Abstract
Trypanosoma cruzi host cell entry depends on lysosomes for the formation of the parasitophorous vacuole. Lysosome internal surface is covered by two major proteins, highly sialilated, Lysosome Associated Membrane Proteins 1 and 2. T. cruzi, on the other hand, needs to acquire sialic acid from its host cell through the activity of trans-sialidase, an event that contributes to host cell invasion and later for parasite vacuole escape. Using LAMP1/2 knock out cells we were able to show that these two proteins are important for T. cruzi infection of host cells, both in entrance and intracellular development, conceivably by being the major source of sialic acid for T. cruzi.
Keywords: T. cruzi, Lysosomes, LAMP, Cell invasion
1. Introduction
Trypanosoma cruzi is the causative agent of Chagas disease, a serious debilitating illness with a variable clinical course that affects millions of people throughout America [1]. T. cruzi is able to invade a wide variety of cell types, especially non-professional phagocytic cells, using a series of molecules that will trigger host intracellular signaling [2–4]. This signaling will culminate with lysosome recruitment and fusion with the plasma membrane either at the site of parasite attachment to the host cell or, alternatively, to the site of invagination of host cell plasma membrane [5,6]. Either way, lysosomal association and fusion has been demonstrated to be essential for formation of a viable parasitophorous vacuole, without which parasites can exit host cells [7]. Then, lysosomes work as anchors for the parasite within host cells, likely as a result from the tight interactions of lysosomal membrane proteins directly with molecules on the parasite plasma membrane. Following cell invasion T. cruzi trypomastigotes escape into the cytosol and differentiate into the replicative amastigote form [8,9].
Lysosomal associated membrane proteins 1 and 2 (LAMP1 and 2) are the major integral membrane proteins of lysosomes [10]. They are highly glycosilated proteins, rich in sialic acid, and estimated to cover approximately 80% of the lysosome luminal surface [10,11]. Interestingly, T. cruzi is not able to synthesize sialic acid, but uses a specialized enzyme, trans-sialidase, to transfer it from host derived glycoconjugates to parasite mucin-like glycoproteins [12], favoring parasite invasion [13]. Cells lacking sialic acid are less permissive to T. cruzi infection, and form looser vacuoles in comparison to what is seen in wild type control cells [14,15]. Furthermore, LAMP1 exposure at the cell surface can enhance T. cruzi invasion [16]. These data together with the fact that lysosomes are essential for T. cruzi retention in host cells, raise the question of whether luminal moieties of LAMP might be acting as a T. cruzi “receptor” when exposed to parasites during lysosomal fusion events for the formation of the parasitophorous vacuole, subsequently playing a role in retaining the parasite inside the host cell. We set out to investigate this issue, by studying T. cruzi infection in LAMP1 and 2 double knock out (LAMP1/2−/−) fibroblasts [11].
2. Materials and methods
2.1. Antibodies and reagents
Anti-mouse LAMP1 mAb (1D4B) was obtained from the Developmental Studies Hybridoma Bank. Anti-T. cruzi polyclonal antibodies were generated by immunizing a rabbit with T. cruzi trypomastigotes, and anti-Ssp-4 mAbs were generated as described previously [17]. Secondary antibodies, anti-rat IgG-Alexa fluor 488 and anti-rabbit IgG-Alexa fluor 546 were obtained from Invitrogen®.
2.2. Cells and parasites
Mouse fibroblast cell lines, derived from wild type (WT) and LAMP1 and 2 knock out (LAMP1/2−/−) mice, were generated in Dr. Paul Saftig’s laboratory by spontaneous immortalization of primary fibroblasts in culture around passages 10–20 (#5 and #9-WT and LAMP1/2−/−, respectively) or by immortalization of primary fibroblasts using a plasmid containing the Simian Virus 40 (SV40) large T antigen (PS1 and SV48, WT and LAMP1/2−/−, respectively) [11]. Cells were maintained in culture by consecutive passages in high glucose DMEM (Invitrogen®) supplemented with 10% FBS, 1% penicillin–streptomycin and 1% glutamine.
Tissue culture trypomastigotes from the T. cruzi Y (T. cruzi II) and CL (T. cruzi VI) strains were obtained from the supernatant of infected LLC-MK2 monolayers and purified as described by Andrews et al. (1987).
2.3. T. cruzi invasion assay
WT fibroblasts as well as LAMP1/2−/− fibroblasts were plated at 1.25 × 105 (experiments longer than 24 h) or 2.5 × 105 cells/dish in high glucose DMEM supplemented with 10% FBS, on 3-cm tissue culture dishes containing 12-mm round coverslips and grown for 24 h at 37 °C in a humidified atmosphere containing 5% CO2 until infection.
Infection of fibroblast cell lines with purified trypomastigotes from Y or CL strains was performed for 20 min at 37 °C at a multiplicity of infection (MOI) of 50 (unless otherwise stated). Immediately after exposure to trypomastigotes, monolayers were washed four times with PBS, and either fixed in 4% (wt/vol) paraformaldehyde/PBS overnight at 4 °C for immunocytochemistry or re-incubated with media for different times (24, 48, or 72 h) before fixation. After fixation coverslips were processed for immunofluorescence. All conditions were analyzed in triplicates and experiments were repeated at least three times. Media from cells, 96 h post-infection, was also collected to determine the number of trypomastigote forms released. In order to do so media from the same conditions were pooled together and the number of trypomastigotes determined in a counting chamber at a light microscope.
2.4. Immunofluorescence and quantification of parasite invasion
After fixation, coverslips with attached cells were washed three times in PBS, incubated for 20 min with PBS containing 2% BSA and processed for an inside/outside immunofluorescence invasion assay as described previously [17].
After the inside/outside immunofluorescence staining, host cell lysosomes were also immunostained using a 1:50 dilution of either rat anti-mouse LAMP1 hybridoma supernatant (1D4B) or rat anti-mouse LAMP-2 hybridoma supernatant (Abl 93) in PBS/BSA/saponin and the appropriate fluorescent labelled secondary antibody, as described previously [7]. After that, the DNA of host cells and parasites was stained for 1 min with DAPI (Sigma), mounted, and examined on a Ziess Axioplan microscope equipped with an Axiocam HRC camera controlled by Axiovision Software (Zeiss).
3. Results
3.1. LAMP1/2−/− cells are less permissive to T. cruzi invasion
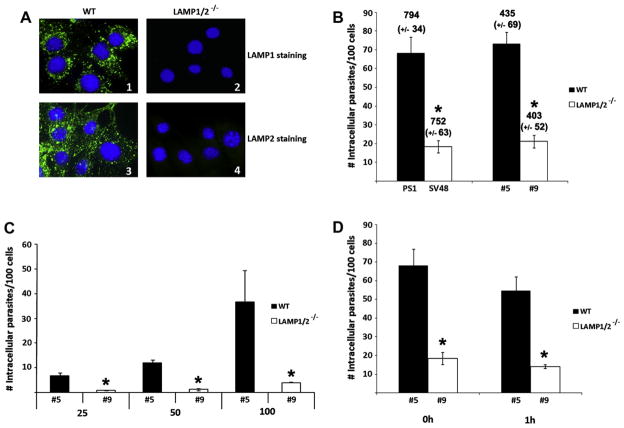
To test the influence of the lysosomal integral membrane proteins LAMP1 and 2 in T. cruzi cell infection, we performed cell invasion assays using fibroblast cell lines generated from a LAMP1/2 double knock out mouse (LAMP1/2−/− fibroblasts). Two independent pairs of WT and LAMP1/2−/− fibroblast cell lines were used. Pair #5 and #9 (WT and LAMP1/2−/− fibroblast lines, respectively) spontaneously immortalized in culture and pair PS1 and SV48 (WT and LAMP1/2−/− fibroblast lines, respectively) immortalized by transformation with SV40 large T antigen. First we confirmed the absence of both LAMP1 and 2 isoforms in LAMP1/2−/− fibroblasts, through immunostaining of these cells and their wild type counterparts with both anti-LAMP-1 and LAMP-2 antibodies. Fig. 1A shows the results obtained for WT #5 and LAMP1/2−/− #9 fibroblast lines. Same result was obtained for PS1 and SV48 (data not shown). Then we investigated the influence of these two proteins in the process of T. cruzi invasion in host cells. We observed a significant reduction of infection in both LAMP1/2−/− fibroblast lines, SV48 and #9, when compared to WT fibroblast lines, PS1 and #5. The number of internalized parasites per 100 counted cells in LAMP1/2−/− fibroblast cultures was about 3 times lower when compared to their WT counterparts (Fig. 1B). The decreased rate of invasion of LAMP1/2−/− fibroblasts was unlikely to be due to loss of infected cells, since the average number of total cells per coverslip did not vary between WT and LAMP1/2−/− in both pairs of fibroblast lines (Fig. 1B, number above bars). Also, reduced invasion of LAMP1/2−/− cells was observed regardless of the MOI used (Fig. 1C). Thus, trypomastigote entry into LAMP1/2−/− was less efficient, indicating a determinant role for these lysosomal proteins in the invasion process.
Fig. 1.
(A) Evaluation of LAMP1 and 2 presence in WT and LAMP1/2−/− cells, through immunofluorescence with anti-LAMP1 (1 and 2) or anti-LAMP-2 (3 and 4) antibodies and secondaries labelled with Alexa Fluor 488®. Cell nucleus is labelled with DAPI. LAMP staining, is observed only in WT cells, while cell nuclei can be seen in all panels. (B) Quantitative analyses of parasite infection in two pairs of Wild type (PS1 and #5) and LAMP1/2−/− (SV48 and #9) fibroblasts. Fibroblasts monolayers were exposed to Tissue Culture derived Trypomastigotes (TCT) from Y strain at an MOI of 50, and then analyzed by immunofluorescence staining. Infection was determined by the number of internalized parasites per 100 counted cells. The total number of fibroblasts per 15 counted fields (with the standard deviation values) is shown on the top of each bar on the graph. (C) Quantitative analysis of parasite infection in #5 (WT) and #9 (LAMP1/2−/−) fibroblasts using different MOI of T. cruzi Y strain (25; 50; 100). Infection was determined as described above. (D) Quantitative analyses of parasite infection in #5 (WT) and #9 (LAMP1/2−/−) fibroblasts, right after or 1 h post-invasion. In this case invasion assays were performed as described in material and methods, however after parasite wash cells were either fixed or re-incubated in media for an additional hour before fixation. Infection was determined as described above. The data in B–D correspond to the mean of triplicates ±SD. Asterisks indicate statistically significant differences (P < 0.05, Student’s t test) between WT and LAMP1/2−/− cells, under the same conditions.
3.2. LAMP deficiency does not lead to T. cruzi loss from host cell
It was demonstrated before that lysosomal fusion impairment, through wortmannin pre-treatment of host cells, leads to parasite loss from host cells, which occurred as early as 15 min and peaking at 1 h post-T. cruzi invasion [7]. To determine if absence of LAMP would lead to impaired fusion of lysosomes with plasma membrane for the formation of the parasitophorous vacuole, we exposed WT and LAMP1/2−/− fibroblasts to T. cruzi Y trypomastigotes for 15 min, washed out non-internalized parasites, and either fixed cells immediately or re-incubated in media for 1 h before fixation. In both cell types, no change in the amount of internalized trypomastigotes, right after parasite exposure or 1 h later, was observed (Fig. 1D). This result suggested, even though LAMP1/2−/− cells were less permissive to infection with T. cruzi Y strain, they were apparently still able to retain some parasites intracellularly.
3.3. LAMP deficiency augments T. cruzi intracellular development
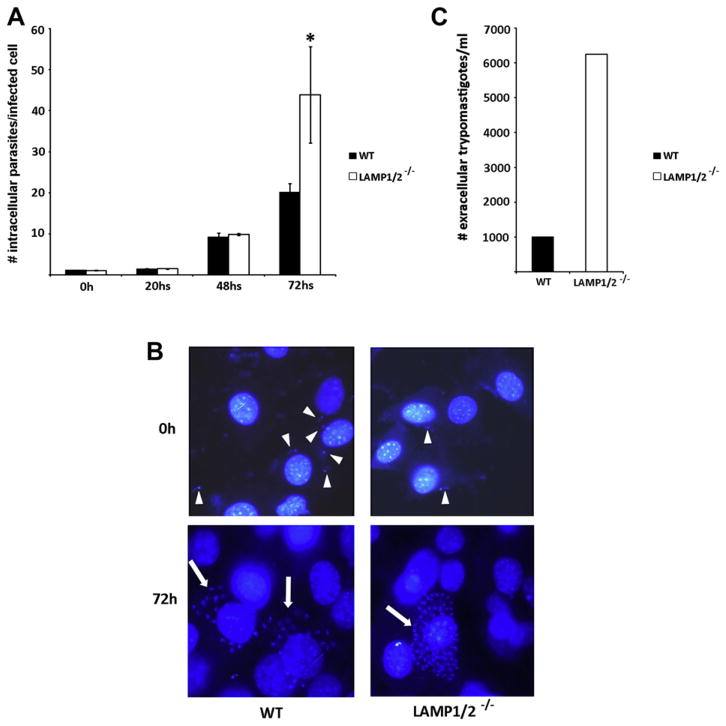
During T. cruzi intracellular development, it escapes from its parasitophorous vacuole and transforms into the amastigote replicative form, continuing its life cycle in the cytosol. To study the influence of the absence of LAMP in parasite intracellular survival and development, an infection time course was performed, in which cells were exposed to parasites for 20 min, washed to eliminate extracellular parasites, and either fixed or re-incubated in media for increasing intervals (20, 48, 72 and 96 h). Since fibroblasts continue to replicate in culture, in order to evaluate the intracellular development of the parasites, only the number of intracellular parasites per infected cell were counted in these experiments. Right after parasite exposure or 20 h later (a point when intracellular parasites have transformed into the amastigote form but not started replicating yet), even though the number of infected cells was much lower for LAMP1/2−/− cultures (Fig. 1B), the number of internalized parasites per infected cell was about the same for both WT and LAMP1/2−/− fibroblasts (Fig. 2A). After 48 h, when parasites have already started to replicate intracellularly, the number of parasites per infected cell increased but remained similar between both cell types. However, 72 h post-infection, LAMP1/2−/− cells showed three times more intracellular parasites than in WT cell cultures, indicating a faster intracellular development of T. cruzi Y strain in the absence of LAMP (Fig. 2A and B). We further followed the infection for 96 h and were able to observe the release of trypomastigote forms from infected cells, confirming the completion of parasite life cycle in both cell types. However, since the LAMP1/2−/− showed a much higher number of intracellular parasites 72 h post-infection, we also observed a higher number of trypomastigotes being released from these cultures when compared to WT cultures (Fig. 2C).
Fig. 2.
(A) Quantitative analyses of T. cruzi Y strain infection in WT as well as LAMP1/2−/− fibroblasts exposed to TCTs for 20 min (MOI 50). Monolayers were washed to remove extracellular parasites and either fixed after wash (PFA 4%) or re-incubated in media for 20 h, 48 h and 72 h before fixing. Cells were stained and analysed in a fluorescence microscope. Parasite load was determined by the number of internalized parasites divided by the total number of infected cells. The data correspond to the mean of triplicates ±SD. Asterisks indicate statistically significant differences (P < 0.05, Student’s t test) between WT and LAMP1/2−/− cells, under the same conditions. (B) Representative images of WT and LAMP1/2 KO cells infected with Y strain TCTs at an MOI of 50. Arrowheads indicate intracellular T. cruzi trypomastigotes and arrows indicate amastigote nests. (C) Total number of T. cruzi Y strain TCTs obtained from the pool of parasites released from WT or LAMP1/2−/− fibroblasts, 96 h post-infection.
3.4. LAMP influence is observed for different T. cruzi strains
Since there have been numerous studies in the literature showing intraspecific variation among T. cruzi strains, we decided to investigate whether the effects of LAMP absence on T. cruzi infection was specific for the T. cruzi Y strain, a member of the T. cruzi II major lineage [18]. For comparison we used the CL strain, a member of the T. cruzi IV major lineage, which is considered to be a polar strain in relation to the Y strain on several of its biological properties, such as morphology as well as pathogenicity [19–21]. Previous in vitro comparative studies of infectiveness of CL and Y strains in Vero cells have shown a significantly less infective rate for CL strain [20], which was also observed in our experiments for both used WT cells (Fig. 3A). The overall number of infected WT fibroblast was 10 times less then that observed for cultures exposed to T. cruzi Y strain, making it difficult to evaluate possible differences between the two cell types (LAMP1/2−/− and WT). In fact, assays performed with CL strain showed no statistically significant differences in invasion rates between LAMP1/2−/− and WT fibroblasts (data not shown). However, when we followed the course of cell infection by this strain, we also observed a faster intracellular development of these parasites in LAMP1/2−/− cultures (Fig. 3B), suggesting that independent of the T. cruzi strain tested, the absence of LAMP leads to a faster intracellular development.
Fig. 3.
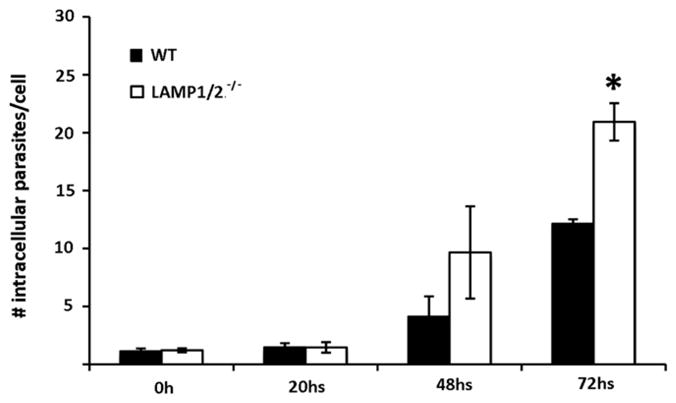
Quantitative analyses of T. cruzi CL strain infection in WT as well as LAMP1/2−/− fibroblasts exposed to parasite for 20 min (MOI 50). Monolayers were washed to remove extracellular parasites and either fixed with paraformaldehyde 4% after wash or re-incubated in media for 20 h, 48 h and 72 h before fixing. Cells were stained and analysed in a fluorescence microscope, as described before. 15 fields were counted for each coverslip and the number of parasites per infected cell is shown. The data correspond to the mean of triplicates ±SD. Asterisks indicate statistically significant differences (P < 0.05, Student’s t test) between WT and LAMP1/2−/− cells, under the same conditions.
4. Discussion
In this present work we showed that LAMP1 and 2, the two main integral membrane proteins from lysosomes, play a major role during T. cruzi infection of host cells. T. cruzi entry in non-professional phagocytic cells depends on host cell lysosome recruitment and fusion with plasma membrane for formation of a stable parasitophorous vacuole, which then holds parasites intracellularly [7]. However, how lysosomes acted as “anchors” for T. cruzi in host cells still remained unclear. Our findings have clarified this issue, by showing that cells lacking two major lysosomal integral membrane proteins, LAMP1 and 2 (LAMP1/2−/− cells), show a significant decrease in cell invasion by T. cruzi, at least for the Y strain. The number of infected cells was much lower and invasion was about three times less efficient in LAMP1/2−/− cells.
LAMP1 and 2 are highly glycosilated proteins, rich in sialic acid on its surface [10]. On the other hand, trans-sialidase, an enzyme present at the surface of T. cruzi infective forms and capable of transferring sialic acid from host surface molecules to parasite surface antigen (such as Ssp3), has an important role during T. cruzi entry in host cells [12,13]. Therefore, LAMP1 and 2 could be a source of sialic acid during T. cruzi attachment and invasion, consequently leading to a closer interaction between parasite and vacuolar membrane. In fact, Kima and co-workers have shown previously that LAMP1 expression at the cell surface increased parasite attachment and invasion, possibly due to exposure of the highly glycosilated N-terminal portion of this protein [16]. Even though it has been shown that lysosome fusion with phagosomes is somewhat disturbed in LAMP1/2−/− cells [22], there is no evidence that lysosomal exocytosis is impaired in these cells. According to our data, decreased T. cruzi invasion in the absence of LAMP proteins also did not seem to be due to lysosome–plasma membrane fusion impairment. It was previously shown that blocking lysosomal fusion with PI-3 kinase inhibitors leads to loss of intracellular T. cruzi trypomastigotes from cells as early as 15 min post-infection [7]. However, in our assays no parasite loss was observed when we infected cells for 20 min, washed and fixed cells 1 h later. This suggests that even in the absence of LAMP, the few trypomastigotes that enter cells reside in parasitophorous vacuoles that still interact with the host cell cytoskeleton. Therefore, it is most likely that the decreased invasion observed in LAMP1/2−/− cells is a consequence of a weaker interaction of parasite membrane with internal lysosomal surface during the invasion process.
We have also followed the intracellular development of T. cruzi in WT and LAMP1/2−/− cells. Counting the number of intracellular parasites per cell in the course of 72 h revealed a much higher parasite doubling rate in LAMP1/2−/− cells, when compared to WT cells, since the number of intracellular parasites in LAMP1/2−/− cells was two times higher. This also happened with other T. cruzi strains tested. Trypomastigote trans-sialidase activity and host cell sialic acid have also been implicated in parasite vacuole escape after entry. T. cruzi escape from its parasitophorous vacuole is thought to be mediated by a pore-forming protein, TcTox, which is secreted by the parasite and at low pH inserts into the vacuolar membrane causing its rupture [9,23]. Insertion of TcTox seems to be more efficient in parasitophorous vacuoles lacking sialic acid [14]. Moreover, infection studies with T. cruzi metacyclic trypomastigotes over-expressing trans-sialidase showed that increase in trans-sialidase activity is associated with faster parasite escape from vacuoles [24]. After trypomastigote release into the cytosol, parasites initiate its transformation into the amastigote replicative form. It is possible that in LAMP1/2−/− cells, the decreased amount of sialic acid available in lysosomal internal surface facilitates parasite vacuole escape. Such earlier release into the cell cytosol might then trigger faster replication of the parasites inside host cells. Whether reaching the cytosol earlier induces faster differentiation into the amastigote form, needs to be investigated. However, the fact that the difference in intracellular development is only noticeable 72 h post-invasion indicates that other factors, like intracellular signaling after exposure to the host cell cytosol, than exclusively an earlier differentiation into the amastigote replicative form, are involved in this process. Importantly, however, parasites infecting LAMP1/2−/− cells were able to complete their life cycle and differentiate back into the trypomastigote infective form and be released extracellularly. Corroborating our findings, the number of trypomastigotes released from LAMP1/2−/− cells was much higher. We also demonstrated that LAMP1/2 influence intracellular development regardless of the strain of T. cruzi used for infecting the cell. Our results show that the requirement for LAMP1/2 in the successful infection of host cells by T. cruzi is not determined exclusively by a role of these lysosomal membrane glycoproteins during invasion. The effect we observed on the levels of intracellular multiplication indicates an important role for LAMP1/2 proteins in determining T. cruzi interactions with intracellular environment, particularly the parasitophorous vacuole, that are critical for regulating their subsequent development.
Acknowledgments
We are especially grateful to Dr. Paul Saftig for providing the LAMP1/2 Knock out cells that have been used in this work. We are also grateful to the supporting agencies, Fundacao de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Cientéfico e Tecnologico (CNPq), the National Institutes of Health (NIH) and World Health Organization (WHO-TDR).
References
- 1.Coura JR, Dias JC. Epidemiology, control and surveillance of Chagas disease–100 years after its discovery. Mem Inst Oswaldo Cruz. 2009;104:31–40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- 2.Caler EV, Vaena de Avalos S, Haynes PA, Andrews NW, Burleigh BA. Oligopeptidase B-dependent signaling mediates host cell invasion by Trypanosoma cruzi. EMBO J. 1998;17:4975–4986. doi: 10.1093/emboj/17.17.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez A, Martinez I, Chung A, Berlot CH, Andrews NW. cAMP regulates Ca2+-dependent exocytosis of lysosomes and lysosome-mediated cell invasion by trypanosomes. J Biol Chem. 1999;274:16754–16759. doi: 10.1074/jbc.274.24.16754. [DOI] [PubMed] [Google Scholar]
- 4.Scharfstein J, Schmitz V, Morandi V, Capella MM, Lima AP, Morrot A, Juliano L, Muller-Esterl W. Host cell invasion by Trypanosoma cruzi is potentiated by activation of bradykinin B(2) receptors. J Exp Med. 2000;192:1289–1300. doi: 10.1084/jem.192.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tardieux I, Webster P, Ravesloot J, Boron W, Lunn JA, Heuser JE, Andrews NW. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992;71:1117–1130. doi: 10.1016/s0092-8674(05)80061-3. [DOI] [PubMed] [Google Scholar]
- 6.Woolsey AM, Sunwoo L, Petersen CA, Brachmann SM, Cantley LC, Burleigh BA. Novel PI 3-kinase-dependent mechanisms of trypanosome invasion and vacuole maturation. J Cell Sci. 2003;116:3611–3622. doi: 10.1242/jcs.00666. [DOI] [PubMed] [Google Scholar]
- 7.Andrade LO, Andrews NW. Lysosomal fusion is essential for the retention of Trypanosoma cruzi inside host cells. J Exp Med. 2004;200:1135–1143. doi: 10.1084/jem.20041408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews NW. The acid-active hemolysin of Trypanosoma cruzi. Exp Parasitol. 1990;71:241–244. doi: 10.1016/0014-4894(90)90027-a. [Review] [DOI] [PubMed] [Google Scholar]
- 9.Ley V, Robbins ES, Nussenzweig V, Andrews NW. The exit of Trypanosoma cruzi from the phagosome is inhibited by raising the pH of acidic compartments. J Exp Med. 1990;171:401–413. doi: 10.1084/jem.171.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howe C, Granger BL, Hull M, Green SA, Gabel CA, Helenius A, Mellman I. Derived protein sequence, oligosacharides, and membrane insertion of the 120 kDa lysosomal membrane glyocprotein (lgp120): identification of a highly conserved family of lysosomal membrane glycoproteins. Proc Natl Acad Sci USA. 1988;85 doi: 10.1073/pnas.85.20.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskelinen EL, Tanaka Y, Saftig P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 2003;13:137–145. doi: 10.1016/s0962-8924(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 12.Schenkman S, Eichinger D. Trypanosoma cruzi trans-sialidase and cell invasion. Parasitol Today. 1993;9:218–222. doi: 10.1016/0169-4758(93)90017-a. [DOI] [PubMed] [Google Scholar]
- 13.Schenkman S, Kurosaki T, Ravetch JV, Nussenweig V. Evidence for the participation of the Ssp-3 antigen in the invasion of non-phagocytic mammalian cells by Trypanosoma cruzi. J Exp Med. 1992;175:1635–1641. doi: 10.1084/jem.175.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall BF, Webster P, Ma AK, Joiner KA, Andrews NW. Desialylation of lysosomal membrane glycoproteins by Trypanosoma cruzi: a role for the surface neuraminidase in facilitating parasite entry into the host cell cytoplasm. J Exp Med. 1992;176:313–325. doi: 10.1084/jem.176.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez M, Huynh C, Andrade LO, Pypaert M, Andrews NW. Role for sialic acid in the formation of tight lysosome-derived vacuoles during Trypanosoma cruzi invasion. Mol Biochem Parasitol. 2002;119:141–145. doi: 10.1016/s0166-6851(01)00399-1. [DOI] [PubMed] [Google Scholar]
- 16.Kima P, Burleigh B, Andrews NW. Surface-targeted lysosomal glycoprotein-1 (Lamp-1) enhances lysosome exocytosis and cell invasion by Trypanosoma cruzi. Cell Microbiol. 2000;2:477–486. doi: 10.1046/j.1462-5822.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 17.Andrews NW, Hong KS, Robbins ES, Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp Parasitol. 1987;64:474–484. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- 18.Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 19.Alcantara A, Brener Z. The in vitro interaction of Trypanosoma cruzi bloodstream forms and mouse peritoneal macrophages. Acta Trop. 1978;35:209–219. [PubMed] [Google Scholar]
- 20.Bertelli MS, Brener Z. Infection of tissue culture cells with bloodstream trypomastigotes of Trypanosoma cruzi. J Parasitol. 1980;66:992–997. [PubMed] [Google Scholar]
- 21.Melo RC, Brener Z. Tissue tropism of different Trypanosoma cruzi strains. J Parasitol. 1978;64:475–482. [PubMed] [Google Scholar]
- 22.Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007;26:313–324. doi: 10.1038/sj.emboj.7601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews NW, Abrams CK, Slatin SL, Griffiths G. A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore-forming activity at low pH. Cell. 1990;61:1277–1287. doi: 10.1016/0092-8674(90)90692-8. [DOI] [PubMed] [Google Scholar]
- 24.Rubin-de-Celis SS, Uemura H, Yoshida N, Schenkman S. Expression of trypomastigote trans-sialidase in metacyclic forms of Trypanosoma cruzi increases parasite escape from its parasitophorous vacuole. Cell Microbiol. 2006;8:1888–1898. doi: 10.1111/j.1462-5822.2006.00755.x. [DOI] [PubMed] [Google Scholar]