Abstract
Monoclonal antibody against the CD45RB protein induces stable transplantation tolerance to multiple types of allograft. We have previously established that this tolerance protocol relies on the regulatory function of B lymphocytes for its effect. B lymphocytes have also been reported to participate in immune regulation in several other settings. In most of these systems, the regulatory function of B lymphocytes depends on the production of IL-10. Therefore, we investigated the role of IL-10 in the anti-CD45RB model of B-cell-mediated transplantation tolerance. Surprisingly, using antibody-mediated neutralization of IL-10, IL-10-deficient recipients and adoptive transfer of IL-10-deficient B lymphocytes, we found that IL-10 actually counter-regulates tolerance induced by anti-CD45RB. Furthermore, neutralization of IL-10 reduced the development of chronic allograft vasculopathy compared to anti-CD45RB alone and reduced the production of graft reactive alloantibodies. These data suggest that the participation of regulatory B lymphocytes in transplantation tolerance may be distinct from how they operate in other systems. Identifying the specific B lymphocytes that mediate transplantation tolerance and defining their mechanism of action may yield new insights into the complex cellular network through which antigen-specific tolerance is established and maintained.
Keywords: B lymphocyte, IL-10, regulation, tolerance, transplantation
Introduction
B lymphocytes are well known for their central role in coordinating the immune response and host defense. Among their unique properties is their dual capacity to both secrete defensive antibodies and activate antigen-reactive T cells. Because of their central role in immune activation, they are also important factors in the development of multiple autoimmune diseases. Although clinical depletion of B cells protects against some of these deleterious immune responses, it does not appear to restore stable immune tolerance. In fact, in animal models, B-cell depletion often exacerbates these diseases (1–3). Collectively, the data suggest that at least some B lymphocytes possess the capacity to regulate the immune response.
We have recently reported that transplantation tolerance induced by treatment with the monoclonal antibody anti-CD45RB is absolutely dependent on the presence and participation of B lymphocytes (4–6). B-cell-deficient animals are completely resistant to tolerance induction by this method. When reconstituted with B cells by adoptive transfer, the susceptibility to tolerance is restored, but the transferred B cells must also express CD45RB, suggesting that they receive active signaling during tolerance induction by antibody treatment. Our studies have further demonstrated that B lymphocytes in this model must express CD40, CD80/86 (B7-1/2) and ICAM-1 to serve as effective regulators. Some of these molecules, particularly CD40 and CD80/86, have been implicated in other models of B-cell-mediated regulation (7). In most of these cases, there is an obligatory role of IL-10 in the tolerance mechanism (8).
We had not previously investigated the role of cytokine production in this unique model of transplantation tolerance. We report herein a systematic investigation of the role of IL-10 in the model, hypothesizing a role as a pro-regulatory cytokine. Surprisingly, we have found that IL-10 expressed by B lymphocytes inhibits B-cell-mediated tolerance induction in this model. Neutralization of IL-10 enhances tolerance induction and improves the long-term outcome of cardiac allografts.
Materials and Methods
Mice
Mice (C57BL/6, C3H, B6µMT−/−) were purchased from the Jackson Laboratories (Bar Harbor, ME). B6.129P2-Il10tm1Cgn/J mice (IL-10−/− mice on C57BL/6 background) were also obtained from the Jackson Laboratories. All mice were housed under specific pathogen-free barrier conditions. All procedures detailed below were performed under the principles of laboratory animal care and approved by the Institutional Animal Care and Use Committee (IACUC) at Massachusetts General Hospital.
Cardiac allografts
Experiments were performed according to a protocol approved by the IACUC at Massachusetts General Hospital. Transplantation was performed according to the Ono–Lindsey model as adapted for mice (9).
Antibody therapies
Animals were treated with intraperitoneal injection of 100 µg of rat anti-mouse CD45RB antibody (clone: MB23G2, ATCC, Rockville, MD) on days 0, 1, 3, 5 and 7 following transplant. For IL-10 neutralization, 200 µg of rat anti-mouse IL-10 antibody (clone: JES5–2A5) was administered every other day post transplantation for a total of 5 doses. These antibodies and all control antibodies (rat IgG2a, IgG1) were purchased from Bio Express, Inc. (West Lebanon, NH).
Cell sorting and transfer
B-cell-deficient hosts were reconstituted with mature syngeneic B cells by intravenous injection of 5 × 106 purified B cells (or 20 × 106 splenocytes) from normal or IL-10−/− mice. For B-cell sorting, B cells were negatively selected using Miltenyi MACS Beads (Germany). Purity of the sorted B-cell population exceeded 95%.
Analysis of alloantibody response
Donor-reactive alloantibodies were detected by flow cytometry. Sera (1:10 dilution) were incubated with 1 million naïve C3H splenocytes for 1 h at 4°C. Cells were washed and incubated with rat anti-mouse IgM-PE (BD Biosciences) and rat anti-mouse IgG-FITC (ebioscience). Test samples were compared with matched rat IgG1-FITC isotype and rat IgG2a-PE isotype, respectively. The median channel fluorescence was determined by flow cytometry.
Morphometric analysis
Morphometric analysis was performed similarly to what has been described previously (10). In brief, grafted hearts were removed at 130–150 days after transplantation and cut into two parts (base and apex). The base part was frozen in OCT compound and stored at −20°C. Cross-sections including the coronary arteries near the ostia or near the middle of grafts were cut at 4–6 µm and stained with Weigert’s elastic tissue stain. An image of the most representative section was captured by digital light microscopy at 200× or 400× magnification. Software for image processing and analysis in Java (Image J; NIH, Bethesda, MD, USA) was used to demarcate the borders of the lumen and intima of the coronary artery. One evaluator, who was blinded as to the diagnosis and the treatment of the hearts, demarcated the areas in all the sections. Tangential arterial sections were demarcated similarly to arterial cross-sections, but in tangential sections, demarcation was made on the coronary artery only at the junction of the coronary artery and the aorta. The software then quantitated the manually demarcated luminal and intimal areas. From these area values the "neointimal index," defined as [(luminal − intimal area)/luminal area] ×100, was calculated.
Statistical analysis
For survival studies, Kaplan–Meier survival curves were generated and a statistical analysis was performed by use of the log-rank test. For neointimal index and alloantibody data analyses, statistical significance was determined by t-test. All data were analyzed by the Prism 5 Program (Graph Pad, San Diego, CA). A value of p < 0.05 was considered significant.
Results
The contribution of IL-10 in anti-CD45RB-induced tolerance
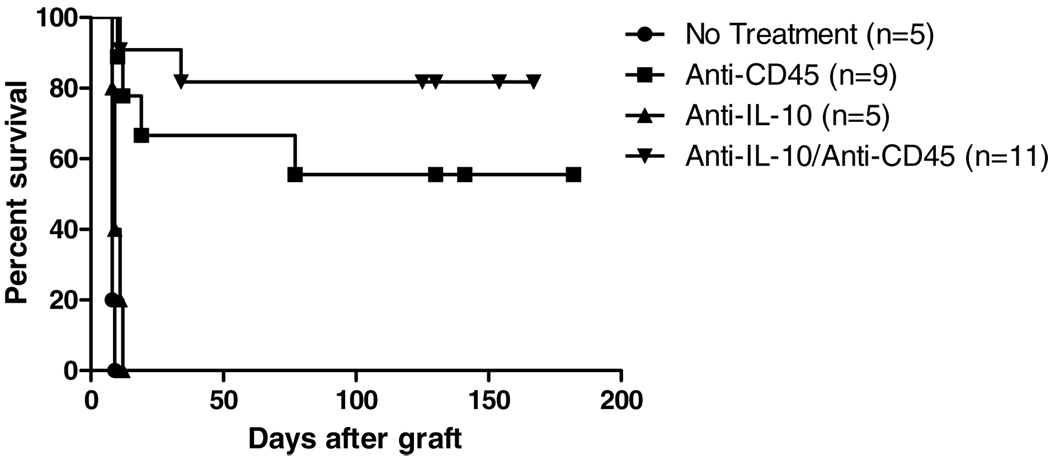
Studies of immune tolerance in transplantation and other immune-mediated tolerance settings have suggested key contributions of proregulatory cytokines. Among these regulatory mediators, IL-10 has been the most consistently reported and best characterized. This cytokine has also been thought to participate in the mechanism of regulatory B-cell function in autoimmune models where Bregs can protect against disease (8). Therefore, we investigated the role of this mediator in a B-cell-dependent transplantation model by using antibody-mediated neutralization. We found that neutralizing antibody against IL-10 alone did not alter graft survival and conferred no protection against rejection As previously reported, anti-CD45RB induced long-term survival of cardiac allografts in about 60% of recipients (Figure 1). Neutralization of IL-10 improved tolerance induction so that more than 80% of recipients demonstrated long-term survival. Increasing the dose of anti-cytokine antibody up to 1 mg for 5 doses produced similar results.
Figure 1. Effect of neutralizing IL-10 on anti-CD45RB-induced tolerance.
C57BL/6 animals received C3H heart allografts in the presence of anti-CD45RB. Treatment with anti-CD45RB-induced long-term survival in about 55% of recipients. Addition of neutralizing antibody against IL-10 improved outcomes such that 80% of recipients showed long-term survival (p = 0.436).
IL-10 deficiency enhances graft survival and tolerance induction
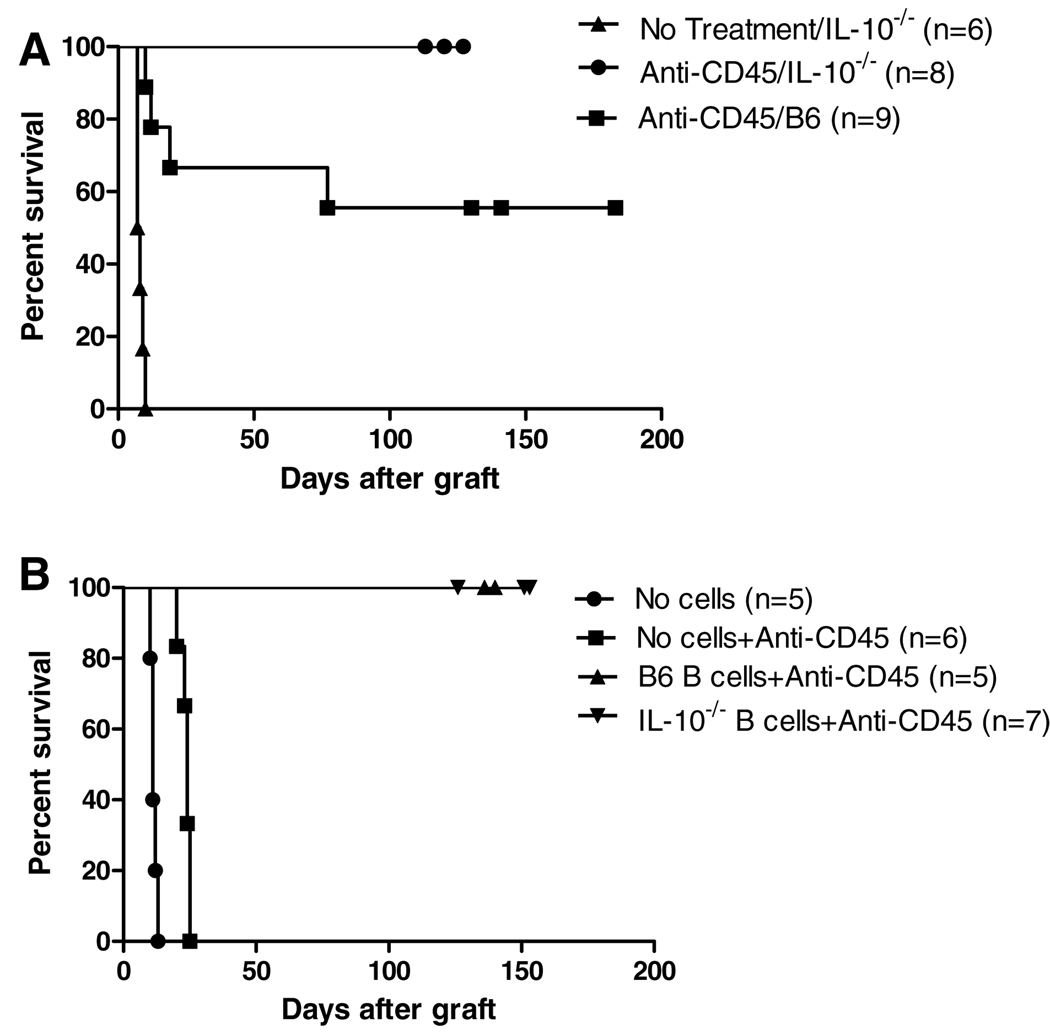
To further dissect the role of IL-10 in the tolerance mechanism, we utilized animals deficient in production of this cytokine to rule out the presence of residual IL-10 production as a contributor to tolerance. In the absence of anti-CD45RB, IL-10-deficient animals rapidly rejected cardiac allografts with an MST of 8 days (Figure 2A). Addition of a short course of anti-CD45RB in the IL-10-deficient animals led to long-term tolerance in 100% of animals, a significant improvement over IL-10-sufficient comparison animals (C57BL6) in which long-term survival did not exceed 60%. In the case of transplantation into IL-10-deficient animals, all lymphocytes are unable to produce this cytokine. We hypothesized that B-cell production of IL-10 was important in determining graft outcome. Therefore, we utilized our previously characterized model of adoptive transfer into B-cell-deficient recipients. By transferring IL-10-deficient splenocytes into B-cell-deficient recipients, only the B-cell compartment remains completely deficient in IL-10 production. While B-cell-deficient animals could not be tolerized by anti-CD45RB, transfer of splenocytes either capable or incapable of producing IL-10 led to long-term tolerance induction in all animals, confirming that IL-10 is dispensable and likely deleterious for tolerance induction (Figure 2B).
Figure 2. Insufficiency of IL-10 in B cells enhances anti-CD45RB-induced tolerance.
(A) IL-10-deficient animals received C3H cardiac allografts in the presence or absence of anti-CD45RB. IL-10 deficiency enhanced induction of long-term allograft acceptance induced by anti-CD45RB as compared to IL-10 sufficient controls (p = 0.037). (B) B-cell-deficient animals were reconstituted with 5 million B lymphocytes from IL-10-deficient or -sufficient donors. In the absence of B cells, tolerance is not induced. IL-10-sufficient and -deficient cells were equally competent in their ability to induce tolerance.
Blockade or elimination of IL-10 improves chronic allograft vasculopathy (CAV)
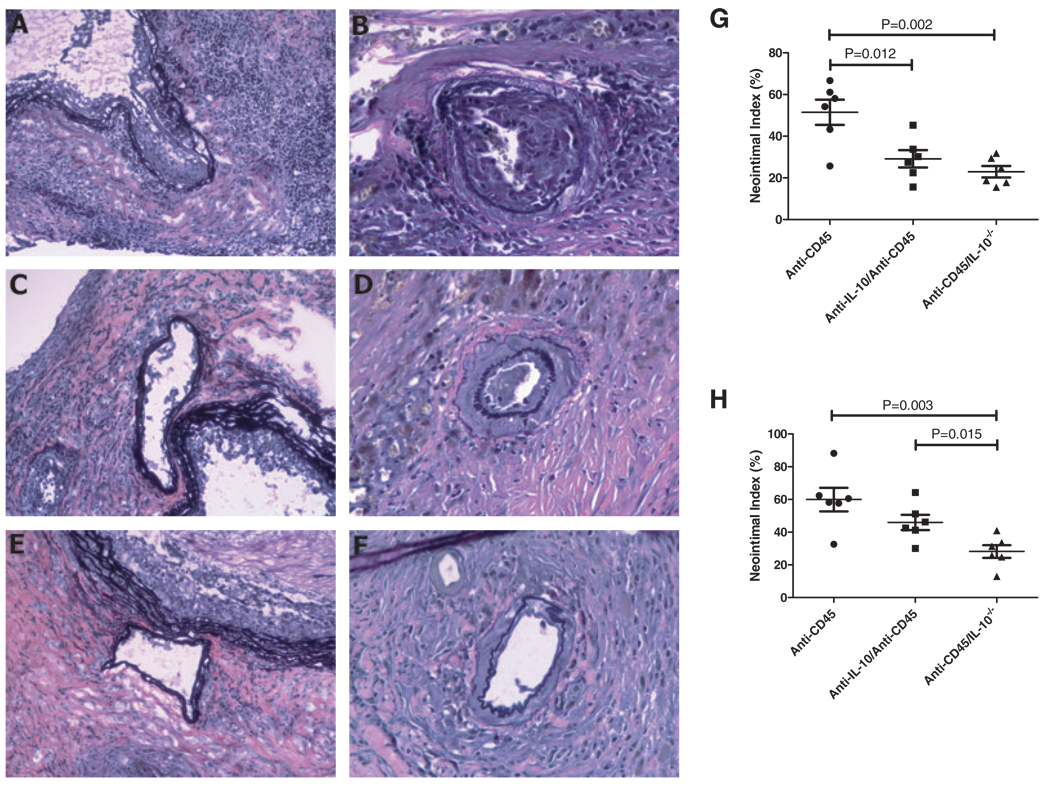
Because neutralization of IL-10 led to improved outcomes in long-term graft survival, we also considered whether blocking this cytokine would improve chronic graft vasculopathy CAV is a particularly important long-term outcome in clinical transplantation, but chronic arterial injury may not be readily noticed in graft-survival studies in animal models. Thus, we undertook detailed histologic assessment of grafts demonstrating long-term survival (Figure 3A–F). Although all grafts showed long-term survival, recipients treated with anti-CD45RB alone showed increased graft vasculopathy as measured by the neointimal index in both proximal and more distal sections of the coronary artery (Figure 3G–H) as compared to animals treated with neutralizing anti-IL-10 antibody and to IL-10-deficient recipients. Complete deficiency in IL-10 demonstrated the most significant effect, which was most pronounced when arterial sections near the middle of the graft were analyzed (Figure 3H).
Figure 3. Inhibition of IL-10 improves chronic allograft vasculopathy (CAV) over anti-CD45RB alone.
Histology of CAV formation and morphometric analysis of CAV lesions in C3H cardiac allografts transplanted in naïve C57BL/6 recipients of anti-CD45RB treatment alone and combined treatment of anti-CD45RB and anti-IL-10, and in IL-10−/− recipients of anti-CD45RB treatment are shown. Panels A, C and E demonstrate coronary artery emerging from the aorta, ×200; Panels B, D and F show the coronary artery near the middle of grafts, ×400. Elastic tissue staining shows apparent intimal thickening of the coronary artery in a recipient treated with anti-CD45RB alone on postoperative day +137 (A and B); the grafts in the recipients treated with a combination of anti-CD45RB and anti-IL-10 show diminished neointimal proliferation (C and D). Moreover, scant evidence of CAV lesions is seen in grafts from IL-10−/− recipients treated with anti-CD45RB (E and F). Morphometric analysis of the coronary artery emerging from the aorta demonstrates that the severity of CAV lesions in anti-CD45RB-treated animals was significantly greater than in anti-CD45RB- and anti-IL-10-treated animals, or in anti-CD45RB-treated IL-10−/− mice (G). Neointimal indices from the coronary artery near the middle of graft show CAV lesion in anti-CD45RB-treated IL-10−/− mice is much less serious than that in the animals of other two groups (H).
Neutralizing IL-10 decreases alloantibody production
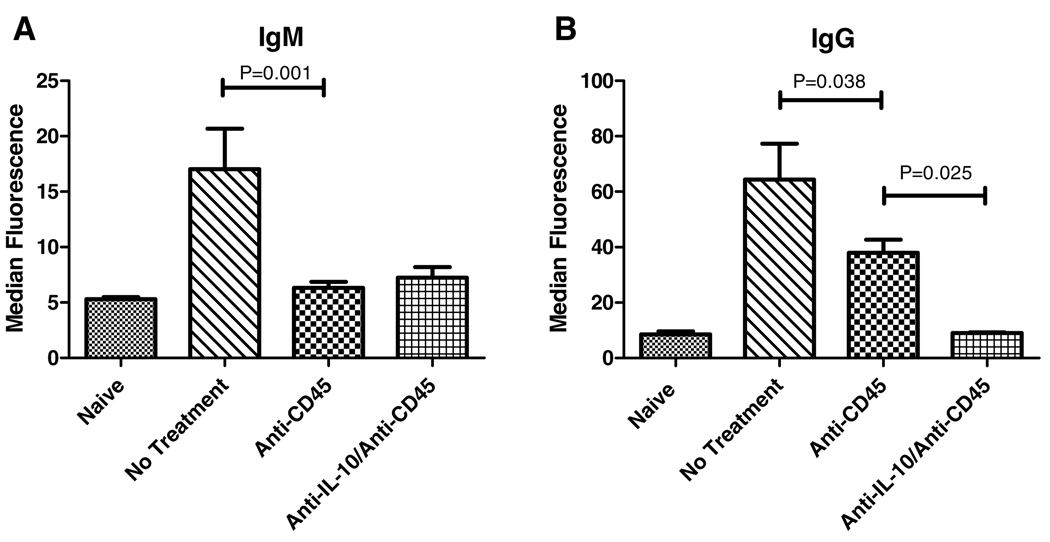
Chronic vasculopathy may result from long-standing immune activation that is insufficient to mediate clinically appreciable acute rejection episodes. This activation may result in the production of alloantibody. In addition, these alloantibodies may themselves participate in the development of vasculopathy (11,12). Because we had demonstrated improvement in arterial neointimal lesions in the absence of IL-10, we investigated the effect of anti-CD45RB in the presence or absence of IL-10 neutralization on alloantibody production. Anti-CD45RB markedly reduced the production of graft reactive antibody (both IgM and IgG) compared to untreated mice (Figure 4). Neutralization of IL-10 led to a further decrease in class-switched IgG alloantibody compared to anti-CD45RB alone (Figure 4B).
Figure 4. Neutralization of IL-10 further reduces alloantibody production compared to anti-CD45RB alone.
Sera were collected from animals on day 14 following allograft placement and anti-CD45RB treatment in the presence or absence of neutralizing anti-IL-10 antibody. Levels of alloreactive IgM and IgG were assessed. IgM levels were decreased by anti-CD45RB alone with no further reduction on the addition of anti-IL-10 (p = 0.001) (A). Alloreactive IgG levels were decreased by anti-CD45RB as well, although they remained elevated above naïve levels (p = 0.038); addition of anti-IL-10 led to a further reduction such that there was no difference from baseline, untransplanted animals (B).
Discussion
Anti-CD45RB induces robust, antigen-specific transplantation tolerance that is absolutely dependent on the presence of B lymphocytes (4,5). As we have previously reported, the mechanism requires CD45RB expression on the surface of B lymphocytes, suggesting that antibody treatment may activate the regulatory potential of B lymphocytes or induce the differentiation of regulatory B cells (4). As anti-CD45RB induces regulatory T cells, we have concluded that B cells may participate in Treg generation (13–15). As far as we are aware, this model represents the only example of regulatory B-cell participation in induced transplantation tolerance.
Nonetheless, the regulatory capacity of B lymphocytes and their action as ‘Bregs’ has been described in several other settings. In particular, autoimmune models and infectious illness have generated the preponderance of the evidence. In autoimmune studies, models of arthritis, SLE and colitis have all been examined. Although B-cell depletion has shown clinical benefit in these diseases in human patients, it has not been curative. The animal models suggest that B-cell depletion may exacerbate disease in certain settings. In models of SLE, loss of the marginal zone B-cell population is associated with a more aggressive disease course (16). In the TCRα−/− model of colitis, coincident B-cell deficiency also renders the disease more active (1). Even in models where colitis still develops, such as the Giα2−/− mice, B cells appear to attempt to stave off the progress of disease (17). Finally, in models of arthritis, CD40-activated B cells appear capable of blocking and reversing disease (7). In all these models, however, IL-10 appears to play the central role as the protective B lymphocytes demonstrate increased IL-10 expression. Neutralization of IL-10 levels seems to prevent disease protection.
This characteristic of Bregs in most other models contrasts strikingly with the findings here. Indeed, inhibition of IL-10 enhanced tolerance generation and improved graft outcomes as determined by long-term assessment of vasculopathy. This finding is most in keeping with the previous report of naïve B-cell-stimulated, induced Tregs (so-called bTregs) (18). These cells were able to inhibit cardiac allograft rejection and were IL-10 independent. While there is functional similarity with our system, our previous data suggest that anti-CD45RB exposed B lymphocytes are not naïve. In fact, our earlier data show enhanced proliferation of B cells with the addition of anti-CD45RB in ex vivo analysis of antigen stimulation (6). Our data presented here do indicate that anti-CD45RB decreases alloantibody production and that this decrease is enhanced by neutralizing IL-10. Interestingly, a recent report of regulatory B cells generated by exposure to an IL-15/GM-CSF fusokine noted a B-cell phenotype with features of both marginal zone precursors and plasmocytes (19). This description suggested the possibility of arrest in the generation of mature antibody-secreting cells during regulatory B-cell induction. Exposure to anti-CD45RB may thus modify B-cell differentiation by enhancing proliferation while inhibiting antibody secretion. Whether the enhanced proliferation leads to unlocking of pro-regulatory B-cell programs remains an active area of investigation.
While IL-10 clearly counter-regulates tolerance induction in the model described here, how this function is mediated, particularly when IL-10 demonstrates immunoregulatory potential in most other settings, is not yet known. Likely considerations include a direct effect on regulatory T cells or an effect on the generation of B lymphocytes with regulatory function. Our data, which show that IL-10 deficiency is a stronger enhancer of tolerance than IL-10 neutralization, suggest that IL-10 may exert its deleterious effect in this model via a paracrine or autocrine interaction since neutralizing antibody is generally less effective against these close interactions. As there is no prior evidence suggesting that IL-10 can override Treg function or formation, it is more likely that IL-10 acts directly on the B lymphocytes in this model. IL-10 is known for its B lymphocyte stimulatory capacity, and this activation may prevent regulatory B-cell development during anti-CD45RB therapy. Since at least some regulatory B cells appear to secrete IL-10, it may also be considered that local IL-10 levels could exert negative feedback on the formation of new regulatory B cells. Finally, IL-10 may result in the up-regulation of other molecules that are not compatible with the anti-CD45RB-induced tolerance mechanism.
Since IL-10 is not involved in this tolerance mechanism, it also remains of interest as to what the tolerogenic mediators may be. We have previously demonstrated that anti-CD45RB-induced tolerance requires B-cell expression of CD40 and CD80/86 (4). This finding is in keeping with the function of regulatory B cells in models of arthritis and EAE where lack of CD40 expression prevents the disease-ameliorating effects of Bregs. Since blockade of CD40L is also generally therapeutic in murine models, these findings have been reconciled by suggesting that CD40 engagement on B cells may depend on B-cell developmental stage or on other signals. As CD45RB is a cell surface phosphatase that modulates the strength of signal available through the BCR, antibody-targeting of the CD45RB molecule may alter these signals to unlock regulatory B-cell programs. At present, it is not known precisely how anti-CD45RB modifies intracellular signaling in B cells following contact with either antigen or cognate T cells. In addition to cognate cellular interactions, other regulatory cytokines may also play a role in B-cell-mediated tolerance following anti-CD45RB. As Treg generation has also been linked to the action of TGF-beta, the role of this cytokine in this model remains an area for further investigation.
Overall, our data expand on our knowledge of the role of regulatory B cells in induced transplantation tolerance. These data suggest that Bregs in this setting may not be functionally identical to what has been described in other models, as these Bregs are IL-10 independent. Whether these cells are similar to previously described Bregs in other ways and whether Bregs may exert different regulatory functions depending on the immunologic setting are fertile areas for further investigation.
Acknowledgment
This work was supported by grants DK54215, AI-057851 and P01-DK-49814 from the National Institutes of Health and NNSF30772042.
References
- 1.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ono S, Shao D, Yamada S, Yang Y, Yamashita M, Hamaoka T. A novel function of B lymphocytes from normal mice to suppress autoimmunity in (NZB × NZW)F1 mice. Immunology. 2000;100:99–109. doi: 10.1046/j.1365-2567.2000.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 4.Deng S, Moore DJ, Huang X, et al. Cutting edge: Transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol. 2007;178:6028–6032. doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 5.Huang X, Moore DJ, Mohiuddin M, et al. Rapid Communication: Inhibition of ICAM-1/LFA-1 interactions prevents B cell dependent anti-CD45RB induced tolerance. Transplantation. 2008;85:675–680. doi: 10.1097/TP.0b013e3181663422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore DJ, Huang X, Lee MKT, et al. Resistance to anti-CD45RB-induced tolerance in NOD mice: Mechanisms involved. Transpl Int. 2004;17:261–269. doi: 10.1007/s00147-004-0698-3. [DOI] [PubMed] [Google Scholar]
- 7.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;57:225–229. [PubMed] [Google Scholar]
- 10.Armstrong AT, Strauch AR, Starling RC, Sedmak DD, Orosz CG. Morphometric analysis of neointimal formation in murine cardiac allografts. Transplantation. 1997;63:941–947. doi: 10.1097/00007890-199704150-00006. [DOI] [PubMed] [Google Scholar]
- 11.Gareau A, Hirsch GM, Lee TD, Nashan B. Contribution of B cells and antibody to cardiac allograft vasculopathy. Transplantation. 2009;88:470–477. doi: 10.1097/TP.0b013e3181b076cc. [DOI] [PubMed] [Google Scholar]
- 12.Kaczmarek I, Deutsch MA, Kauke T, et al. Donor-specific HLA alloantibodies: Long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant. 2008;6:229–235. [PubMed] [Google Scholar]
- 13.Gao Z, Zhong R, Jiang J, et al. Adoptively transferable tolerance induced by CD45RB monoclonal antibody. J Am Soc Nephrol. 1999;10:374–381. doi: 10.1681/ASN.V102374. [DOI] [PubMed] [Google Scholar]
- 14.Fecteau S, Basadonna GP, Freitas A, Ariyan C, Sayegh MH, Rothstein DM. CTLA-4 up-regulation plays a role in tolerance mediated by CD45. Nat Immunol. 2001;2:58–63. doi: 10.1038/83175. [DOI] [PubMed] [Google Scholar]
- 15.Deng S, Moore DJ, Huang X, et al. Antibody-induced transplantation tolerance that is dependent on thymus-derived regulatory T cells. J Immunol. 2006;176:2799–2807. doi: 10.4049/jimmunol.176.5.2799. [DOI] [PubMed] [Google Scholar]
- 16.Amano H, Amano E, Moll T, et al. The Yaa mutation promoting murine lupus causes defective development of marginal zone B cells. J Immunol. 2003;170:2293–2301. doi: 10.4049/jimmunol.170.5.2293. [DOI] [PubMed] [Google Scholar]
- 17.Wei B, Velazquez P, Turovskaya O, et al. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci U S A. 2005;102:2010–2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichardt P, Dornbach B, Rong S, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–1529. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 19.Rafei M, Hsieh J, Zehntner S, et al. A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B-cell population with immune suppressive properties. Nat Med. 2009;15:1038–1045. doi: 10.1038/nm.2003. [DOI] [PubMed] [Google Scholar]