open-angle glaucoma, and t-test procedures were used to evaluate the associations of continuous variables (intraocular pressure, corneal power, and axial length). Univariate analysis was performed to calculate the odds ratio of factors affecting the prevalence of OAG. The resulting statistically significant (at p-value ≦ 0.1) factors were then entered in a multivariate model. MRE was analyzed using the refractive error of the worse eye (more myopic) for participants who had bilateral glaucoma or who did not have glaucoma. For participants with unilateral glaucoma, the refractive error used in the analysis was from the glaucomatous eye. Axial lengths were analyzed in a similar manner. The axial length of the longer eye was used for participants with bilateral glaucoma or bilateral non-glaucomatous eyes. For those with unilateral glaucoma, the axial length was chosen for the glaucomatous eye. The association between MRE, AL and the prevalence of OAG was calculated using a logistic regression model, adjusting for those covariates that were significant in the univariate analyses. The independent associations between AL, MRE, CP, and OAG were assessed. A LOWESS22 plot (a locally weighted polynomial regression) was created to examine the relationship comparing prevalence of OAG to AL or CP, after adjusting for age, gender, IOP, diabetes, family history, and NO. A correlation coefficient was calculated between AL and MRE to evaluate the association between both variables. All statistical testing was conducted using SAS software (SAS Institute, Cary, NC).
Results
Of the 6357 participants who were examined, 5927 (93%) had complete data and were included in the analysis. Complete baseline characteristics have been reported previously.19 More women (58%) than men (42%) were included in the study (P = 0.007). Among the entire study population, 799 participants (13.5%) were myopes. Equal percentages of women (13.5%) and men (13.4%) were myopic. Additionally, we have previously shown that the prevalence of myopia was greater in older Latinos compared to younger Latinos (P < 0.0001).13 Clinical and demographic characteristics of persons with and without OAG are presented in Table 1. In the univariate analyses, older age, male gender, presence of diabetes, family history of OAG, myopic refractive error, higher mean IOP, greater NO, flatter corneas, and longer mean AL were associated with OAG. CCT was not significant in the univariate analyses. The variables that were significantly associated with prevalence of OAG in the univariate analyses were entered into a multivariate analysis. Because AL and MRE were moderately correlated (R=0.67), each was entered in a separate model and adjusted for significant covariates. In the multivariate analyses, age, IOP, CP, AL, and MRE were independently and significantly (p<0.05) associated with the prevalence of OAG. (Table 2).
Table 1.
Frequency Distribution of Risk Factors and Clinical Characteristics for Open-angle Glaucoma in Participants of the Los Angeles Latino Eye Study
| Risk Factors | All (N=5927) n (%)* |
OAG (N = 252) n (%)* |
No OAG (N = 5675) n (%)* |
P Value± |
|---|---|---|---|---|
|
Age (years) 40–49 50–59 60–61 70+ |
2337 (39.4) 1831 (30.9) 1151 (19.4) 608 (10.3) |
29 (11.5) 54 (21.4) 84 (33.3) 85 (33.7) |
2308 (40.7) 1777 (31.3) 1067 (18.8) 523 (9.2) |
<0.001 |
| Gender (male) | 2479 (41.8) | 126 (50) | 2353 (41.5) | 0.01 |
| Presence of Diabetes | 252 (4.3) | 86 (34.1) | 166 (2.9) | <0.0001 |
|
Family History of Glaucoma |
264 (4.5) | 31 (12.3) | 203 (3.6) | 0.01 |
|
Refractive Error (D) > −1 ≤ −1 and > −3 ≤−3 |
5128 (86.5) 485 (8.2) 314 (5.3) |
187 (74.2) 42 (16.7) 23 (9.1) |
4941(87.1) 443 (7.8) 291 (5.1) |
<0.001 |
|
Presence of Nuclear Opacity (NO) |
215 (3.6) | 31 (12.3) | 184 (3.2) | <0.0001 |
| Mean IOP (mmHg) | 14.6 (± 3.3) | 17.1 (±4.7) | 14.5 (±3.1) | <0.0001 |
| Mean CCT (mm) | 0.554 (±0.04) | 0.553 (±0.04) | 0.554 (±0.03) | 0.47 |
| Mean Corneal Power (D) | 43.6 (±1.6) | 43.2 (±1.7) | 43.6 (±1.6) | 0.01 |
| Mean Axial Length (mm) | 23.3 (±1.1) | 23.6 (±1.3) | 23.3 (±1.1) | 0.01 |
n(%)=number and column percent, or mean (±standard deviation); ± Pvalue=chi-square test of association for categorical variables, and t-test procedures for continuous variables.
D = diopters, OAG = open-angle glaucoma, IOP = intraocular pressure, CCT = central corneal thickness, LOCS = Lens Opacities Classification System, Nuclear Opacity = LOCS score grading of NII or greater
Table 2.
Multivariate models assessing the association between various risk factors and the prevalence of Open-angle Glaucoma in Participants of the Los Angeles Latino Eye Study
| Prevalence of Open-Angle Glaucoma | ||||
|---|---|---|---|---|
| Model with MRE | Model with AL | |||
| Risk Factors | OR (95% CI) | P Value | OR (95% CI) | P Value |
|
Age 40–49 50–59 60–61 70+ |
1.0 1.36 (0.62–2.97) 4.20 (2.11–8.36) 8.06 (3.95–16.44) |
<0.0001 |
1.0 1.25 (0.58–2.71) 3.85 (1.94–7.64) 7.45 (3.66–15.18) |
<0.0001 |
| Gender (male) | 1.35 (0.94–1.95) | 0.11 | 1.27 (0.88–1.84) | 0.20 |
| Presence of Diabetes | 1.14 (0.79–1.66) | 0.48 | 1.15 (0.79–1.67) | 0.47 |
|
Family History of Glaucoma |
1.68 (0.96–2.96) | 0.07 | 1.67 (0.95–2.95) | 0.07 |
|
Presence of Nuclear Opacity (NO) |
1.11 (0.72–1.72) | 0.63 | 1.30 (0.85–1.99) | 0.23 |
| IOP (1 mmHg increase) | 1.19 (1.13–1.25) | <0.0001 | 1.20 (1.14–1.27) | <0.0001 |
|
Corneal Power (1D flatter)* |
1.21 (1.08–1.35) | 0.0007 | 1.14 (1.01–1.28) | 0.04 |
| MRE (≤ −1 D vs > −1 D) | 1.82 (1.20–2.77) | 0.005 | - | - |
| AL (1 mm increase) | - | - | 1.25 (1.03–1.50) | 0.02 |
Multivariate model includes all risk factors significant (p<0.05) in the univariate analysis
Significant P values in bold
OR = odds ratio, CI = confidence interval, D = diopters, IOP = intraocular pressure, MRE = myopic refractive error, AL = axial length, LOCS = Lens Opacities Classification System, Nuclear Opacity = LOCS score grading of NII or greater;
Corneal Power measurements were available in only 2594 subjects.
Myopic Refractive Error and Prevalence of Open-Angle Glaucoma
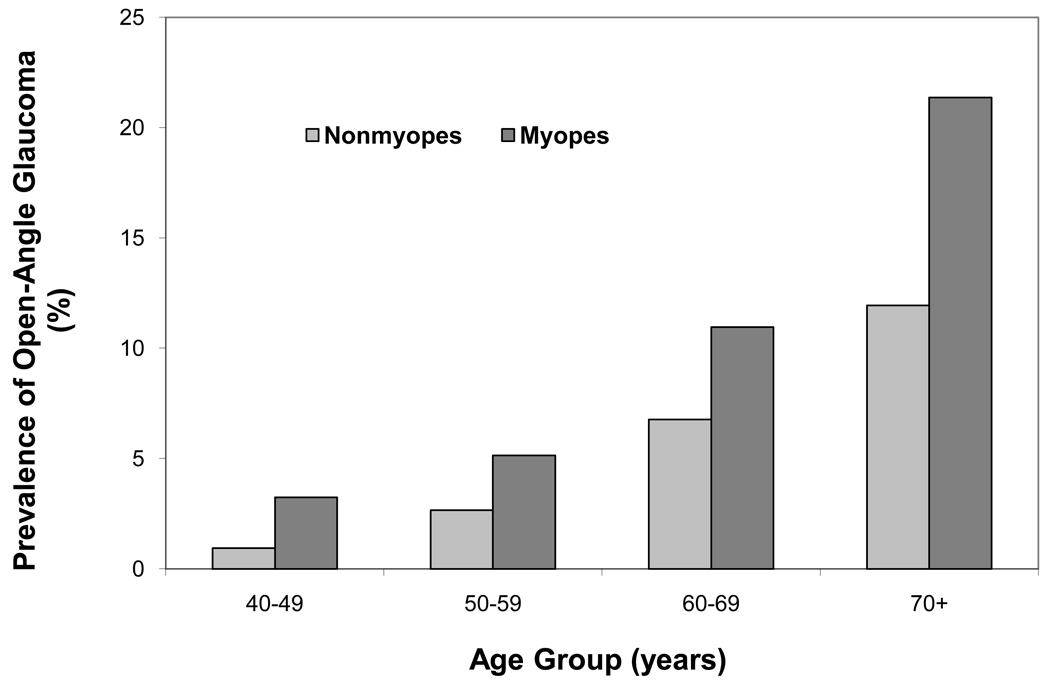
Myopes were significantly more likely to have OAG than were non-myopes. The unadjusted prevalence of OAG among myopes was 8.1%, compared to 3.7% among non-myopes (Odds Ratio [OR]: 2.34, 1.7 – 3.1, P<0.0001). After stratifying age, the prevalence of OAG was higher among myopes in every age group compared to non-myopes (p <0.03) (Figure 1). After adjusting for covariates of age, gender, IOP, diabetes, and family history, myopes were still nearly twice as likely to have OAG as were non-myopes (OR: 1.89, 1.36–2.62; P=0.0002). This association remained significant after adjusting for severity of NO (OR 1.86, 1.32–2.59; P=0.0003).
Figure 1.
Prevalence of Open Angle Glaucoma stratified by age groups in myopes and non-myopes in Los Angeles Latino Eye Study Participants
When myopes were further stratified into a low myopia group (MRE of ≤ −1D and ≥ −3D) and a moderate to high myopia group (MRE ≤ −3D), the adjusted odds ratios for the association between myopia and the prevalence of OAG was significant only for the moderate/high myopia group [Low Myopia OR 1.6 (0.9 – 2.6) and Moderate/High Myopia 2.0 (1.1 – 3.7), respectively]. Finally, there was no significant difference in the prevalence of ocular hypertension between myopes and non-myopes (P=0.07) or moderate to high myopes (MRE ≤ −3) and non-myopes (P=0.39).
Axial Length and Prevalence of Open-Angle Glaucoma
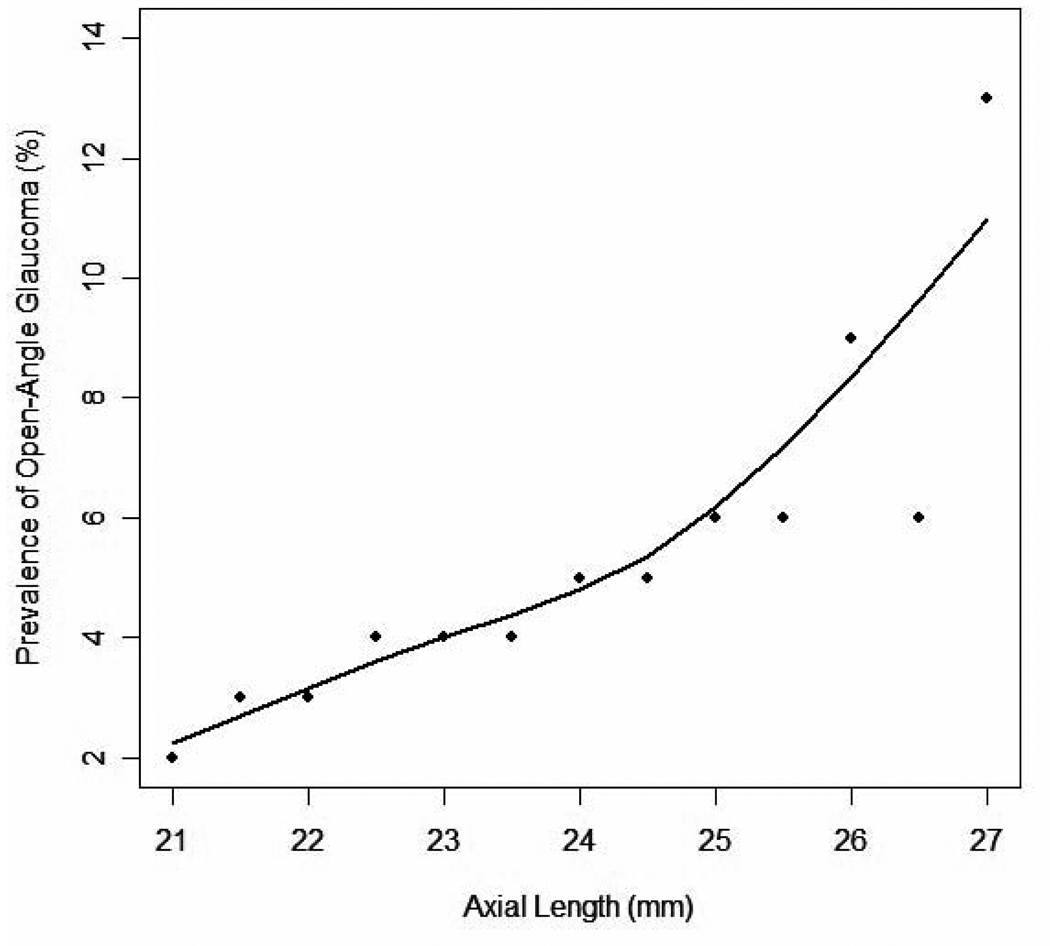
As myopic refractive error and axial length were moderately correlated (r = 0.67), we assessed the relationship between AL and prevalence of OAG in a model that excluded MRE. Taken as a continuous variable, each millimeter longer axial length was associated with a 26% higher prevalence of OAG (OR 1.26, 1.1–1.4, P<0.0001), independent of MRE. After adjusting for covariates, the association remained significant (OR 1.28, 1.15–1.44, P<0.0001).. A LOWESS plot exploring the relationship between the prevalence of OAG and AL revealed that the prevalence of OAG increases exponentially in eyes with an AL greater than 25 mm (Figure 2).
Figure 2.
A LOWESS plot (a locally weighted polynomial regression) of the relationship between axial length and adjusted prevalence of Open Angle Glaucoma in participants in the Los Angeles Latino Eye Study. The LOWESS21 plot uses an iterative, locally weighted, least-squares method to plot the best-fit line and has been adjusted for age, gender, IOP (intraocular pressure), diabetes, family history, nuclear opacity, and corneal power.
Corneal Power and Prevalence of Open-Angle Glaucoma
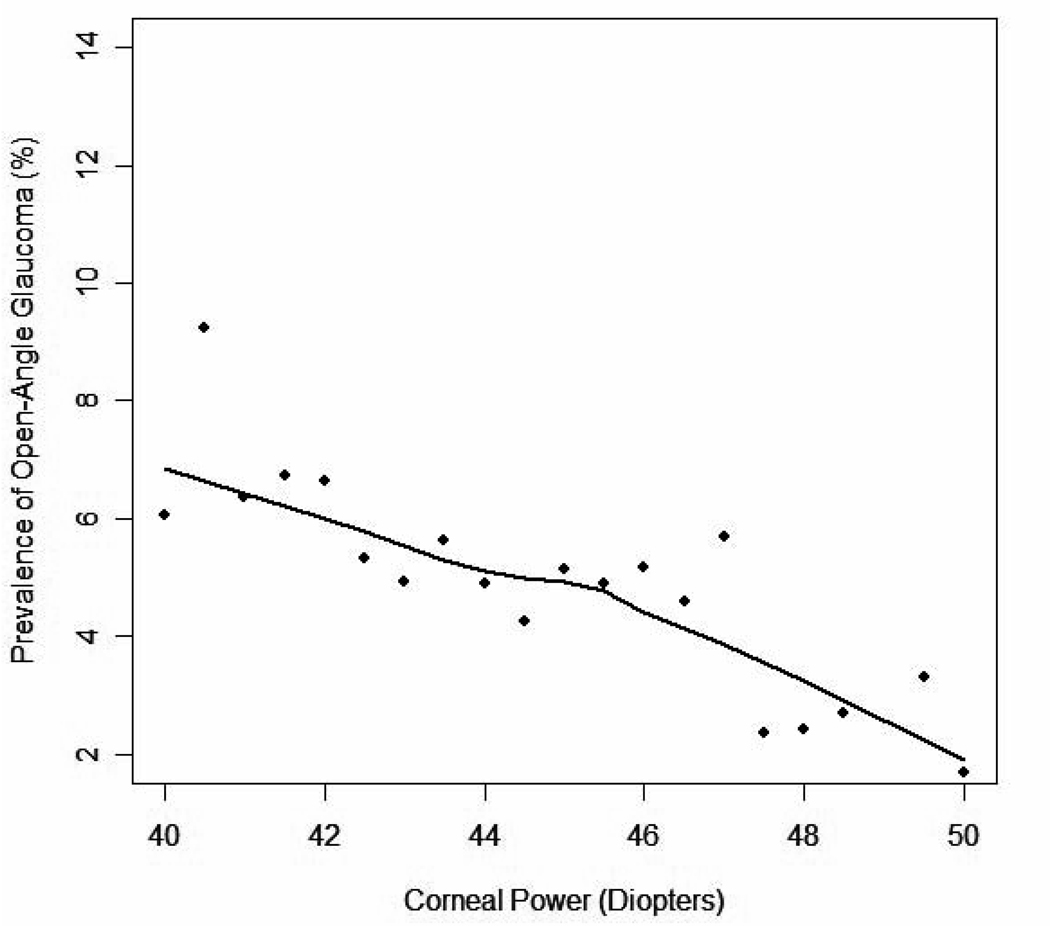
Corneal power was analyzed in a subset analysis of all 2594 participants (41%) who had CP measurements. These individuals were similar to those persons in the whole cohort. Each diopter of decreasing corneal power was associated with a 15% higher prevalence of OAG (OR 1.15, 1.04–1.27, p=0.006). After adjusting for age, gender, IOP, diabetes, family history, NO, and MRE or AL, the association remained significant (In the model with MRE or AL, OR 1.21, 1.08–1.35; P=0.0007 and OR 1.14, 1.01–1.28; P=0.04, respectively)(Table 2). A LOWESS plot exploring the independent relationship between corneal power and adjusted prevalence of OAG reveals that prevalence the rises in a linear manner as corneal power decreases (Figure 3).
Figure 3.
A LOWESS plot (a locally weighted polynomial regression) of the relationship between corneal power and adjusted prevalence of Open Angle Glaucoma in the Los Angeles Latino Eye Study participants. The LOWESS21 plot uses an iterative, locally weighted, least-squares method to plot the best-fit line and has been adjusted for age, gender, intraocular pressure, diabetes, family history, nuclear opacity, and axial length.
Discussion
Our study is the first large population-based study to show that myopia is a risk factor for OAG in Latinos. Participants with myopia of −1 D or worse have an 86% higher risk of developing OAG than do their non-myopic counterparts. Other population-based studies among both white and non-white populations have found an association between myopia and OAG (See Table 3). Our study introduces two novel analyses for elucidating the relationship between OAG and myopia by assessing myopia using a surrogate measure – AL and by adjusting for the impact of nuclear opalescence on myopic refractive error. In addition, we have found a new biometric measure (CP) that for the first time has been shown to have a significant association with the prevalence of OAG.
Table 3.
Comparison of the relationship between myopic refractive error and prevalence of open-angle glaucoma in major population-based studies3–10
| Study | Race/Ethnicity | N | Definition of Myopia | OR (95% CI) |
|---|---|---|---|---|
| Beaver Dam Eye Study3 | White | 4630 | ≤ −1 D | 1.6 (1.1–2.3) |
| ≤−1 and > −3 D | 1.6 (1.1–2.4) | |||
| ≤ −3 D | 1.5 (0.8–2.6) | |||
| Blue Mountains Eye Study4 | White | 3654 | ≤ −1 and > −3 D | 2.3 (1.3–4.1) |
| ≤ −3 D | 3.3 (1.7–6.4) | |||
| Malmö Eye Survey10 | White | 32918 | ≤ −1 D | 2.1* (1.7 – 2.6) |
| Barbados Eye Study8 | Afro-Caribbean | 4036 | < 0.5 D | 1.5 (1.1–2.0) |
|
Aravind Comprehensive Eye Survey5 |
Indian | 5150 | Mild myopia¶ | 2.9 (1.3–6.9) |
| Severe myopia¶ | 3.9 (1.6–9.5) | |||
| Tajimi Study6 | Japanese | 2874 | < −1 and > −3 D | 1.9 (1.0–3.3) |
| ≤ −3 D | 2.6 (1.6–4.4) | |||
| Beijing Eye Study7 | Chinese | 4340 | <−0.5 and ≥ −3 D | 0.8** (0.4–1.6) |
| < −3 and ≥−6 D | 0.5** (0.2–1.0) | |||
| < −6 D | 3.8** (1.8–8.1) | |||
|
Los Angeles Latino Eye Study |
Latino | 5927 | ≤−1 D | 1.8 (1.2–2.8) |
| ≤ −1 and > −3 D | 1.6 (0.9–2.6) | |||
| ≤ −3 D | 2.0 (1.1–3.7) |
CI = confidence interval, D = diopters, OR = odds ratio
Unadjusted odds ratio calculated from data contained in article10
Odds ratio with persons with glaucomatous optic nerves and hyperopia as reference group
Severity of myopia was not defined in the article. Moderate myopia was not significantly associated with prevalence of open-angle glaucoma. Overall Myopia was defined as <0.5D
This study is the first population-based study to evaluate AL directly as a risk factor for having OAG. We found that people with longer eyes are more likely to have OAG. Moreover, the prevalence of glaucoma increases exponentially in eyes longer than 25 mm (Figure 2). Myopic refractive error may be the result of excess CP, NO, or increased AL. By adjusting for CP and NO in addition to known covariates in this population such as age, gender, diabetes, family history, and IOP, we believe the present study provides the most compelling population-based data to date that AL may be one of the most important factors in the higher prevalence of OAG in myopes relative to non-myopes.
Our analysis adjusts for NO by LOCS score -- a factor our previous work has shown to be the primary reason for increased myopic refractive error among Latinos. Because the prevalence of both nuclear opalescence (NO) and OAG is greater in older Latinos compared to younger Latinos, NO should be accounted for when evaluating MRE as an independent risk factor for glaucoma. Absent an adjustment for NO, the relationship between MRE and prevalence of OAG is likely to be artificially higher than its true biological association.
Our study also found a higher prevalence of OAG with increasing MRE. The pattern of increased risk of having OAG in persons with more severe myopia has also been noted in other population-based assessments including the Blue Mountains Eye Study4, the Aravind Eye Study5, the Tajimi Eye Study6, and the Beijing Eye Study.7 However, the Beaver Dam Eye Study3 did not show a dose-response pattern with increasing severity of myopia. These differences could be explained by racial variations, differences in the definition of glaucoma, or differences in the analytic approaches particularly with using different covariates in the multivariate analyses. As expected, there is a high correlation between MRE and AL (r = 0.67). However, the strength of association between AL and glaucoma (OR: 1.25) is less than the adjusted association between MRE and glaucoma (OR: 1.82). This suggests that while AL is an important contributor to the greater risk of having OAG in myopes, other factors related to MRE also contribute to the increased risk of having OAG in myopes.
Many hypotheses have attempted to explain the association between myopia or increased axial length and glaucoma. One explanation is that increased cup-to-disc ratio found in myopes may increase risk for damage to ganglion cell axons23 24 Additionally, alterations in connective tissue and scleral rigidity, as well as exaggerated shearing forces across the lamina cribrosa found in myopes, may contribute to the greater susceptibility of the optic nerve in myopes.25 26 27 However, one must be cautious when making this association as artifactual visual field defects as well as tilted and anomalous appearing optic nerves may be present in persons with myopia. 28
Finally, the Los Angeles Latino Eye Study is the first large population-based study to show a significant association between CP and OAG. Flatter corneal curvature was associated with a higher prevalence of glaucoma in both the univariate and multivariate analyses. Furthermore, CP was shown (Figure 3) to have a linear relationship with prevalence of OAG. One other study, the Tajimi Eye Study6, assessed the relationship between corneal curvature and prevalence of glaucoma but found no significant association. While there are differences between persons with glaucoma the two populations (Mean IOP in Tajimi and LALES were 15.4mmHG and 44.5D, Mean CP in persons with glaucoma in Tajimi and LALES 17.1mmmHG and 43.2D) the reason for a difference in the association between CP and prevalence of glaucoma remains unclear.
Since the Ocular Hypertension Treatment Study published data relating CCT to risk of glaucoma29, many other studies have assessed how not only CCT but other corneal properties might be associated with the risk of having or developing glaucoma. A recent study reported that lower corneal hysteresis, as well as increased AL, was a significant factor in progressive visual field loss in patients with glaucoma.16 Additionally, some studies have indicated a relationship between lower hysteresis/rigidity and flatter corneas.17 18 In our study we found that flatter corneas are associated with increased prevalence of glaucoma. It is possible that the risk for having glaucoma is at least partially due to differences in corneal hysteresis as a more distensible corneoscleral shell may be more sensitive to the stress of IOP and fluctuation. Further studies are needed to examine whether CP is significantly related to a higher risk of having glaucoma even after adjusting for corneal hysteresis or rigidity.
In summary, the data from the Los Angeles Latino Eye Study confirms the relationship between myopia and glaucoma in the Latino population. This association was independent of other risk factors such as age and IOP. In addition, we have shown for the first time that axial length and corneal curvature are two additional factors associated with OAG in a dose-dependent pattern. Both biometric measurements may also have a significant relationship to the biologic mechanisms responsible for the pathogenesis of glaucoma. These factors, both of which are easily measured in a clinical setting should be considered when assessing persons who are at risk of having OAG.
Acknowledgments
Funding and Support: Grants EY11753 and EY03040, the National Eye Institute and National Center on Minority Health and Health Disparities, and an unrestricted grant from Research to Prevent Blindness, New York, New York. RV is a Research to Prevent Blindness Sybil B. Harrington Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Association for Research in Vision and Ophthalmology Meeting 5/8/07.
References
- 1.Knapp A. Glaucoma in myopic eyes. Trans Am Ophthalmol Soc. 1925:61–70. [PMC free article] [PubMed] [Google Scholar]
- 2.Podos SM, Becker B, Morton WR. High myopia and primary open-angle glaucoma. Am J Ophthalmol. 1966;62:1038–1043. [PubMed] [Google Scholar]
- 3.Wong TY, Klein BE, Klein R, et al. Refractive errors, intraocular pressure, and glaucoma in a white population. Ophthalmology. 2003;110:211–217. doi: 10.1016/s0161-6420(02)01260-5. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–2015. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishnan R, Nirmalan PK, Krishnadas R, et al. Glaucoma in a rural population of southern India: the Aravind Comprehensive Eye Survey. Ophthalmology. 2003;110:1484–1490. doi: 10.1016/S0161-6420(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki Y, Iwase A, Araie M, et al. Tajimi Study Group. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study. Ophthalmology. 2006;113:1613–1617. doi: 10.1016/j.ophtha.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Wang Y, Wang S, et al. High myopia and glaucoma susceptibility: the Beijing Eye Study. Ophthalmology. 2007;114:216–220. doi: 10.1016/j.ophtha.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 8.Wu SY, Nemesure B, Leske MC. Glaucoma and myopia. Ophthalmology. 2000;107:1026–1027. doi: 10.1016/s0161-6420(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 9.Leske MC, Connell AM, Wu SY, et al. Barbados Eye Study Group. Risk factors for open-angle glaucoma: the Barbados Eye Study. Arch Ophthalmol. 1995;113:918–924. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- 10.Grødum K, Heijl A, Bengtsson B. Refractive error and glaucoma. Acta Ophthalmol Scand. 2001;79:560–566. doi: 10.1034/j.1600-0420.2001.790603.x. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Census Bureau. [Accessed November 30, 2008];(NP-T5-G) Projections of the resident population by race, Hispanic origin and nativity: middle series, 2050 to 2070. Available at: http://www.census.gov/population/projections/nation/summary/np-t5-g.txt.
- 12.Varma R, Ying-Lai M, Francis BA, et al. Los Angeles Latino Eye Study Group. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–1448. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Tarczy-Hornoch K, Ying-Lai M, Varma R Los Angeles Latino Eye Study Group. Myopic refractive error in the adult Latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2006;47:1845–1852. doi: 10.1167/iovs.05-1153. [DOI] [PubMed] [Google Scholar]
- 14.Hahn S, Azen S, Ying-Lai M, Varma R Los Angeles Latino Eye Study Group. Central corneal thickness in Latinos. Invest Ophthalmol Vis Sci. 2003;44:1508–1512. doi: 10.1167/iovs.02-0641. [DOI] [PubMed] [Google Scholar]
- 15.Shufelt C, Fraser-Bell S, Ying-Lai M, et al. Los Angeles Latino Eye Study Group. Refractive error, ocular biometry, and lens opalescence in an adult population: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2005;46:4450–4460. doi: 10.1167/iovs.05-0435. [DOI] [PubMed] [Google Scholar]
- 16.Congdon N, Broman AT, Bandeen-Roche K, et al. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006;141:868–875. doi: 10.1016/j.ajo.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Francis BA, Hsieh A, Lai MY, et al. Los Angeles Latino Eye Study Group. Effects of corneal thickness, corneal curvature, and intraocular pressure level on Goldmann applanation tonometry and dynamic contour tonometry. Ophthalmology. 2007;114:20–26. doi: 10.1016/j.ophtha.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 18.Lim L, Gazzard G, Chan YH, et al. Cornea biomechanical characteristics and their correlates with refractive error in Singaporean children. Invest Ophthalmol Vis Sci. 2008;49:3852–3857. doi: 10.1167/iovs.07-1670. [DOI] [PubMed] [Google Scholar]
- 19.Varma R, Paz SH, Azen SP, et al. Los Angeles Latino Eye Study Group. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–1131. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Ferris FL, III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 21.Chlyack LT, Jr, Leske MC, McCarthy D, et al. Lens Opacities Classification System II (LOCS II) Arch Ophthalmol. 1989;107:991–997. doi: 10.1001/archopht.1989.01070020053028. [DOI] [PubMed] [Google Scholar]
- 22.Cleveland WS, Grosse E. Computational methods for local regression. Stat Comput. 1991;1:47–62. [Google Scholar]
- 23.Jonas JB, Dichtl A. Optic disc morphology in myopic primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1997;235:627–633. doi: 10.1007/BF00946938. [DOI] [PubMed] [Google Scholar]
- 24.Jonas JB, Gusek GC, Naumann GO. Optic disk morphometry in high myopia. Graefes Arch Clin Exp Ophthalmol. 1988;226:587–590. doi: 10.1007/BF02169209. [DOI] [PubMed] [Google Scholar]
- 25.Cahane M, Bartov E. Axial length and scleral thickness effect on susceptibility to glaucomatous damage: a theoretical model implementing Laplace’s law. Ophthalmic Res. 1992;24:280–284. doi: 10.1159/000267179. [DOI] [PubMed] [Google Scholar]
- 26.Quigley HA. Reappraisal of the mechanisms of glaucomatous optic nerve damage. Eye (Lond) 1987;1:318–322. doi: 10.1038/eye.1987.51. [DOI] [PubMed] [Google Scholar]
- 27.Curtin BJ, Iwamoto T, Renaldo DP. Normal and staphylomatous sclera of high myopia: an electron microscopic study. Arch Ophthalmol. 1979;97:912–915. doi: 10.1001/archopht.1979.01020010470017. [DOI] [PubMed] [Google Scholar]
- 28.Brazitikos PD, Safran AB, Simona F, Zulauf M. Threshold perimetry in tilted disc syndrome. Arch Ophthalmol. 1990;108:1698–1700. doi: 10.1001/archopht.1990.01070140052027. [DOI] [PubMed] [Google Scholar]
- 29.Gordon MO, Beiser JA, Brandt JD, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]