Abstract
Background
Allogeneic tolerance can be reliably obtained with monoclonal antibody therapy targeting CD45RB. Although regulatory T cells play an important role in the mechanism, we have recently demonstrated the active participation of host B lymphocytes. After anti-CD45RB therapy, B lymphocytes demonstrate phenotypic alterations that include up-regulation of CD54 (intercellular adhesion molecule [ICAM]-1). We have investigated the hypothesis that alteration in ICAM-1 expression is required for tolerance induction.
Materials and Methods
Recipients of heterotopic allogeneic cardiac grafts (C3H donors into B6 recipients) were treated with anti-CD45RB, anti-ICAM, anti-lymphocyte function-associated antigen-1 (LFA), or the combination of these agents. These data were extended by performing allogeneic cardiac transplants into ICAM−/− or LFA−/− recipients treated with a 5-day course of anti-CD45RB. Finally, B-cell-deficient animals were reconstituted with ICAM−/− splenocytes to create a recipient with a selective deficiency of ICAM-1 restricted to the B-cell compartment.
Results
Anti-CD45RB alone or the combination of anti-LFA/anti-ICAM reliably induced transplantation tolerance. However, the triple combination was routinely unsuccessful and induced long-term graft survival in no recipients. ICAM-deficient or LFA-deficient recipients were also resistant to tolerance induced by anti-CD45RB. Finally, transfer of control splenocytes to B-cell-deficient recipients permitted anti-CD45RB-induced tolerance, whereas transfer of ICAM−/− cells was unable to support tolerance induction.
Conclusions
Expression of ICAM-1 by B lymphocytes and interaction with LFA-1 form a central aspect of transplantation tolerance induced by anti-CD45RB therapy. These data further elucidate the cellular mechanisms used by B lymphocytes in the induction of transplantation tolerance.
Keywords: Tolerance, Transplantation, ICAM, LFA-1, Anti-CD45RB
The success of transplantation for numerous life-threatening illnesses is currently predicated on the efficacy of immunosuppressive therapies. Given the toxicities of these regimens, there is a strong impetus to develop new strategies that induce permanent donor-specific tolerance. Despite the great theoretical advantages of such therapies, translating them into the clinical setting remains a significant challenge. A better understanding of the mechanistic underpinnings of induced tolerance may facilitate this transition and may also reveal tolerance-adverse interactions that could occur in the setting of polypharmacy.
Monoclonal antibody therapy against the CD45RB molecule is an attractive target, given the short course of therapy needed for tolerance induction and the growing body of literature elucidating the mechanism of action of this reagent (1–4). Previous studies have demonstrated the induction of donor-specific regulatory T cells, the importance of cytotoxic T lymphocyte antigen 4 (CTLA-4), the modulation of peripheral T cell ratios, and the requisite role of the thymus (5–9). Unexpectedly, we have also recently demonstrated an absolute requirement for B lymphocytes in this model of transplantation tolerance (10).
Our finding that B cells are required for anti-CD45RB-mediated tolerance has important implications, given a recent surge in interest in B-cell-depleting immunotherapies in the settings of both transplantation and autoimmunity. B lymphocytes are the most numerous antigen presenting cells and have the unique capacity to concentrate circulating antigen several hundred fold through the surface Ig receptor, a capability that suggests a prominent role in indirect allopresentation. The requirement for B cell participation in tolerance induction opens a number of new investigational opportunities. If this tolerance-promoting pathway can be discerned, it is possible that it could be replaced by tailored pharmacotherapy or that the pathway in recipient B cells could be specifically enhanced during tolerance induction. Moreover, given the role of regulatory T cells in anti-CD45RB and other immunotherapies, there may be important insights into B cell-Treg interactions.
During our prior investigation, we have demonstrated that treatment with anti-CD45RB induces B cells to up-regulate CD54 (intercellular adhesion molecule [ICAM]-1) during therapy (11). Because the ICAM-1 adhesion molecule is important for lymphocyte migration and may also have co-stimulatory function, we have extended these studies to determine the function of ICAM-1/lymphocyte function-associated antigen-1 (LFA-1) interactions in tolerance induced by anti-CD45RB. Herein, we report that this pathway is also required for long-term tolerance induction.
MATERIALS AND METHODS
Mice
Mice (C57BL/6, C3H, B6.ICAM-1−/−, B6.LFA-1−/−, and B6.μMT−/−) were purchased from the Jackson Laboratories (Bar Harbor, ME). All mice were housed under specific pathogen-free barrier conditions. All procedures detailed below were performed under the principles of laboratory animal care and approved by the IACUC committee at the University of Pennsylvania.
Heart Grafting
Experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Transplantation was performed according to the Ono-Lindsey model as adapted for mice (12). Kaplan-Meier survival curves were generated and statistical analysis performed by use of the log-rank test.
Antibody Therapies
Animals were treated with intraperitoneal (IP) injection of 100μg of rat anti-mouse CD45RB antibody (clone: MB23G2, ATCC, Rockville, MD) on days 0, 1, 3, 5, and 7 after transplant. Treatments with either anti-ICAM-1 (clone: YN-1/1.7.4, ATCC) or anti-LFA-1 (clone: M17/4.411.9, ATCC) consisted of daily injection of 50 μg IP for a total of 7 days. All these antibodies and the control antibodies (rat IgG2a, IgG2b) were purchased from Bio Express, Inc. (West Lebanon, NH).
Flow Cytometry
One million cells were suspended in biotin-free RPMI containing 0.1% azide and 3% FCS and surface-stained in 96-well plates with the appropriate mAbs (PharMingen, San Diego, CA or eBioscience, San Diego, CA): anti-CD3 (145-2C11-FITC or -PE), anti-CD45R/B220 (RA3-6B2-biotin), anti-CD11a (M17/4-FITC), or anti-CD54 (3E2-PE). All samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA) using CellQuest software. Differences were detected by changes in mean fluorescence intensity (MFI) and analyzed by unpaired t-tests.
Adoptive Transfer Study
Ten million splenocytes from the indicated background strain were adoptively transferred to B-cell-deficient hosts by tail vein injection (10).
RESULTS
Anti-CD45RB Immunotherapy Leads to Specific Alteration of ICAM-1 and LFA-1 Expression
We have recently reported that anti-CD45RB-induced tolerance on both the B6 and BALB/c background depends on the presence of B lymphocytes. Prior characterization of the B cell requirement during tolerogenesis revealed consistent up-regulation of CD54 (ICAM-1) in B cells during exposure to anti-CD45RB (10). To elucidate further the underlying mechanisms for this B-cell-dependent T-cell tolerance, we analyzed the expression profiling and change of both ICAM-1 and its primary ligand LFA-1 on immune cells in mice treated with anti-CD45RB antibody.
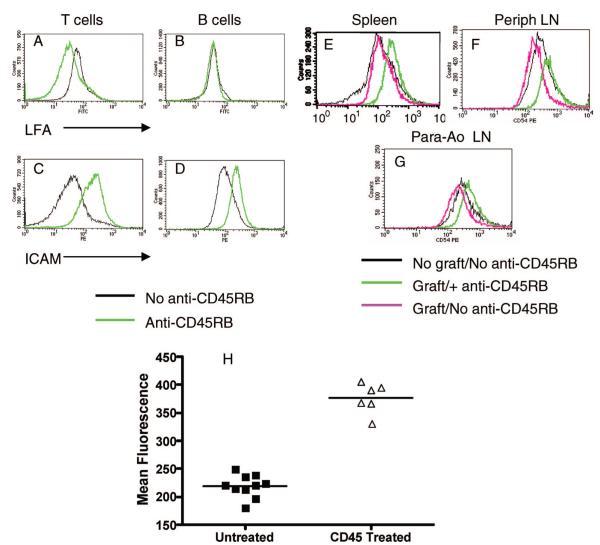
As shown in Figure 1, T cells and B cells express both ICAM-1 and LFA-1 molecules. In naive animals, anti-CD45RB treatment results in up-regulation of ICAM-1 on both T and B cells, and down-regulation of LFA-1 on T cells but not on B cells (Fig. 1A–D). To extend this observation, we analyzed the ICAM-1 expression on B cells at different sites of the transplanted recipients. As seen in Figure 1, exposure of B6 mice to an allogeneic cardiac transplant along with anti-CD45RB treatment leads to consistent up-regulation of CD54 at multiple sites of B lymphocyte accumulation, including the spleen (Fig. 1E–H), the peripheral lymph nodes (Fig. 1F), and the para-aortic nodes draining the graft (Fig. 1G). This increase in ICAM-1 expression was not caused by depletion of B lymphocytes with lower expression of ICAM as there was no significant depleting effect detected in splenocytes (52±20×106 in control vs. 60±11×106 in anti-CD45RB-treated mice, n=5) immediately after completion of the anti-CD45RB treatment.
FIGURE 1.
Expression of CD54 by B lymphocytes after anti-CD45RB therapy. Naive B6 animals were given a routine course of anti-, and the expression of LFA-1 (A and B) and intercellular adhesion molecule (ICAM)-1 (C and D) was analyzed on splenic T and B lymphocytes at the end of therapy. LFA-1 expression was down-regulated on T cells (CD3+, A) but B cell expression was unaffected (CD19+, B). ICAM-1 expression was enhanced on both splenic T (C) and B (D) lymphocytes. (E–F) B6 mice were grafted with allogeneic C3H hearts in the presence or absence of anti-CD45RB administered by the routine protocol. Exposure to anti-CD45RB leads to up-regulation of CD54 on B cells in multiple sites as identified by B220-gating. Up-regulation of B lymphocyte CD54 (ICAM-1) is detected in the spleen (E), peripheral nodes (F), and para-aortic lymph nodes (G). (H) Mean fluorescence intensities of anti-CD54 staining of splenic B lymphocytes from anti-CD45RB treated and untreated animals were compiled from four different experiments. A nearly twofold increase in MFI is seen consistently after anti-CD45RB therapy (P<0.0001, unpaired t test). Similar analysis for draining and para-aortic lymph nodes also reveals a significant increase in ICAM-1 expression (P<0.01, unpaired t test).
Combination Therapy With Anti-CD45RB and Blockade of ICAM-1/LFA-1 Interaction Leads to Disruption of Tolerance Induction
Given the strong association of CD54 up-regulation with CD45RB-directed treatment, we determined whether this change played a positive or negative role in the tolerance process. Because ICAM-1 itself is a potent co-stimulatory molecule and antibody against ICAM-1 has also been used successfully to induce tolerance (13–16), we considered whether up-regulation of the ICAM molecule may inhibit tolerance induction because anti-CD45RB fails to induce tolerance in about 30% of treated recipients in most published series. We hypothesized that blockade of the ICAM-1/LFA-1 interaction could enhance anti-CD45RB-mediated tolerance induction, and therefore, determined whether antibody-mediated disruption of the LFA-1/ICAM-1 interaction would enhance or disrupt tolerance induced by anti-CD45RB in a fully allogeneic C3H to B6 cardiac transplant model.
As can be seen in Figure 2A, anti-CD45RB or combination blockade of both ICAM-1 and LFA-1, but not anti-ICAM-1 or anti-LFA-1 alone, induces tolerance in this transplant model, findings consistent with the published literature (14, 15). However, coadministration of anti-CD45RB with antibody against ICAM-1, LFA-1, or the tolerogenic ICAM-1/LFA-1 combination abrogates tolerance induction (Fig. 2B). Importantly, therapy with anti-ICAM-1 did not lead to B-cell depletion but did result in significant down-regulation of ICAM-1 expression on B cells (data not shown). These data suggest that the up-regulation of ICAM-1 seen after anti-CD45RB treatment plays a crucial role in the pathway leading to tolerance.
FIGURE 2.
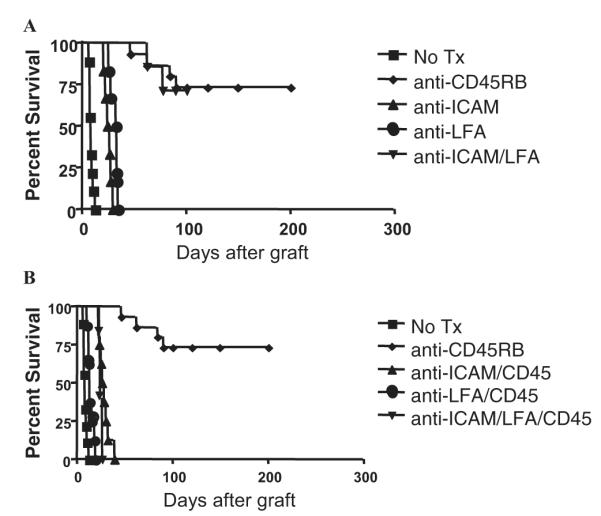
Anti-CD45RB-mediated tolerance is disrupted by blockade of intercellular adhesion molecule (ICAM)-1 or LFA-1. Hearts from C3H mice were transplanted into the abdominal cavity of B6 mice. (A) Mice were untreated (n=9, square) or treated with anti-CD45RB Abs (100 μg intraperitoneally [IP] on days 0, 1, 3, 5, 7; n=15, diamond), anti-ICAM-1 (50 μg IP for 7 days; n=6, triangle), anti-LFA-1 (50 μg for 7 days; n=6, circle), or the combination of anti-ICAM-1/anti-LFA-1 (n=6, inverted triangle). Tolerance was induced in both anti-CD45RB (P<0.0001 vs. untreated) and ICAM-1/LFA-1 combination therapy recipients (P<0.0001 vs. untreated). (B) Coadministration of anti-CD45RB with anti-ICAM-1 (n=8, triangle), anti-LFA-1 (n=8, circle), and anti-LFA-1/ICAM-1 (n=5, inverted triangle) combination abrogated tolerance induction in all cases. The untreated and anti-CD45RB-tolerized groups from (A) are shown for comparison (square).
Expression of ICAM-1 and LFA-1 Is Required in Recipient Animals for Tolerance Induction
Although these initial experiments support an important role for ICAM-1/LFA-1 interactions in anti-CD45RB tolerance, we sought added specificity in our system through the use of recipients genetically deficient in either ICAM-1 or LFA-1. B6-background mice deficient in either ICAM-1 or LFA-1 readily rejected allogeneic C3H hearts, indicating that these recipients were not immunocompromised (Fig. 3). When treated with anti-CD45RB antibody, long-term graft survival was not achieved in either setting; this tolerance resistance confirmed the requisite role played by this molecular interaction in promoting anti-CD45RB-mediated tolerance induction (Fig. 3).
FIGURE 3.
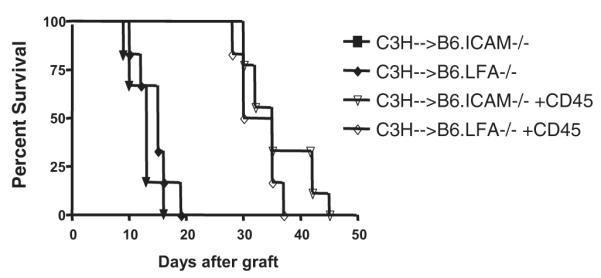
Anti-CD45RB is ineffective in mice that lack expression of intercellular adhesion molecule (ICAM)-1 or LFA-1. Hearts from C3H donors were transplanted heterotopically into the abdominal cavity of B6 mice that were genetically deficient in ICAM-1 or LFA-1 expression. Transplantation of C3H hearts into either ICAM-1−/− (n=6, triangle) or LFA-1−/− (n=6, diamond) recipients leads to rejection in a routine fashion indicating the immunocompetence of the recipients. Treatment of ICAM-1−/− (n=8, open triangle) or LFA-1−/− (n=6, open diamond) deficient animals with anti-CD45RB therapy is unable to induce tolerance in either case although there is a modest prolongation over untreated animals.
Restoration of Tolerance Induction by B-Cell Transfer into B-Cell-Deficient Hosts Requires B-Cell Expression of ICAM-1
Extension of the model into the knock-out systems also afforded the opportunity for further dissection of the cellular mechanisms involved. Because we had demonstrated up-regulation of ICAM-1 in B lymphocytes and a B-cell requirement for tolerance induction, we hypothesized that expression of ICAM-1 on B cells is necessary for anti-CD45RB-mediated tolerance. To test this hypothesis, we used our previously described protocol in which B-cell-deficient hosts are reconstituted with transferred splenocytes (10). As a brief overview of the established model, using the B-cell-deficient (μMT−/−) recipient mice in multiple genetic backgrounds we have previously demonstrated that the B cell deficiency is the single factor that prevents tolerance induction. Reconstitution of these mice with purified B cells restores tolerance susceptibility, indicating that B cells rather than another cell type altered by B cell deficiency is the target of anti-CD4RB immunotherapy. Because splenocyte transfer similarly restores the ability to induce tolerance in B-cell-deficient recipients, we have used this model to minimize the effects of prolonged ex vivo manipulation of the transferred cells. In the current investigation, B-cell-deficient recipients were either left unmanipulated or reconstituted with splenocytes from normal B6 or B6.ICAM−/− donors to generate recipients with a selective deficiency of ICAM-1 in the B-cell compartment (Fig. 4). B-cell-deficient recipients efficiently rejected allogeneic hearts and could not be tolerized by anti-CD45RB therapy. In contrast, tolerance induction was restored by injection of splenocytes from naive B6 donors. Splenocytes from ICAM-1-deficient donors were unable to restore tolerance induction, indicating a critical role for B cell ICAM-1 expression in the development of tolerance.
FIGURE 4.
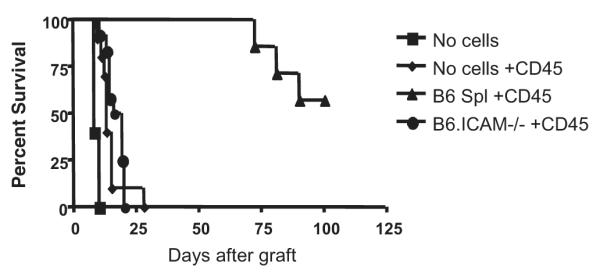
Expression of intercellular adhesion molecule (ICAM)-1 by B lymphocyte is required for tolerance induction. B-cell-deficient B6 hosts were reconstituted with B lymphocytes by intravenous injection of 10 million splenocytes from normal B6 donors. B-lymphocyte-deficient hosts were resistant to tolerance induction by anti-CD45RB (n=10, diamond), and tolerance was restored by transfer of splenocytes from normal B6 donors (n=7, triangle; P<0.001 vs. anti-CD45RB-treated B-deficient control). However, reconstitution with ICAM-1-deficient cells (n=12, circle) was unable to restore tolerance induction.
DISCUSSION
Collaboration of T cells and B cells is a complex and multifactorial process that is central to the development of a robust immune response. In addition to the immunostimulatory capacity for T cells possessed by B lymphocytes, there is an emerging literature demonstrating that B cells also exhibit potent immunoregulatory function (10, 17–20). Consistent with this notion, we recently reported that B cells are required for tolerance induced by anti-CD45RB therapy (10). This finding has been mirrored in other model systems, particularly in certain murine models of autoimmune disease in which B lymphocytes attenuate autoreactivity (18–22).
These new findings place the B lymphocyte into a critical juncture within both the adaptive immune response and the maintenance of self-tolerance. Over the past decade, multiple studies have also demonstrated that B cells are potent activators of autoreactive T cells. In murine models of IDDM and lupus, T lymphocyte activation is impaired and autoimmunity is nearly nonexistent in B-cell-deficient animals (23–26). Nonetheless, in these same animals, the T lymphocytes remain nontolerant and retain disease-provoking ability when transferred to B-cell-sufficient hosts (27), and it is apparent that autoimmune-prone B lymphocytes cannot precipitate autoimmunity by themselves and cannot provoke autoimmunity in otherwise normal hosts (28). Thus, in the absence of B cells, tolerance is not achieved and modification of B cell action may be necessary to induce long-term T-cell tolerance in these contexts.
Overall, the prior data suggest that B lymphocytes could interact with allo- and autoreactive effector cells and that alteration of B lymphocyte function may be able to control effector cell activation and promote tolerance induction. This issue is of critical importance given the recent rise in interest in antibody therapies that deplete B cells for use in autoimmunity and transplant tolerance-inducing therapies (29). Our data suggest that selective manipulation of B cell function rather than depletion may represent the optimal approach to promotion of antigen-specific T-cell tolerance to allografts. Of interest, our model system in which B-cell-deficient recipients can be reconstituted with B cells from knockout donors represents a unique and incisive model to elucidate the cell type with which B lymphocytes interact during tolerance induction. Potential candidates include CD4 and CD8 T cells as well as NKT cells, which can be differentiated by their respective requirements for antigen presentation by MHC class II, class I, and the CD1d molecule. Understanding the cellular mechanism may also reconcile the apparent discrepancy between this work and the recent study by Rayat and Gill in which a combination of anti-LFA-1 and anti-CD45RB more efficiently prevented the rejection of islet xenografts (30). Certainly, the cellular basis of allogeneic and xenogeneic recognition and rejection are distinct as these findings affirm (31–33). Despite this difference, the report by Rayat and Gill did demonstrate robust B lymphocyte tolerance as a principle component of the xenogeneic tolerance mechanism. Our findings suggest that the effect on the B cell compartment must be transferred to another cell type that is involved in allograft but not xenograft rejection and that this mechanism proceeds in part through the function of adhesion molecules. Whether B lymphocyte tolerance is also required in our model of allogeneic tolerance is under investigation.
Because anti-CD45RB leads to up-regulation of ICAM-1, understanding the cellular and molecular mechanisms involved in their function will be critical to clarify their roles in tolerance and rejection in diverse settings. Although the complete mechanism of action of CD45RB is not known, the natural role of the CD45 cell surface molecule is to control the state of lymphocyte sensitivity and responsiveness to antigenic stimuli. We have reported elsewhere that treatment with anti-CD45RB promotes activation-induced cell death (9, 11). In addition to these actions, selective up-regulation of CD54 may enhance the ability of B cells to interact with Tregs. Indeed, Marski et al. have demonstrated that LFA-1 is required for optimal Treg development and function (34). By enhancing CD54 expression, B cells may be able to stimulate Tregs that would not otherwise be recruited into the protective response.
In addition to a candidate mechanism for enhanced Treg interactions, alteration of the adhesion molecule ICAM-1 could affect cell trafficking and could lead to lymphocyte retention in the node similar to that seen with FTY720 immunotherapy (35–37). We have investigated lymphocyte recirculation in this model using adoptive transfer of CFSE-labeled lymphocytes and have not found any differences in B or T cell trafficking patterns, suggesting that there is either no effect or that the effected cellular subsets are too small for identification by this method (data not shown).
Overall, these data demonstrate a pattern of B-cell activation that is required for tolerance induction by anti-CD45RB. Whether a similar role is played by B cells in other immunotherapies requires further investigation, particularly, given current interest in B-cell depletion therapy. There is mounting evidence that B cells can play an effective role in immunoregulation and identifying and enhancing these pathways, such as the ICAM-1/LFA-1 interaction demonstrated here, may lead to more potent and specific therapies for tolerance induction.
Acknowledgments
This work was supported by grants DK54215, AI-057851, and P01-DK-49814 from the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Zhang Z, Lazarovits A, Grant D, Garcia B, Stiller C, Zhong R. CD45RB monoclonal antibody induces tolerance in the mouse kidney graft, but fails to prevent small bowel graft rejection. Transplant Proc. 1996;28:2514. [PubMed] [Google Scholar]
- 2.Auersvald LA, Rothstein DM, Oliveira SC, et al. Indefinite islet allograft survival in mice after a short course of treatment with anti-CD45 monoclonal antibodies. Transplantation. 1997;63:1355. doi: 10.1097/00007890-199705150-00026. [DOI] [PubMed] [Google Scholar]
- 3.Auersvald LA, Rothstein DM, Oliveira SC, Khuong CQ, Basadonna GP. Anti-CD45RB treatment prolongs islet allograft survival in mice. Transplant Proc. 1997;29:771. doi: 10.1016/s0041-1345(96)00478-2. [DOI] [PubMed] [Google Scholar]
- 4.Basadonna GP, Auersvald L, Khuong CQ, et al. Antibody-mediated targeting of CD45 isoforms: A novel immunotherapeutic strategy. Proc Natl Acad Sci USA. 1998;95:3821. doi: 10.1073/pnas.95.7.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Z, Zhong R, Jiang J, et al. Adoptively transferable tolerance induced by CD45RB monoclonal antibody. J Am Soc Nephrol. 1999;10:374. doi: 10.1681/ASN.V102374. [DOI] [PubMed] [Google Scholar]
- 6.Lazarovits AI, Visser L, Asfar S, et al. Mechanisms of induction of renal allograft tolerance in CD45RB-treated mice. Kidney Int. 1999;55:1303. doi: 10.1046/j.1523-1755.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 7.Fecteau S, Basadonna GP, Freitas A, Ariyan C, Sayegh MH, Rothstein DM. CTLA-4 up-regulation plays a role in tolerance mediated by CD45. Nat Immunol. 2001;2:58. doi: 10.1038/83175. [DOI] [PubMed] [Google Scholar]
- 8.Ariyan C, Salvalaggio P, Fecteau S, et al. Cutting edge: Transplantation tolerance through enhanced CTLA-4 expression. J Immunol. 2003;171:5673. doi: 10.4049/jimmunol.171.11.5673. [DOI] [PubMed] [Google Scholar]
- 9.Deng S, Moore DJ, Huang X, et al. Antibody-induced transplantation tolerance that is dependent on thymus-derived regulatory T cells. J Immunol. 2006;176:2799. doi: 10.4049/jimmunol.176.5.2799. [DOI] [PubMed] [Google Scholar]
- 10.Deng S, Moore DJ, Huang X, et al. Cutting edge: Transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol. 2007;178:6028. doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 11.Moore DJ, Huang X, Lee MK, et al. Resistance to anti-CD45RB-induced tolerance in NOD mice: Mechanisms involved. Transpl Int. 2004;17:261. doi: 10.1007/s00147-004-0698-3. [DOI] [PubMed] [Google Scholar]
- 12.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;57:225. [PubMed] [Google Scholar]
- 13.Grazia TJ, Gill RG, Gelhaus HC, JR, Doan AN, Sleater ML, Pietra BA. Perturbation of leukocyte function-associated antigen-1/intercellular adhesion molecule-1 results in differential outcomes in cardiac vs islet allograft survival. J Heart Lung Transplant. 2005;24:1410. doi: 10.1016/j.healun.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Isobe M, Yagita H, Okumura K, Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992;255:1125. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- 15.Isobe M, Suzuki J, Yamazaki S, Horie S, Okubo Y, Sekiguchi M. Assessment of tolerance induction to cardiac allograft by anti-ICAM-1 and anti-LFA-1 monoclonal antibodies. J Heart Lung Transplant. 1997;16:1149. [PubMed] [Google Scholar]
- 16.Nozawa M, Otsu I, Kobayashi H, et al. New immunosuppression with monoclonal antibody to intracellular adhesion molecule 1 (ICAM-1) in rat organ transplantation. Transpl Int. 1992;5(suppl 1):S521. doi: 10.1007/978-3-642-77423-2_153. [DOI] [PubMed] [Google Scholar]
- 17.Bennett SR, Carbone FR, Toy T, Miller JF, Heath WR. B cells directly tolerize CD8(+) T cells. J Exp Med. 1998;188:1977. doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 19.Serra P, Santamaria P. To ‘B’ regulated: B cells as members of the regulatory workforce. Trends Immunol. 2006;27:7. doi: 10.1016/j.it.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Wei B, Velazquez P, Turovskaya O, et al. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci USA. 2005;102:2010. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005;8:25. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 22.Knoechel B, Lohr J, Kahn E, Abbas AK. The link between lymphocyte deficiency and autoimmunity: Roles of endogenous T and B lymphocytes in tolerance. J Immunol. 2005;175:21. doi: 10.4049/jimmunol.175.1.21. [DOI] [PubMed] [Google Scholar]
- 23.Braley-Mullen H, Yu S. Early requirement for B cells for development of spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2000;165:7262. doi: 10.4049/jimmunol.165.12.7262. [DOI] [PubMed] [Google Scholar]
- 24.Noorchashm H, Lieu YK, Noorchashm N, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a check-point in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol. 1999;163:743. [PubMed] [Google Scholar]
- 25.Akashi T, Nagafuchi S, Anzai K, et al. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int Immunol. 1997;9:1159. doi: 10.1093/intimm/9.8.1159. [DOI] [PubMed] [Google Scholar]
- 26.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: Analysis of a new “speed congenic” stock of NOD. Ig mu null mice. J Exp Med. 1996;184:2049. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greeley SA, Moore DJ, Noorchashm H, et al. Impaired activation of islet-reactive CD4 T cells in pancreatic lymph nodes of B cell-deficient nonobese diabetic mice. J Immunol. 2001;167:4351. doi: 10.4049/jimmunol.167.8.4351. [DOI] [PubMed] [Google Scholar]
- 28.Moore DJ, Noorchashm H, Lin TH, Greeley SA, Naji A. NOD B-cells are insufficient to incite T-cell-mediated anti-islet autoimmunity. Diabetes. 2005;54:2019. doi: 10.2337/diabetes.54.7.2019. [DOI] [PubMed] [Google Scholar]
- 29.Eisenberg R, Looney RJ. The therapeutic potential of anti-CD20 “what do B-cells do?”. Clin Immunol. 2005;117:207. doi: 10.1016/j.clim.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Rayat GR, Gill RG. Indefinite survival of neonatal porcine islet xenografts by simultaneous targeting of LFA-1 and CD154 or CD45RB. Diabetes. 2005;54:443. doi: 10.2337/diabetes.54.2.443. [DOI] [PubMed] [Google Scholar]
- 31.Guillet M, Gagne K, Lair D, et al. Different patterns of TCR beta chain regulation following allo- and xeno-transplantation. Xenotransplantation. 2004;11:315. doi: 10.1111/j.1399-3089.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 32.Borenstein SH, Graham J, Zhang XL, Chamberlain JW. CD8+ T cells are necessary for recognition of allelic, but not locus-mismatched or xeno-, HLA class I transplantation antigens. J Immunol. 2000;165:2341. doi: 10.4049/jimmunol.165.5.2341. [DOI] [PubMed] [Google Scholar]
- 33.Gotoh M, Monden M, Yamamoto H, et al. Roles of CD4+ and CD8+ cells in islet allo- and xeno-graft rejection. Horm Metab Res Suppl. 1990;25:173. [PubMed] [Google Scholar]
- 34.Marski M, Kandula S, Turner JR, Abraham C. CD18 is required for optimal development and function of CD4+CD25+ T regulatory cells. J Immunol. 2005;175:7889. doi: 10.4049/jimmunol.175.12.7889. [DOI] [PubMed] [Google Scholar]
- 35.Kunisawa J, Kurashima Y, Gohda M, et al. Sphingosine 1-phosphate regulates peritoneal B cell trafficking for subsequent intestinal IgA production. Blood. 2007;18:18. doi: 10.1182/blood-2006-08-041582. [DOI] [PubMed] [Google Scholar]
- 36.Vaessen LM, van Besouw NM, Mol WM, Ijzermans JN, Weimar W. FTY720 treatment of kidney transplant patients: A differential effect on B cells, naive T cells, memory T cells and NK cells. Transpl Immunol. 2006;15:281. doi: 10.1016/j.trim.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Quesniaux VF, Menninger K, Kunkler A, et al. The novel immunosuppressant FTY720 induces peripheral lymphodepletion of both T- and B-cells in cynomolgus monkeys when given alone, with cyclosporine Neoral or with RAD. Transpl Immunol. 2000;8:177. doi: 10.1016/s0966-3274(00)00025-3. [DOI] [PubMed] [Google Scholar]