Abstract
Oxidant damage from reactive oxygen species (ROS) and reactive nitrogen species (RNS) is a major contributor to the cellular damage seen in numerous types of renal injury. Resveratrol (trans-3, 4′, 5-trihydroxystilbene) is a phytoalexin found naturally in many common food sources. The antioxidant properties of resveratrol are of particular interest because of the fundamental role that oxidant damage plays in numerous forms of kidney injury. To examine whether resveratrol could block damage to the renal epithelial cell line, mIMCD-3, cells were exposed to the peroxynitrite donor 5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride (SIN-1). Resveratrol produced a concentration-dependent inhibition of cytotoxicity induced by SIN-1. To examine the mechanism of protection, resveratrol was incubated with authentic peroxynitrite and found to block nitration of bovine serum albumin with an EC50 value of 22.7 μM, in contrast to the known RNS scavenger, N-acetylcysteine, which inhibited nitration with an EC50 value of 439 μM. These data suggested that resveratrol could provide functional protection by directly scavenging peroxynitrite. To examine whether resveratrol was a substrate for peroxynitrite oxidation, resveratrol was reacted with authentic peroxynitrite. Resveratrol nitration products and dimers were detected using liquid chromatograph with tandem electrospray mass spectrometry. Similar products were detected in the media of cells treated with SIN-1 and resveratrol. Taken collectively, the data suggest that resveratrol is able to provide functional protection of renal tubular cells, at least in part, by directly scavenging the RNS peroxynitrite. This property of resveratrol may contribute to the understanding of its antioxidant activities.
Keywords: resveratrol, peroxynitrite, nitration, SIN-1, cytotoxicity
1. Introduction
There is growing evidence both in vitro and in vivo to suggest that the generation of reactive oxygen species (ROS) and nitric oxide-derived reactive nitrogen species (RNS) play critical roles in the development of diverse causes of acute kidney injury (AKI). These include renal ischemia/reperfusion [1-3], transplant rejection [4], cisplatin nephrotoxicity [5, 6], and sepsis-induced AKI [7, 8]. These findings have lead to increased efforts to test clinically anti-oxidant therapeutic approaches to treating AKI [9, 10].
Resveratrol (trans-3, 4′, 5-trihydroxystilbene) is a phytoalexin found naturally in many common food sources including grapes, mulberries, and peanuts and reported to have numerous beneficial effects on human health (reviewed in ref [11]). In addition to its anti-cancer properties, resveratrol is anti-inflammatory, anti-aging, and thought to improve overall cardiovascular health. While resveratrol interacts with many important enzymes and receptor signaling pathways, the anti-oxidant properties of resveratrol are of particular interest because of the fundamental role that oxidant damage plays in numerous pathological conditions. In rodents, resveratrol preserves anti-oxidant enzyme activities such as superoxide dismutase, glutathione peroxidase and catalase [12-14]. It has also been shown in in vitro systems to scavenge superoxide [15] and block oxidation of low-density lipoproteins (LDL) by metals [15-17] and the RNS, peroxynitrite (ONOO−) [16], generated in biological systems by the reaction of NO and superoxide [18]. Collectively, these studies suggest that resveratrol may have multiple activities that reduce cellular oxidative stress.
There is now substantial evidence that RNS like peroxynitrite play an important role in many of the diseases and conditions reported to be ameliorated by resveratrol [11, 19] including those in the kidney, where resveratrol has been shown to decrease lipid peroxidation during cisplatin nephrotoxicity [20] and decrease the inflammatory response and RNS generation in glycerol-induced renal injury [21]. While the interactions between resveratrol and RNS have not been thoroughly studied, reports by Brito et al. showing that resveratrol blocks oxidation of LDL by peroxynitrite [16] and by Olas et al. showing that resveratrol blocks peroxynitrite-mediated oxidation and nitration of serum [22] and platelets proteins [23] suggest that resveratrol may be capable of detoxifying peroxynitrite.
In addition to lipid and protein oxidation, some of the toxic effects of peroxynitrite are mediated by nitration of protein tyrosine residues and inactivation of key enzymes, such as superoxide dismutase [24]. Since phenols are substrates for nitration by peroxynitrite [25], polyphenols like resveratrol may effectively scavenge peroxynitrite and thereby reduce peroxynitrite toxicity. The goal of our study was to examine whether resveratrol can act as a functional scavenger and protect renal cells from the lethal effects of peroxynitrite. We also used analytical approaches to examine, in detail, the reactions of resveratrol with peroxynitrite to propose a mechanism of resveratrol's RNS scavenging activity.
2. Material and Methods
2.1. Chemicals and Reagents
Trans-3, 4′, 5-trihydroxystilbene (trans-resveratrol) and 5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride (SIN-1) were purchased from Cayman Chemical Company (Ann Arbor, MI). Bovine serum albumin (BSA) fraction V and N-acetyl-L-cysteine (NAC), were purchased from Sigma-Aldrich (St. Louis, MO). Authentic peroxynitrite and degraded peroxynitrite were purchased from Millipore (Temecula, CA). Fetal bovine serum (FBS) was purchased from Thermo Fisher Scientific (Walthan, MA). Dulbecco's Modified Eagles Medium (DMEM):F12 medium was purchased from Invitrogen Molecular Probes (Eugene, OR).
2.2. Reaction of resveratrol and peroxynitrite
A stock solution of resveratrol was dissolved in 100% ethanol and then diluted in phosphate buffer (0.1 M, pH = 7.4) to a final concentration of 50 μM containing 0.2% ethanol. The concentration (~ 100 mM) of the peroxynitrite stock solution in 0.3M NaOH was determined prior to each reaction by measuring the absorbance at 302 nm using a molar extinction coefficient of 1670 M−1cm−1 [26]. Peroxynitrite in 0.3 M NaOH was diluted 1:100 with 0.3 M NaOH to yield final concentrations of 100 μM to 1 mM in the reaction solution. Additions of peroxynitrite to the resveratrol solution were made under continuous vortexing and incubated for 5 minutes. The addition of peroxynitrite did not change the pH of the 0.1 M phosphate buffer.
2.3. Nitration of BSA
A solution of bovine serum albumin (1 mg/ml) containing resveratrol (0-100 μM) was made by dissolving resveratrol in 100% ethanol followed by dilution with phosphate buffer containing BSA to the desired concentration. The concentration of resveratrol was determined by measuring the absorbance at 312 nm using a molar extinction coefficient of 33,400 M−1cm−1 [28]. Peroxynitrite was added under continual vortexing to the solution at room temperature to obtain a final concentration of 1 mM. After 10 min, the reaction was then dialyzed for 24 h at 4°C against three changes of phosphate buffer (800 ml) using Slide-A-Lyzer dialysis cassettes (Thermo Fisher Scientific, Rockford IL). Each condition within an experiment was performed in triplicate. The nitrotyrosine content of BSA following dialysis was determined by measuring absorbance at 438 nm at pH 9.0 using a molar extinction coefficient of 4,300 M−1cm−1 [27]. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Walthan, MA)
2.4. Cell Culture
The mIMCD-3 cell line (obtained from American Type Culture Collection, Manassas, VA) is a polarized inner medullary collecting duct epithelial cell line derived from a mouse transgenic for the early region of SV40 [Tg(SV40E)bri/7] [29]. Cultures were maintained in DMEM:F12 Medium containing 5% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. The mIMCD-3 cells were grown in 48-well plates to a density of 80% confluence. This cell line has been used to study the mechanisms of oxidant-induced injury to the renal epithelium [30]. Experiments were performed on cells from passages 10-17.
2.6. Assessment of cell viability
Cell viability was determined by measuring the percentage of total lactate dehydrogenase (LDH) release from cells into the media using the LDH Cytotoxicity Assay Kit (Cayman Chemical, Ann Arbor, MI) as directed by the manufacturer. Cells (105) were seeded in 48-well culture plates and grown to 80% confluence and then treated with SIN-1 (5 mM) and varying concentrations of resveratrol (0-100 μM). Deactivated SIN-1 was prepared by incubating a stock solution of SIN-1 in 0.1 M phosphate buffer (pH 7.4) for 36 h at room temperature prior to use. After a 6-h incubation period with cells, the supernatant (100μl) was transferred to a new 96-well plate. Cells were then treated with 0.1% Triton X-100 and gently shaken on an orbital shaker according to the manufacturer's protocol to release intracellular LDH. Each condition within each experiment was performed in triplicate. Total cellular LDH activity was defined as LDH activity in the cell lysate plus LDH activity released into the media after subtracting LDH activity contributed by FBS.
2.7. LC/MS and LC/MS/MS analysis of reaction products
Prior to analysis, reaction products present in aqueous reaction buffer or cell culture media were extracted with an equal volume of ethyl acetate then dried with MgSO4. HPLC analyses were performed using a Waters Acquity UPLC system (Waters Co., Milford, MA) with a Phenomenex Luna C18 column (150 ×2mm, 3μm, Torrance, CA), operating at a flow rate of 0.15 ml/min. A mobile phase consisting of water (solvent A) and acetonitrile (solvent B) was utilized. Gradient elution started from 30% B and was increased linearly to 100% B over 10 min and maintained at 100% B for 1 min before returning to the initial conditions (30% B). Total run time was 14 min. Injection volumes were 10 μl. A Quattro Micro triple quadrupole mass spectrometer (Waters Co., Milford, MA) equipped with an electrospray interface was used for all analyses. Negative ions were acquired in both full scan ([M-H]− ions for resveratrol, mono-nitro, di-nitro, and dimer = m/z 227, 272, 317, 453, respectively) and product ion modes with a desolvation temperature of 350°C, a source temperature of 100°C and a collision gas pressure of 0.00417 mbar. A UV detector (AD20; Dionex Co., Sunnyvale, CA) was placed in-line with the mass spectrometer to monitor column effluent using 346 nm detection. Nitrogen was used as the desolvation gas (750 l/hr). Full scans were obtained using a cone voltage of 35 V and scanning from m/z 100-600. Product ion scans were obtained using a cone voltage of 35 V, collision energy of 25 eV and scanning from m/z 50-500. Product ion mass spectra were acquired and the major transitions from molecular species were monitored: mono-nitroresveratrol ([m/z 272 → 225), di-nitroresveratrol (m/z 317→ 254) and resveratrol dimers (m/z 453→ 265). The presence of multiple peaks with similar mass spectra for each product were consistent with the formation of isomeric mononitro, di-nitro, and dimeric species.
2.8. Statistical analysis
Nitration and cell viability data are presented at mean ± SEM. Data were analyzed by Prism 5c for Mac (GraphPad Software Inc., San Diego, CA) and statistical differences were determined using a one-way ANOVA followed by Newman-Keuls post-test. A P value < 0.05 was considered significant.
3. Results
3.1. Effects of resveratrol on SIN-1 cytotoxicity in mIMCD-3 cells
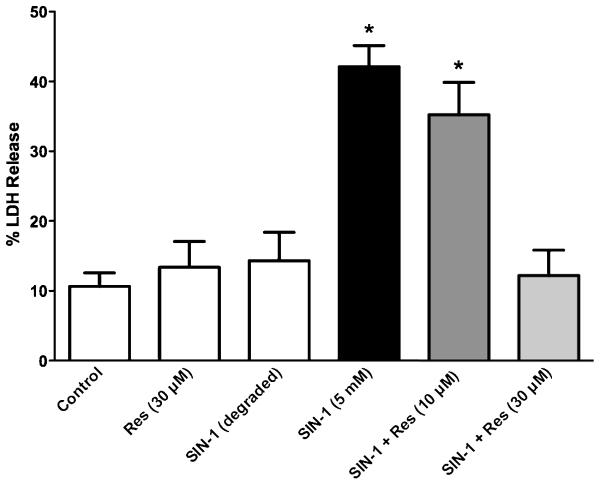
The peroxynitrite generator, SIN-1 releases NO and superoxide over time in equal molar amounts to generate ONOO− [31]. mIMCD-3 cells were exposed to SIN-1 (5 mM) for 6 h in the absence or presence of varying concentration of resveratrol (Fig. 1). SIN-1 caused a significant increase in LDH release. Deactivated SIN-1 was without effect. Resveratrol (30 μM) alone also had no effect on LDH release. However, resveratrol produced a concentration-dependent inhibition of SIN-1-induced LDH release.
Fig. 1. Protective effects of resveratrol on peroxynitrite-induced cell death.
The peroxynitrite generator, SIN-1 produced significant LDH release, an indicator of cell death, from mIMCD-3 cells at 6 h. Degraded SIN-1 was without effect. Resveratrol produced a concentration-dependent protection against peroxynitrite-induced cell death. Data are mean ± SEM (n = three independent experiments). *P < 0.05 compared to control, Res (30 μM), SIN-1 (degraded) and SIN-1 + Res (30 μM).
3.2. Effects of resveratrol on peroxynitrite-induced nitration of BSA
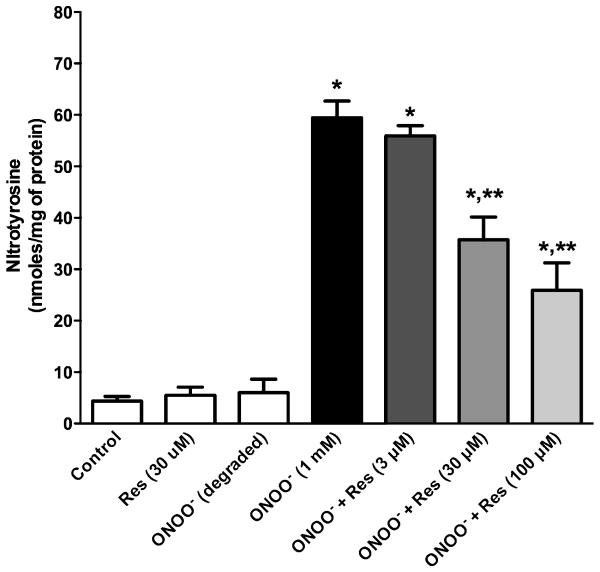
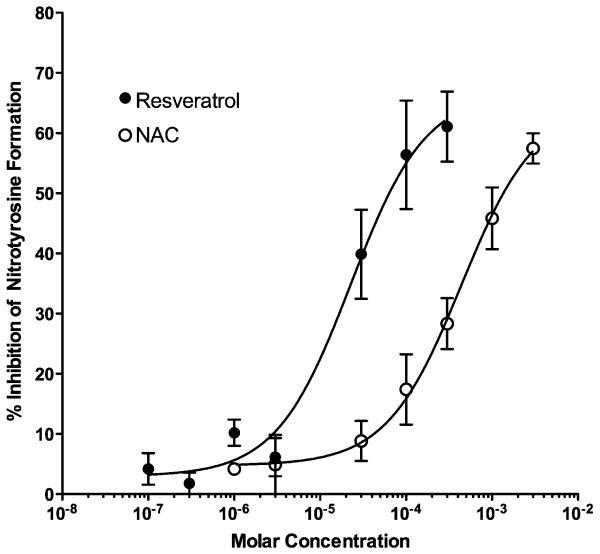
The findings that resveratrol prevented the cytotoxicity of the peroxynitrite generator SIN-1, suggested that resveratrol may act as a functional peroxynitrite scavenger. Peroxynitrite modifies protein tyrosine residues through a nitration reaction to form nitrotyrosine, which is considered to be a biomarker for ONOO− oxidation [32]. To address the scavenging activity of resveratrol more directly, the ability of resveratrol to block nitration of protein tyrosine by peroxynitrite was assed by incubating authentic peroxynitrite with BSA in the presence of varying concentrations of resveratrol. Peroxynitrite (1 mM) produced approximately a 10-fold increase in nitrotyrosine compared to degraded peroxynitrite (Fig. 2). The amount of nitrotyrosine generated corresponded to 4.1 ± 0.1 moles nitrotyrosine/mole BSA and is consistent with values of 3-6 moles nitrotyrosine/mole BSA reported by others [27]. Resveratrol produced a concentration-dependent inhibition of nitration. To evaluate the potency of resveratrol to block nitration, a full concentration-response curve for the inhibition of nitrotyrosine formation was generated and compared against that of a known peroxynitrite scavenger, NAC [33]. Concentration-response curves (Fig. 3) revealed that resveratrol (EC50 = 22.7 μM; 95% confidence interval = 9.0 to 57.5 μM) was a more potent inhibitor of nitration than NAC (EC50 = 439.3 μM; 95% confidence interval = 215 to 894 μM).
Fig. 2. Effects of resveratrol on peroxynitrite-induced nitration.
Peroxynitrite produced nitration of BSA equivalent to 4.1 ± 0.1 moles nitrotyrosine/mole BSA. Neither resveratrol alone nor degraded peroxynitrite produced nitrated BSA. Resveratrol significantly inhibited peroxynitrite-induced nitration in a concentration-dependent manner. Data are mean ± SEM (n = three independent experiments). *P < 0.05 compared to control; **P < 0.05 compared to control and ONOO− alone.
Fig. 3. Comparison of the inhibitory effects of resveratrol and NAC on peroxynitrite-induced nitration.
Both resveratrol and NAC produced concentration-dependent inhibition of peroxynitrite (1 mM)-induced nitration of BSA. Resveratrol (EC50 = 22.7 μM; 95% confidence interval = 9.0 to 57.5 μM) was a more potent inhibitor of nitration than NAC (EC50 = 439.3 μM; 95% confidence interval = 215 to 894 μM). Data points are the mean ± SEM from three independent experiments.
3.3. Covalent modification of resveratrol by peroxynitrite
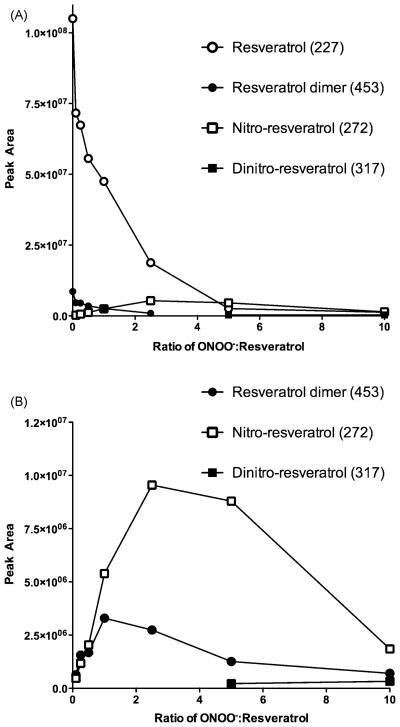
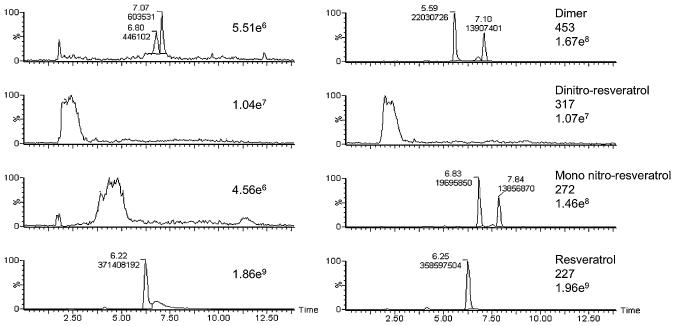
Phenols such as tyrosine are known substrates for oxidation and nitration by peroxynitrite. The finding that resveratrol blocked nitration of BSA suggested that, as a polyphenol, resveratrol may act as a competing substrate for oxidation by peroxynitrite. To address this, resveratrol (50 μM) was reacted with varying concentrations of peroxynitrite at pH 7.4 to yield reaction ratios (ONOO−:resveratrol) from 0 (resveratrol alone) to 10. As the ratio increased, the amount of resveratrol decreased. For example, with a ONOO−:resveratrol ratio of 5, the resveratrol was completely consumed. While unreacted resveratrol contained low levels of dimers, no nitrated resveratrol was detected. Isomers of mono- (m/z 272) and di-nitroresveratrol (m/z 317), as well as resveratrol dimers (m/z 453), were detected following reaction with all peroxynitrite concentrations (Fig. 4A and 4B). As the amount of peroxynitrite in the reaction mixture increased, peak areas for the daughter ions of the resveratrol dimers and the mono-nitroresveratrol isomers increased. Di-nitroresveratrol isomers were observed only for peroxynitrite concentration ratios above 5.
Fig. 4. LC/MS/MS analysis of resveratrol-peroxynitrite reaction products.
Increasing ratios of ONOO−:resveratrol resulted in loss of resveratrol and the appearance of isomeric mono- and di-nitroresveratrol and resveratrol dimers (panel A). Analysis of product ions (panel B) showed that as the ONOO−:resveratrol ratios increased, peak areas of the major product ion transitions for the resveratrol dimers and the mono-nitroresveratrol isomers increased. The peaks for mono-nitroresveratrol isomers (m/z 272 → 225) and resveratrol dimers (m/z 453 → 265) were observed at all ONOO− concentration ratios. The peak for di-nitroresveratrol isomers ((m/z 317 → 254) ) was observed only for ONOO−:resveratrol ratios above 5. Data are representative of three separate experiments.
3.4. Modification of resveratrol in mIMCD-3 cells treated with SIN-1-treated
Since resveratrol was shown to be oxidized and nitrated by peroxynitrite concomitant with blocking the cytotoxicity of SIN-1, we examined resveratrol reaction products in the media from cells treated with SIN-1. LC/MS/MS analysis found mono- and di-nitroresveratrol products as well as resveratrol dimers in the media of cells treated with SIN-1 (5 mM, 6 h) but not in cells treated with resveratrol alone. Representative chromatograms are presented in Fig. 5. These data provide strong evidence that the protective effects of resveratrol in SIN-1-treated cells were due to the scavenging of peroxynitrite.
Fig. 5. Analysis of resveratrol products in the media of mIMCD-3 cells treated with SIN-1.
Resveratrol (50 μM) was incubated with mIMCD-3 cells in the absence or presence of SIN-1 (5 mM) for 6h. LC/MS/MS analysis (product ion scans) of media extracts showed peaks for at least two isomeric forms of mono-nitroresveratrol and resveratrol dimers. No di-nitroresveratrol was observed. Data are representative of three independent experiments. The numerical superscripts above each integrated peak indicate retention time (min) and relative intensity. The numerical entry to the right of each chromatogram indicates the scaling of maximum intensity.
Discussion
It is clear from both in vitro and in vivo studies that resveratrol has anti-oxidant properties [11]. While resveratrol can modulate endogenous oxidant defenses, there is suggestive evidence that resveratrol may also directly scavenge certain oxidants. RNS like peroxynitrite play an important role in many of the diseases and conditions reported to be ameliorated by resveratrol [11, 19]. The data are especially compelling in the kidney [34], where resveratrol has been shown to decrease lipid peroxidation products produced by cisplatin [20] and gentamicin [35], decrease oxidative stress and injury caused by renal ischemia/reperfusion [36] and lipopolysaccharide [14], and decrease the inflammatory response and RNS generation in glycerol-induced renal injury [21]. Moreover, scavengers of peroxynitrite are known to be protective in the kidney. For example, ebselen [37, 38] and superoxide/peroxynitrite decomposition catalysts such as Mn (III) meso-tetrakis-(N-methlypyridinium-2-yl) porphyrin (MnTE-2-PyP) and Mn (III) meso-tetrakis-(1,3-diethylimidazolium-2-yl) porphyrin (MnTDE-2-ImP5+) [32, 39] have been shown to be protective in oxidative stress models of kidney injury [40, 41] as well as in other models of cardiovascular disease [42, 43], sepsis [44] and neuroinflammation [45].
Peroxynitrite is produced by the reaction of NO and superoxide with diffusion-limited kinetics [46, 47]. At physiological pH, peroxynitrite is protonated to generate peroxynitrous acid (ONOOH) or can react with CO2 to form nitrosoperoxycarbonate (ONOOCO2−). Both species can nitrate phenols, such as tyrosine [48]. The appearance of 3-nitrotyrosine is considered a biomarker for peroxynitrite-mediated oxidation of proteins [32, 49] and has been detected in the kidney following ischemia/reperfusion injury [2] and sepsis [7].
Studies by Brito et al. [16] showing that resveratrol could block oxidation of LDL by peroxynitrite and by Olas et al. showing that resveratrol reduced peroxynitrite-mediated oxidation and nitration of serum proteins [22] and platelet proteins [23] led us to examine the peroxynitrite scavenging activity of resveratrol. We show here that resveratrol blocked nitration of BSA by authentic peroxynitrite with an EC50 of approximately 20 μM. To our knowledge this is the first reported EC50 value for resveratrol to scavenge peroxynitrite and is in the concentration range most often reported for the in vitro effects of resveratrol [50]. Resveratrol is a potent scavenger of peroxynitrite relative to the thiol NAC, an antioxidant [32] shown to block peroxynitrite-mediated protein nitration [33]. We found that resveratrol was more that 20-fold more potent than NAC at blocking nitration of BSA. Mn and Fe porphyrins can decompose peroxynitrite in a catalytic manner while thiols like NAC and glutathione scavenge nitrogen dioxide generated from peroxynitrite as a result of peroxide bond homolysis [48]. However, to label resveratrol a true peroxynitrite scavenger, it must effectively compete with cellular targets of peroxynitrite [32].
We demonstrated here that resveratrol not only blocks protein tyrosine nitration but also acts as a functional scavenger to prevent peroxynitrite-medicated cell death. SIN-1 generates equimolar ratios of superoxide and nitric oxide, which react to generate peroxynitrite [31]. Using SIN-1 to test the functional scavenging activity of resveratrol, we showed that resveratrol was able to protect renal epithelial cells from SIN-1-induced cytotoxicity. The finding that resveratrol protected against SIN-1 cytotoxicity in this same concentration range that it blocked peroxynitrite-mediated nitration further supports the scavenging of peroxynitrite as the likely mechanism of cytoprotection. This was confirmed by the observation that decomposed SIN-1 was not cytotoxic.
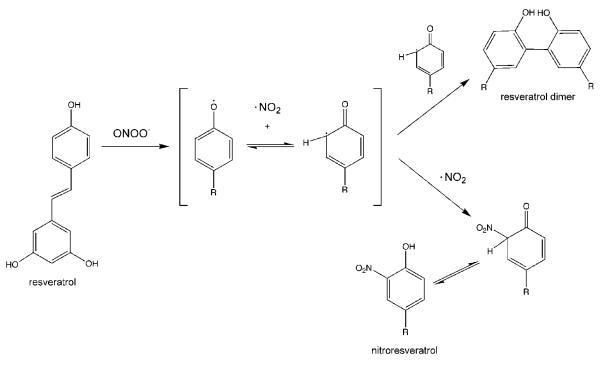
Peroxynitrite scavengers react rapidly with peroxynitrite and/or quench reactive intermediates [32]. To our knowledge, the present studies are to the first to analyze resveratrol-peroxynitrite reaction products. As a polyphenol, resveratrol has multiple potential sites of nitration. Resveratrol could be nitrated via direct reaction with peroxynitrous acid or via nitrogen dioxide. Analysis of reaction products formed during the reaction of resveratrol with authentic peroxynitrite at pH 7.4 identified isomeric forms of mono- and di-nitro derivatives of resveratrol, as well as resveratrol dimers. The formation of both dimers and mono-nitro derivatives with similar concentration ratio profiles (see Fig. 4) is consistent with the generation of a phenoxy radical intermediate (Fig. 6) via a stepwise radical mechanism and nitration via the nitrogen dioxide radical [48]. At lower ONOO−:resveratrol reaction ratios, mono-nitration and dimerization were prominent, whereas higher peroxynitrite concentrations were required for di-nitro resveratrol formation. Importantly, a similar profile of products was detected in the media from cells when SIN-1 was used as a peroxynitrite generator. The experiments evaluating SIN-1 cytotoxicity in renal epithelial cells were particularly important because they demonstrated that the scavenging ability of resveratrol actually protected the cells from the lethal effects of peroxynitrite.
Fig. 6.
Hypothetical pathway for nitroresveratrol and resveratrol dimer formation by peroxynitrite
Taken collectively, these data suggest that resveratrol may provide functional protection in microenvironments where NO and superoxide are produced simultaneously by competing with peroxynitrite for endogenous targets. This activity may be especially important in the kidney where even relatively low levels of peroxynitrite generation can reduce renal epithelial cell adhesion to the extracellular matrix proteins [51]. Thus, the ability of resveratrol to directly scavenge peroxynitrite may help to explain some of its protective effects reported in models of renal diseases associated with induction of nitric oxide synthase, superoxide generation, and peroxynitrite formation. These new findings suggest another novel mechanism of action to explain the antioxidant properties of this widely consumed neutraceutical.
Acknowledgements
This work was supported by the Nation Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grants F30 DK085705 (JHH) and R01 DK075991 (PRM). The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration.
List of abbreviations
- trans-resveratrol
Trans-3, 4′, 5-trihydroxystilbene
- SIN-1
5-amine-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride
- BSA
bovine serum albumin
- NAC
N-acetyl-L-cysteine
- FBS
fetal bovine serum
- DMEM
Dulbecco's Modified Eagles Medium
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vinas JL, Sola A, Hotter G. Mitochondrial NOS upregulation during renal I/R causes apoptosis in a peroxynitrite-dependent manner. Kidney Int. 2006;69:1403–9. doi: 10.1038/sj.ki.5000361. [DOI] [PubMed] [Google Scholar]
- 2.Walker LM, Walker PD, Imam SZ, Ali SF, Mayeux PR. Evidence for peroxynitrite formation in renal ischemia-reperfusion injury: studies with the inducible nitric oxide synthase inhibitor L-N6-(1-iminoethyl)-lysine. J Pharmacol Exp Ther. 2000;295:417–22. [PubMed] [Google Scholar]
- 3.Walker LM, York JL, Imam SZ, Ali SF, Muldrew KL, Mayeux PR. Oxidative stress and reactive nitrogen species generation during renal ischemia. Toxicol Sci. 2001;63:143–8. doi: 10.1093/toxsci/63.1.143. [DOI] [PubMed] [Google Scholar]
- 4.MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Rad Biol Med. 2001;31:1603–8. doi: 10.1016/s0891-5849(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 5.Baldew GS, Boymans AP, Mol JG, Vermeulen NP. The influence of ebselen on the toxicity of cisplatin in LLC-PK1 cells. Biochem Pharmacol. 1992;44:382–7. doi: 10.1016/0006-2952(92)90024-d. [DOI] [PubMed] [Google Scholar]
- 6.Chirino YI, Hernandez-Pando R, Pedraza-Chaverri J. Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol. 2004;4:20. doi: 10.1186/1471-2210-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L, Gokden N, Mayeux PR. Evidence for the role of reactive nitrogen species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. J Am Soc Nephrol. 2007;18:1807–15. doi: 10.1681/ASN.2006121402. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, Mayeux PR. Effects of the inducible nitric oxide synthase inhibitor L-N6-(1-iminoethyl)-lysine on microcirculation and reactive nitrogen species generation in the kidney following lipopolysaccharide administration in mice. J Pharmacol Exp Ther. 2007;320:1061–7. doi: 10.1124/jpet.106.117184. [DOI] [PubMed] [Google Scholar]
- 9.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–65. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 10.Koyner JL, Sher Ali R, Murray PT. Antioxidants. Do they have a place in the prevention or therapy of acute kidney injury? Nephron Experimental Nephrology. 2008;109:e109–e17. doi: 10.1159/000142935. [DOI] [PubMed] [Google Scholar]
- 11.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 12.Martinez J, Moreno JJ. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol. 2000;59:865–70. doi: 10.1016/s0006-2952(99)00380-9. [DOI] [PubMed] [Google Scholar]
- 13.Sebai H, Ben-Attia M, Sani M, Aouani E, Ghanem-Boughanmi N. Protective effect of resveratrol in endotoxemia-induced acute phase response in rats. Arch Toxicol. 2008 doi: 10.1007/s00204-008-0348-0. [DOI] [PubMed] [Google Scholar]
- 14.Sebai H, Ben-Attia M, Sani M, Aouani E, Ghanem-Boughanmi N. Protective effect of resveratrol on acute endotoxemia-induced nephrotoxicity in rat through nitric oxide independent mechanism. Free Radic Res. 2008;42:913–20. doi: 10.1080/10715760802555577. [DOI] [PubMed] [Google Scholar]
- 15.Hung LM, Su MJ, Chu WK, Chiao CW, Chan WF, Chen JK. The protective effect of resveratrols on ischaemia-reperfusion injuries of rat hearts is correlated with antioxidant efficacy. Br J Pharmacol. 2002;135:1627–33. doi: 10.1038/sj.bjp.0704637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brito P, Almeida LM, Dinis TC. The interaction of resveratrol with ferrylmyoglobin and peroxynitrite; protection against LDL oxidation. Free Radic Res. 2002;36:621–31. doi: 10.1080/10715760290029083. [DOI] [PubMed] [Google Scholar]
- 17.Frankel EN, Kanner J, German JB, Parks E, Kinsella JE. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–7. doi: 10.1016/0140-6736(93)90206-v. [DOI] [PubMed] [Google Scholar]
- 18.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do Amaral CL, Francescato HD, Coimbra TM, Costa RS, Darin JD, Antunes LM, et al. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch Toxicol. 2008;82:363–70. doi: 10.1007/s00204-007-0262-x. [DOI] [PubMed] [Google Scholar]
- 21.de Jesus Soares T, Volpini RA, Francescato HD, Costa RS, da Silva CG, Coimbra TM. Effects of resveratrol on glycerol-induced renal injury. Life Sci. 2007;81:647–56. doi: 10.1016/j.lfs.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Olas B, Nowak P, Kolodziejczyk J, Ponczek M, Wachowicz B. Protective effects of resveratrol against oxidative/nitrative modifications of plasma proteins and lipids exposed to peroxynitrite. J Nutr Biochem. 2006;17:96–102. doi: 10.1016/j.jnutbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Olas B, Wachowicz B, Nowak P, Stochmal A, Oleszek W, Glowacki R, et al. Comparative studies of the antioxidant effects of a naturally occurring resveratrol analogue -- trans-3,3′,5,5′-tetrahydroxy-4′-methoxystilbene and resveratrol -- against oxidation and nitration of biomolecules in blood platelets. Cell Biol Toxicol. 2008;24:331–40. doi: 10.1007/s10565-007-9045-7. [DOI] [PubMed] [Google Scholar]
- 24.MacMillan-Crow LA, Crow JP, Kerby JK, Beckman JS. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93:11853–8. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Rad Biol Med. 2001;30:463–88. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 26.Ischiropoulos H, Al-Mehdi AB. Peroxynitrite-mediated oxidative protein modifications. FEBS Letter. 1995;364:279–82. doi: 10.1016/0014-5793(95)00307-u. [DOI] [PubMed] [Google Scholar]
- 27.Khan J, Brennan DM, Bradley N, Gao B, Bruckdorfer R, Jacobs M. 3-Nitrotyrosine in the proteins of human plasma determined by an ELISA method. Biochem J. 1998;331:795–801. doi: 10.1042/bj3300795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto MC, Garcia-Barrado JA, Macias P. Resveratrol is a potent inhibitor of the dioxygenase activity of lipoxygenase. J Agric Food Chem. 1999;47:4842–6. doi: 10.1021/jf990448n. [DOI] [PubMed] [Google Scholar]
- 29.Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol. 1993;265:F416–F24. doi: 10.1152/ajprenal.1993.265.3.F416. [DOI] [PubMed] [Google Scholar]
- 30.Pathak E, Mayeux PR. In vitro model of sepsis-induced renal epithelial reactive nitrogen species generation. Toxicol Sci. 2010 doi: 10.1093/toxsci/kfq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ischiropoulos H, Duran D, Horwitz J. Peroxynitrite-mediated inhibition of DOPA synthesis in PC12 cells. J Neurochem. 1995;65:2366–72. doi: 10.1046/j.1471-4159.1995.65052366.x. [DOI] [PubMed] [Google Scholar]
- 32.Crow JP. Peroxynitrite scavenging by metalloporphyrins and thiolates. Free Rad Biol Med. 2000;28:1487–94. doi: 10.1016/s0891-5849(00)00249-5. [DOI] [PubMed] [Google Scholar]
- 33.Fontana M, Pecci L, Macone A, Cavallini D. Antioxidant properties of the decarboxylated dimer of aminoethylcysteine ketimine: assessment of its ability to scavenge peroxynitrite. Free Radic Res. 1998;29:435–40. doi: 10.1080/10715769800300481. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigo R, Bosco C. Oxidative stress and protective effects of polyphenols: comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142:317–27. doi: 10.1016/j.cbpc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Morales AI, Buitrago JM, Santiago JM, Fernandez-Tagarro M, Lopez-Novoa JM, Perez-Barriocanal F. Protective effect of trans-resveratrol on gentamicin-induced nephrotoxicity. Antioxid Redox Signal. 2002;4:893–8. doi: 10.1089/152308602762197434. [DOI] [PubMed] [Google Scholar]
- 36.Sener G, Tugtepe H, Yuksel M, Cetinel S, Gedik N, Yegen BC. Resveratrol improves ischemia/reperfusion-induced oxidative renal injury in rats. Arch Biochem Biophys. 2006;37:822–9. doi: 10.1016/j.arcmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Briviba K, Toussyn I, Sharov VS, Sies H. Attenuation of oxidation and nitration reactions of peroxynitrite by selenomethionine, selenocystine and ebselen. Biochem J. 1996;319:13–5. doi: 10.1042/bj3190013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabuchi Y, Sugiyama N, Horiuchi T, Furusawa M, Furuhama K. Ebselen, a seleno-organic compound protects against ethanol-induced murine gastric mucosal injury in both in vivo and vitro systems. Eur J Pharmacol. 1995;272:195–210. doi: 10.1016/0014-2999(95)90819-u. [DOI] [PubMed] [Google Scholar]
- 39.Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Neta P, Okado-Matsumoto A, et al. New class of potent catalysts of O2.-dismutation. Mn(III) ortho-methoxyethylpyridyl- and di-ortho-methoxyethylimidazolylporphyrins. Dalton Trans. 2004:1696–702. doi: 10.1039/b400818a. [DOI] [PubMed] [Google Scholar]
- 40.Noiri E, Nakao A, Uchida K, Tsukahara H, M O, Fujita T, et al. Oxidative nitrosative stress in acute renal ischemia. Am J Physiol. 2001;281:F948–F57. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Jittikanont S, Falk SA, Li P, Feng L, Gengaro PE, et al. Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am J Physiol. 2003;284:F532–F7. doi: 10.1152/ajprenal.00323.2002. [DOI] [PubMed] [Google Scholar]
- 42.Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, et al. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Cir Res. 2004;94:377–84. doi: 10.1161/01.RES.0000111802.09964.EF. [DOI] [PubMed] [Google Scholar]
- 43.Gealekman O, Brodsky SV, Zhang F, Chander PN, Friedli C, Nasjletti A, et al. Endothelial dysfunction as a modifier of angiogenic response in Zucker diabetic fat rat: amelioration with Ebselen. Kidney Int. 2004;66:2337–47. doi: 10.1111/j.1523-1755.2004.66035.x. [DOI] [PubMed] [Google Scholar]
- 44.Nin N, Cassina A, Boggia J, Alfonso E, Botti H, Peluffo G, et al. Septic diaphragmatic dysfunction is prevented by Mn(III)porphyrin therapy and inducible nitric oxide synthase inhibition. Intensive Care Med. 2004;30:2271–8. doi: 10.1007/s00134-004-2427-x. [DOI] [PubMed] [Google Scholar]
- 45.Crow JP. Catalytic antioxidants to treat amyotropic lateral sclerosis. Expert Opin Investig Drugs. 2006;15:1383–93. doi: 10.1517/13543784.15.11.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huie RE, Padmaja S. The reaction of NO with superoxide. Free Rad Res Comms. 1993;18:195–9. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 47.Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 48.Gunaydin H, Houk KN. Mechanisms of peroxynitrite-mediated nitration of tyrosine. Chem Res Tox. 2009;22:894–8. doi: 10.1021/tx800463y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Tox. 1996;9:836–44. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 50.Pirola L, Frojdo S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60:323–32. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 51.Wangsiripaisan A, Gengaro PE, Nemenoff RA, Ling H, Edelstein CL, Schrier RW. Effect of nitric oxide donors on renal tubular epithelial cell-matrix adhesion. Kidney Int. 1999;55:2281–8. doi: 10.1046/j.1523-1755.1999.00484.x. [DOI] [PubMed] [Google Scholar]