Abstract
Background
In Canada, vaccination coverage for seasonal influenza among health care personnel remains below 50%. The objective of this review was to determine which seasonal influenza vaccination campaign or campaign components in health care settings were significantly associated with increases in influenza vaccination among staff.
Methods
We identified articles in eight electronic databases and included randomized controlled trials, controlled before-and-after studies and studies with interrupted time series designs in our review. Two reviewers independently abstracted the data and assessed the risk of biases. We calculated risk ratios and 95% confidence intervals for randomized controlled trials and controlled before-and-after studies and described interrupted time series studies.
Results
We identified 99 studies evaluating influenza vaccination campaigns for health care workers, but only 12 of the studies were eligible for review. In nonhospital health care settings, including long-term care facilities, campaigns with a greater variety of components (including education or promotion, better access to vaccines, legislation or regulation and/or role models) were associated with higher risk ratios (i.e, favouring the intervention group). Within hospital settings, the results reported for various types of campaigns were mixed. Many of the criteria for assessing risk of bias were not reported.
Interpretation
Campaigns involving only education or promotion resulted in minimal changes in vaccination rates. Further studies are needed to determine the appropriate components and combinations of components in influenza vaccination campaigns for health care personnel.
Health care personnel can act as vectors of influenza and may transmit the disease to patients who are at risk for influenza-related complications or death.1 A Cochrane review2 of three studies showed that vaccination of health care personnel, combined with vaccination of patients, was 86% efficacious (95% confidence interval [CI] 40%–97%) in preventing influenza-like illnesses among elderly patients. It is recommended that all health care personnel (i.e., minimum 90% coverage) receive the seasonal influenza vaccine for protection from the virus.3
Rates of vaccination against seasonal influenza among health care personnel are often below targeted levels and vary across health care organizations in Canada and internationally. In 2003, vaccination coverage was 46% among Canadians employed in ambulatory care settings, hospitals and long-term care facilities.4 In a survey of Canadian long-term care facilities, the average vaccination rate among workers was 35%.5 Similarly, in the United States, vaccination coverage for health care personnel was about 40%,6 and in European countries, reported vaccine uptake has ranged from 14% to 48%.7
The Canadian National Advisory Committee on Immunization encourages all organizations to actively promote the influenza vaccine and to provide education aimed at health care personnel.3 The US Healthcare Infection Control Practices Advisory Committee and the Advisory Committee on Immunization Practices have recommended that all organizations employing health care personnel use evidence-based approaches that may overcome barriers to vaccine uptake as part of their influenza vaccination campaigns.6 These two committees identified five categories of components of influenza vaccination campaigns aimed at improving immunization rates among health care personnel (Table 1).
Table 1.
Components of influenza vaccination campaigns to improve uptake of influenza vaccine by health care personnel6
| Component | Operational definition | Examples |
|---|---|---|
| Education or promotion | Organized effort to raise awareness and/or increase knowledge about influenza and influenza vaccination | Educational sessions and materials, material or events promoting vaccine, incentives |
| Improved access to vaccine | Strategies to allow for easier access to vaccination for health care personnel | Mobile vaccine carts, peer-to-peer vaccination, additional or extended vaccine clinics |
| Legislation or regulation | Interventions involving changes in vaccination policy for health care personnel | Staff vaccination policy, mandatory vaccination programs, declination forms |
| Measurement and feedback | Tracking of vaccination rates of health care personnel and dissemination of results | Regular monitoring of vaccination coverage rates, reporting of coverage rates to administrators and health care personnel |
| Role models | Activities that involve leaders and/or senior staff to encourage vaccination | Vaccination advocates and champions, public support from leaders, visible vaccination of senior staff |
No systematic reviews have been conducted on interventions aimed at increasing influenza vaccination coverage among staff of health care organizations. Previous relevant reviews included a Cochrane review for improving vaccination rates among patient groups,8 a summary of 32 studies examining staff perceptions of the influenza vaccine and vaccination coverage9 and a systematic review of interventions to improve influenza vaccination coverage among high-risk adults.10 A narrative review on use of declination forms concluded that the intervention might lead to modest increases in vaccination rates, depending on the content and language of the forms.11 The primary objective of the current review was to determine which influenza vaccination campaign or campaign components in health care settings were significantly associated with higher rates of influenza vaccination among staff. The focus of our systematic review was seasonal influenza vaccination campaigns; we did not consider pandemic influenza vaccination programs.
Methods
Literature search
We identified potentially eligible reports by searching the following databases with the OvidSP interface on Apr. 29, 2008: MEDLINE (January 1950 to present), EMBASE (1980–2008) and CINAHL, the Cumulative Index to Nursing and Allied Health Literature (1982–2008). Our search terms included “health personnel,” “influenza vaccine” and “health facilities.” We applied the methodologic search filters provided by the Cochrane Effective Practice and Organisation of Care Group.12 The complete search strategies are presented in Appendix 1 (available at www.cmaj.ca/cgi/content/full/cmaj.091304/DC1). We did not apply any language or date restrictions. We consulted infection control experts and hand-searched bibliographies of relevant reports for additional studies. The MEDLINE and EMBASE databases were last searched on Sept. 22, 2009. We searched the following additional databases on Sept. 27, 2009: Science Citation Index Expanded (Web of Science 1899–2009), Database of Abstracts of Reviews of Effects, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials and Proquest (for dissertations and theses). We keyed the titles of relevant articles into the PubMed “related articles” feature to identify similar reports.
Selection of studies
We selected for our analysis any studies evaluating influenza vaccination campaigns for health care personnel. We defined such campaigns as organized efforts to promote greater vaccination coverage among staff members. An eligible study had to report the percentage or number of health care personnel who received the influenza vaccine as an outcome measure. We excluded studies that did not describe the study population or did not report ascertainment of vaccination status. Because the influenza vaccine is administered annually and because health care personnel have specific attitudes toward this vaccine, we excluded studies involving other vaccines.
We applied study design criteria only to randomized controlled trials, cluster randomized controlled trials, controlled before-and-after studies and interrupted time series designs. To be included, a controlled before-and-after study had to have at least one comparison group, with one observation point before and another point after implementation of the intervention. All included interrupted time series studies had to have a clear time point at which the intervention was implemented. For interrupted time series, a minimum of five pre-intervention observations must have been recorded or, for studies with a shorter duration, a minimum of three pre- and post-intervention points must have been recorded.
Assessment of risk of bias and extraction of data
Two reviewers (P.L. and D.M.P.M.) independently assessed the risk of bias with the quality assessment checklist of the Cochrane Effective Practice and Organisation of Care Group.13 Each study design had its own assessment criteria.13 Study quality criteria that were explicitly reported were marked as “done.” Items reported as not completed were marked as “not done.” If a study characteristic was not reported, the reviewers checked off “not clear.”
The same two reviewers used a data collection form to independently abstract data from the studies. The reviewers discussed any discrepancies in their results to reach agreement. The form covered information about study design, participants’ characteristics, setting, interventions assessed, components of influenza vaccination campaigns and vaccination uptake.
Statistical analysis
We synthesized the data abstracted from the studies and then stratified them by health care settings. For randomized controlled trials and before-and-after studies with a control group, we used the post-intervention vaccination rates to calculate risk ratios and 95% confidence intervals. We summarized the results in a forest plot. We summarized interrupted time series studies descriptively in the text.
Results
Search yield
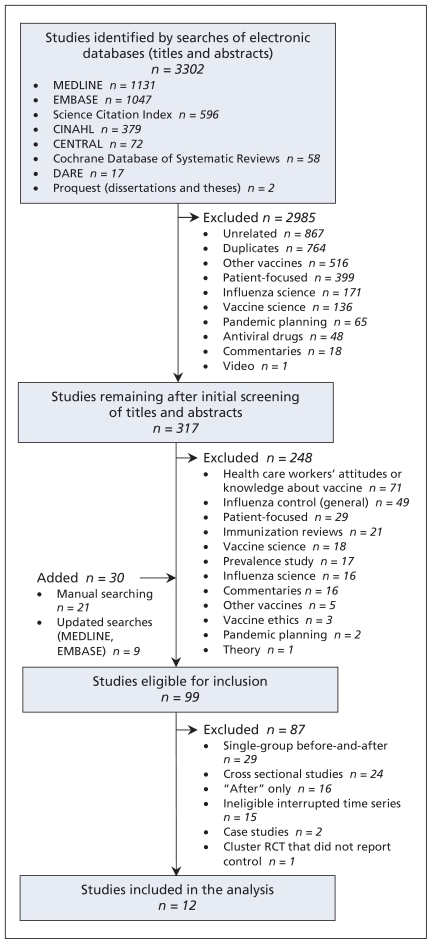
The search strategy yielded 3302 citations (Figure 1). Of these, 99 studies reported an organized effort to increase influenza vaccination among staff and evaluated implemented strategies and were therefore eligible for inclusion. However, only 12 studies met our study design criteria. The characteristics of the studies deemed ineligible because of study design (n = 87) are summarized in Appendices 2 to 5 (available at www.cmaj.ca/cgi/content/full/cmaj.091304/DC1).
Figure 1.
Selection of studies for systematic review. CENTRAL = Cochrane Central Register of Controlled Trials, CINAHL = Cumulative Index to Nursing and Allied Health Literature, DARE = Database of Abstracts of Reviews of Effects, RCT = randomized controlled trial.
Studies included in analysis
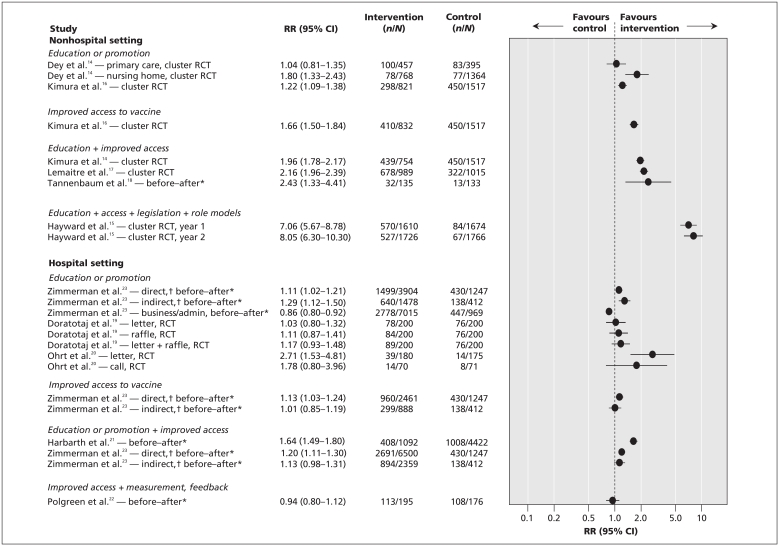
Table 2 summarizes the characteristics of and results from the 12 studies14–25 included in the analysis. For randomized controlled trials and controlled before-and-after studies, differences in influenza vaccination coverage across comparison groups are presented in Figure 2.14–23 The included studies were published from 1992 to 2009 and were conducted in long-term care facilities, hospitals and primary health care settings. The studies were based in the United States, Canada, the United Kingdom, Germany and Switzerland.
Table 2.
Characteristics of studies included for analysis
| Study | Population | Ascertainment | Intervention group | Comparison group |
|---|---|---|---|---|
| Nonhospital setting | ||||
| Cluster randomized controlled trials | ||||
| Dey et al.14 |
|
Vaccine provider submitted claim forms for reimbursement for vaccine |
|
|
| Hayward et al.15 |
|
Not reported |
|
|
| Kimura et al.16 |
|
Self-administered questionnaire to all health care workers |
|
|
| Lemaitre et al.17 |
|
|
|
|
| Before-and-after with control | ||||
| Tannenbaum et al.18 |
|
|
|
|
| Hospital setting | ||||
| Randomized controlled trial | ||||
| Doratotaj et al.19* |
|
Vaccination rates reported by on-site vaccine clinics | Comparison campaign plus one or more of the following:
|
|
| Randomized controlled trial | ||||
| Ohrt and McKinney20 |
|
Nurses recorded vaccination rates | Comparison campaign plus one or more of the following:
|
|
| Before-and-after with control | ||||
| Harbarth et al.21 |
|
Not reported | Comparison campaign plus:
|
|
| Polgreen et al.22 |
|
Not reported | Comparison campaign plus:
|
|
| Zimmerman et al.23 |
|
Records from paper logs at each site | Comparison campaign plus one or more of the following:
|
|
| Interrupted time series | ||||
| Bertin et al.24† |
|
Rates (as captured by declination forms) reported by vaccine clinics | After Oct. 17, 2005:
|
Before intervention (1997–2005):
|
| Wicker25 |
|
Not reported | After Jan 7, 2009 — comparison campaign plus:
|
2003/2004 to Jan 7, 2009:
|
Nonsignificant differences across comparison groups (p = 0.66).
The authors reported two campaigns, in 2004 and 2005. Only the 2005 campaign, for which the authors compared the difference in coverage rates between 2005 and the previous 9-year period, was considered for the current analysis.
Figure 2.
Results from randomized controlled trials and controlled before-and-after studies showing the effectiveness of strategies to improve immunization coverage among health care personnel. CI = confidence interval, RCT = randomized controlled trial, RR = risk ratio. *With control. †Refers to contact with patients.
Nonhospital health care settings
We identified five studies from nonhospital health care settings (Table 2): four cluster randomized controlled trials14–17 and one controlled before-and-after study.18 All of the studies were conducted in long-term care facilities. The study by Dey and colleagues14 included a separate study arm involving primary health care teams. The five studies reported a total of nine comparisons. The populations targeted in the campaigns included physicians, nurses, nursing assistants, housekeeping staff, technicians, other professionals and administrators. Ascertainment of vaccination status relied primarily on self-reporting and reporting by the vaccine provider.
Various types of campaigns were used in studies from non-hospital settings: education or promotion, improved access to the vaccine, legislation or regulation, and/or role models (Table 2). In eight of the nine campaigns, the health care personnel in the intervention groups were more likely to be vaccinated than those in the control groups. Campaigns with more components had higher risk ratios (i.e., favouring the intervention group).
Hospital settings
We identified seven studies conducted in a hospital setting: two randomized controlled trials,19,20 three controlled before-and-after studies21–23 and two interrupted time series studies.24,25 The seven studies reported a total of 16 comparisons. The study populations included medical residents, nurses, physicians, other professionals, administrators, housekeeping staff and volunteers (Table 2). Vaccination rates were collected through tracking by the vaccine provider and/or mandatory self-reporting. The interventions used included education or promotion, improved access to the vaccine, measurement with feedback, and legislation or regulation.
The results across the various campaigns were mixed (Figure 2). In three of the eight comparisons involving educational or promotional campaigns alone,20,23 the results favoured the intervention group. In two of the three comparisons involving campaigns with educational or promotional components combined with improved access to the vaccine,21,23 staff in the intervention group were more likely to be vaccinated than those in the control group. In the two interrupted time series studies,24,25 legislation or regulation components were integrated into the overall campaigns. In one campaign, in which staff completed a mandatory electronic declination form,24 vaccination coverage increased to 55%. This was an improvement over the previous nine years, during which rates had ranged from 21% to 38%. When unvaccinated personnel were required to wear masks,25 vaccination rates increased from 33% to 52%, but the authors did not report the statistical significance.
Assessment of risk of bias
Of the six randomized controlled trials, five had concealment of allocation,14–17,20 and four protected against contamination by using the study sites as the unit of allocation.14–17 However, other assessment criteria were not well reported. None of the studies involved follow-up with staff. Two of the six studies compared baseline measures between the intervention and comparison groups,16,17 and only one study had a reliable method of ascertaining vaccination status.19
In the controlled before-and-after studies and the interrupted time series studies, reporting was not clear for many of the criteria for assessing risk of bias. Comparison of baseline measures between groups was reported for three of the four controlled before-and-after studies.18,21–23 However, these studies did not report follow-up with staff or a reliable method of ascertaining vaccination status. In the two interrupted time series studies,24,25 the authors explained the intervention effect but did not report many of the other assessment criteria, such as analysis of data, reason for number of observation points, completeness of the data set and reliability of the primary outcome measure.
Interpretation
In this review, we identified 12 studies that evaluated interventions to increase influenza vaccination coverage among health care personnel in long-term care facilities, hospitals and primary health care settings. Of the five recommended campaign components to increase vaccination rates among health care personnel (Table 1), the most common strategies were education or promotion and improving access to the vaccine. None of the campaigns in the included studies reached the recommended level of 90% uptake of vaccine among health care personnel.
In nonhospital health care settings, campaigns involving only education or promotion resulted in small increases in vaccination rates relative to other interventions. A combination of education or promotion and improved access to the vaccine yielded greater increases in coverage among long-term care workers. Coverage was highest in the study in which each worker had a personal interview session with a member of the study team.17 Only one campaign had more than two components, so no conclusions can be drawn about campaigns using other combinations of components.
In hospital settings, education or promotion resulted in small improvements in coverage. Only Ohrt and McKinney20 found a substantial improvement, which might have been due in part to low vaccine uptake at baseline. Similarly, campaigns involving only improved access to the vaccine had minimal impact. Conversely, campaigns involving legislative or regulatory components (e.g., mandatory declination form, mandatory masks for unvaccinated personnel) achieved higher rates than other interventions.
The major shortcomings of the reviewed studies were failure to report the number of health care personnel exposed to the campaign and the number of health care personnel for whom there was no follow-up. Ascertainment of vaccination status often excluded off-site vaccination, which resulted in underestimation of coverage. To assess the association between a campaign and the subsequent vaccination rate, health care personnel should be tracked for their exposure to the intervention and their resulting vaccination status. Rates stratified by level of direct contact with patients may inform future efforts to target specific high-risk groups.
Among the excluded studies, single-group before-and-after studies and cross-sectional studies were the most common study designs. Such studies are more logistically feasible than more rigorously designed studies, but they do not control for factors outside the “intervention” that may inflate or diminish the observed outcome. Organizers conducting campaign evaluations for before-and-after studies should consider having a comparable control group. Organizations are encouraged to monitor and report annual vaccination rates for health care personnel over time, to improve the accuracy of observed outcomes and to provide multiple observation points for an interrupted time series design.
Limitations
The limitations of this review included inability to pool data across studies because of heterogeneity in study methods and campaign components. In addition, the study methods had several risks of bias that might have generated misleading results, such as lack of comparable baseline characteristics across study groups. In our review, we did not assess the impact of pandemic influenza programs. Three of the excluded studies26–28 incorporated “pandemic vaccination drills” as part of their respective seasonal campaigns. The effect of pandemic influenza on vaccination coverage for seasonal influenza among health care personnel is unknown.
Conclusion
This review revealed gaps in the literature about the appropriate campaign components for increasing influenza vaccination among health care personnel. To determine the appropriate design and components of influenza vaccination campaigns for health care personnel, rigorously designed studies assessing the effect of various campaign components design are needed.
Supplementary Material
Footnotes
Funding: The systematic review was supported by the Ontario Ministry of Health and Long-Term Care. Additional support was provided by the Élisabeth Bruyère Research Institute, The Ottawa Hospital, the Ottawa Hospital Research Institute, the Canadian Center for Vaccinology, the University of Ottawa and the Canadian Institutes of Health Resarch (CIHR). In 2008, Ms. Lam received a Frederick Banting and Charles Best Canada Graduate Scholarship from CIHR. While doing this work, she also was enrolled in the Ontario Training Centre in Health Services and Policy Research, which is funded in part by the Canadian Health Services Research Foundation.
Previously published at www.cmaj.ca
Competing interests: None declared.
Contributors: All authors were involved in developing the review protocol and identifying the search strategy. Po-Po Lam and Donna Pierrynowski MacDougall retrieved the articles and extracted data. Po-Po Lam, Larry Chambers and Donna Pierrynowski MacDougall analyzed and interpreted the data. Po-Po Lam and Larry Chambers drafted the manuscript. All authors contributed to the development, review and revision of the manuscript, and all authors approved the final version for publication.
This article has been peer reviewed.
REFERENCES
- 1.Nichol KL. Efficacy and effectiveness of influenza vaccination. Vaccine. 2008;26(Suppl 4):D17–22. doi: 10.1016/j.vaccine.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 2.Thomas RE, Jefferson T, Demicheli V, et al. Influenza vaccination for healthcare workers who work with the elderly [review] Cochrane Database Syst Rev. 2006;3:CD005187. doi: 10.1002/14651858.CD005187.pub2. [DOI] [PubMed] [Google Scholar]
- 3.National Advisory Committee on Immunization. Statement on influenza vaccination for the 2008–2009 season. An Advisory Committee statement. Can Commun Dis Rep. 2008;34(ACS-3):1–46. [PubMed] [Google Scholar]
- 4.Johansen H, Sambell C, Zhao W. Flu shots—national and provincial/territorial trends. Health Rep. 2006;17:43–8. [PubMed] [Google Scholar]
- 5.Stevenson CG, McArthur MA, Naus M, et al. Prevention of influenza and pneumococcal pneumonia in Canadian long-term care facilities: How are we doing? CMAJ. 2001;164:1413–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson ML, Bridges CB, Harper SA Healthcare Infection Control Practices Advisory Committee (HICPAC); Advisory Committee on Immunization Practices (ACIP) Influenza vaccination of health-care personnel: recommendations of the healthcare infection control practices advisory committee (HICPAC) and the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-2):1–16. [PubMed] [Google Scholar]
- 7.Mereckiene J, Cotter S, Nicoll A, et al. National seasonal influenza vaccination survey in Europe, 2008. Euro Surveill. 2008;13(43) doi: 10.2807/ese.13.43.19017-en. pii:19017. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson VJ, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates [review] Cochrane Database Syst Rev. 2005;(3):CD003941. doi: 10.1002/14651858.CD003941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann F, Ferracin C, Marsh G, et al. Influenza vaccination of healthcare workers: a literature review of attitudes and beliefs. Infection. 2006;34:142–7. doi: 10.1007/s15010-006-5109-5. [DOI] [PubMed] [Google Scholar]
- 10.Ndiaye SM, Hopkins DP, Shefer AM, et al. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(Suppl):248–79. doi: 10.1016/j.amepre.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Talbot TR. Do declination statements increase health care worker influenza vaccination rates? Clin Infect Dis. 2009;49:773–9. doi: 10.1086/605554. [DOI] [PubMed] [Google Scholar]
- 12.EPOC methodological search filters. Ottawa (ON): University of Ottawa, Cochrane Effective Practice and Organisation of Care Group; [(accessed 2008 Apr. 29)]. Available: www.epoc.cochrane.org/en/newPage1.html. [Google Scholar]
- 13.EPOC-specific resources for review authors. Ottawa (ON): University of Ottawa, Cochrane Effective Practice and Organisation of Care Group; [(accessed 2008 Apr. 29)]. Available: www.epoc.cochrane.org/en/handsearchers.html. [Google Scholar]
- 14.Dey P, Halder S, Collins S, et al. Promoting uptake of influenza vaccination among health care workers: a randomized controlled trial. J Public Health Med. 2001;23:346–8. doi: 10.1093/pubmed/23.4.346. [DOI] [PubMed] [Google Scholar]
- 15.Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ. 2006;333:1241. doi: 10.1136/bmj.39010.581354.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura AC, Nguyen CN, Higa JI, et al. The effectiveness of vaccine day and educational interventions on influenza vaccine coverage among health care workers at long-term care facilities. Am J Public Health. 2007;97:684–90. doi: 10.2105/AJPH.2005.082073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemaitre M, Meret T, Rothan-Tondeur M, et al. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster-randomized trial. J Am Geriatr Soc. 2009;57:1580–6. doi: 10.1111/j.1532-5415.2009.02402.x. [DOI] [PubMed] [Google Scholar]
- 18.Tannenbaum TN, Thomas D, Baumgarten M, et al. Evaluation of an influenza vaccination program for nursing home staff. Can J Public Health. 1993;84:60–2. [PubMed] [Google Scholar]
- 19.Doratotaj S, Macknin ML, Worley S. A novel approach to improve influenza vaccination rates among health care professionals: a prospective randomized controlled trial. Am J Infect Control. 2008;36:301–3. doi: 10.1016/j.ajic.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Ohrt CK, McKinney WP. Achieving compliance with influenza immunization of medical house staff and students. A randomized controlled trial. JAMA. 1992;267:1377–80. [PubMed] [Google Scholar]
- 21.Harbarth S, Siegrist CA, Schira JC, et al. Influenza immunization: improving compliance of healthcare workers. Infect Control Hosp Epidemiol. 1998;19:337–42. doi: 10.1086/647825. [DOI] [PubMed] [Google Scholar]
- 22.Polgreen PM, Pottinger J, Polgreen LA, et al. Influenza vaccination rates, feedback and the hawthorne effect. Infect Control Hosp Epidemiol. 2006;27:98–9. doi: 10.1086/499393. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman RK, Nowalk MP, Lin CJ, et al. Factorial design for improving influenza vaccination among employees of a large health system. Infect Control Hosp Epidemiol. 2009;30:691–7. doi: 10.1086/598343. [DOI] [PubMed] [Google Scholar]
- 24.Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control. 2007;35:33–7. doi: 10.1016/j.ajic.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Wicker S. Unvaccinated health care workers must wear masks during flu season —a possibility to improve influenza vaccination rates? Vaccine. 2009;27:2631–2. doi: 10.1016/j.vaccine.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Kuntz JL, Holley S, Helms CM, et al. Use of a pandemic preparedness drill to increase rates of influenza vaccination among healthcare workers. Infect Control Hosp Epidemiol. 2008;29:111–5. doi: 10.1086/526434. [DOI] [PubMed] [Google Scholar]
- 27.Stubbe KK, Luebbert PP, Madison L. Drill down: increasing influenza vaccination rates while testing for pandemic preparedness. Am J Infect Control. 2007;35:E43. [Google Scholar]
- 28.Yang KS, Fong YT, Koh D, et al. High coverage of influenza vaccination among healthcare workers can be achieved during heightened awareness of impending threat. Ann Acad Med Singapore. 2007;36:384–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.