Abstract
Background
Significant alterations in γ-aminobutyric acid (GABA) and glutamate levels have been previously reported in symptomatic and remitted major depressive disorder (MDD); however, no studies to date have investigated potential associations between these amino acid neurotransmitters and treatment-resistance.
Methods
The objective of this study was to compare occipital cortex (OCC) and anterior cingulate cortex (ACC) GABA and glutamate+glutamine (“Glx”) levels measured in vivo by proton magnetic resonance spectroscopy (1H MRS) in 15 medication-free treatment-resistant depression (TRD) patients with those in 18 non-treatment-resistant MDD (nTRD) patients and 24 healthy volunteers (HVs).
Results
Levels of OCC GABA relative to voxel tissue water (W) were decreased in TRD patients compared to both HV (20.2% mean reduction; p=.001; Cohen’s d=1.3) and nTRD subjects (16.4% mean reduction; p=.007; Cohen’s d=1.4). There was a similar main effect of diagnosis for ACC GABA/W levels (p=.047; Cohen’s d=0.76) with TRD patients exhibiting reduced GABA in comparison to the other two groups (22.4–24.5% mean reductions). Group differences in Glx/W were not significant in either brain region in primary ANOVA analyses. Only GABA results in OCC survived correction for multiple comparisons.
Conclusions
Our findings corroborate previous reports of decreased GABA in MDD and provide initial evidence for a distinct neuronal amino acid profile in patients who have failed to respond to several standard antidepressants, possibly indicative of abnormal glutamate/glutamine/GABA cycling. Given interest in novel antidepressant mechanisms in TRD that selectively target amino acid neurotransmitter function, the translational relevance of these findings awaits further study.
Keywords: glutamate, GABA, magnetic resonance, spectroscopy, depression
INTRODUCTION
Previous research using proton magnetic resonance spectroscopy (1H MRS) has documented decreased concentrations (20–50%) of the inhibitory amino acid neurotransmitter γ-aminobutyric acid (GABA) in the occipital cortex (OCC) of medication-free patients with major depressive disorder (MDD), as well as smaller reductions (8.5–11%) in remitted MDD patients (1–4). Reductions in GABA levels of approximately 10% have been observed in the anterior cingulate cortex (ACC) of remitted unipolar depressed patients (4) and decreased prefrontal GABA [in a dorsomedial/dorsal-anterolateral region] was observed in unmedicated symptomatically depressed patients (5). Alterations in glutamate and/or glutamate+glutamine (“Glx”) concentrations have also been reported in MDD samples relative to healthy volunteers, with increases reported in OCC and decreases reported in prefrontal regions encompassing the ACC (2–5).
Agents that initially target amino acid neurotransmitter receptors have been experimentally tested in treatment-resistant depression (TRD) patients who have failed to respond to conventional antidepressant medications (6–8). The neurobiological substrates of TRD are obscure, in part due to definitional differences across studies and confounds of concurrent medication use. The clinical course and long-term prognosis for these patients could suggest a distinct neurobiology contributing to insufficient therapeutic response to monoaminergic antidepressants. While previous studies have classified MDD subgroups based on recovery status (remitted, symptomatic) and DSM-IV subtypes (melancholic, atypical), no study to our knowledge has examined subgroups determined by antidepressant medication resistance.
Strategies for operationally defining TRD vary widely and there is no well-validated consensus definition (9–11). We selected a categorical cut-off of insufficient response to at least three adequate antidepressant trials in the current episode based on especially poor remission rates for these patients following subsequent antidepressant treatment in a large outpatient MDD sample (12). Capturing treatment-resistance as a categorical rather than a continuous variable has both clinical and regulatory relevance, and reflects the observation that patients who surpass a threshold of treatment failures appear markedly more likely to fail subsequent treatment trials. OCC and ACC were selected as ROIs based on the above MRS findings, feasibility of obtaining high-quality spectroscopic data in these regions, and the integral role of ACC’s subregions in mood disorder pathophysiology (13). We predicted that TRD patients, in comparison to healthy volunteers and non-TRD patients with MDD (nTRD), would exhibit decreased GABA levels in OCC and ACC. Glutamate+glutamine (Glx) levels were hypothesized to be increased in OCC and decreased in ACC of TRD patients.
MATERIALS AND METHODS
Study Subjects
Treatment-seeking MDD patients were recruited via media advertisement or clinician referral. All participants signed informed consent after being given a complete description of the study, which was approved by the Institutional Review Boards of Mount Sinai School of Medicine and Weill Cornell Medical College. Diagnoses of MDD, including characterization of atypical, melancholic, and psychotic features during the current episode, and comorbid Axis I conditions were made according to DSM-IV-TR criteria, as established by the Structured Clinical Interview for DSM-IV (SCID-I/P) (14) and by independent interview by a board-certified psychiatrist. SCID interviewers were master’s-level or higher clinicians trained to criterion for diagnostic reliability. Eligible participants were psychotropic medication-free for ≥ 2 weeks prior to scan; free of substance abuse/dependence for ≥ 6 months; had no lifetime history of psychotic disorder, mania or hypomania; had no pervasive developmental disorder or mental retardation; had no current eating disorder; and had no clinically unstable medical or neurologic conditions. Of 35 MDD patients receiving scans, 33 participants had interpretable data from the OCC ROI while data from 2 patients were discarded due to poor spectral quality. An additional 5 MDD patients had unusable data from the ACC ROI and were included in OCC analyses only. Healthy volunteers (HVs) were recruited via media advertisement and were eligible if, in addition to exclusion criteria outlined above, they had no lifetime axis I disorder (per SCID-I/NP). Of 26 HVs scanned, data of adequate spectral quality was obtained from 24 participants in OCC and 21 participants in ACC. Subjects with unusable data had significantly greater age (p=.01) and body mass index (p=.048) than subjects with complete datasets, but did not differ on sex distribution or clinical variables (all p’s>.4).
The number of past antidepressant trials was determined by a physician-conducted phone or in-person interview using the Antidepressant Treatment History Form (ATHF), a validated instrument for assessing the strength and adequacy of past FDA-approved treatments with respect to type and dosage of treatments and adherence (15,16). Consistent with the recommendations of the ATHF manual, and in light of evidence that accuracy in patient-reported treatment history declines as a function of time (17), we focused on the current episode only. Three failed adequate antidepressant trials in the current episode was the minimum threshold for the designation of TRD (n=15); the nTRD group included treatment-naïve patients and those with <3 past trials (n=18).
Day-of-scan clinical assessments were performed using the Hamilton Rating Scale for Depression (HRSD17), Quick Inventory of Depressive Symptomatology (QIDS-SR16), Hamilton Rating Scale for Anxiety (HAM-A), Sheehan Disability Scale (SDS), Hollingshead Four-Factor Index of Social Position (HH-SES), and Family History Screen (18–23). Clinical and demographic characteristics of the sample are presented in Table 1.
Table 1.
Descriptive and Clinical Characteristics of Patients and Healthy Volunteers
| Healthy Volunteers (n=24)† | nTRD MDD (n=18)† | TRD (n=15)† | |
|---|---|---|---|
| Age, mean (SD), y | 37.25 (13.5); range: 21–60 | 38.3 (12.3) range: 23–65 | 46.80 (11.9)a range: 28–68 |
| Female, No. (%) | 13 (54%) | 6 (33%) | 7 (47%) |
| Non-hispanic Caucasian, No. (%) | 13 (54%) | 8 (44%) | 12 (80%) |
| IQ, mean (SD) | 114.95 (10.3) | 111.12 (16.0) | 112.64 (12.1) |
| Years of education, mean (SD) | 16.38 (1.9) | 15.38 (2.9) | 14.00 (2.6)a |
| Median household annual income | $35,000–49,999 | $15,000–34,999 | $15,000–34,999 |
| HH-SES, mean (SD) | 50.29 (10.7) | 43.67 (15.3) | 46.47 (9.8) |
| Body Mass Index, mean (SD), kg/m2 | 26.27 (4.6) | 26.83 (4.3) | 27.72 (4.9) |
| Family History of Mood Disorder, No. (%) | 1 (4%) | 5 (31%) c | 6 (40%) a |
| Any Comorbid Anxiety Disorder, No. (%) | - | 13 (72%) | 11 (73%) |
| Social Phobia | 9 (50%) | 5 (33%) | |
| Obsessive Compulsive Disorder | 2 (11%) | 1 (7%) | |
| Post-Traumatic Stress Disorder | 2 (11%) | 2 (13%) | |
| Panic Disorder | 0 (0%) | 1 (7%) | |
| Specific Phobia | 1 (6%) | 0 (0%) | |
| Generalized Anxiety Disorder | 5 (28%) | 1 (7%) | |
| Anxiety Disorder Not Otherwise Specified | 3 (17%) | 5 (33%) | |
| MDD Subtypes, No. (%) | - | ||
| Atypical | 1 (6%) | 3 (20%) | |
| Melancholic | 1 (6%) | 1 (7%) | |
| Psychotic | 2 (11%) | 1 (7%) | |
| Antidepressant NaÏve, No. (%) | - | 9 (50%) | 0 (0%)b |
| 2-week Washout Period, No. (%) | - | 2 (11%) | 7 (47%)b |
| Time Since Illness Onset, mean (SD), y | - | 21.80 (16.4) | 26.93 (10.8) |
| Age-of-Onset, mean (SD), y | - | 17.73 (14.3) | 22.20 (15.4) |
| Number of Episodes, mean (SD) | - | 2.73 (4.8) | 2.00 (2.1) |
| Single Episode (Non-remitting), No. (%) | - | 11 (61%) | 10 (67%) |
| Duration of Current Episode >= 2y, No. (%) | - | 15 (83%) | 15 (100%) |
| Number of Failed Trials in Current Episode, mean (SD) | - | 0.89 (0.9) | 5.43 (2.8)b |
| HRSD17, mean (SD) | 0.84 (0.9) | 17.94 (5.5)c | 24.40 (5.1)a,b |
| QIDS-SR16, mean (SD) | 1.34 (1.5) | 13.11 (4.0)c | 18.13 (4.1)a,b |
| HAM-A, mean (SD) | 1.42 (1.3) | 19.44 (6.9)c | 27.00 (7.9)a,b |
| SDS, mean (SD) | - | 17.06 (5.7) | 19.14 (10.5) |
Note: Continuous variables compared by ANOVA w/LSD post-hoc comparisons, dichotomous variables compared by Fisher’s exact test, 2-tailed; HV = Healthy Volunteer; nTRD MDD = Non-Treatment-Resistant Major Depressive Disorder; TRD = Treatment-Resistant Depression; HH-SES = Hollingshead Four-Factor Index of Social Position; HRSD17 = Hamilton Rating Scale for Depression, 17-item; QIDS-SR16 = Quick Inventory of Depressive Symptoms-Self-Report; HAM-A = Hamilton Anxiety Rating Scale; SDS = Sheehan Disability Scale (18–22)
Descriptives are presented for subjects with interpretable occipital lobe MRS data; for ACC analyses, 3 HVs, 2 nTRD MDDs, and 3 TRD participants had uninterpretable data due to motion-degraded spectral quality
TRDs differ from HVs, p < .05
TRDs differ from nTRD MDDs, p < .05
nTRD MDDs differ from HVs, p < .05
1H MRS Data Acquisition Methods
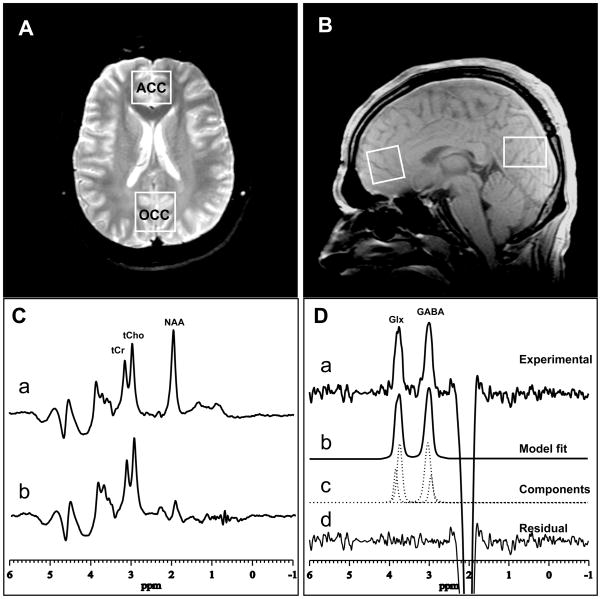
The GABA and Glx 1H spectra were recorded on a 3.0T GE ‘EXCITE’ MR system, using the standard J-edited spin echo difference method (24), as modified by Sailasuta et al (25), and a manufacturer-supplied 8-channel phased-array head coil to enhance detection sensitivity, as recently described (26). Our implementation of the J-editing sequence consisted of incorporating a pair of frequency-selective cosine-modulated Shinar-LeRoux inversion or “editing” rf pulses—flanked by spoiler gradients of opposite signs—before and after the second 180° rf pulse of the standard PRESS sequence, to turn it into a volume-selective J-edited spin echo difference spectroscopy method (24,25). Application of these frequency-selective editing pulses at 1.9 ppm (the resonance frequency of the GABA C-3 protons) on alternate scans inverts the outer lines of the C-4 GABA triplet proton resonance at 3.0 ppm after a spin echo time, TE, of 68 ms on alternate scans by, respectively, inhibiting and allowing its J-modulation (Fig. 1C). Subtracting the pair of sub-spectra thus acquired yields the desired GABA difference spectra, consisting of the outer lines of the GABA C-4 triplet at 3.0 ppm, while the much stronger overlapping Cr resonance—a singlet that is not J-modulated—is eliminated (Fig, 1D). Although this pulse sequence is optimized for GABA detection, it also achieves detection of the combined resonance for glutamate and glutamine (Glx) at 3.7 ppm (Fig. 1D), due to the high structural, chemical and magnetic similarities between these neurometabolites.
FIGURE 1.
(A) axial and (B) sagittal images showing ACC and OCC voxel sizes and locations; (C) PRESS 1H MR spectra with “editing” rf pulse (a) off and (b) on. (D) The difference of the spectra in (C) showing: (a) the detected GABA and Glx peaks, with (b–d) best-fit model curves and residuals, which yield the areas under the peaks. The depicted data were acquired in 13 min from a 2.5×2.5×3.0 cm3 ACC voxel, using TE/TR 68/1500 ms, and 256 interleaved excitations (total 512) with editing pulse on or off.
In the present study, the described editing sequence was implemented to record the GABA and Glx spectra in 13 min from a 2.0×3.0×3.0-cm3 OCC voxel (Fig. 1A,B) and from a 2.5×2.5×3.0-cm3 ACC voxel (Fig. 1A,B), using TE/TR 68/1500 ms, a 5-KHz spectral widths, 1024 sample points, and 256 interleaved excitations, or a total 512 excitations with the editing pulses on or off.
Spectral Data Processing, Analysis and Quantitation
The derived raw 8-channel phased-array coil time-domain free-induction decay (FID) signals were combined into a single regular FID using a published time-domain reconstruction algorithm (27), with the unsuppressed voxel tissue water signal from each receiver channel providing a strong and reliable reference signal to compute the relative sensitivities of the eight array coil elements. The single-voxel 1D spectra obtained by Fourier transformation of the combined signals were model-fitted in the frequency domain to a pseudo-Voight function (Fig. 1D), which permits more precise analysis of lineshapes that consist of mixtures of Lorentzian and Gaussian functions (28). Finally, the derived GABA and Glx peak areas were expressed semi-quantitatively as ratios relative to the area of the unsuppressed ACC or OCC voxel tissue water resonance (W), which is automatically and simultaneously acquired as part of single-voxel MRS acquisitions on our MR system. Tissue water resonance values did not differ across the three groups in either the OCC (p=.70) or ACC (p=.51). All reported values represent GABA/W and Glx/W ratios, which will simply be referred to as GABA and Glx, henceforth.
Statistical Analysis
MDD vs. HV group comparisons were made by unpaired t-tests, and 3-group comparisons (TRDs/nTRDs/HVs) were made by one-way analysis of variance (ANOVA). For post-hoc analysis, Tukey’s HSD adjustment for multiple comparisons was used unless otherwise noted. Based on previously observed sex and age effects on MDD amino acid neurotransmitter levels (1) and non-equivalencies across our groups in age and ethnic distribution, additional analyses of covariance (ANCOVA) were performed for each group comparison with age, sex, and a dichotomous ethnicity variable (non-Hispanic Caucasian vs. any other ethnicity) entered as covariates. Effect sizes (Cohen’s d) were calculated for all statistically significant results and trends (29). Tests of associations between continuous variables were performed using Pearson’s product-moment correlations and rank-order correlations were performed with Spearman’s rho. Multiple linear and logistic regression analyses were performed to investigate relationships between the TRD/nTRD dichotomous categorization and MDD patients’ OCC GABA levels, while adjusting for clinical and demographic factors. Multicollinearity among predictors was assessed using a conventional tolerance threshold of (1-r2)>.20 (30); all predictors showed tolerance values above this threshold. All tests were two-tailed, with alpha level set at .05, unadjusted. A Bonferroni correction for multiple comparisons was subsequently applied to the four primary ANOVA and ANCOVA analyses, yielding a critical p value of 0.05/4=0.0125.
RESULTS
Comparisons of TRD, nTRD MDD, and HV participants
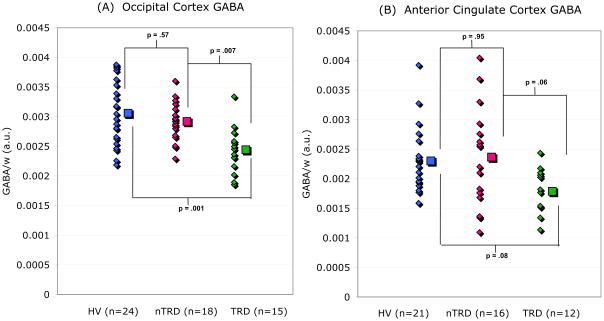
A one-way ANOVA revealed a main effect of group (HV/TRD/nTRD) on OCC GABA levels (F(2,54)=9.76; p<.001). Post hoc pairwise comparisons showed that the TRD group (n=15; M=2.44×103; SD=0.39×103) had significantly reduced GABA in comparison to both nTRDs (n=18; M=2.92×103; SD=0.33×103; p=.007; Cohen’s d=1.3) and HVs (n=24; M=3.06×103; SD=0.52×103; p<.001; d=1.3), while nTRDs and HVs did not differ (p=.57) (Figure 2). ANCOVA controlling for age, sex, and ethnicity did not alter significance for the main effect of group [F(2,51)=10.62; p<.001] and revealed no significant main effects of any covariate.
FIGURE 2.
GABA/W ratios [arbitrary units (a.u.)] in (A) the occipital cortex (OCC) and (B) the anterior cingulate (ACC) of healthy volunteers (HV), non-treatment-resistant major depression patients (nTRD), and treatment-resistant depression patients (TRD). Group means are displayed to the right of data from individual subjects. In the OCC, TRD patient values are 20.2% lower than HVs (p=.001; Cohen’s d=1.3) and 16.4% lower than nTRD subjects (p=.007; Cohen’s d=1.4). In the ACC, TRD values are 22.4% lower than HVs (p = .08, Cohen’s d=1.03) and 24.5% lower than nTRDs (p = .06, Cohen’s d=0.88). P-values derived by Tukey’s HSD post-hoc tests.
A one-way ANOVA revealed a similar main effect of group on ACC GABA [F(2,46)=9.76; p=.047]. The TRD group (n=12; M=1.79×103; SD=0.38×103) showed reduced GABA in comparison to both nTRDs (n=16; M=2.36×103; SD=0.85×103) and HVs (n=21; M=2.30×103; SD=0.57×103), although the differences did not reach the threshold for significance (TRDs vs. nTRDs: p=.06, d=0.88; TRDs vs. HVs: p=.08, d=1.03; nTRDs vs. HVs: p=.95) (Fig. 2). ANCOVA controlling for age, sex, and ethnicity did not alter significance for the main effect of group [F(2,43)=3.26; p=.048] and revealed no significant main effects of any covariate.
Identical one-way ANOVAs performed on Glx revealed no significant main effect of group in either the OCC (p=.46) or ACC (p=.22) regions. In the OCC, ANCOVA revealed a main effect of age which was negatively related to OCC Glx [F(1,51)=6.38; p=.02]; however the significance of group was unaltered by the covariates. In ACC, no single covariate was significant in the ANCOVA (p’s>.12); however removal of the combined variance from these sources increased the explanatory power of group [F(2,43)=3.70; p=.03], revealing decreased Glx levels in the TRD group only in comparison to HVs (TRDs vs. HVs: p=.03; TRDs vs. nTRDs: p=.18; nTRDs vs. HVs: p=.99].
Applying a Bonferonni correction to the four primary ANOVAs and the four secondary ANCOVAs did not alter significance for the main effect of group on OCC GABA (adjusted p’s<.004), but resulted in loss of significance for group effects on ACC GABA and Glx. Exclusion of antidepressant treatment-naïve patients (n=9) from the nTRD group altered significance in ACC GABA only, where the main effect of group was slightly reduced [F(2,40)=3.21; p=.052].
A confirmatory 2×3 ANOVA (with region as a within-subjects variable and group as a between-subjects variable) was performed on GABA levels from the 49 participants who had interpretable data for both ACC and OCC. There were main effects of region [F(1,46)=39.82; p<.001] and group [F(2,46)=7.98; p=.001]. The TRD group showed GABA reductions in comparison to both nTRDs (p=.005, d=1.30) and HVs (p=.001, d=1.59), while nTRDs and HVs did not differ (p=.97). The group × region interaction was not significant [F(2,46)=.307; p=.74], suggesting the pattern of GABA decreases was relatively consistent across regions.
Ratios of Glx:GABA levels were computed and compared across the three groups with one-way ANOVAs. In the OCC only, there was a main effect of group on Glx:GABA ratios [F(2,54)=3.28; p=.045]. Glx:GABA ratios were elevated in the TRD group (M=.84; SD=.22) in comparison to HVs (M=.71; SD=.15; p=.04; d=0.74) while nTRDs did not differ significantly from either group (p’s>0.15). Glx:GABA ratios in the ACC did not differ across the three groups [F(2,46)=.51; p=.60].
Comparisons of MDD and HV participants
In the full sample of 33 MDD patients (TRD and nTRD) compared to the 24 HVs, unpaired t-tests revealed significantly decreased OCC GABA in MDD participants (M=2.70×10−3; SD=0.43×103) compared to controls (M=3.06×103; SD=0.52×103) [t(55)=2.85; p=.006; d=0.77]. No significant group differences were found for ACC GABA, OCC Glx, ACC Glx, or Glx:GABA ratios (all p’s>=.10). ANCOVAs controlling for age, sex, and ethnicity did not alter significance in any analysis.
Correlation Analyses
In the full sample of patients and controls (n=57 for OCC; n=49 for ACC), age was negatively associated with Glx in the OCC (r=−.30; p=.02), but not ACC Glx (r=.21; p=.16), OCC GABA (r=−.09; p=.45), or ACC GABA (r=−.13; p=.36). Sex was not related to any MRS variable (p>.40). GABA and Glx were positively correlated in the ACC across all participants (r=.34; p=.02) as well as within the MDD patients only (r=.42, p=.02), but not in the OCC in either the full sample (r=.13; p=.32) or the patients alone (r=.31; p=.10). GABA, Glx, and Glx:GABA in both ROIs were unrelated to depression or anxiety rating scales in either the MDD patient sample or the HV sample (p’s>.10).
In MDD patients, a rank-order correlation between the number of failed trials and OCC GABA revealed a negative relationship (Spearman’s rho=−.44; p=.01). No other MRS variable was related (p’s>.09). The relationship between specific numbers of failed trials and OCC GABA is further explored in the Supplementary Material.
Regression Analyses in MDD Participants
Follow-up regression analyses in the 33 MDD participants were designed to explore the robustness of the TRD vs. nTRD dichotomous categorization in predicting OCC GABA levels when additional clinical and demographic features were added to the model (Table 2). In the full sample of 33 MDD patients, the TRD vs. nTRD dichotomous categorization was a highly significant predictor (β= −0.57; p=.001) of occipital GABA levels. In each of the four models tested, TRD status was entered in step 1. In Model 1, measures of clinical symptom severity (HRSD17, HAM-A) and anxiety disorder comorbidity were entered in step 2. In Model 2, measures of sociodemographic status (Caucasian vs. non-Caucasian; HH-SES; years of education) were entered in step 2. In Model 3, measures of patient life history (age, age of illness onset, single-episode vs. recurrent depression) were entered at step 2. In Model 4, dichotomous measures of psychotropic exposure [antidepressant treatment-naïve vs. non-treatment-naïve; 2-week psychotropic washout (the study minimum) vs. > 2-week washout period] were entered at step 2. The change in R2 at step 2 was not significant for any model [Model 1: F(3,28)=.15; p=.93; Model 2: F(3,27)=.36; p=.79; Model 3: F(3,28)=.23, p=.88; Model 4: F (2,29) =.43; p=.66], and TRD status was the only significant predictor in each final model.
Table 2.
Multiple Regression Statistics for Models Predicting Occipital GABA in Patients with Major Depressive Disorder
| β | Adjusted R2 | ΔR2 | |
|---|---|---|---|
| Step 1: All Models | .301 (p = .001) | .323 (p = .001) | |
| TRD vs. nTRD | −.57 (p =.001) | ||
| Step 2: Model 1 | .238 (p = .019) | .011 (p = 0.93) | |
| TRD vs. nTRD | −.59 (p = .003) | ||
| HRSD17 | −.09 (p = 0.72) | ||
| HAM-A | .14 (p = 0.57) | ||
| Comorbid anxiety disorder | −.06 (p = .73) | ||
| Step 2: Model 2 | .233 (p = .024) | .026 (p = 0.79) | |
| TRD vs. nTRD | −.60 (p = .001) | ||
| Non-hispanic Caucasian vs. any other ethnicity | .16 (p = 0.38) | ||
| HH-SES | .03 (p = 0.88) | ||
| Years of education | .05 (p = 0.84) | ||
| Step 2: Model 3 | .245 (p = .017) | .016 (p = 0.88) | |
| TRD vs. nTRD | −.58 (p = .001) | ||
| Age | .10 (p = 0.60) | ||
| Age of illness onset | −.14 (p = 0.44) | ||
| Single episode vs. recurrent | −.02 (p = 0.93) | ||
| Step 2: Model 4 | .274 (p = .006) | .019 (p = 0.66) | |
| TRD vs. nTRD | −.49 (p = .01) | ||
| 2-week washout vs. > 2-week washout | .05 (p = 0.81) | ||
| ADM-naïve vs. any exposure | −.14 (p = 0.42) | ||
Note: TRD = Treatment-Resistant Depression; nTRD = Non-Treatment-resistant Major Depressive Disorder; HRSD17 = Hamilton Rating Scale for Depression, 17-item; HAM-A = Hamilton Anxiety Rating Scale; HH-SES = Hollingshead Four-Factor Index of Social Position; ADM = antidepressant medication
A logistic regression predicting TRD status showed that OCC GABA levels correctly classified 83% of nTRDs and 73% of TRDs (Model χ2=12.64; p<.001). In addition, OCC GABA significantly improved the prediction of TRD/nTRD group status when clinical and demographic features that differed across groups (age, ethnic distribution, HAM-A, HRSD17) were entered at Step 1. While these control variables correctly classified 94% of nTRDs and 80% of TRDs (Step 1 χ2=21.25; p<.001), the addition of OCC GABA significantly improved the model (Step 2 χ2=9.02; p=.003) resulting in correct classification of 94% of nTRD and 93% of TRD patients [Model χ2=30.27; p<.001]. OCC GABA (Wald’s χ2=4.38; p=.04) and ethnicity (Wald’s χ2=4.12; p=.04) were the only significant predictors in the final model.
DISCUSSION
Consistent with predictions, GABA levels in OCC were decreased in medication-free TRD patients. Reductions were evident compared to two concurrently recruited groups–a healthy volunteer sample as well as a medication-free MDD sample without history of treatment-resistance. Notably, 14 of 15 TRD patients exhibited GABA levels below the group mean for nTRD (Fig. 2). Reductions in TRD patients could not be explained by any clinical or demographic factor examined, including age, sex, ethnicity, SES, symptom severity, comorbid anxiety, age of illness onset, number of episodes, treatment-naïve status, or duration of psychotropic washout. Though a similar pattern of GABA reduction was present in the ACC region, with TRD mean percentage decreases (22.4–24.5%) larger than those observed in OCC (16.4–20.2%), the effect was statistically less robust, and did not survive correction for multiple comparisons.
Our data suggest that stratification based on systematic assessment of prior antidepressant treatment response is a salient variable for biological investigations of MDD and reliably distinguishes patients with more pronounced GABA deficits from those who resemble healthy participants. Previous 1H MRS studies of occipital GABA in medication-free MDD patients (Table 3) have not distinguished TRD from nTRD patients. The largest published effect size to date was observed in a primarily inpatient MDD sample, 57% of whom (8 of 14) were identified as failing 3 or more antidepressant trials (1). In a follow-up investigation combining data from this inpatient sample with a larger sample of outpatients, GABA decreases were most pronounced in patients exhibiting melancholic features (2). In our outpatient sample, the low frequency of patients meeting specific DSM-IV subtype criteria did not permit similar analyses.
Table 3.
Occipital GABA Findings in Medication-Free MDD samples
| Study | Measurement | Field Strength | MDD Characteristics | Samples Compared | Effect Size: Cohen’s d [95% CI] |
|---|---|---|---|---|---|
| Sanacora et al. 1999 (1) | GABA/Creatine | 2.1 Tesla | primarily inpatient, 57% TRD | 14 MDD vs. 18 HV | d=2.28 [1.42–3.25] |
| Sanacora et al. 2004 (2) | GABA/Creatine | 2.1 Tesla | primarily outpatient, 24% TRD | 33 MDD vs. 38 HV | d=0.64 [0.13–1.08] |
| Bhagwagar et al. 2007 (3) | GABA/Creatine | 3.0 Tesla | fully remitted, recurrent | 15 MDD vs. 18 HV | d=0.89 [0.14–1.58] |
| Current study | GABA/Water | 3.0 Tesla | outpatient, categorized as TRD or nTRD by ATHF | 15 TRD vs. 24 HV | d=1.32 [0.59–2.01] |
| 15 TRD vs. 18 nTRD MDD | d=1.35 [0.57–2.10] | ||||
| 18 nTRD vs. 24 HV | d= 0.31[−0.31–0.92] | ||||
| 33 MDD vs. 24 HV | d=.77 [0.22–1.30] |
Although the occipital cortex has not been given a prominent role in neural circuitry models of MDD (13,31), serotonin1A receptor binding and glucose metabolism abnormalities in this region have been reported in PET studies (32–34). Examination of additional brain regions will be needed to determine whether GABA abnormalities are specific to occipital and prefrontal ROIs, or more broadly distributed throughout the brain, though at present technological limitations constrain the number of voxels that can be assessed via MRS within a feasible time frame. GABA decreases previously reported in the plasma and cerebrospinal fluid of depressed patients (35–37) might suggest a broad distribution of decreases. Decreased GABA in TRD patients is consistent with amino acid neurotransmitter dysfunction in mood disorder pathophysiology (7,38). Disruption of glutamate-glutamine neuronal-glial cycling in MDD is thought to result in excessive build-up of extracellular glutamate and decreased glutamate release, leading to a decrease in cortical GABA (39), a hypothesis consistent with the elevated OCC Glx:GABA ratios observed in our TRD group. However, the extent to which alterations in steady-state GABA and Glx levels relate to alterations in neurotransmitter function remains to be clarified, as altered MRS levels might reflect changes occurring in neurons or glia, and could index diverse physiological abnormalities including altered neurotransmitter metabolism, recycling/production rates, and/or synaptic or extra-synaptic accumulation (40).
We did not find strong evidence of Glx alterations in either our full sample of MDD patients or in our TRD subgroup. Diminished ACC Glx levels in TRD patients emerged when age, sex, and ethnicity were covaried, but the effect did not survive Bonferonni correction. Consistent with a previous report (4), we found that Glx and GABA were positively correlated in ACC but not OCC. OCC Glx levels, unlike GABA levels, were negatively correlated with age across all participants, which might have influenced results given that age-matched samples were not obtained, although we adjusted for age in ANCOVA analyses. At the current study’s field strength (3.0T), the Glx resonance is predominately comprised of glutamate, with a smaller contribution from glutamine. Glutamate and glutamine alterations in MDD samples have been more variable than GABA findings in previous 1H MRS studies conducted at differing field strengths (1.5T-3T), with inconsistent direction of change reported across cortical regions (2–5,41,42). Additional studies are therefore needed to clarify the nature of regional glutamate/glutamine alterations in MDD.
Conventional antidepressants such as selective serotonin reuptake inhibitors (SSRIs) have broad effects on amino acid neurotransmission and have been reported to increase occipital GABA in MDD (43). These findings have generated interest in developing novel antidepressants that normalize amino acid neurotransmitter metabolism and neurotransmission (44) and enhance neuronal plasticity and cellular resilience in patients who do not respond to conventional approaches (38,45). Our data suggest that regional GABA dysfunction may be particularly prominent in MDD patients with a history of non-response to conventional antidepressants. Though our cross-sectional study does not permit us to disambiguate cause from effect in the relationship between treatment-resistance and GABA reductions, our findings support the potential utility of direct targeting of amino acid neurotransmitter function in TRD patients. We cannot rule out the possibility that GABA reductions in TRD are a cumulative result of previous antidepressant medication exposure, though the previous finding that chronic administration of SSRIs increases occipital GABA is inconsistent with this interpretation (43). Thus, our findings might suggest that decreased GABA levels confer risk for poor response to conventional antidepressants or, alternatively, that a third factor results in both GABA reductions and treatment-resistance. It is important to note that the present nTRD sample likely comprises both patients who would respond well to standard antidepressants and those who would emerge as TRD given adequate exposure, particularly given the inclusion of antidepressant treatment-naïve participants for whom treatment responsivity is not determinable. Our principle findings were maintained when treatment-naïve participants were excluded from analysis. However, a randomized, placebo-controlled, prospective design exploring the relationship between cortical GABA levels and subsequent specific antidepressant response would be necessary to corroborate a role for GABAergic deficits in increasing the risk of treatment-resistance.
The present study examined well-characterized, medication-free cohorts using high field-strength MRS and quantification methods with demonstrated reliability (46). The use of unsuppressed voxel tissue water resonance as a referencing standard, as opposed to total creatine ratios as in previous studies, permits more precise inference to be made regarding amino acid neurotransmitter levels per se. The present samples of TRD and nTRD patients were notably well-matched in terms of anxiety disorder comorbidity, sex distribution, IQ, BMI, SES, family history of mood disorder, self-reported disability level, illness duration, age of onset, chronicity, and number of episodes. However, differential sample characteristics included age, ethnicity, modal duration of washout period, and current anxiety and depression symptom severity. Regression analyses including these factors, as well as additional demographic and clinical predictors selected for conceptual or empirical relevance to TRD and GABA, consistently failed to reduce the predictive power of TRD status. However, it should be noted that regional GABA alterations are not specific to MDD and have been observed in other disorders, including bipolar disorder, social phobia, panic disorder, and alcohol dependence (3,47–50), suggesting that GABA decreases may relate to clinical features that cut across diagnostic boundaries. Future studies should aim to delineate the relationships between specific clinical characteristics with relevance to TRD status and potential neurochemical substrates of treatment non-response.
Our conclusions are further limited by small sample size, which constrained power to detect small and medium effects. The effects of partial volume averaging cannot be ruled out given that tissue segmentation was not performed. The generalizability of the present findings may be limited to patients sharing the specific clinical characteristics of our TRD and nTRD samples, such as high rates of illness chronicity, anxiety disorder comorbidity, and self-reported disability. Although we used a validated instrument (ATHF) to quantify failed antidepressant trials in the current episode, retrospective assessment of treatment response is constrained by the accuracy of patient reports (17) and medical records. In addition, our TRD classification did not take into account trials occurring during previous depressive episodes, the total duration of exposure, nor exposure to medications not FDA-approved for MDD.
In summary, occipital GABA levels were reduced in TRD patients in comparison to both HVs and MDD patients without history of treatment-resistance. A similar main effect of diagnosis was present in the ACC, though evidence for TRD-specific reductions was less conclusive. These findings are possibly consistent with a role for occipital GABA reductions in conferring risk of inadequate antidepressant medication response and may suggest the potential utility of amino acid neurotransmitter-modulating agents for TRD. Large-scale prospective designs are necessary to corroborate this interpretation.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants R01-MH07895 (DCS), K23-MH-069656 and MO1-RR-00071 (SJM), and in part by an Investigator-initiated research grant from the CFIDS Association of America, Inc (DCS), and by Weill Cornell Medical College New Faculty Development Funds (DCS). We thank the staff of the Citigroup Biomedical Imaging Center, Mount Sinai General Clinical Center, and the Mood & Anxiety Disorders Program for their assistance with the study.
Footnotes
FINANCIAL DISCLOSURES
Ms. Price, Dr. Shungu, Ms. Mao, Mr. Nestadt, Ms. Kelly, Ms. Collins, and Dr. Murrough report no biomedical financial interests or potential conflicts of interest. Dr. Mathew has received lecture or consulting fees from AstraZeneca, Jazz Pharmaceuticals, and Roche, and has received research support from Alexza Pharmaceuticals, GlaxoSmithKline Pharmaceuticals, and Novartis Pharmaceuticals. Dr. Charney discloses consultant activities with the following during the past two years: Astra Zeneca, Bristol-Myers Squibb Company, Cyberonics, Neurogen, Neuroscience Education Institute, Novartis Pharmaceuticals Corporation, Orexigen, and Unilever UK Central Resources Limited. Drs. Charney and Mathew have been named as an inventor on a use-patent of ketamine for the treatment of depression. If ketamine were shown to be effective in the treatment of depression and received approval from the Food and Drug Administration (FDA) for this indication, they could benefit financially.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 2.Sanacora G, Gueorguieva R, Epperson CN, Wu Y-T, Appel M, Rothman DL, et al. Subtype-specific alterations of c-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 3.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, et al. Reduction in occipital cortex GABA concentrations in medication free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 4.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, Matthews PM, et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharm. 2008;11:255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 5.Hasler G, Van der Veen J, Tuonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and c-aminobutryric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 6.aan het Rot M, Charney DS, Mathew SJ. Intravenous ketamine for treatment-resistant major depressive disorder. Prim Psychiatry. 2008;15:39–47. [Google Scholar]
- 7.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 9.Souery D, Amsterdam J, de Montigny C, Lecrubier Y, Montgomery S, Lipp O, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9:83–91. doi: 10.1016/s0924-977x(98)00004-2. [DOI] [PubMed] [Google Scholar]
- 10.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 11.Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58S13:23–29. [PubMed] [Google Scholar]
- 12.McGrath PJ, Stewart JW, Fava M, Trivedi MH, Wisniewski SR, Nierenberg AA, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163:1531–1541. doi: 10.1176/ajp.2006.163.9.1531. [DOI] [PubMed] [Google Scholar]
- 13.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured clinical interview for DSM-IV Axis I disorders. patient. New York: New York Psychiatric Institute; 1995. [Google Scholar]
- 15.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62S16:10–17. [PubMed] [Google Scholar]
- 16.Prudic J, Haskett RF, Mulsant B, Malone KM, Pettinati HM, Stephens S, et al. Resistance to antidepressant medications and short-term clinical response to ECT. Am J Psychiatry. 1996;153:985–992. doi: 10.1176/ajp.153.8.985. [DOI] [PubMed] [Google Scholar]
- 17.Posternak MA, Zimmerman M. How accurate are patients in reporting their antidepressant treatment history? J Affect Disord. 2003;75:115–124. doi: 10.1016/s0165-0327(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), Clinician-Rating (QIDS-C), and Self-Report (QIDS-SR): A Psychometric Evaluation in Patients with Chronic Major Depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 21.Sheehan DV. The Anxiety Disease. New York: Scribner’s; 1983. [Google Scholar]
- 22.Hollingshead AB. Four Factor Index of Social Position. New Haven, CT: Yale University; 1975. [Google Scholar]
- 23.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57:675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- 24.Rothman DL, Petroff OAC, Behar KL, Mattson RH. Localized 1H NMR measurements of g-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sailasuta N, LeRoux P, Hurd Ralf, Wang P, Sachs N, Ketter Detection of cerebral Gamma-aminobutyric acid (GABA) in Bipolar Disorder Patients and Healthy Volunteers at 3 T. Proc Intl Soc Mag Reson Med. 2001;6:1011. [Google Scholar]
- 26.Shungu DC, Mao X, Kegeles LS. Evaluation of GABA detection sensitivity gains achieved with an 8-channel phased-array head coil at 3.0 T in the human dorsolateral prefrontal cortex using the J-editing technique. Proc Intl Soc Mag Reson Med. 2006;14:488. [Google Scholar]
- 27.Brown MA. Time-domain combination of MR spectroscopy data acquired using phased-array coils. Magn Reson Med. 2004;52:1207–1213. doi: 10.1002/mrm.20244. [DOI] [PubMed] [Google Scholar]
- 28.Marshall I, Bruce SD, Higinbotham J, MacLullich A, Wardlaw JM, Ferguson KJ, et al. Choice of spectroscopic lineshape model affects metabolite peak areas and area ratios. Magn Reson Med. 2000;44:646–649. doi: 10.1002/1522-2594(200010)44:4<646::aid-mrm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 30.Menard S. Applied Logistic Regression Analysis: Sage University Series on Quantitative Applications in the Social Sciences. Thousand Oaks, CA: Sage; 1995. [Google Scholar]
- 31.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 32.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 33.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin-1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 34.Saxena S, Brody AL, Ho ML, Alborzian S, Maidment KM, Zohrabi N, et al. Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder vs major depression. Arch Gen Psychiatry. 2002;59:250–261. doi: 10.1001/archpsyc.59.3.250. [DOI] [PubMed] [Google Scholar]
- 35.Petty F, Kramer GL, Gullion CM, Rush AJ. Low plasma γ-aminobutyric acid levels in male patients with depression. Biol Psychiatry. 1992;32:354–363. doi: 10.1016/0006-3223(92)90039-3. [DOI] [PubMed] [Google Scholar]
- 36.Kasa K, Otsuki S, Yamamoto M, Sato M, Kuroda H, Ogawa N. Cerebrospinal fluid γ-aminobutyric acid and homovanillic acid in depressive disorders. Biol Psychiatry. 1982;17:877–883. [PubMed] [Google Scholar]
- 37.Roy A, Dejong J, Ferraro T. CSF GABA in depressed patients and normal controls. Psychol Med. 1991;21:613–618. doi: 10.1017/s0033291700022248. [DOI] [PubMed] [Google Scholar]
- 38.Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;225:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- 39.Sanacora G, Rothman DL, Mason GF, Krystal JH. Clinical studies implicating glutamate neurotransmission in mood disorders. Ann NY Acad Sci. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- 40.Batra NA, Seres-Mailo J, Hanstock C, Seres P, Khudabux J, Bellavance F, et al. Proton magnetic resonance spectroscopy measurement of brain glutamate levels in premenstrual dysphoric disorder. Biol Psychiatry. 2008;63:1178–1184. doi: 10.1016/j.biopsych.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, et al. Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. J Am Acad Child Adolesc Psychiatry. 2004;43:341–348. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg DR, Macmaster FP, Mirza Y, Smith JM, Easter PC, Banerjee SP, et al. Reduced anterior cingulate glutamate in pediatric major depression: a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:700–707. doi: 10.1016/j.biopsych.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 44.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.106. Published Online: 30 September 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008;33:2080–2092. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- 46.Kegeles LS, Mao X, Dyke J, Gonzales R, Soones TN, Shungu DC. Test-retest reliability of dorsolateral prefrontal cortical GABA measurement using an 8-channel phased-array head coil with the J-editing technique at 3T. Proc Intl Soc Mag Reson Med. 2006;14:489. [Google Scholar]
- 47.Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: response to treatment with levetiracetam. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:739–743. doi: 10.1016/j.pnpbp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OA, et al. Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Arch Gen Psychiatry. 2001;58:556–561. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- 49.Goddard AW, Mason GF, Appel M, Rothman DL, Gueorguieva R, Behar KL, Krystal JH. Impaired GABA neuronal response to acute benzodiazepine administration in panic disorder. Am J Psychiatry. 2004;161:2186–2193. doi: 10.1176/appi.ajp.161.12.2186. [DOI] [PubMed] [Google Scholar]
- 50.Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, et al. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.