Abstract
Background
West Nile virus (WNV) is a neurotropic flavivirus transmitted to humans by mosquito vectors. Homozygosity for CCR5Δ32, a complete loss-of-function mutation in the chemokine receptor CCR5, has been previously associated with severe symptomatic WNV infection in patients presenting with clinical disease, however whether it acts at the level of initial infection or in promoting clinical progression is unknown.
Methods
Here we address this gap in knowledge by comparing CCR5Δ32 distribution in US blood donors identified through a comprehensive blood supply screening program (n=34,766,863 donations from 2003–2008) as either WNV true positive (WNV+ cases; n=634) or false positive (WNV− controls; n=422).
Results
No difference was observed in CCR5Δ32 homozygous frequency between the WNV+ cases and WNV− controls, suggesting that CCR5 deficiency is not a risk factor for initial infection. However, CCR5Δ32 homozygosity was associated with worse clinical outcome, with these individuals developing more clinical symptoms as determined by a standardized questionnaire (P=0.0016).
Conclusions
Thus CCR5 deficiency is not a risk factor for WNV infection per se, but is a risk factor for both early and late clinical manifestations after infection. Our study also illustrates the power of large scale databases to answer important clinical questions in man.
Keywords: CCR5, polymorphism, West Nile virus, genetic risk, blood donations, clinical outcome, early symptomatology
Introduction
West Nile virus (WNV) is a mosquito-borne flavivirus that has caused annual outbreaks since its entry into the US in 1999 [1]. As of November 18, 2008, 28,906 confirmed cases of symptomatic WNV infection have been reported to the US Centers for Disease Control and Prevention (CDC) with 1121 (~4%) fatalities (www.cdc.gov). Currently, there are no specific treatment options or licensed vaccines for humans approved by the FDA [2, 3]. Transmission of WNV can occur through blood transfusion and organ transplantation, and by 2003, universal screening for WNV RNA was implemented for essentially all blood donations in the US [4–6]. These studies have shown that WNV infection in the general population remains rare, with seropositivity being found in approximately 1 in 10,000 blood donors [7, 8].
It is estimated that most WNV-infected individuals remain asymptomatic, with 20% to 30% developing symptoms after a 2–14 day latent period [9]. Clinical features can range from mild flu-like symptoms (West Nile fever; WNF) to more severe neuroinvasive disease (WNND) [9]. Clinical features of WNF typically include fever, headache, fatigue, skin rash, swollen lymph glands, and eye pain (www.cdc.gov). WNND manifests primarily as meningitis, encephalitis, and/or acute-flaccid paralysis, and associated severe clinical features commonly include diarrhea/vomiting, generalized weakness, bone/joint pain, severe muscle aches, tremors, seizures, new difficulty thinking, and changes in mental status [9]. Thus, outcome of WNV infection is heterogeneous and identification of risk factors affecting outcome is an important goal.
In a mouse model of WNV disease, CCR5 was found to be a strong host defense factor, with CCR5 deficient mice displaying markedly increased viral titers in the CNS and mortality [10]. We have extended these findings in mice to humans by demonstrating a strong epidemiologic association between symptomatic WNV disease and homozygosity for a common, complete loss-of-function mutation named CCR5Δ32 in the chemokine receptor CCR5 (OR=4.2, 95% CI [2.1–8.3], P<0.0001) [11, 12]. Although these studies showed a strong, reproducible effect using WNV positive patients with a clinical diagnosis of WNF or WNND from 4 geographically and temporally distinct US collections, they were unable to distinguish whether the association was with susceptibility or with severity of clinical presentation since our previous analyses were retrospective, did not include individuals who remained asymptomatic or mildly symptomatic, and did not record specific clinical symptoms. To evaluate whether all CCR5Δ32 individuals have higher susceptibility to infection and/or a different clinical presentation compared to individuals with a wild type allele, we retrospectively screened a non-concurrent prospective cohort of nearly 35 million blood donors from the American Red Cross to identify asymptomatic and symptomatic WNV+ Caucasians, and compared CCR5Δ32 genotypic frequencies and self-reported symptoms associated with WNV disease.
Materials and Methods
Study populations
The study was approved by the Office of Human Subjects Research of the National Institutes of Health. All blood samples from random blood donors collected by the American Red Cross (ARC) between May 2003 and July 2008 were tested for reactivity to a WNV-nucleic acid amplification testing (NAT) as previously described [7, 13]. Upon identification of a WNV NAT reactive donation, donors were contacted for enrollment/participation in the follow-up study, which included a questionnaire regarding symptoms. Prior to learning their true WNV infection status, all donors were administered a follow-up standardized check-box questionnaire by trained personnel in the course of an in-person interview. Blood donors that tested reactive on the initial screen were further tested and defined as either WNV true positive (n=656) if repeat WNV NAT and WNV-specific antibody test were positive, or WNV false positive controls (n=431) if repeat WNV NAT and WNV-specific antibody results were negative. The questionnaire provided information for all donors regarding age, race, sex, date of sample collection, and the presence or absence of 15 listed symptoms consistent with WNV infection and other self-reported symptoms occurring during the two weeks following donation. The 15 listed symptoms were fever, chills, headache, painful eyes, severe muscle pain, swollen glands, new rash, seizures, tremors, new difficulty thinking, generalized weakness, joint pain, bone pain, vomiting or diarrhea, and abdominal pain. All blood components associated with a donation that was reactive on the initial WNV NAT were quarantined and the plasma unit was retrieved for further testing.
DNA isolation and genotyping
DNA from 250 μl of index plasma samples was purified and eluted into 100 μl of recommended buffer using NucliSENS EasyMAG technology (Biomerieux, Inc). Genotyping was performed by methods described previously [12]. Briefly, 2 μl of DNA was amplified by PCR using primers that flank the site of the 32 bp deletion: 5'-TGTTTGCGTCTCTCCCAG -3' and 5'- CACAGCCCTGTGCCTCTT -3'. PCR products were analyzed by electrophoresis on a 3.0% agarose/TBE gel with known wild type CCR5 and CCR5Δ32 amplicon controls (233 and 201 bp, respectively) and visualized with Gelstar (Cambrex, Walkersville, MD) DNA staining. Each sample was tested in two independent PCR reactions, and results were concordant, as determined by 2 independent investigators.
Statistical analysis
Odds Ratios (OR) and 95% Confidence Intervals (CI) were calculated using a recessive genetic model (Graphpad Software, San Diego, CA). Significance was determined by the Fisher's exact test using a two-sided test. Hardy-Weinberg equilibrium was judged using a chi-square test and 2 degrees of freedom to calculate expected frequencies of the three genotypes. The two-sided unpaired t-test was used to calculate statistical significance of the number of symptoms reported according to genotype. Logistic regression analysis was used to estimate the probability of homozygosity as a function of the square root of the number of symptoms. A likelihood ratio test was used to test the null hypothesis.
Results
From May 2003 through July 2008, a total of 34,766,863 blood donations were screened by the nationwide WNV blood screening program of the American Red Cross (ARC) by WNV nucleic acid amplification test (NAT). As shown in Figure 1, the initial screening of these samples revealed 1,892 donations of the nearly 35 million blood donations to be reactive by WNV NAT. Since this initial WNV NAT screening has a high false positivity rate, these samples were further tested through serology and a follow-up WNV NAT to determine true or false WNV positivity. Of the 1,892 donations identified as potentially positive, 1,065 donations were confirmed as WNV true positives (WNV+ cases; 1 in 32,645) through serology and a second WNV NAT; the remaining 827 donations were WNV false positive (WNV− controls), defined as negative by both serology and a follow-up WNV NAT. Based on sample availability and the inclusion criteria for this study (defined as self-reported Caucasian/non-Hispanic race, information available regarding age and gender, and questionnaire completed), we were able to analyze data from 656 unique WNV+ cases representing 35 states and 431 unique WNV− controls also from 35 states. CCR5 genotypes were obtained for 634 WNV+ cases and 422 WNV− control samples, with an overall success rate of 96.1%. .
Figure 1.
Identification of WNV+ cases and WNV− controls in the American Red Cross national blood donor screening program, 2003–2008. NAT, nucleic acid amplification test; serology was tested using IgM and/or IgM/IgG antibody test; inclusion criteria were self-reported Caucasian/non-Hispanic race, information available regarding age and gender, and questionnaire completed.
The characteristics of the study subjects, including symptoms experienced two weeks post-donation are shown in Table 1. Cases and controls were similar in age, but were more likely to be male, as previously observed [9]. The frequency of individuals reporting no symptoms by questionnaire after donation was significantly greater in cases compared to controls (75.6% vs. 33.6%; OR=5.42, 95% CI [4.15–7.09], P<0.0001). When each symptom was evaluated separately, cases were more likely than controls to report symptoms (Table 1), as expected and as previously reported [13].
Table 1.
Characteristics of WNV+ cases and WNV− control study subjects.
| WNV+ Cases | WNV− Controls | OR | 95% CI | p value | |
|---|---|---|---|---|---|
| N | 634 | 422 | |||
| Mean Age (y) | 48.37±13.93 | 46.74±15.18 | 0.08 | ||
| Male Sex (%) | 207 (58.4) | 370 (49.1) | 1.46 | 1.136–1.865 | 0.0036 |
| No Symptoms (%) | 169 (26.7) | 280 (66.4) | 0.18 | 0.141–0.241 | <0.0001 |
| Fever (%) | 24.4 | 13.4 | 3.18 | 2.182–4.628 | <0.0001 |
| Chills (%) | 18.8 | 9.6 | 3.25 | 2.111–5.009 | <0.0001 |
| Headache (%) | 46.7 | 30.2 | 3.32 | 2.507–4.407 | <0.0001 |
| Painful Eyes (%) | 18.8 | 7.2 | 4.41 | 2.725–7.145 | <0.0001 |
| Severe Muscle Pain (%) | 22.4 | 8.9 | 4.40 | 2.835–6.816 | <0.0001 |
| Swollen Glands (%) | 11.0 | 6.2 | 2.79 | 1.634–4.750 | 0.0002 |
| New Rash (%) | 31.9 | 7.2 | 8.93 | 5.583–14.28 | <0.0001 |
| Seizures (%) | 0.0 | 0.0 | - | - | - |
| Tremors (%) | 0.8 | 0.7 | 1.67 | 0.3222–8.648 | 0.8 |
| New Difficulty Thinking (%) | 6.9 | 2.7 | 3.86 | 1.798–8.285 | 0.0004 |
| Generalized Weakness (%) | 39.4 | 14.4 | 5.89 | 4.124–8.412 | <0.0001 |
| Joint Pain (%) | 22.6 | 7.6 | 5.30 | 3.316–8.455 | <0.0001 |
| Bone Pain (%) | 7.9 | 3.4 | 3.53 | 1.768–7.038 | 0.0003 |
| Vomiting/Diarrhea (%) | 13.1 | 7.9 | 2.61 | 1.618–4.221 | <0.0001 |
| Abdominal Pain (%) | 8.5 | 6.9 | 1.87 | 1.103–3.175 | 0.0256 |
| Other Symptom (%) | 26.3 | 13.4 | 3.51 | 2.416–5.104 | <0.0001 |
Data on symptoms experienced 2 weeks post-donation were self-assessed and based on a face-to-face interview and questionnaire. OR, odds ratio; CI, confidence interval; WNV, West Nile virus.
To test whether CCR5Δ32 is associated with susceptibility to WNV infection, we compared genotypic frequencies of cases to controls. Within these groups, the CCR5 genotypes were in Hardy-Weinberg equilibrium (P=0.13 and P=0.16, respectively). In control samples, ten of 422 (2.4%) were CCR5Δ32 homozygotes (Table 2). This value was identical to the frequency of CCR5Δ32 homozygotes observed in WNV+ cases, with fifteen of the 634 informative cases (2.4%) homozygous for this allele (OR=1.0, 95% CI [0.44–2.24], P=0.99; Table 2). These data indicate that CCR5Δ32 homozygosity is not associated with WNV infection per se. It is important to note that there is a fundamental difference in the way participants were identified in the present study versus our previous studies reporting an association between CCR5Δ32 homozygosity and symptomatic WNV disease. The previous studies selected for individuals seeking medical attention for symptomatic disease, whereas the ARC donors in the present study were accrued as voluntary blood donors independent of symptoms, and thus lacking selection bias. Therefore, we next hypothesized that CCR5Δ32 homozygosity is associated with symptoms in early WNV infection, which the design of the present study uniquely allowed us to test by examining the distribution of the average number of symptoms according to genotype. The symptoms evaluated here are based on a previously published and validated follow-up questionnaire [13] that was administered to all individuals that were reactive on the initial WNV NAT screening test (both cases and controls) prior to learning their WNV infection status. As shown in Figure 2A, CCR5Δ32 homozygotes experienced more symptoms on average (5.47 symptoms) versus heterozygotes (2.81 symptoms) and wild type individuals (3.05 symptoms; P=0.002). This increase in symptom number was not observed among the CCR5Δ32 homozygotes identified in the WNV− control group (P=0.49; Figure 2A) and was not due to increased age among the CCR5Δ32 homozygotes, since the average age for WNV seropositive CCR5 wild type (48.5±14), CCR5Δ32 heterozygotes (48.0±13), and CCR5Δ32 homozygotes (47.5±11) is very similar. To further understand the relationship between CCR5Δ32 homozygosity and symptoms, we stratified the ARC cases into 2 groups, those with or without symptoms. As shown in Table 2, we identified no CCR5Δ32 homozygotes among the 169 asymptomatic WNV+ cases, which was less than expected based on the overall frequency observed in the ARC cohort (4.4 CCR5Δ32 homozygotes expected), and significantly decreased when compared to the frequency observed among the 280 asymptomatic WNV− controls, where 7 CCR5Δ32 homozygotes were identified (P<0.05). In contrast, when the frequency of this value was compared to that for cases who developed symptoms (n=465), a significant difference was observed (Figure 2B; 0% vs. 3.2%, respectively; P=0.015). No significant differences were observed when the asymptomatic and symptomatic controls were compared (P=1.0), or when symptomatic WNV+ cases were compared to symptomatic WNV− controls (P=0.78). Finally, we performed a logistic regression analysis plotting the predicted probability curve of CCR5Δ32 homozygosity as a function of the number of symptoms (Figure 2C) and found a significant positive correlation (P=0.0016).
Table 2.
CCR5 genotypes of study subjects.
| WNV+ Cases | WNV− Controls | |
|---|---|---|
| All Blood donors (n) | 634 | 422 |
| CCR5/CCR5 (%) | 515 (81.2) | 348 (82.4) |
| CCR5/Δ32 (%) | 104 (16.4) | 64 (15.2) |
| Δ32/Δ32 (%) | 15 (2.4) | 10 (2.4) |
|
| ||
| No Symptoms (n) | 169 | 280 |
| CCR5/CCR5 (%) | 139 (82.2) | 230 (82.1) |
| CCR5/Δ32 (%) | 30 (17.8) | 43 (15.4) |
| Δ32/Δ32 (%) | 0 (0) | 7 (2.5) |
|
| ||
| ≥ Symptoms (n) | 465 | 142 |
| CCR5/CCR5 (%) | 376 (80.9) | 118 (83.1) |
| CCS5/Δ32 (%) | 74 (15.9) | 21 (14.8) |
| Δ32/Δ32 (%) | 15 (3.2) | 3 (2.1) |
Symptoms were self-assessed and determined based on a face-to-face interview and questionnaire. WNV, West Nile virus; CCR5, wild type CCR5 allele; Δ32, CCR5 Δ32 allele.
Figure 2.
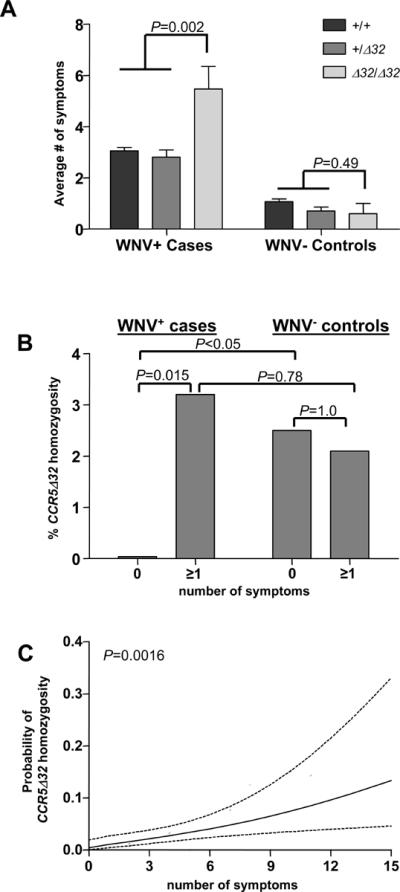
Association of CCR5Δ32 homozygosity and West Nile virus-related symptoms. (A) The number of symptoms reported among WNV+ cases and WNV− controls was averaged (±SEM) according to CCR5 genotype. An unpaired t-test was used to calculate significance of the difference between CCR5Δ32 homozygotes versus CCR5Δ32 heterozygotes and CCR5 wild-type individuals. +, CCR5 wild-type allele; Δ32, CCR5Δ32 allele. (B) CCR5Δ32 homozygous frequency among WNV+ cases or WNV− controls with 0 symptoms was compared to individuals reporting ≥1 symptoms. (C) The predicted probability curve (solid line) along with upper and lower 95% confidence intervals (dotted lines) of CCR5Δ32 homozygotes is plotted as a function of the number of symptoms.
To understand whether any specific symptoms were associated with CCR5Δ32 homozygosity, we evaluated the symptom prevalence among the cases in the CCR5Δ32 homozygotes versus carriers of the wild type allele. As shown in Table 3, significant increases were found for CCR5Δ32 homozygotes reporting painful eyes (P=0.003), swollen glands (P=0.018), generalized weakness (P=0.013), vomiting/diarrhea (P=0.035), and abdominal pain (P=0.032). Of note, painful eyes, which could indicate photophobia, headache, tremors, and new difficulty thinking, all of which could be associated with neurologic disease, were increased, although only the first reached statistical significance. Together, these data demonstrate that CCR5Δ32 homozygosity is associated with a more aggressive clinical presentation compared to carriers of a normal allele.
Table 3.
Symptom prevalence according to CCR5 genotype.
| Symptom Frequency among +/+ and +/Δ32 individuals | Symptom Frequency among Δ32/Δ32 individuals | OR | 95% CI | P value | |
|---|---|---|---|---|---|
| Fever (%) | 24.1 | 40.0 | 2.10 | 0.74–6.01 | 0.219 |
| Chills (%) | 18.4 | 33.3 | 2.22 | 0.84–6.61 | 0.174 |
| Headache (%) | 46.2 | 66.7 | 2.33 | 0.79–6.89 | 0.126 |
| Painful Eyes (%) | 17.9 | 53.3 | 5.23 | 1.86–14.7 | 0.003 |
| Severe Muscle Pain (%) | 22.1 | 33.3 | 1.76 | 0.59–5.23 | 0.345 |
| Swollen Glands (%) | 10.5 | 33.3 | 4.26 | 1.41–12.9 | 0.018 |
| New Rash (%) | 31.7 | 40.0 | 1.44 | 0.51–4.10 | 0.576 |
| Seizures (%) | 0.0 | 0.0 | 0.00 | - | - |
| Tremors (%) | 0.6 | 6.7 | 10.98 | 0.208–119.5a | 0.113 |
| New Difficulty Thinking (%) | 6.8 | 13.3 | 2.11 | 0.46–9.68 | 0.280 |
| Generalized Weakness (%) | 38.6 | 73.3 | 4.37 | 1.38–13.9 | 0.013 |
| Joint Pain (%) | 22.1 | 40.0 | 2.35 | 0.82–6.71 | 0.118 |
| Bone Pain (%) | 7.8 | 13.3 | 1.83 | 0.40–8.35 | 0.334 |
| Vomiting/Diarrhea (%) | 12.6 | 33.3 | 3.47 | 1.15–10.4 | 0.035 |
| Abdominal Pain (%) | 8.1 | 26.7 | 4.14 | 1.27–13.5 | 0.032 |
| Other Symptom (%) | 26.0 | 40.0 | 1.90 | 0.66–5.41 | 0.240 |
Values were calculated by dividing the number of individuals of a certain genotype reporting the symptom by the total number of individuals with that genotype. P values were not corrected for multiple testing because there is only one independent hypothesis;
CI was calculated using the exact method for tremors due to small numbers.
+, CCR5 wild type allele; Δ32, CCR5 Δ32 allele; OR, odds ratio, CI, confidence interval.
Discussion
In this study we show, using samples collected from random blood donors, that CCR5 deficiency is not associated with increased susceptibility to infection, but rather a more aggressive early clinical presentation. Our study not only suggests that WNV infection of a CCR5Δ32 homozygote will result in early symptom development, but also that CCR5Δ32 homozygotes are more likely to experience more aggressive disease, with a higher frequency of individual symptoms and more total symptoms, compared to CCR5 sufficient individuals. WNV infection in CCR5Δ32 homozygous individuals were more likely to present with multisystem symptomatology; including lymphadenopathy, neurologic and gastrointestinal symptoms. The newly found role of CCR5Δ32 homozygosity as a predictor of severity of clinical presentation is consistent with the hypothesis that functional CCR5 is critical for control of WNV in man.
It is important to note that the present study is a retrospective analysis of a non-concurrent prospective cohort, with the WNV+ cases identified prior to the onset of symptoms and thus lacking selection bias, whereas our previous studies of WNV-related symptoms were retrospective and selected for individuals seeking medical attention for clinical manifestations of WNV disease. The current study also captures a wide range of clinical presentation of initial infection, including individuals who remained healthy and those who experienced symptoms. Thus, unlike our previous study that analyzed patients at a later stage of disease, the current study evaluates WNV-infected individuals at an early stage of disease. The current study design allowed us to directly compare CCR5Δ32 frequency in symptomatic WNV+ cases to both asymptomatic WNV+ cases and uninfected WNV− controls, and therefore to evaluate the role of CCR5Δ32 in both susceptibility to infection and severity of disease. Our data show no evidence for increased susceptibility or symptoms associated with CCR5Δ32 heterozygotes since the heterozygous frequency was similar between cases and controls (Table 2), identical to wild-type individuals in terms of number of symptoms experienced (Figure 2A), and identical to wild-type individuals with regard to individual symptom prevalence (data not shown). These data suggest that normal CCR5 expression is in excess relative to what is needed for normal host defense against WNV, and that a mutation in both alleles is needed to confer a deficit. The mechanism by which CCR5 functions at the biochemical level in vivo is not known and may involve dimerization with another chemokine receptor. Potential partners based on previously reported biochemical data include CXCR4 and CCR2 [14, 15].
The CCR5Δ32 homozygous frequency observed in the WNV false positive controls (2.4%) was slightly elevated compared to previous reports for this genotype in US Caucasians, which range from 0.7–1.7% [11, 16–21]. It is important to note that the study populations tested in these previous studies were sampled from one region/city of the US, whereas the control population tested in the current study is the first to our knowledge to evaluate CCR5Δ32 homozygous frequency based on large scale sampling of the entire nation. In any case, the frequency we found is only 0.7% greater than the upper range previously reported. Other virtues of the control group used in the present study are that they: 1.) were temporally and geographically matched to the cases; 2.) were accrued in the same manner; and 3.) had filled out and submitted the same symptom questionnaire as the cases. The only other potential control group available from among the ~35 million donations is one composed of those who screened negative at the time of blood donation (true negatives); however these donors would not have filled out a questionnaire since they were non-reactive for the initial WNV NAT screening test and thus were not enrolled in the WNV follow-up study. Even if these patients had been enrolled in the study, they would have answered the questionnaire regarding symptoms with the knowledge that they were WNV negative, which might bias the response regarding symptoms.
It is also important to consider potential limitations to our study. First, there may be recall bias, since all individuals were aware of their potential infection with WNV and may have been more likely to report symptoms. However, both cases and controls answered the questionnaire prior to learning the results of their true WNV infection status and thus a similar bias would be expected. Since the latent period prior to symptom onset has been estimated to be 2–14 days, it is possible that a donor could have tested positive for WNV but already have experienced the majority of their symptoms, causing an underreporting of symptoms on the questionnaire for this group. Second, evaluation of symptoms was based on participant response, rather than objective measurements, and therefore could be imprecise. Finally, our results do not address the mechanism of action of CCR5 during WNV pathogenesis. Our study of WNV in an excellent mouse model has suggested it works in part at the level of leukocyte trafficking, at least for neuroinvasive disease; however mechanisms in man could differ [10].
WNV is the first human disease for which normal CCR5 has clearly been shown to be beneficial [11, 12, 22]. Prior to these studies, CCR5 deficient individuals were believed to have minimal, if any, health defects [23]. In contrast, they were well-known to have strong resistance to HIV infection, because normal CCR5 is used as a critical coreceptor for cell entry. This finding led to the development of Maraviroc, an effective CCR5 antagonist used in the treatment of HIV/AIDS [24]. Although Maraviroc had an excellent safety profile in clinical trials, as it is marketed and used on a larger scale it will be important to maintain a high index of suspicion in HIV-infected individuals who present with signs and symptoms compatible with WNV infection, particularly since these patients are already immunocompromised. Our results suggest that if a patient who is currently taking Maraviroc does test positive for WNV infection or displays the characteristic symptoms during an outbreak that the drug be held until the infection is cleared. A more general caution regarding WNV risk may apply to any CCR5 blocking agent that might be developed, and any disease in which it might be used. It will also be interesting to investigate the susceptibility of CCR5 deficient individuals to other types of flaviviruses. In this regard, homozygous CCR5Δ32 has recently been associated in one study with increased susceptibility to Tick-borne encephalitis virus [25].
Although our study specifically highlights the importance of a single genetic risk factor in a specific infectious disease, it also illustrates the power of large scale databases to answer important clinical questions in man, particularly for rare diseases and/or rare genetic mutations.
Supplementary Material
Acknowledgements
The authors would like to thank Xiao Liu for statistical assistance, Elisa Saba for assistance in nucleic acid extraction, and Christopher Obara and Engin Erdoğan for assistance with figure preparation. This research was supported by the Intramural Research Program of the National Institutes of Health, NIAID/DIR.
(2) This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- WNV
West Nile virus
- OR
Odds Ratio
- CI
Confidence Interval
Footnotes
(3) This work has not been presented previously.
(5) J.L., no conflict; D.M., no conflict; A.L., no conflict; G.F., no conflict; D.K., no conflict; D.F., no conflict; S.S., no conflict; and P.M., no conflict
(1) Potential conflicts of interest: none reported.
References
- 1.Asnis DS, Conetta R, Teixeira AA, Waldman G, Sampson BA. The West Nile Virus outbreak of 1999 in New York: the Flushing Hospital experience. Clin Infect Dis. 2000;30:413–8. doi: 10.1086/313737. [DOI] [PubMed] [Google Scholar]
- 2.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45:1039–46. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 3.Throsby M, Ter Meulen J, Geuijen C, Goudsmit J, de Kruif J. Mapping and analysis of West Nile virus-specific monoclonal antibodies: prospects for vaccine development. Expert Rev Vaccines. 2007;6:183–91. doi: 10.1586/14760584.6.2.183. [DOI] [PubMed] [Google Scholar]
- 4.Busch MP, Caglioti S, Robertson EF, et al. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N Engl J Med. 2005;353:460–7. doi: 10.1056/NEJMoa044029. [DOI] [PubMed] [Google Scholar]
- 5.Iwamoto M, Jernigan DB, Guasch A, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 6.Pealer LN, Marfin AA, Petersen LR, et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–45. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 7.Stramer SL, Fang CT, Foster GA, Wagner AG, Brodsky JP, Dodd RY. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451–9. doi: 10.1056/NEJMoa044333. [DOI] [PubMed] [Google Scholar]
- 8.Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–4. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 9.Hayes EB, Gubler DJ. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 2006;57:181–94. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- 10.Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–98. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass WG, McDermott DH, Lim JK, et al. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203:35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim JK, Louie CY, Glaser C, et al. Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J Infect Dis. 2008;197:262–5. doi: 10.1086/524691. [DOI] [PubMed] [Google Scholar]
- 13.Orton SL, Stramer SL, Dodd RY. Self-reported symptoms associated with West Nile virus infection in RNA-positive blood donors. Transfusion. 2006;46:272–7. doi: 10.1111/j.1537-2995.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal L, Lu X, Qingwen J, et al. Role for CCR5Delta32 protein in resistance to R5, R5X4, and X4 human immunodeficiency virus type 1 in primary CD4+ cells. J Virol. 2004;78:2277–87. doi: 10.1128/JVI.78.5.2277-2287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Asmar L, Springael JY, Ballet S, Andrieu EU, Vassart G, Parmentier M. Evidence for negative binding cooperativity within CCR5-CCR2b heterodimers. Mol Pharmacol. 2005;67:460–9. doi: 10.1124/mol.104.003624. [DOI] [PubMed] [Google Scholar]
- 16.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–62. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–3. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Hwangbo Y, Holte S, et al. Analysis of genetic polymorphisms in CCR5, CCR2, stromal cell-derived factor-1, RANTES, and dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin in seronegative individuals repeatedly exposed to HIV-1. J Infect Dis. 2004;190:1055–8. doi: 10.1086/423209. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Nerurkar VR, Dashwood WM, et al. Genotype and allele frequency of a 32-base pair deletion mutation in the CCR5 gene in various ethnic groups: absence of mutation among Asians and Pacific Islanders. Int J Infect Dis. 1999;3:186–91. doi: 10.1016/s1201-9712(99)90022-x. [DOI] [PubMed] [Google Scholar]
- 20.Michael NL, Louie LG, Sheppard HW. CCR5-delta 32 gene deletion in HIV-1 infected patients. Lancet. 1997;350:741–2. doi: 10.1016/S0140-6736(05)63552-0. [DOI] [PubMed] [Google Scholar]
- 21.Prahalad S. Negative association between the chemokine receptor CCR5-Delta32 polymorphism and rheumatoid arthritis: a meta-analysis. Genes Immun. 2006;7:264–8. doi: 10.1038/sj.gene.6364298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim JK, Glass WG, McDermott DH, Murphy PM. CCR5: no longer a “good for nothing” gene--chemokine control of West Nile virus infection. Trends Immunol. 2006;27:308–12. doi: 10.1016/j.it.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen GT, Carrington M, Beeler JA, et al. Phenotypic expressions of CCR5-delta32/delta32 homozygosity. J Acquir Immune Defic Syndr. 1999;22:75–82. doi: 10.1097/00042560-199909010-00010. [DOI] [PubMed] [Google Scholar]
- 24.Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–41. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kindberg E, Mickiene A, Ax C, et al. A deletion in the chemokine receptor 5 (CCR5) gene is associated with tickborne encephalitis. J Infect Dis. 2008;197:266–9. doi: 10.1086/524709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



