Abstract
The insulin-like growth factors (IGFs; IGF-1 and IGF-2) play central roles in cell growth, differentiation, survival, transformation and metastasis. The biologic effects of the IGFs are mediated by the IGF-1 receptor (IGF-1R), a receptor tyrosine kinase with homology to the insulin receptor (IR). Dysregulation of the IGF system is well recognized as a key contributor to the progression of multiple cancers, with IGF-1R activation increasing the tumorigenic potential of breast, prostate, lung, colon and head and neck squamous cell carcinoma (HNSCC). Despite this relationship, targeting the IGF-1R has only recently undergone development as a molecular cancer therapeutic. As it has taken hold, we are witnessing a robust increase and interest in targeting the inhibition of IGF-1R signaling. This is accentuated by the list of over 30 drugs, including monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs) that are under evaluation as single agents or in combination therapies [1]. The IGF binding proteins (IGFBPs) represent the third component of the IGF system consisting of a class of six soluble secretory proteins. They represent a unique class of naturally occurring IGF antagonists that bind to and sequester IGF-1 and IGF-2, inhibiting their access to the IGF-1R. Due to their dual targeting of the IGFs without affecting insulin action, the IGFBPs are an untapped “third” class of IGF-1R inhibitors. In this commentary, we highlight some of the significant aspects of and prospects for targeting the IGF-1R and describe what the future may hold.
Keywords: IGF-1 receptor, receptor tyrosine kinase, targeted therapeutics, resistance
Introduction
The IGF system is comprised of the IGF-1R, IGF-1, IGF-2, insulin and six soluble IGF-binding proteins (IGFBP-1-6; Fig. 1a). The IGF-1R (and insulin receptor; IR) is a type 1 receptor tyrosine kinase (RTK) initially synthesized as a single chain precursor that is cleaved and disulfide bonded within the Golgi complex to yield a heterodimer and finally a heterotetramer. The immediate downstream target of the IGF-1R tyrosine kinase is the insulin receptor substrate protein (IRS-1; [2]), which uniquely serves as a scaffold for binding downstream targets of the IGF-1R. Once IRS-1 (or IRS-2) becomes tyrosine phosphorylated, effectors are recruited and bind via their SH2 or PTB domains to mediate IGF-1R actions. This differs from other receptor tyrosine kinases (RTKs), which directly bind to their immediate downstream effectors. There are exceptions to this modality with signaling to extracellular signal regulated kinases (Erks 1/2) triggered by the binding of Src homologous and collagen containing (Shc) signaling protein, Shc-66, to the IGF-1R [3].
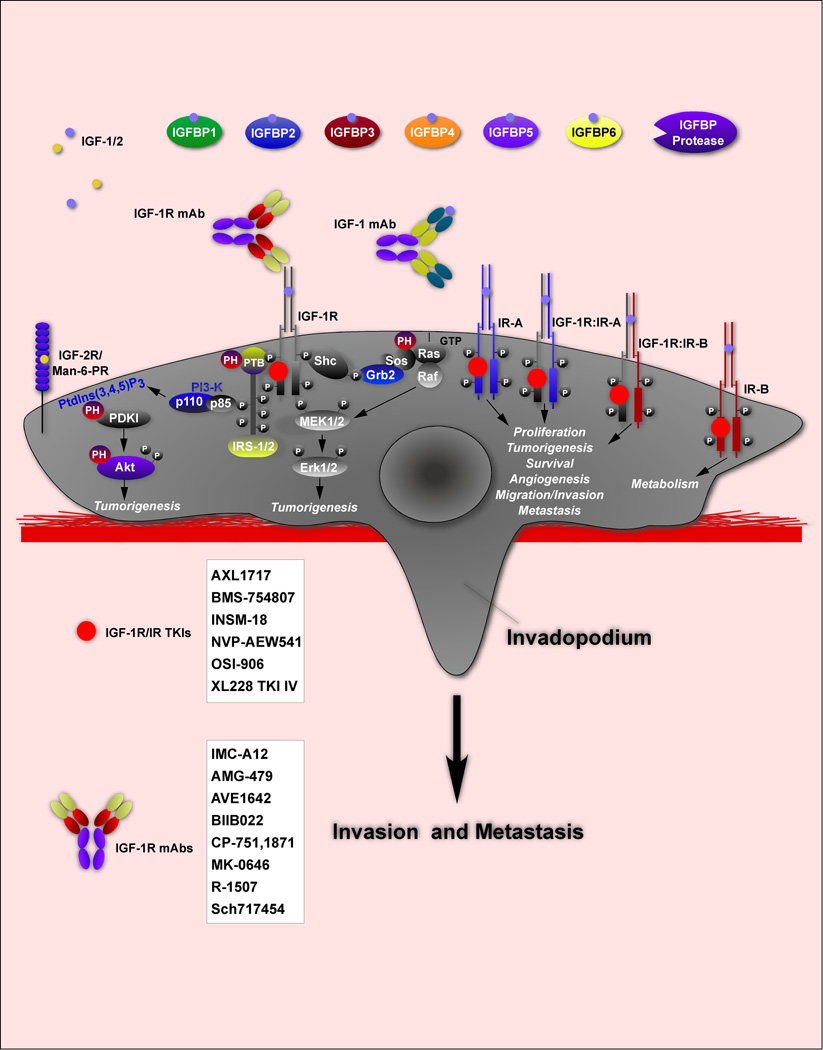
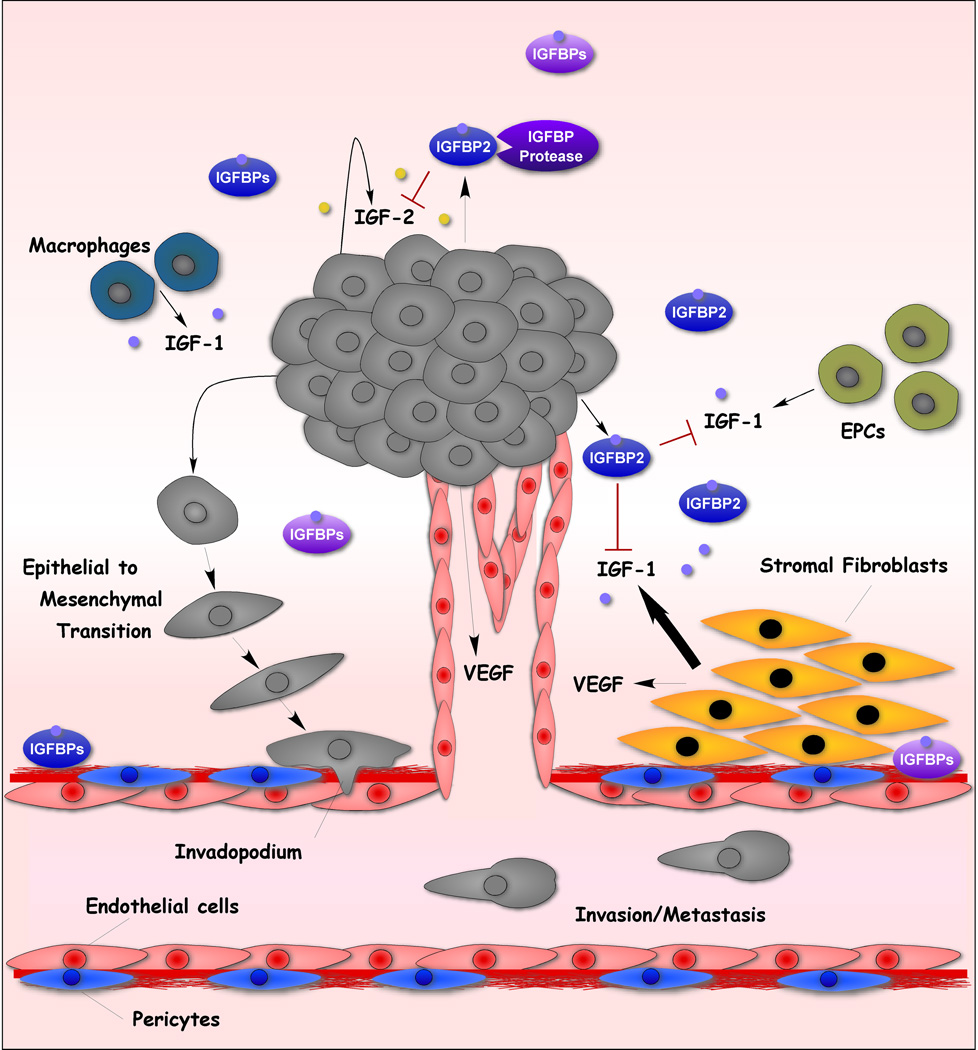
Figure 1. The IGF System in Cancer.
(a) IGF System Components. Shown are the ligands, IGF-1/IGF-2, the receptors, IGF-1R, IR-A, IR-B and hybrid IGF-1R:IR-A and IGF-1R:IR-B receptors and IGFBPs. The mAbs and TKIs targeting the IGF-1R is an incomplete list.
(1b) Targeting the Tumor Microenvironment IGF System. Within the tumor microenvironment, multiple cell types secrete IGF-1 that diffuses to tumor cells to enhance tumorigenesis. IGFBP inhibition of IGF-1 and IGF-2 is well suited for the tumor microenvironment where multiple sources of IGF-1 exist. Also shown is increased angiogenesis mediated by VEGF downstream of IGF-1R action [99, 102, 103].
The IGFBPs consist of a unique class of proteins capable of binding IGF-1 and IGF-2 with higher affinity than their interactions with the IGF-1R, but do not bind to insulin. This sequestration of IGF-1 and IGF-2 results in reduced IGF-1R signaling. It is likely that such fine-tuning of IGF-1R action provided a distinct evolutionary advantage that is unique to the IGF system. The role of the IGF system in normal physiology, development, aging, pathology and cancer provide a rationale in support of such tight control. This, in turn, begs the question “how did the IGFBPs evolve their own IGF-independent actions”, many of which run counter to the effects of inhibiting IGF-1R signaling [4]. The IGFBPs appear to lack cell surface receptors for regulating IGF-independent cellular functions. In this regard, IGFBP-3 and IGFBP-5 are most similar to one another and each have within their structures, a peptide stretch that is cell membrane permeable [5] providing a mechanism whereby these proteins may enter cells to elicit IGF-independent effects. In that context, IGFBP-3 has had the most IGF-independent actions and sites of actions described [6].
Signaling by the IGF-1R and its dysregulation has been noted to be contributory to a variety of diseases including, diabetic retinopathy [7], diabetic nephropathy [8], age-related macular degeneration [9], cardiovascular disease, aging and in a variety of cancers [10]. Due to space considerations, we will focus our discussion on cancer and review the current state of IGF-1R targeting by mAb and TKI approaches. We will then make the case for considering IGFBP-2 as a tractable inhibitor of IGF-1R signaling for clinical use given its ability to block both IGF ligands and to potentially lack the side effects and toxicities common to the current inhibitory drugs.
The IGF system in cancer
It is generally accepted that the growth promoting activities of IGF-1, IGF-2 and insulin are mediated by the IGF-1R and that the IGF-2R serves as a clearance receptor, removing IGF-2 from the cell surface. Elevated IGF-1R expression and activity has been associated with multiple aspects of cancer progression including enhanced carcinogenesis, tumorigenesis, metastasis, resistance to chemotherapeutics and other molecularly targeted drugs and to transformation [11]. In addition to the aforementioned IGF system components, two isoforms of the IR exist, IR-A and IR-B resulting from the alternative splicing of the 22 exon human IR gene [12]. As a result of skipping exon 11 (36 bp) during development and tissue-specific expression, IR-A differs from IR-B by a 12 amino acid truncation (residues 717–728) at the C-terminal end of the α-subunit. While IR-A is widely distributed across tissues, IR-B is present in liver, muscle, adipose and kidney and regulates metabolism and glucose uptake [12]. IR-A is expressed in fetal tissues and in cancer cells, preferentially binds to IGF-2 and regulates growth-promoting actions [13, 14]. Hybrid receptors consisting of IGF-1R:IR-A or IGF-1R:IR-B heterotetramers bind to IGF-2 or insulin and IGF-1, respectively, and participate in cancer cell signaling paradigms [15]. It is in this context that the ability of IGF-1R TKIs to inhibit both the IGF-1R and IR or dual specificity IGFBPs (blocking IGF-1 and IGF-2) would be most beneficial.
An important clue to the essential role of the IGF-1R in cell function was uncovered by Baserga and co-workers [16] who reported that IGF-I signaling was an absolute requirement for viral transformation of cells. These findings and subsequent analyses revealed that many oncogenes require IGF-1R signaling to be effective [2, 17, 18]. This is consistent with the well studied prosurvival signaling properties of the IGF-1R mediated by Akt [19]. Engagement of this signaling pathway increases the propensity of cells harboring cancerous mutations to survive rather than undergo apoptosis.
The growth promoting effects of the IGF-1R are related to the permissive nature of IGF-1R signaling, which supports the microenvironment in a manner that can enhance tumorigenesis. The autocrine and paracrine functions of its two principal ligands appear to be dysregulated in cancer. IGF-2 is imprinted and only expressed from the paternal allele. When imprinting is lost the result is IGF-2 overexpression [20]. The IGF-2 gene is the most overexpressed gene in colorectal cancer [21] consistent with signaling by this ligand being capable of enhancing tumorigenesis [17] including β-cell tumorigenesis [22]. Baserga and coworkers [16] were the first to demonstrate that oncogenic transformation of cells required functional IGF-1Rs, underscoring the importance of autocrine and paracrine IGF-2 and IGF-1 in tumors and the tumor microenvironment, respectively, in supporting tumorigenic progression. An example of the tight regulation of these pathways by the IGFBPs is evident from studies on colonic myofibroblasts where MMP-7 cleavage of IGFBP-5 releases bound IGF-2 which then acts as a myofibroblast mitogen [23].
It has been pointed out that the IGF-1R alone does not mediate growth and transforming activities, but rather the pathway itself, which is administered by IRS-1, signals to growth promoting and antiapoptotic pathways [2]. IRS-1 has 18 potential sites of tyrosine phosphorylation that serve as SH2 domains for docking downstream effectors; constitutively phosphorylated IRS-1 has been found in a number of cancers [24]. This has led to the hypothesis that IRS-1 may be the preferred target for cancer therapeutics, given that it is regulated by IGF-1R, IR, cytokine receptors [25] and EGFRs [11]. It is clear that IRS-1 is a key hub overseeing downstream signaling actions of the IGF-1R. Consistent with its central role in survival signaling, Baserga has referred to IRS-1 as an anti-tumor suppressor acting as an anti-p53 protein [18]. In cancer, there are numerous alterations in signaling pathways that modify the standard signaling nodes. For example, breast cancer cell resistance to estrogen deprivation results in alternative signaling pathways and association of estrogen receptor-α with Shc, Src, EGFR and the IGF-1R [26]. Recent studies on IGF-1R and EGFR signal crosstalk revealed that phosphoinositide-dependent kinase 1 (PDK1) is tyrosine phosphorylated by and binds directly to the IGF-1R [27].
Targeting the IGF-1R: Early reluctance of this strategy
While a number of non-receptor tyrosine kinases (non-RTKs) and RTKs have been targeted since FDA approval of the HER2/neu inhibitory monoclonal antibody (mAb) trastuzumab (Herceptin®) in 1998 and the Bcr-Abl tyrosine kinase inhibitor (TKI) imatinib (Gleevec®) in 2001, targeting the IGF-1R has been slow to catch on. This reluctance has largely been due to concerns that inhibition of this key system that is so crucial to normal physiology might have too many side effects and toxicities. This engendered a view that targeting the IGF-1R was an unattractive proposition [28] or at the very least, a delicate balancing act [29]. This primarily stems from the well known ubiquitous distribution of IGF-1Rs in normal tissues [30] and the inhibition of IR signaling exhibited by IGF-1R directed RTKIs. Given that excellent specificity has been retained for TKIs and notwithstanding the fact that tyrosine kinase domains of RTKs and non-RTKs share upwards of 90% sequence homology and identity, targeting the IGF-1R tyrosine kinase domain has become an active area of research, as has the development of IGF-1R targeting monoclonal antibodies [1].
Why the IGF-1R and why now?
The IGF-1R is an essential regulator of transformation, pro-survival anti-apoptotic signaling and is known to have a role in the resistance to chemotherapeutic and radiation therapies, all of which serve to underscore the attractiveness of its targeting as a means of killing tumor cells. Yet, targeting IGF-1R had been of less interest despite its well known role in contributing to the cancer cell autocrine growth regulation as the second hallmark of cancer and its role in invasion and metastasis, the sixth hallmark [31]. The principal reason was concern that IGF-1R TKIs would also target the IR as these receptors share ∼60% overall sequence identity and ∼80% kinase domain sequence identity [32]. Accordingly, one would predict that inhibition of the IGF-1R using a TKI would likely inhibit IR signaling, resulting in a diabetogenic state characterized by hyperglycemia. Indeed, hyperglycemia is the primary adverse effect of the IGF-1R TKIs currently being evaluated in clinical trials. It has been treated by administering metformin [33]. This introduces an additional rationale for why there should be increased interest in targeting the IGF system. Despite their expression in multiple cancer types, IGF-1Rs were overlooked as viable targets due to their lack of amplification/mutation, unlike other receptors, such as HER2/Neu, which exhibits overexpression and ligand-independent activation as a result of gene amplification [34].
An additional aspect relevant to the targeting of IGF-1Rs is the mounting link between diabetes and cancer incidence as described for colon, pancreatic and post menopausal breast cancer [35]. Moreover, recent epidemiologic studies have raised concern over the use of long-acting insulin (glargine insulin, Lantus®, Sanofi-Aventis) based on its potential to increase cancer incidence [36]. There are two aspects to consider: (1) will IGF-1R TKI therapy result in hyperglycemia and a diabetic state and; (2) does having type 2 diabetes predispose patients to being more cancer-prone [36]. A recent study in Germany comparing diabetic patients taking human insulin, short acting analogs or long-acting glargine insulin revealed a greater than expected increase in cancer incidence in the glargine group compared to those taking human insulin [37]. Related to the diabetes and cancer connection, Goodwin and coworkers [38] reported that high levels of fasting insulin led to poor breast cancer outcomes and that these women were candidates for new and more effective treatment strategies. Here is where the use of alternative drug species, such as IGFBP-2 may provide a benefit. IGFBP-2 is the second most abundant IGFBP in the circulation after IGFBP-3. Its levels are relatively stable and unaffected by meals or glucose levels [39] with serum IGFBP-2 levels being inversely proportional to insulin levels; IGFBP-2 transgenic mouse studies have revealed minimal adverse effects (reviewed in [40], see Anti-diabetic effects of IGFBP-2 below).
Ready, aim, fire: the IGF-1R is a target
Despite the many barriers to targeting the IGF-1R, numerous pharmaceutical and biotechnology companies have developed molecularly targeted reagents against this receptor, primarily employing mAb and TKI approaches (Fig. 1; Table 1; reviewed in [1, 33]). One of the common occurrences seen with mAb and TKI therapies directed against RTKs is toxicity. A case in point for mAbs is trastuzumab (Herceptin), which is associated with congestive heart failure [41], likely the result of targeted receptors being present on cardiac myocytes. The issue of receptor localization also holds true for TKIs as does the fact that these small molecules gain access to the large set of intracellular proteins with which they interact and modify functionally, consistent with their additional toxicities and side effects [42]. Such generalized toxicities have been observed in the early testing of IGF-1R targeted monoclonal antibodies and RTKIs [17, 42] leading to considerable disappointment. This has occurred despite the high targeting/receptor specificity of these agents [1, 33]. The precise mechanisms responsible for these negative outcomes are currently unclear. It is because of these confounding effects, alternate means of inhibiting this receptor should be considered, including the use of the IGFBPs.
Table 1.
Drugs Targeting the IGF System
| (A) Anti-IGF-1R mAbs | ||
|---|---|---|
| Inhibitor | Isotype | Company |
| IMC-A12 | fully human IgG1 (cixutumumab) | Imclone, Inc., NY, NY |
| AMG-479 | fully human IgG1 | Amgen, Thousand Oaks, CA |
| AVE1642 | humanized IgG1 | Sanofi-Aventis, Paris, France |
| BIIB022 | human non-glycosylated IgG4 | Biogen, Cambridge, MA |
| CP-751,1871 | fully human IgG2 (figitumumab) | Pfizer, NY, NY |
| MK-0646 | humanized IgG1 | Merck, Whitehouse Station, NJ |
| R-1507 | fully human IgG1 (robatumumab) | Roche, Basel, Switzerland |
| Sch717454 | fully human IgG1, (19D12) | Schering-Plough, Kenilworth, NJ |
| (B) IGF-1R TKIs | |
|---|---|
| Inhibitor | Company |
| AXL1717 | Axelar, Stockholm, Sweden (picropodophyllin) |
| BMS-754807 | Bristol-Myers Squibb, Princeton, NJ |
| INSM-18 | Insmed, Richmond, VA; IGF-1R and HER2/Neu inhibitor) |
| OSI-906 | OSI Pharmaceuticals, Melville, NY |
| NVP-AEW541 | Novartis, Basel, Switzerland |
| XL228 TKI IV | Exelixis, South San Francisco, CA, (IGF-1R, Src, FGFR, Bcr-Abl) |
| IGF-1R Anti-Sense Oligos/siRNA | Company |
|---|---|
| ATL1101 | Antisense Therapeutics, LTD |
| (C) IGF-1 and IGF-2 | |
|---|---|
| Ligand Sequestration Strategy | Reference |
| rhIGFBP3 (mecasermin) | Insmed, Inc., Richmond, VA (formerly Celtrix) |
| IGFBP-1 | Yee [104, 105] |
| IGFBP-2 | Horney [8]; Kibbey [76] |
| IGFBP-mimetics | Sidhu [106]; Clemmons [107]; Robinson [108] |
| Anti-IGF-1/IGF-2 antibodies | IGF-1/IGF-2 [109]; IGF-2 [110] |
| Soluble IGF-1R (decoy) | IGF-1R 486 stop [111, 112] |
There are currently an estimated 30 drugs in various stages of development that target IGF-1R signaling. Of those targeting the IGF-1R, approximately half are receptor directed mAbs and the other half are TKIs. These represent two distinct classes of drugs of the standard four categories of cancer therapeutics which include (1) small molecule inhibitors, (2) mAbs, (3) natural products from plants and (4) natural products from microorganisms [43], each of which have their pros and cons with respect to selectivity/specificity, toxicities and probability of inducing resistance to long term therapy.
IGF system targeting strategies: IGFBPs are a novel class of natural product IGF antagonists
The prevalence of toxicities to IGF-1R directed mAbs and TKIs begs the question of whether targeting the ligands, IGF-1 and IGF-2 might be a viable alternative with the potential of reduced toxicities other than hyperglycemia. A successful ligand-targeting strategy comes from the vascular endothelial growth factor (VEGF) targeting mAbs, bevacizumab (avastin) and ranibizumab (lucentis, [44]). To date, there has been limited development of IGF-1 or IGF-2 directed antibody therapeutics (Table 1). Along these lines, the IGFBPs are viable alternatives to mAbs in ligand targeting, with the added advantages that they bind to both IGF-1 and IGF-2 and are natural products. Although they are natural products, it is largely unknown whether toxicities would be associated with their therapeutic use, unlike the use of mAbs and receptor tyrosine kinase inhibitors [42]. To date, the only IGFBP applied therapeutically is IGFBP-3, which is available for clinical use both as a single agent (mecasermin) and in a complex with IGF-1 as mecasermin rinfabate or iPlex™ [45]. The formulated complex is designed to reduce the adverse affects of IGF-1 treatment, such as hypoglycemia. The application and proof of concept of introducing mutations into natural proteins for the development of novel, more effective protein-based therapeutics is already well developed with the introduction of shorter acting (lispro insulin) and longer acting insulin molecules (glargine, [46], see Building a better IGFBP-2 below).
IGFBPs as cancer chemopreventive agents
It is worth mentioning a chemoprevention approach to therapeutics, given that many agents have the potential of up-regulating the IGFBPs. Vitamin D increases IGFBP-3 expression [47] and has been under investigation for use in colorectal [48] and prostate cancers [49]. The tumor suppressor p53 induces IGFBP-3 expression [50] providing insight into one of the multiple ways p53 blocks cell growth. Retinoids induce IGFBP-5 [51] and IGFBP-3 [17, 52] as do antiestrogens and TGF-β [53], the flavonoid silibinin from milk thistle [54], the green tea flavonoid, epigallocatechin gallate EGCG [55], and grape seed extract [56]. On the negative side of this approach, IGFBP-2 was shown to be downstream of the PI3K/Akt pathway, with loss of function PTEN mutants increasing IGFBP-2 in glioblastoma and prostate cancer and correlating with a poor prognosis [57]. The opposite preventive approach to up-regulating IGFBP levels is to block their proteolysis by the administration of proteinase inhibitors. An example of the therapeutic use of a proteinase inhibitor is the oral hypoglycemic agent sitagliptin. It is a dipeptidyl peptidase-IV inhibitor that raises the level of circulating incretin by reducing its proteolysis; it is administered either as a monotherapy or in combination with insulin and/or metformin in type 2 diabetics [58].
Building a better IGFBP-2
The IGFBPs, numbered IGFBP-1-6, have molecular masses in the range of 22–31 kDa (216–289 amino acids; [10, 59]). Two important structural features in this protein family are: (1) the presence of three distinct domains (denoted as N-terminal, central and C-terminal) and (2) presence of 16–18 cysteines (20 in IGFBP-4) that are distributed within the N- and C-terminal domains and that form 8–9 disufide bonds [10, 59]. The cysteines are predominantly located in the N-terminal domain (10–12 in number) with the C-terminal domain containing of 6 cysteines. The overall sequence similarity between the IGFBPs ranges from 45–60% with conserved residues present primarily in the N-and C-terminal domains. A considerable variation exists in the central domain thus proving that this domain isn't essential to IGF binding activity
The biological activities of the IGFBPs can be broadly classified as IGF-dependent and IGF-independent [60, 61]. The former involves the modulation of IGF-1/2 activity by competition with the IGF-1R for ligand binding. IGFBPs bind strongly to the IGFs (KD ∼ 300–700 pM) ensuring that all circulating IGF in the blood stream is sequestered and that the access of the IGFs to IGF-1Rs is effectively attenuated when an IGFBP is present. The binding affinity of IGF-1 for the IGFBPs is greater than its affinity towards the IGF-1R [10, 59–61]. However, the relative affinities of IGF-1 and IGF-2 vary for the different IGFBPs with IGFBP-1,3,4 having higher affinities for IGF-1 compared to IGF-2 and vice-versa for IGFBP-2,5,6 [62, 63]. Once bound, ligands are released upon proteolysis of the IGFBPs [64]. Free ligand is then available to subsequently bind to and activate the cell surface receptor. It is now understood that the binding sites for IGF-1 are located in both the N-terminal and C-terminal domains, with the central domain having sites for proteolysis and post-translational modifications [10, 59–61]. The IGF-independent actions of the IGFBPs involve activities that are independent of their IGF-binding properties. A number of additional IGF-independent actions have been reported for the IGFBPs and a comprehensive discussion of these actions have been reported elsewhere (reviewed in [4, 60, 61]. Germane to this discussion is the presence of an RGD motif within the C-terminal domain of IGFBP-2 (and IGFBP-1) that engages of α5β1 integrins, thereby representing the most physiologic and molecularly defined IGF-independent action of IGFBP-2 [65]. The net effect of α5β1 integrin engagement by IGFBP-2 is stimulation of a signaling cascade leading to Akt activation independent of IGF-1R signaling. Accordingly, elevated IGFBP-2 levels are a negative prognostic risk factor for invasive glioma due to this signaling paradigm and its ability to enhance cell migration and invasion [40, 65].
While the biological actions of the IGF1-IGFBP-IGF1R axis have been extensively studied, an understanding of the IGF-IGFBP interactions on a structural level is incomplete. The three-dimensional (3D) structures of full-length IGFBPs have not yet been determined, though considerable structural information is available from studies carried out on individual domains from IGFBP-1-6 [66–73]. A critical challenge in the structural characterization of full-length IGFBPs has been the difficulty in expressing these proteins at levels suitable for NMR/X-ray crystallography analysis. We recently reported the first high-yield expression and structural characterization of full-length recombinant human IGFBP-2 (rhIGFBP-2) in E. coli [74]. This opens up new avenues to carry out structure-based functional studies in this protein family. Table 2 lists the high-resolution 3D structures obtained thus far for the individual domains from different IGFBPs and their complexes with IGF-1 using NMR or X-ray crystallography. Based on their structures and disulfide-bonding pattern, the IGFBPs are known to be thyroglobulin type-1 domain homologues. Both the N-terminal and the C-terminal domains are of α/β type consisting of 30–40% of residues in regular secondary structural elements and 60–70% in unstructured regions. Figure 2 depicts the 3D structure of the N-terminal domain of IGFBP-4 and the C-terminal domain of IGFBP-2 determined by X-ray crystallography and NMR, respectively. Also shown is a ternary complex involving the N-and C-terminal domains of IGFBP-4 and IGF-1 [72]. The central or ‘linker’ domain in all IGFBPs is largely unstructured and contains sites of post-translational modification and proteolysis.
Table 2.
Current IGFBP 3D structures and their complexes with IGF-1
| Protein | Structural Domain |
Complex | Experimental Method |
PDB code |
|---|---|---|---|---|
| IGFBP-1 | C-domain | Uncomplexed | X-ray | 1ZT3, 1ZT5 |
| IGFBP-2 | C-domain | Uncomplexed | NMR | 2H7T |
| IGFBP-4 | N-domain | Complex with IGF-1 |
X-ray | 2DSP |
| 1WQJ | ||||
| Ternary complex with IGF-1 and C- domain of IGFBP-1 |
X-ray | 2DSQ | ||
| N- and C-domain | Ternary complex with IGF-1 |
X-ray | 2DSR | |
| IGFBP-5 | N-domain (Residues 58–111) |
Complex with IGF-1 |
X-ray | 1H59 |
| N-domain (Residues 40–92) |
Uncomplexed | NMR | 1BOE | |
| IGFBP-6 | N-domain (Residues 25–69) |
Uncomplexed | NMR | 2JM2 |
| C-domain | Uncomplexed | NMR | 1RMJ | |
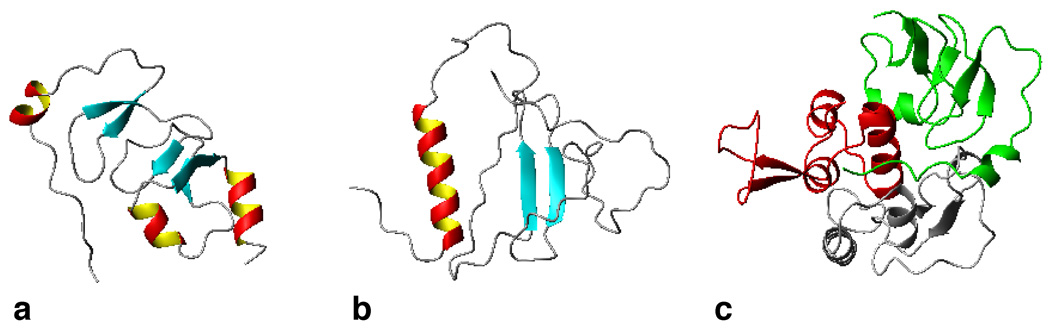
Figure 2. IGFBP Structures Obtained by NMR or X-ray Crystallography.
(a) 3D structure of the N-terminal domain of IGFBP-4 (PDB code: 2DSP) and (b) the C-terminal domain of IGFBP-2 (PDB code: 2H7T). (c) Also shown is a ternary complex involving the N-and C-terminal domains of IGFBP-4 with IGF-1 (PDB code: 2DSR).
Studies involving site directed mutagenesis have identified key residues in IGFBPs that are required for binding the IGFs (reviewed in [75]). These studies have also revealed that both the N- and C-terminal domains in IGFBPs are essential for IGF-1/2 binding. It has been shown that truncated IGFBP molecules lacking the N- or C-terminal domains have considerably reduced binding affinity for the IGFs compared to the intact full-length protein. One such study in our laboratories focused on the binding affinities of truncation mutants of IGFBP-2 for IGF-1 [76]. This study has provided valuable insights into IGF-binding and is briefly discussed below.
Analysis of IGF-1 binding by IGFBP-2 truncation mutants
All six IGFBPs contain a CWCV motif in their C-terminal domain. In a previous study involving IGFBP-2, residues both up- and down-stream of this motif were identified to be involved in IGF-1 binding [77]. Hence to test the contribution of the polypeptide segment of downstream of the CWCV motif in IGFBP-2 (residues 247–250), three different truncation mutants comprising residues 1–190, 1–248 and 249–289 were cloned and expressed in E. coli [76]. Although high (KD = 7 nM), IGFBP-21–248 exhibited a 20 fold reduction in binding affinity compared to the full-length IGFBP-2 (KD = 0.35 nM) and IGFBP-21–190 had a binding affinity indistinguishable from that of IGFBP-21–248. An important result was borne out by kinetic studies, which revealed that dissociation of bound IGF-1 from IGFBP-21–248 was considerably faster than the full-length protein. This implied that the region downstream of the CWCV motif provides stability to the IGFBP-2/IGF-1 complex, accounting for l-length IGFBP-2 as compared to IGFBP-21–248. .
Self-assembly of IGFBP-2 truncation mutants into nanotubular structures
To further understand the structural basis of the above observation, IGFBP-2249–289 was subjected to structural analysis using NMR spectroscopy. While the native form of IGFBP-2249–289 has two cysteines (C-249 and C-270), the polypeptide fragment used in our study had an additional cysteine at position 281 (R281C). Under reducing conditions such as in the presence of 1 mM β-mercaptoethanol the protein remained a monomer. However, upon removal of β-mercaptoethanol by ultrafiltration, it was found to spontaneously self-assemble into nanotubular structures several micrometers long [78]. This was investigated in detail using transmission electron microscopy, NMR and fluorescence microscopy and found to be the result of inter-molecular disulfide bonds formed due to the presence of an odd number of cysteines in the polypeptide fragment. This observation opens up avenues for novel biomedical applications and at the same time raises some important questions. For instance, is it possible that polypeptides resulting from proteolysis of IGFBPs also undergo such ordered aggregation if they end up with an odd number of cysteines? Could IGFs play any role in stabilizing or de-stabilizing such aggregates influencing, in turn, the efficiency of proteolysis? There are many instances where the IGFBP fragments resulting from proteolysis contain an odd number of cysteine residues [64]. The structural properties of such IGFBP fragments remain to be investigated.
A potential application of the nanotubes described above lies in targeting integrin positive tumors, taking advantage of the fact that IGFBP-2249–289 contains an RGD motif, known to be recognized by α5β1 integrin [65]. In recent years, highly efficient tumor targeting systems based on carbon nanotubes have been proposed [79, 80]. In these applications, soluble carbon nanotubes were functionalized with an RGD peptide in order to target cell surface integrins. The presence of nanotubes in vivo is probed using Raman imaging [80]. The IGFBP-2249–289 nanotubes we have described may be similarly used and their inherent tyrosine fluorescence [78] exploited for detection or monitoring. Work in this direction is currently in progress in our laboratories.
IGF-antagonist based cancer therapeutics
A few peptides have been developed which mimic the IGF binding domain of IGFBPs [81]. One of these peptides blocked IGF-1 stimulated insulin receptor autophosphorylation [81]. This peptide had a structure similar to the IGF-binding domain of IGFBP-5 [66]. Further developments can benefit from structural details of how the N-and the C-terminal domains of IGFBPs together bind IGF thereby allowing the use of rational and combinatorial protein engineering approaches. A recent review on developing therapeutic proteins by engineering ligand–receptor interactions discusses these approaches [82]. The recent structural studies on the ternary complex of N- and C-terminal domains of IGFBP-4 with IGF-1 are a significant step in this direction (Fig. 2; [72]). However, two major challenges will have to be tackled for developing IGFBPs as IGF-antagonist based therapeutics: (1) the IGFBPs should be protease resistant so as to be more effective in inhibiting IGF-1R signaling and (2) IGF-independent actions should be handled so that they do not stall the beneficial effects of IGF-1 binding, including integrin engagement by IGFBP-1 and IGFBP-2 through their RGD motifs. The first objective can be achieved by developing protease-resistant forms of IGFBP. A number of proteases regulate IGFBP levels extracellularly, dissociating the IGFBP-IGF complex thereby increasing IGF-1/2 available for interacting with the IGF-1R [64]. This is based on the differential effects of IGFBP-3 in tumor vs. normal prostate cells, wherein IGF-1 bioavailability is increased via PSA-mediated IGFBP proteolysis [83]. Thus, there is a need for understanding the structural mechanisms involved in proteolysis in order to develop protease resistant IGFBPs with enhanced IGF-inhibitory actions. The second objective is more difficult as a number of IGF-1 independent actions have been reported [60, 61]. However, a first step in the case of IGFBP-2 is to modify its RGD motif by mutagenesis to abrogate integrin binding capacity.
Increasing the IGF binding affinity of the IGFBPs
Developing IGFBPs as IGF-antagonists for cancer therapeutics also leads to the question of whether the IGFBP binding affinity for the IGFs can be further enhanced. One starting point for engineering improved antagonists is to introduce mutations with the goal of enhancing their IGF binding affinity [82]. An alternative approach is to develop novel chimeric protein constructs containing the N- and C- terminal domains taken from different IGFBPs. This exploits the variations in affinity of N- and C-terminal domains of different IGFBPs in binding IGFs [75]. For instance, the C-terminal domain of IGFBP-2 can be combined with the N-terminal domain of IGFBP-3 or vice-versa. A chimeric protein produced this way may bind to IGF-1 more efficaciously than either individual protein. Chimeric proteins have been used in the past in drug development to impart properties from each of the parent proteins to the resulting drug [84].
Anti-diabetic effects of IGFBP-2
Little is known about the potential toxicities associated with the use of IGFBPs as therapeutic agents in cancer or other diseases. What is known comes from studies in mice where IGFBP2 overexpression can promote glioma development and progression [85]. Although this may be true for other cancers [86], this effect is likely due to integrin engagement by the IGFBP-2 RGD motif. However, IGFBP3 was found to suppress cancer, by inhibiting prostate cancer xenograft growth [87]. IGFBP1 and IGFBP2 impact metabolic regulation [40] with the levels of these two proteins decreasing in type 2 diabetes as a result of hyperinsulinemia [88]. Consistent with this, compared to wild type animals, IGFBP-2 transgenic mice exhibit a lean phenotype, are protected against developing age-related glucose intolerance, insulin resistance and high blood pressure and are resistant to developing obesity and insulin resistance when fed high-energy or high-fat diets [89]. In addition to being obesity-resistant, these animals have decreased leptin levels, all of which suggest that IGFBP2 may be a factor in obesity prevention [89]. A recent microarray analysis of leptin action revealed up-regulation of IGFBP2 in the livers of leptin vs. vehicle treated ob/ob mice [90]. This observation was further validated by acute IGFBP2 overexpression in ob/ob, type 1 and type 2 diabetic mice, using adenoviral infection, and in all cases plasma glucose and insulin levels were reduced. This improved glycemic control, which was independent of weight loss and food intake, was also observed in leptin-resistant animals, confirming that IGFBP2 acts downstream of leptin action [90]. These findings provide additional rationale for considering IGFBP-2 as a cancer therapeutic by underscoring the reduced diabetic symptoms IGFBP-2 therapy would likely have.
Conclusion and perspectives
It is clear from the increased number of drugs under development, that targeting the IGF-1R/IGF system is a viable approach to therapeutics for cancer and other diseases. The success of these approaches will be related to their limited toxicities and lack of induction of significant resistance to treatment. This raises the question of how best to monitor patients for therapeutic outcome and whether pre-selecting patients for those who would be predictably more sensitive to therapeutic intervention. Given that IRS-1 involvement is an absolute requirement for IGF-1R signaling in cancer, it has been suggested that it may serve as a biomarker. The question of utility and cost effectiveness of biomarker analyses in phase I clinical trials has recently been the subject of multiple reviews [91]. In addition to monitoring biomarkers during therapy, there are also predictive biomarkers to consider. As we progress into the era of personalized medicine, pre-selection of patient populations representing those expected to be the highest responders to a given drug regimen will be possible [92–95]. In this context, Yee and colleagues demonstrated that IRS protein expression is required for mAb down-regulation of the IGF-1R to yield an inhibitory response [96]. IRS-1 has been considered to be the biomarker of choice in cancer, with its presence indicating a cell’s sensitivity to IGF-1R targeting drugs [2]. Recently, tissue microarray analysis of patients with invasive breast cancer revealed that elevated levels of phosphorylated IGF-1R/IR were prognostic of poor survival, while total IGF-IR levels were not [97] supporting the contention that evaluating phosphorylated IGF-1R and IR might serve as a predictive biomarker for response to IGF-1R TKIs.
Finally, resistance to mAbs and RTKIs targeting the IGF-1R including compensatory activation of other growth factor RTK pathways, such as the EGFR pathway [98], have been and will continue to be present on the horizon. As newer drugs and potential combination therapies based on the identification of novel molecular targets come into existence, the toxicities of many of the current drugs may become less problematic. Additional, novel targeting opportunities exist based on crosstalk that occurs between IGF-1Rs and EGFRs [99], VEGFRs [100] and highly druggable GPCRs [101]. There is much to look forward to in the context of targeting the IGF system and developing personalized therapies to reduce the metastatic potential of many cancers.
Acknowledgments
Financial support – Supported in part, by NIH R01 CA-134845, P30 CA138313, the American Health Assistance Foundation Award number M2009086 (SAR) and the Association for International Cancer Research (HSA).
Abbreviations
- EGCG
epigallocatechin gallate
- EGFR
epidermal growth factor receptor
- Erk
extracellular signal regulated kinase
- GPCR
G-protein coupled receptor
- HNSCC
head and neck squamous cell carcinoma
- IGF-1
Insulin-like growth factor-1
- IGFBP
IGF binding protein
- IR
insulin receptor
- IRS
insulin receptor substrate
- mAb
monoclonal antibody
- MMP
matrix metalloproteinase
- NMR
nuclear magnetic resonance
- PDB
protein data bank
- PDK1
phosphoinositide-dependent kinase 1
- PI3K
phosphatidylinositol 3-kinase
- PSA
prostate specific antigen
- PTEN
Phosphatase and tensin homolog deleted on chromosome 10
- RGD
arginyl-glycyl-aspartyl tripeptide
- RTK
receptor tyrosine kinase
- RTKI
receptor tyrosine kinase inhibitor
- Shc
Src homologous and collagen containing
- TGF-β
transforming growth factor-β
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weroha S, Haluska P. IGF-1 receptor inhibitors in clinical trials—Early lessons. J Mammary Gland Biol Neoplasia. 2008;13:471–483. doi: 10.1007/s10911-008-9104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baserga R. The insulin receptor substrate-1: A biomarker for cancer? Exp Cell Res. 2009;315:727–732. doi: 10.1016/j.yexcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricort J-M. Insulin-like growth factor binding protein (IGFBP) signalling. Growth Horm IGF Res. 2004;14:277–286. doi: 10.1016/j.ghir.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Goda N, Tenno T, Inomata K, Shirakawa M, Tanaka T, Hiroaki H. Intracellular protein delivery activity of peptides derived from insulin-like growth factor binding proteins 3 and 5. Exp Cell Res. 2008;314:2352–2361. doi: 10.1016/j.yexcr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Yamada PM, Lee K-W. Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am J Physiol Cell Physiol. 2009;296:C954–C976. doi: 10.1152/ajpcell.00598.2008. [DOI] [PubMed] [Google Scholar]
- 7.Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 8.Horney MJ, Shirley DW, Kurtz DT, Rosenzweig SA. Elevated glucose increases mesangial cell sensitivity to insulin-like growth factor I. Am J Physiol. 1998;274:F1045–F1053. doi: 10.1152/ajprenal.1998.274.6.F1045. [DOI] [PubMed] [Google Scholar]
- 9.Slomiany MG, Rosenzweig SA. IGF-1-induced VEGF and IGFBP-3 secretion correlates with increased HIF-1 alpha expression and activity in retinal pigment epithelial cell line D407. Invest Ophthalmol Vis Sci. 2004;45:2838–2847. doi: 10.1167/iovs.03-0565. [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig SA. What's new in the IGF-binding proteins? Growth Horm IGF Res. 2004;14:329–336. doi: 10.1016/j.ghir.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knowlden JM, Jones HE, Barrow D, Gee JM, Nicholson RI, Hutcheson IR. Insulin receptor substrate-1 involvement in epidermal growth factor receptor and insulin-like growth factor receptor signalling: implication for Gefitinib ('Iressa') response and resistance. Breast Cancer Res Treat. 2008;111:79–91. doi: 10.1007/s10549-007-9763-9. [DOI] [PubMed] [Google Scholar]
- 12.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 13.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sciacca L, Prisco M, Wu A, Belfiore A, Vigneri R, Baserga R. Signaling differences from the A and B isoforms of the insulin receptor (IR) in 32D cells in the presence or absence of IR substrate-1. Endocrinol. 2003;144:2650–2658. doi: 10.1210/en.2002-0136. [DOI] [PubMed] [Google Scholar]
- 15.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 16.Sell C, Rubini M, Rubin R, Liu J, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993;90:11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollak M. Targeting insulin and insulin-like growth factor signalling in oncology. Curr Opin Pharmacol. 2008;8:384–392. doi: 10.1016/j.coph.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Baserga R. Customizing the targeting of IGF-1 receptor. Future Oncol. 2009;5:43–50. doi: 10.2217/14796694.5.1.43. [DOI] [PubMed] [Google Scholar]
- 19.Tamburini J, Chapuis N, Bardet V, Park S, Sujobert P, Willems L, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–382. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 20.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 22.Christofori G, Naik P, Hanahan D. Deregulation of both imprinted and expressed alleles of the insulin-like growth factor 2 gene during beta-cell tumorigenesis. Nat Genet. 1995;10:196–201. doi: 10.1038/ng0695-196. [DOI] [PubMed] [Google Scholar]
- 23.Hemers E, Duval C, McCaig C, Handley M, Dockray GJ, Varro A. Insulin-like growth factor binding protein-5 is a target of matrix metalloproteinase-7: Implications for epithelial-mesenchymal signaling. Cancer Res. 2005;65:7363–7369. doi: 10.1158/0008-5472.CAN-05-0157. [DOI] [PubMed] [Google Scholar]
- 24.Chang Q, Li Y, White MF, Fletcher JA, Xiao S. Constitutive activation of insulin receptor substrate 1 is a frequent event in human tumors: therapeutic implications. Cancer Res. 2002;62:6035–6038. [PubMed] [Google Scholar]
- 25.Yenush L, White MF. The IRS-signalling system during insulin and cytokine action. Bioessays. 1997;19:491–500. doi: 10.1002/bies.950190608. [DOI] [PubMed] [Google Scholar]
- 26.Song RX, Chen Y, Zhang Z, Bao Y, Yue W, Wang JP, et al. Estrogen utilization of IGF-1-R and EGF-R to signal in breast cancer cells. J Steroid Biochem Mol Biol. 2009;118:219–230. doi: 10.1016/j.jsbmb.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberobello AT, D'Esposito V, Marasco D, Doti N, Ruvo M, Bianco R, et al. Selective disruption of insulin-like growth factor (IGF)-1 signalling via phosphoinositide-dependent kinase-1 prevents the protective effect of IGF-1 on human cancer cell death. J Biol Chem. 2009 doi: 10.1074/jbc.M109.097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartog H, Wesseling J, Boezen HM, van der Graaf WTA. The insulin-like growth factor 1 receptor in cancer: Old focus, new future. Eur J Cancer. 2007;43:1895–1904. doi: 10.1016/j.ejca.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Kamrava M, Gius D, Casagrande G, Kohn E. Will targeting insulin growth factor help us or hurt us?: An oncologist's perspective. Ageing Res Rev. 2009 doi: 10.1016/j.arr.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yee D. Targeting insulin-like growth factor pathways. Br J Cancer. 2006;94:465–468. doi: 10.1038/sj.bjc.6602963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 32.Wu J, Li W, Craddock BP, Foreman KW, Mulvihill MJ, Ji Q-s, et al. Small-molecule inhibition and activation-loop trans-phosphorylation of the IGF1 receptor. EMBO J. 2008;27:1985–1994. doi: 10.1038/emboj.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28:3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 34.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 35.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 36.Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52:1699–1708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- 37.Hemkens L, Grouven U, Bender R, Günster C, Gutschmidt S, Selke G, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 39.Clemmons DR, Snyder DK, Busby WH., Jr Variables controlling the secretion of insulin-like growth factor binding protein-2 in normal human subjects. J Clin Endocrinol Metab. 1991;73:727–733. doi: 10.1210/jcem-73-4-727. [DOI] [PubMed] [Google Scholar]
- 40.Wheatcroft SB, Kearney MT. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends Endocrinol Metab. 2009;20:153–162. doi: 10.1016/j.tem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Orphanos GS, Ioannidis GN, Ardavanis AG. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 2009;48:964–970. doi: 10.1080/02841860903229124. [DOI] [PubMed] [Google Scholar]
- 42.Garber K. IGF-1: Old Growth Factor Shines as New Drug Target. J Natl Cancer Inst. 2005;97:790–792. doi: 10.1093/jnci/97.11.790. [DOI] [PubMed] [Google Scholar]
- 43.Bailly C. Ready for a comeback of natural products in oncology. Biocheml Pharmacol. 2009;77:1447–1457. doi: 10.1016/j.bcp.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Lien S, Lowman HB. Therapeutic anti-VEGF antibodies. Handb Exp Pharmacol. 2008:131–150. doi: 10.1007/978-3-540-73259-4_6. [DOI] [PubMed] [Google Scholar]
- 45.Kemp SF, Fowlkes JL, Thrailkill KM. Efficacy and safety of mecasermin rinfabate. Expert Opin Biol Ther. 2006;6:533–538. doi: 10.1517/14712598.6.5.533. [DOI] [PubMed] [Google Scholar]
- 46.Vajo Z, Fawcett J, Duckworth WC. Recombinant DNA Technology in the Treatment of Diabetes: Insulin Analogs. Endocrine Reviews. 2001;22:706–717. doi: 10.1210/edrv.22.5.0442. [DOI] [PubMed] [Google Scholar]
- 47.Peng L, Malloy PJ, Feldman D. Identification of a functional vitamin D response element in the human insulin-like growth factor binding protein-3 promoter. Mol Endocrinol. 2004;18:1109–1119. doi: 10.1210/me.2003-0344. [DOI] [PubMed] [Google Scholar]
- 48.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz GG. Vitamin D and intervention trials in prostate cancer: From theory to therapy. Ann Epidemiol. 2009;19:96–102. doi: 10.1016/j.annepidem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR, et al. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 51.Higo H, Duan C, Clemmons DR, Herman B. Retinoic acid inhibits cell growth in HPV negative cervical carcinoma cells by induction of insulin-like growth factor binding protein-5 (IGFBP-5) secretion. Biochem Biophys Res Commun. 1997;239:706–709. doi: 10.1006/bbrc.1997.7499. [DOI] [PubMed] [Google Scholar]
- 52.Han GR, Dohi DF, Lee HY, Rajah R, Walsh GL, Hong WK, et al. All-trans-retinoic acid increases transforming growth factor-beta2 and insulin-like growth factor binding protein-3 expression through a retinoic acid receptor-alpha-dependent signaling pathway. J Biol Chem. 1997;272:13711–13716. doi: 10.1074/jbc.272.21.13711. [DOI] [PubMed] [Google Scholar]
- 53.Gucev ZS, Oh Y, Kelley KM, Rosenfeld RG. Insulin-like growth factor binding protein 3 mediates retinoic acid- and transforming growth factor beta2-induced growth inhibition in human breast cancer cells. Cancer Res. 1996;56:1545–1550. [PubMed] [Google Scholar]
- 54.Singh RP, Deep G, Blouin MJ, Pollak MN, Agarwal R. Silibinin suppresses in vivo growth of human prostate carcinoma PC-3 tumor xenograft. Carcinogenesis. 2007;28:2567–2574. doi: 10.1093/carcin/bgm218. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu M, Deguchi A, Hara Y, Moriwaki H, Weinstein IB. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem Biophys Res Commun. 2005;334:947–953. doi: 10.1016/j.bbrc.2005.06.182. [DOI] [PubMed] [Google Scholar]
- 56.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 57.Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, et al. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci U S A. 2007;104:5563–5568. doi: 10.1073/pnas.0609139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pratley RE. Overview of glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors for type 2 diabetes. Medscape J Med. 2008;10:171. [PMC free article] [PubMed] [Google Scholar]
- 59.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocrine Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 60.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–E976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 61.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocrine Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 62.Roghani MLC, Zapf J, Povoa G, Binoux M. Two insulin-like growth factor (IGF)-binding proteins are responsible for the selective affinity for IGF-II of cerebrospinal fluid binding proteins. J Clin Endocrinol Metab. 1991;73:658–666. doi: 10.1210/jcem-73-3-658. [DOI] [PubMed] [Google Scholar]
- 63.Kiefer MC, Schmid C, Waldvogel M, Schlapfer I, Futo E, Masiarz FR, et al. Characterization of recombinant human insulin-like growth factor binding proteins 4, 5, and 6 produced in yeast. J Biol Chem. 1992;267:12692–12699. [PubMed] [Google Scholar]
- 64.Bunn RC, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab. 2003;14:176–181. doi: 10.1016/s1043-2760(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 65.Jones JI, Gockerman A, Busby WH, Jr, Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci U S A. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalus W, Zweckstetter M, Renner C, Sanchez Y, Georgescu J, Grol M, et al. Structure of the IGF-binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): implications for IGF and IGF-I receptor interactions. EMBO J. 1998;17:6558–6572. doi: 10.1093/emboj/17.22.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeslawski W, Beisel HG, Kamionka M, Kalus W, Engh RA, Huber R, et al. The interaction of insulin-like growth factor-I with the N-terminal domain of IGFBP-5. EMBO J. 2001;20:3638–3644. doi: 10.1093/emboj/20.14.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Headey SJ, Leeding KS, Norton RS, Bach LA. Contributions of the N- and C-terminal domains of IGF binding protein-6 to IGF binding. J Mol Endocrinol. 2004;33:377–386. doi: 10.1677/jme.1.01547. [DOI] [PubMed] [Google Scholar]
- 69.Siwanowicz I, Popowicz GM, Wisniewska M, Huber R, Kuenkele K-P, Lang K, et al. Structural basis for the regulation of insulin-like growth factors by IGF binding proteins. Structure. 2005;13:155–167. doi: 10.1016/j.str.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Sala A, Capaldi S, Campagnoli M, Faggion B, Labo S, Perduca M, et al. Structure and properties of the C-terminal domain of Insulin-like growth factor-binding protein-1 isolated from human amniotic fluid. J Biol Chem. 2005;280:29812–29819. doi: 10.1074/jbc.M504304200. [DOI] [PubMed] [Google Scholar]
- 71.Kuang Z, Yao S, Keizer DW, Wang CC, Bach LA, Forbes BE, et al. Structure, dynamics and heparin binding of the C-terminal domain of insulin-like growth factor-binding protein-2 (IGFBP-2) J Mol Biol. 2006;364:690–704. doi: 10.1016/j.jmb.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Sitar T, Popowicz GM, Siwanowicz I, Huber R, Holak TA. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci USA. 2006;103:13028–13033. doi: 10.1073/pnas.0605652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chandrashekaran IR, Yao S, Wang CC, Bansal PS, Alewood PF, Forbes BE, et al. The Nterminal subdomain of insulin-like growth factor (IGF) binding protein 6. Structure and interaction with IGFs. Biochemistry. 2007;46:3065–3074. doi: 10.1021/bi0619876. [DOI] [PubMed] [Google Scholar]
- 74.Swain M, Slomiany MG, Rosenzweig SA, Atreya HS. High-yield expression and structural characterization of recombinant human insulin-like growth factor binding protein-2. Archives of Biochemistry and Biophysics. 2010 doi: 10.1016/j.abb.2010.06.006. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clemmons DR. Use of mutagenesis to probe IGF-binding protein structure/function relationships. Endocrine Rev. 2001;22:800–817. doi: 10.1210/edrv.22.6.0449. [DOI] [PubMed] [Google Scholar]
- 76.Kibbey MM, Jameson MJ, Eaton EM, Rosenzweig SA. Insulin-like growth factor binding protein-2: contributions of the C-terminal domain to insulin-like growth factor-1 binding. Mol Pharmacol. 2006;69:833–845. doi: 10.1124/mol.105.016998. [DOI] [PubMed] [Google Scholar]
- 77.Horney MJ, Evangelista CA, Rosenzweig SA. Synthesis and characterization of insulinlike growth factor (IGF)-1 photoprobes selective for the IGF-binding proteins (IGFBPs). photoaffinity labeling of the IGF-binding domain on IGFBP-2. J Biol Chem. 2001;276:2880–2889. doi: 10.1074/jbc.M007526200. [DOI] [PubMed] [Google Scholar]
- 78.Swain M, Thirupathi R, Krishnarjuna B, Eaton EM, Kibbey MM, Rosenzweig SA, et al. Spontaneous and reversible self-assembly of a polypeptide fragment of insulin-like growth factor binding protein-2 into fluorescent nanotubular structures. Chem Commun (Camb) 2010;46:216–218. doi: 10.1039/b915775a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 80.Zavaleta C, de la Zerda A, Liu Z, Keren S, Cheng Z, Schipper M, et al. Noninvasive Raman spectroscopy in living mice for evaluation of tumor targeting with carbon nanotubes. Nano Lett. 2008;8:2800–2805. doi: 10.1021/nl801362a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deshayes K, Schaffer ML, Skelton NJ, Nakamura GR, Kadkhodayan S, Sidhu SS. Rapid identification of small binding motifs with high-throughput phage display: discovery of peptidic antagonists of IGF-1 function. Chemistry & Biol. 2002;9:495–505. doi: 10.1016/s1074-5521(02)00129-1. [DOI] [PubMed] [Google Scholar]
- 82.Jones DS, Silverman AP, Cochran JR. Developing therapeutic proteins by engineering ligand-receptor interactions. Trends Biotechnol. 2008;26:498–505. doi: 10.1016/j.tibtech.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 83.Miyamoto S, Yano K, Sugimoto S, Ishii G, Hasebe T, Endoh Y, et al. Matrix metalloproteinase-7 facilitates insulin-like growth factor bioavailability through Its proteinase activity on insulin-like growth factor binding protein 3. Cancer Res. 2004;64:665–671. doi: 10.1158/0008-5472.can-03-1916. [DOI] [PubMed] [Google Scholar]
- 84.Buckel P. Recombinant proteins for therapy. Trends Pharmacol Sci. 1996;17:450–456. doi: 10.1016/s0165-6147(96)01011-5. [DOI] [PubMed] [Google Scholar]
- 85.Dunlap SM, Celestino J, Wang H, Jiang R, Holland EC, Fuller GN, et al. Insulin-like growth factor binding protein 2 promotes glioma development and progression. Proc Nat'l Acad Sci, USA. 2007;104:11736–11741. doi: 10.1073/pnas.0703145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoeflich A, Reisinger R, Lahm H, Kiess W, Blum WF, Kolb HJ, et al. Insulin-like growth factor-binding protein 2 in tumorigenesis: protector or promoter? Cancer Res. 2001;61:8601–8610. [PubMed] [Google Scholar]
- 87.Liu B, Lee KW, Anzo M, Zhang B, Zi X, Tao Y, et al. Insulin-like growth factor-binding protein-3 inhibition of prostate cancer growth involves suppression of angiogenesis. Oncogene. 2006 doi: 10.1038/sj.onc.1209977. [DOI] [PubMed] [Google Scholar]
- 88.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 89.Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:285–294. doi: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 91.Ratain MJ, Glassman RH. Biomarkers in phase I oncology trials: signal, noise, or expensive distraction? Clin Cancer Res. 2007;13:6545–6548. doi: 10.1158/1078-0432.CCR-07-2133. [DOI] [PubMed] [Google Scholar]
- 92.Zha J, O'Brien C, Savage H, Huw LY, Zhong F, Berry L, et al. Molecular predictors of response to a humanized anti-insulin-like growth factor-I receptor monoclonal antibody in breast and colorectal cancer. Mol Cancer Ther. 2009;8:2110–2121. doi: 10.1158/1535-7163.MCT-09-0381. [DOI] [PubMed] [Google Scholar]
- 93.Carden CP, Molife LR, de Bono JS. Predictive biomarkers for targeting insulin-like growth factor-I (IGF-I) receptor. Mol Cancer Ther. 2009;8:2077–2078. doi: 10.1158/1535-7163.MCT-09-0641. [DOI] [PubMed] [Google Scholar]
- 94.Buck E, Eyzaguirre A, Rosenfeld-Franklin M, Thomson S, Mulvihill M, Barr S, et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68:8322–8332. doi: 10.1158/0008-5472.CAN-07-6720. [DOI] [PubMed] [Google Scholar]
- 95.Rodon J, DeSantos V, Ferry RJ, Jr, Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008;7:2575–2588. doi: 10.1158/1535-7163.MCT-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Byron SA, Horwitz KB, Richer JK, Lange CA, Zhang X, Yee D. Insulin receptor substrates mediate distinct biological responses to insulin-like growth factor receptor activation in breast cancer cells. Br J Cancer. 2006;95:1220–1228. doi: 10.1038/sj.bjc.6603354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Law JH, Habibi G, Hu K, Masoudi H, Wang MYC, Stratford AL, et al. Phosphorylated insulin-like growth factor-I/Insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–10246. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 98.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62:200–207. [PubMed] [Google Scholar]
- 99.Slomiany MG, Black LA, Kibbey MM, Tingler MA, Day TA, Rosenzweig SA. Insulin-like growth factor-1 receptor and ligand targeting in head and neck squamous cell carcinoma. Cancer Lett. 2007;248:269–279. doi: 10.1016/j.canlet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 100.Lucas JT, Jr, Salimath BP, Slomiany MG, Rosenzweig SA. Regulation of invasive behavior by vascular endothelial growth factor is HEF1-dependent. Oncogene. 2010 doi: 10.1038/onc.2010.185. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between Insulin/Insulin-like Growth Factor-1 Receptors and G Protein-Coupled Receptor Signaling Systems: A Novel Target for the Antidiabetic Drug Metformin in Pancreatic Cancer. Clinical Cancer Research. 2010;16:2505–2511. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Slomiany MG, Black LA, Kibbey MM, Day TA, Rosenzweig SA. IGF-1 induced vascular endothelial growth factor secretion in head and neck squamous cell carcinoma. Biochem Biophys Res Commun. 2006;342:851–858. doi: 10.1016/j.bbrc.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 103.Lucas JT, Jr, Salimath BP, Slomiany MG, Rosenzweig SA. Regulation of invasive behavior by vascular endothelial growth factor is HEF1-dependent. Oncogene. 2010 doi: 10.1038/onc.2010.185. In Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Van den Berg CL, Cox GN, Stroh CA, Hilsenbeck SG, Weng CN, McDermott MJ, et al. Polyethylene glycol conjugated insulin-like growth factor binding protein-1 (IGFBP-1) inhibits growth of breast cancer in athymic mice. Eur J Cancer. 1997;33:1108–1113. doi: 10.1016/s0959-8049(97)00071-3. [DOI] [PubMed] [Google Scholar]
- 105.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 106.Deshayes K, Schaffer ML, Skelton NJ, Nakamura GR, Kadkhodayan S, Sidhu SS. Rapid identification of small binding motifs with high-throughput phage display: discovery of peptidic antagonists of IGF-1 function. Chem Biol. 2002;9:495–505. doi: 10.1016/s1074-5521(02)00129-1. [DOI] [PubMed] [Google Scholar]
- 107.Sakai K, Lowman HB, Clemmons DR. Increases in free, unbound insulin-like growth factor I enhance insulin responsiveness in human hepatoma G2 cells in culture. J Biol Chem. 2002;277:13620–13627. doi: 10.1074/jbc.M107771200. [DOI] [PubMed] [Google Scholar]
- 108.Robinson SA, Rosenzweig SA. Paradoxical effects of the phage display-derived peptide antagonist IGF-F1-1 on insulin-like growth factor-1 receptor signaling. Biochem Pharmacol. 2006;72:53. doi: 10.1016/j.bcp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 109.Goya M, Miyamoto Si, Nagai K, Ohki Y, Nakamura K, Shitara K, et al. Growth inhibition of human prostate cancer cells in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice by a ligand-specific antibody to human insulin-like growth factors. Cancer Res. 2004;64:6252–6258. doi: 10.1158/0008-5472.CAN-04-0919. [DOI] [PubMed] [Google Scholar]
- 110.Kimura T, Kuwata T, Ashimine S, Yamazaki M, Yamauchi C, Nagai K, et al. Targeting of bone-derived insulin-like growth factor-II by a human neutralizing antibody suppresses the growth of prostate cancer cells in a human bone environment. Clin Cancer Res. 2010;16:121–129. doi: 10.1158/1078-0432.CCR-09-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dunn SE, Ehrlich M, Sharp NJH, Reiss K, Solomon G, Hawkins R, et al. A Dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 1998;58:3353–3361. [PubMed] [Google Scholar]
- 112.Min Y, Adachi Y, Yamamoto H, Ito H, Itoh F, Lee C-T, et al. Genetic blockade of the insulin-like growth factor-I receptor: A promising strategy for human pancreatic cancer. Cancer Res. 2003;63:6432–6441. [PubMed] [Google Scholar]