Abstract
Mutations in the bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9 (GDF-9) genes have been identified in women with primary ovarian insufficiency (POI) and mothers of dizygotic twins. Here, we show that biological activities of the conditioned media from human embryonic kidney 293F cells transfected with two representative BMP-15 and GDF-9 mutants identified in the affected women have significantly reduced biological activities compared with the corresponding wild-type. Moreover, this difference is due to decreased production of the mature proteins, attributed most likely to impaired posttranslational processing of the proprotein. As genetic studies of the BMP-15 and/or GDF-9 genes in ewes established that a reduction of these proteins is associated with an increased ovulation rate, it is conceivable that women affected with these mutations may have an increased probability of bearing dizygotic twins during active reproductive ages before diagnosis with POI at later ages due to an earlier exhaustion of ovarian reserve.
Keywords: BMP, GDF, primary ovarian insufficiency, premature ovarian failure, dizygotic twins, ovary
1. Introduction
Women under 40 years of age that experience more than 4–6 months of amenorrhea and present with elevated serum FSH levels are diagnosed with premature ovarian failure (POF) or premature menopause (Rebar et al., 1990). However, POF differs from menopause, in that more than 50% of patients diagnosed with POF have new follicle growth and 5–10% deliver a child after diagnosis (Nelson et al., 1994; Rebar et al., 1990; Rebar et al., 1982; Taylor et al., 1996), Hence, primary ovarian insufficiency (POI) has been proposed as a preferred term for this condition, as first introduced by Fuller Albright in 1942 (Albright et al., 1942). Since POI affects around 1% of women under 40 years of age (Conway, 2000; Coulam et al., 1986), it is important to uncover the pathogenesis of POI. Genetic linkage analysis of familial POI cases has demonstrated that some POI cases result from chromosomal and genetic abnormalities (Laissue et al., 2008; Simpson, 2008; Skillern et al., 2008). However, the majority of POI cases are clinically idiopathic, though factors such as chemotherapy (Chemaitilly et al., 2006; Meirow, 2000; Sklar et al., 2006), pelvic surgery (Lass, 1999), autoimmune diseases (Bakalov et al., 2005; Hoek et al., 1997), infection and environmental factors (Jick et al., 1977) may play a role.
Bone morphogenetic protein-15 (BMP-15) and growth differentiation factor-9 (GDF-9) have recently been recognized as candidate factors that may be involved in POI (Di Pasquale et al., 2004; Dixit et al., 2005; Dixit et al., 2006; Kovanci et al., 2007; Laissue et al., 2006). Both BMP-15 and GDF-9 are TGF-β superfamily members and produced in oocytes within the ovary (Shimasaki et al., 2004). Like other members of the TGF-β superfamily, BMP-15 and GDF-9 are both produced as a proprotein, comprised of a signal peptide, a proregion and a mature region (Dube et al., 1998; Laitinen et al., 1998). After removal of the signal peptide, the proprotein dimerizes, for which the proregion is essential (Hogan, 1996). The dimerized proprotein then undergoes proteolytic cleavage at a conserved RXXR cleavage site, which separates the proregion from the bioactive mature region (Massagué et al., 1994). A major breakthrough in the field of these oocyte-specific factors occurred when naturally occurring mutations of the BMP-15 and GDF-9 genes were found in ewes (Galloway et al., 2000; Hanrahan et al., 2004; McNatty et al., 2005). Ewes homozygous for the BMP-15 or GDF-9 mutations are infertile due to an arrest in follicle growth at the primary stage, whereas heterozygous carriers are super-fertile displaying increased ovulation rates and litter size compared with wild-type ewes.
BMP-15 also plays an important role in ovarian function in women. The first identified human BMP-15 mutation associated with hypergonadotropic ovarian failure due to ovarian dysgenesis was a non-conserved substitution of a tyrosine with a cysteine at amino acid residue 235 of the proregion of BMP-15 (Di Pasquale et al., 2004). The two patients with the BMP-15 mutation were sisters and heterozygous carriers. Laparoscopic examination indicated that the ovaries of these patients are grossly “streak”, like that observed in the homozygous, but not heterozygous, BMP-15 mutant ewes. As it was shown that the mutant protein antagonizes the mitotic properties of wild-type BMP-15, the simplest interpretation of this finding is that the infertility in those patients could be attributed to the antagonistic nature of the mutant. The same group extended the study to conduct genetic screening in unrelated POI patients, and demonstrated that mutations in the BMP-15 gene are associated with a high incidence of POI (Di Pasquale et al., 2006). Furthermore, they showed that three mutations (BMP-15R68W, BMP-15L148P, and BMP-15R138H) identified in women with overt POI lead to decreased production of mature protein in an in vitro assay (Rossetti et al., 2009). Other groups have also investigated whether BMP-15 mutations are involved in POI (Dixit et al., 2006; Laissue et al., 2006; Ledig et al., 2008; Takebayashi et al., 2000), with two studies (Dixit et al., 2006; Laissue et al., 2006) identifying additional BMP-15 mutations that occur with a higher frequency in POI patients than in normal women. Mutations in the BMP-15 gene have also been identified in mothers of dizygotic twins (Zhao et al., 2008); however, it remains to be determined whether these variants are mutations associated with the pathogenesis of POI and/or dizygotic twins or rare polymorphisms, thus caution is recommended in the interpretation of BMP-15 mutations identified in POI cases (Ledig et al., 2008).
Screening for mutations in the GDF-9 gene in POI patients has identified several missense mutations not found in control women (Dixit et al., 2005; Kovanci et al., 2007; Laissue et al., 2006; Zhao et al., 2007). Moreover, mutations in the GDF-9 gene have also been identified as being significantly more common in mothers of dizygotic twins compared with controls (Zhao et al., 2007). However, the nature and biological impact of these mutations on the GDF-9 gene are unknown because no structure/function studies have been performed.
It is notable that in the majority of BMP-15 and GDF-9 mutations identified in POI patients and/or mothers of dizygotic twins, the mutation site is located in the proregion of the proprotein. Thus, if the processing of these mutant proproteins occurred normally, the resulting mature proteins should be indistinguishable from the wild type, and no functional defects in the mutants would be expected. Therefore, we hypothesize that BMP-15 and GDF-9 mutations described in POI patients and/or mothers of dizygotic twins may result from altered posttranslational processing of these proteins.
To test our hypothesis, we have chosen two representative BMP-15 and GDF-9 mutants identified in women with POI and/or mothers of dizygotic twins (Dixit et al., 2005; Dixit et al., 2006; Kovanci et al., 2007). They are BMP-15R76C, BMP-15R206H, GDF-9K67E and GDF-9P103S, that occur with a high incidence (n=3/133, 1/133, 4/127 and 1/61, respectively) in POI patients, and were not identified in normal women (n=0/197, 0/197, 0/220 and 0/60, respectively) (Dixit et al., 2005; Dixit et al., 2006; Kovanci et al., 2007). These mutations are predicted to be deleterious and thus may have pathogenic effects. Moreover, GDF-9P103S was also identified in mothers of dizygotic twins with a significantly higher frequency than in controls (0.0119 vs 0.0048, p<0.02886) (Palmer et al., 2006). In the current study, we have explored whether and to what extent these mutant proteins affect BMP-15 and GDF-9 biology.
2. Materials and Methods
2.1. Reagents and supplies
Female Sprague Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). A human granulosa cell line (COV-434) and a mouse embryo teratocarcinoma epithelial cell line (P19) were generously provided by Drs. Peter Schrier and Sylvia Evans, respectively. Phospho Smad1/5/8, Phospho Smad2, Smad2/3 and Smad5 antibodies were obtained from Cell Signaling Technology (Beverly, MA).
2.2. Construction of expression plasmids
We previously described the generation of phBMP-15F and phGDF-9F plasmids that have a Flag epitope (F) at the C-terminus (Liao et al., 2004; Otsuka et al., 2000). These plasmids were used as templates to construct expression plasmids of phBMP-15FR76C, phBMP-15FR206H, phGDF-9FK67E, and phGDF-9FP103S by a site-directed mutagenesis technique (Hashimoto et al., 2005; Liao et al., 2003; Liao et al., 2004). Comparison with previously reported data, in regard to the biological activities of rhBMP-15 and rhGDF-9, indicates that the incorporation of the flag epitope tag at the C-terminus does not alter the biological activities of untagged rhBMP-15 and rhGDF-9 (Li et al., 2009; Liao et al., 2004; Mottershead et al., 2008; Otsuka et al., 2000).
2.3. Transfection and cell culture
The recombinant proteins were produced using FreeStyle™ 293 Expression System (Invitrogen, Carlsbad, CA). Briefly, human embryonic kidney 293F cells were cultured in 30 ml FreeStyle serum-free medium (Invitrogen) in a 125 ml Erlenmyer flask on an orbital shaker rotating at 135 rpm in a 37°C CO2 incubator with a humidified atmosphere. The cells were then seeded in 160 µl of the medium in a 48-well plate at a density of 106 cells/ml and transfected with 160 ng of each plasmid using 293fectin™ transfection reagent. (Invitrogen). The transfected cells were cultured on the orbital shaker rotating at 135 rpm in the incubator for 1–4 days, then the conditioned media were collected and the cells lysed for further analysis.
2.4. Bioactivity studies
For the bioactivity studies, primary granulosa cells were harvested from ovaries of Sprague-Dawley rats (23 days old) that had been implanted subcutaneously with a silastic capsule containing 10 mg of diethylstilbestrol for 4 days to increase granulosa cell number. All animal protocols were approved by the University of California San Diego Institutional Animal Care and Use Committee. Primary granulosa cells (2×105 viable cells) were cultured in a 96-well plate containing 200 µl of serum-free McCoy’s 5A medium as described previously (Otsuka et al., 2000) in the presence or absence of 50 µl of conditioned media collected from 293F cells cultured for 4 days after the transfection with either phBMP-15F, phBMP-15FR76C, phBMP-15FR206H, phGDF-9F, phGDF-9F K67E, or phGDF-9FP103S. After 24 h culture, the granulosa cells were incubated with 5-bromo-2’-deoxyuridine (BrdU; 10 µM) for additional 24 h at 37°C. BrdU incorporation was quantified by a BrdU ELISA assay kit purchased from Roche Applied Science (Indianapolis, IN) according to the manufacturer’s instruction.
For analysis of Smad phosphorylation, COV434 and P19 cells were cultured in a 48-well plate in DMEM/F12 containing 10% fetal bovine serum, 100 U/ml penicillin, 1000 mg/ml streptomycin and 2 mM glutamine. When the cells reached approximately 80% confluency the medium was replaced with 200 µl of serum-free DMEM/F12. After a 3 h preculture, 50 µl of conditioned medium from the transfected 293F cells was added and cells were incubated for 1 h. Cells were then washed once in chilled PBS and solubilized in a lysis buffer. The cell lysates were subjected to Western blotting analysis using anti-phospho Smad1/5/8, anti-phospho Smad2, anti-total Smad5 or anti-total Smad2/3 antibodies as reported previously (McMahon et al., 2008). The relative integrated density of each protein band was digitized by NIH image J 1.40 (National Institutes of Health, Bethesda, MD).
2.5. RNA extraction and quantitative real-time PCR
The 293F cells were seeded in 800 µl of medium in a 12-well plate at a density of 106 cells/ml and transfected with 800 ng of each plasmid. After culture for 48 h, the cells were collected by centrifugation and total cellular RNA extracted as described previously (Liao et al., 2003). PCR primer pairs were selected from different exons of the corresponding genes to discriminate PCR products that might arise from possible chromosome DNA contaminants. Specifically, they were derived from the cDNA clones at the following nucleotide numbers: BMP-15, 131 bp (GenBank, accession no. NM_005448, 252–271 and 361–382); GDF-9, 94 bp (NM_005260, 581–600 and 655–674); and housekeeping gene, ribosomal protein L19, 190 bp (NM_000981, 401–420 and 571–590). For the quantification of mRNA expression, real-time PCR was performed using LightCycler-FastStart DNA master SYBR Green I system (Roche Diagnostic Co., Tokyo, Japan) with annealing at 60 °C and the PCR reaction containing 4 mM MgCl2 following the manufacturer’s protocol. Accumulated levels of fluorescence during amplification were analyzed by the second-derivative method after melting-curve analysis (Roche Diagnostic), with expression levels of target genes in each sample standardized to RPL19 expression.
2.6. Protein analysis
For analysis of intracellular proteins, cells were washed once in chilled PBS and solubilized in lysis buffer containing Protease-Arrest, 2% SDS and 4% β-mercaptoethanol. For analysis of proteins secretion, conditioned media were concentrated 5-fold using acetone precipitation. Briefly, conditioned medium was mixed with a 4 times volume of cold acetone (−20°C). After incubation at −80°C for 1 h, a pellet was obtained by centrifugation at 12,000×g for 10 min and dissolved in lysis buffer to make a 5-fold concentrated solution. Western immunoblotting was performed under reducing conditions using the anti-FLAG antibody as described previously (Otsuka et al., 2000).
2.7. Statistical analysis
All results are shown as mean ± SEM of data from at least three separate experiments, each performed with triplicate samples. Differences between groups were analyzed for statistical significance using ANOVA with Fisher’s protected least significant difference test (Stat-View 5.0 software, Abacus Concepts, Inc., Berkeley, CA). P <0.05 was considered to be statistically significant.
3. Results
3.1. Biological activities of the conditioned media containing BMP-15 and GDF-9 mutants
We began this study by evaluating the biological activity of BMP-15 and GDF-9 mutants identified in POI patients and/or mothers of dizygotic twins. For this purpose, we used conditioned media from the transfected 293F cells instead of purified mature proteins because the mutation sites of phBMP-15FR76C, phBMP-15FR206H, phGDF-9FK67E and phGDF-9FP103S were all located in the proregion, thus the mature proteins should be identical to the wild-type counterparts if production and processing of the proproteins occurred normally.
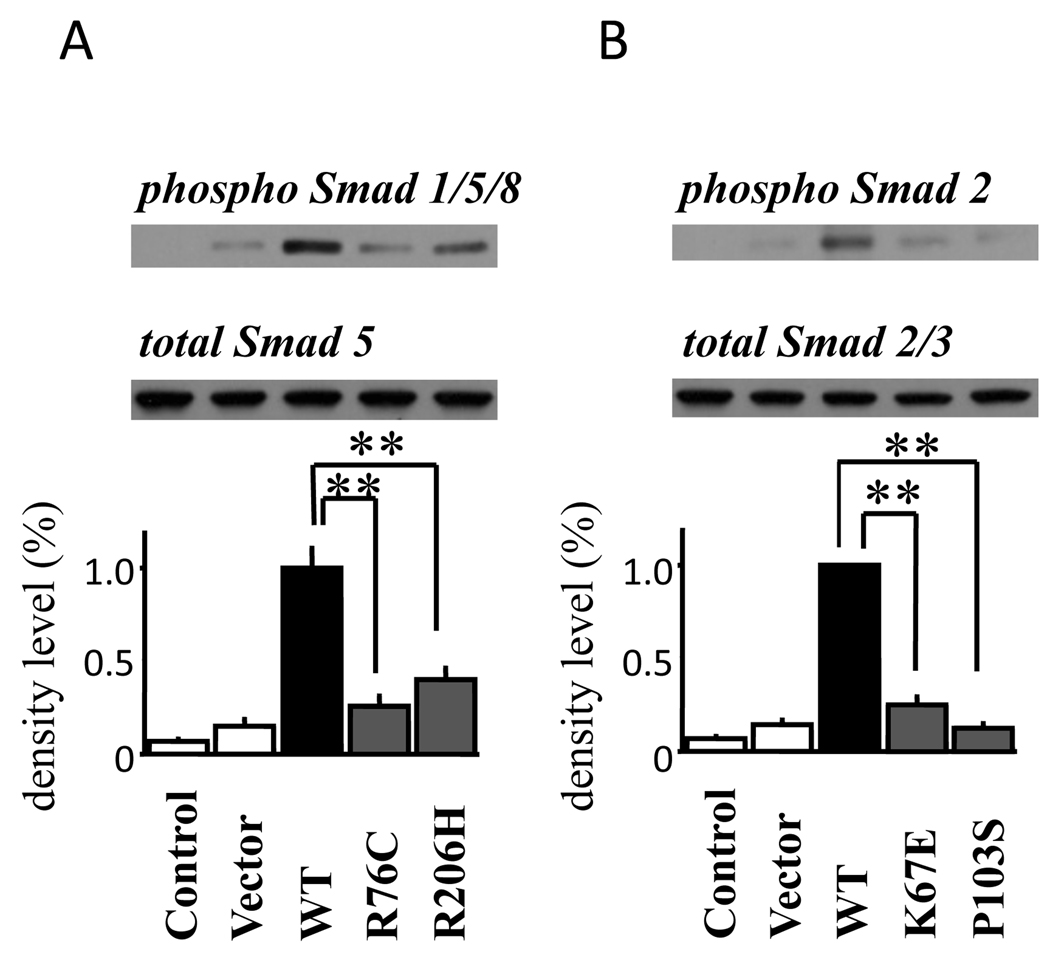
It has been previously reported that BMP-15 stimulates Smad1/5/8 phosphorylation in COV434 cells whereas GDF-9 stimulates Smad2/3 phosphorylation in P19 cells (McMahon et al., 2008). Therefore, we first used these cell lines as a bioassay. As shown in Panel A of Fig. 1, the conditioned media of 293F cells transfected with wild-type BMP-15 potently phosphorylated Smad1/5/8 whereas those transfected with phBMP-15R76C and phBMP-15R206H exhibited much weaker phosphorylation. Similarly, phosphorylation of Smad2/3 by the wild-type GDF-9 media was more prominent than that by conditioned media from GDF-9K67E and GDF-9P103S (Fig. 1B).
Fig. 1.
Effect of wild-type and mutant rhBMP-15 (A) and rhGDF-9 (B) on the Smad signaling pathways. COV-434 (A) and P19 (B) cells were cultured for 1 h with conditioned medium collected from 293F cells cultured for 4 days after the transfection with the indicated plasmids. Cell lysates were then subjected to Western immunoblotting analysis using indicated Smad antibodies. Representative data obtained from three independent experiments are presented in the upper panels. The band intensity of scanned images was statistically analyzed and the cumulative data from three independent experiments are shown with mean ± SEM mean in the lower panels. Each experiment was performed with triplicate samples. *, P <0.05; **, P < 0.01 between the indicated groups.
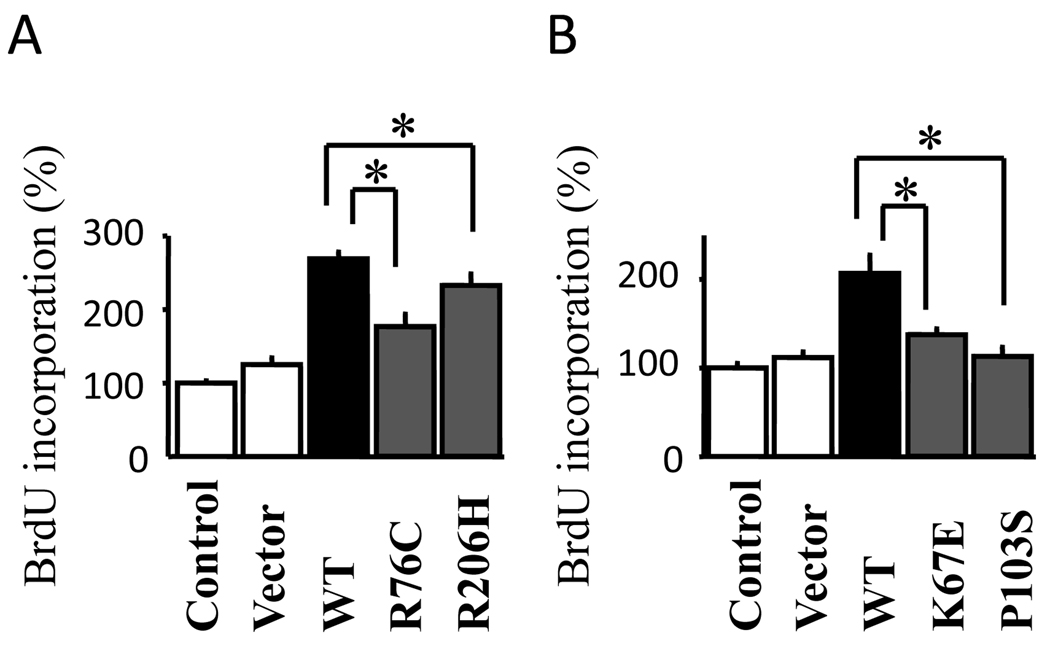
We also compared biological activities of the mutants in primary cultured rat granulosa cells, known to be target cells of these oocyte-derived growth factors. As both BMP-15 and GDF-9 have been shown to be mitogens for granulosa cells (Liao et al., 2004), we compared the effects of the conditioned media from the wild-type and mutant on granulosa cell proliferation using a BrdU assay. Both wild-type and mutants of BMP-15 increased BrdU incorporation; however, the effects of BMP-15R76C and BMP-15R206H were significantly weaker than wild-type (Fig. 2A). Similarly, the effects of GDF-9K67E and GDF-9P103S were also weaker than that of the wild-type (Fig. 2B).
Fig. 2.
Effect of wild-type and mutant rhBMP-15 (A) and rhGDF-9 (B) on rat granulosa cell mitosis. Granulosa cells were cultured for 24 h with conditioned medium collected from 293F cells cultured for 4 days after transfection with the indicated plasmids, followed by additional 24 h culture in the presence of 10 µM BrdU. The BrdU incorporated into the cells was quantified by a BrdU ELISA assay. All results are shown as mean ± SEM of data from at least three separate experiments, each performed with triplicate samples. *, P <0.05; **, P < 0.01 between the indicated groups.
3.2. Effect of mutations in BMP-15 and GDF-9 on gene transcription, protein synthesis and secretion
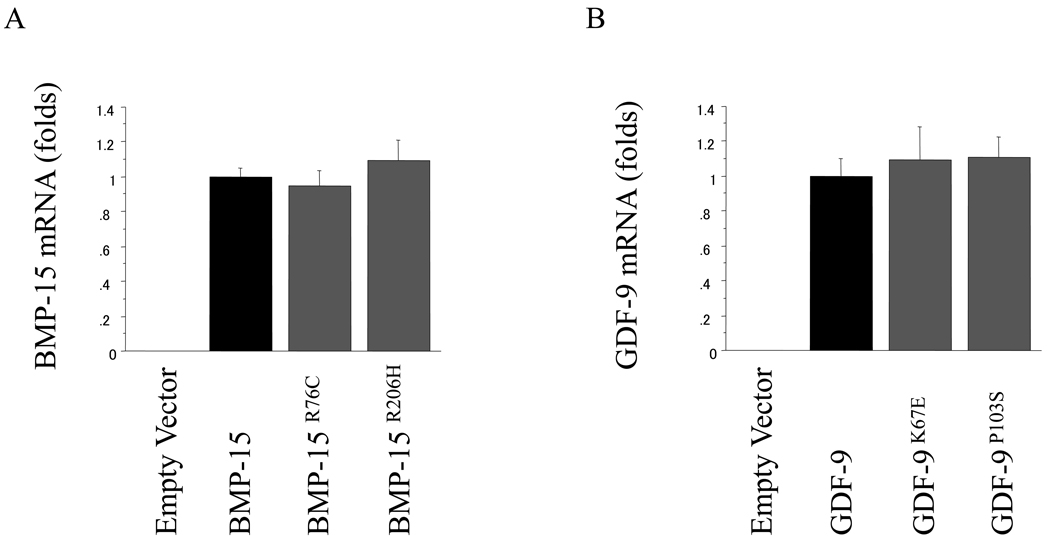
To elucidate the mechanism underlying the reduced bioactivity of the conditioned media from 293F cells transfected with the mutant BMP-15 or GDF-9, we assessed any effects of the mutations from gene transcription through the secretion of bioactive mature protein. Analysis of wild-type and mutant BMP-15 and GDF-9 transcripts showed no effect of the mutations on mRNA levels in transfected cells (Fig. 3).
Fig. 3.
Quantitative RT-PCR analysis for transfected 293F cells. Total cellular RNAs were extracted from 293F cells transfected with the indicated plasmids. For the quantification of BMP-15 (A) and GDF-9 (B) mRNA levels, real-time PCR was performed, and the expression levels of target genes were standardized by RPL19 level in each sample. All results are shown as mean ± SEM of data from at least three separate experiments, each performed with triplicate samples.
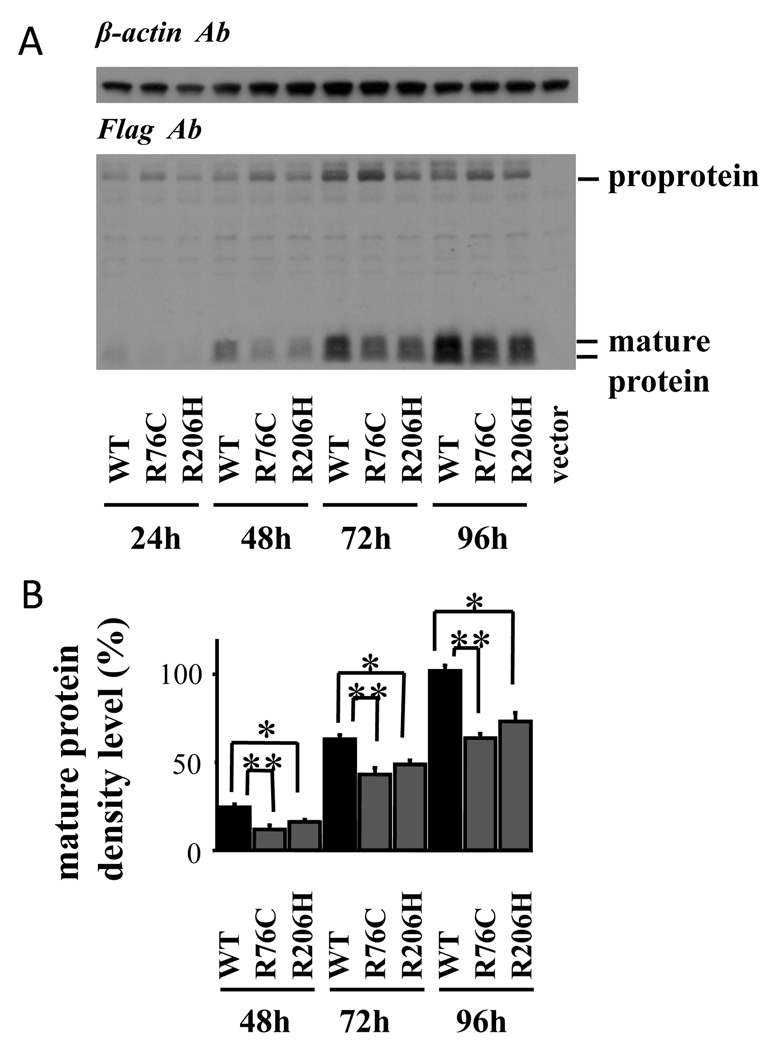
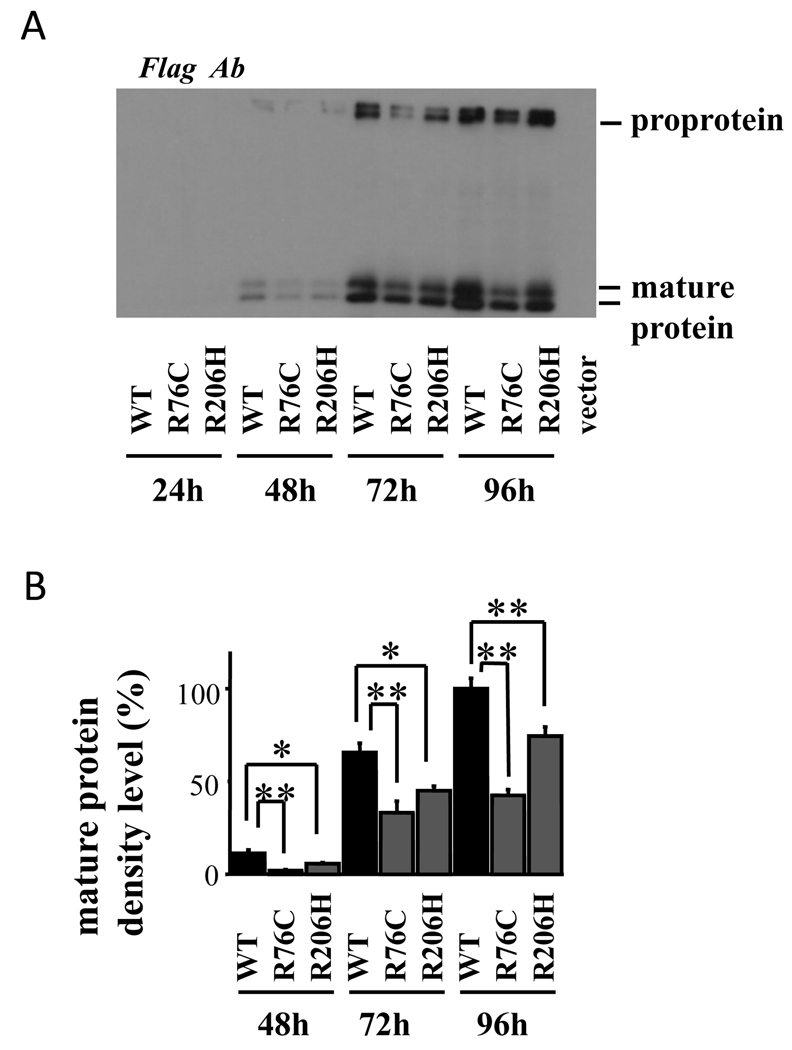
Assessment of wild-type and mutant BMP-15 protein production in the transfected cells revealed that mature protein levels of BMP-15R76C and BMP-15R206 in the cell lysates were remarkably lower than wild-type BMP-15 (Fig. 4). This was consistently observed each day of the 4 day-culture period. Similarly, the secreted protein profile in the conditioned medium of transfected cells also showed that mature protein levels of BMP-15R76C and BMP-15R206 were significantly decreased compared with those of wild-type BMP-15 (Fig. 5).
Fig. 4.
Production of BMP-15 by 293F cells transfected with wild-type and mutant plasmids. The 293F cells transfected with the indicated BMP-15 plasmids were cultured for the indicated hours, and cell lysates were subjected to SDS/PAGE immunoblotting analysis using anti-FLAG antibody (Ab) and anti-β-actin Ab. Note that all expression plasmids have a Flag epitope at the C-terminus. CTRL: control cells transfected with the empty vector. Results are shown as representative of those obtained from three independent experiments in panel A. The band intensity of scanned images was statistically analyzed, and the cumulative data from three independent experiments are presented with mean ± SEM in panel B. Each experiment was performed with triplicate samples. *, P <0.05; **, P < 0.01 between the indicated groups.
Fig. 5.
Production of BMP-15 by 293F cells transfected with wild-type and mutant plasmids. The 293F cells transfected with the indicated plasmids were cultured for the indicated hours, and conditioned media were treated with acetone to precipitate proteins followed by the SDS/PAGE immunoblotting analysis using anti-FLAG Ab. CTRL: control cells transfected with the empty vector. Results are shown as representative of those obtained from three independent experiments in panel A. The band intensity of scanned images was statistically analyzed (panel B). All results are shown as mean ± SEM of data from at least three separate experiments, each performed with triplicate samples. *, P <0.05; **, P < 0.01 between the indicated groups.
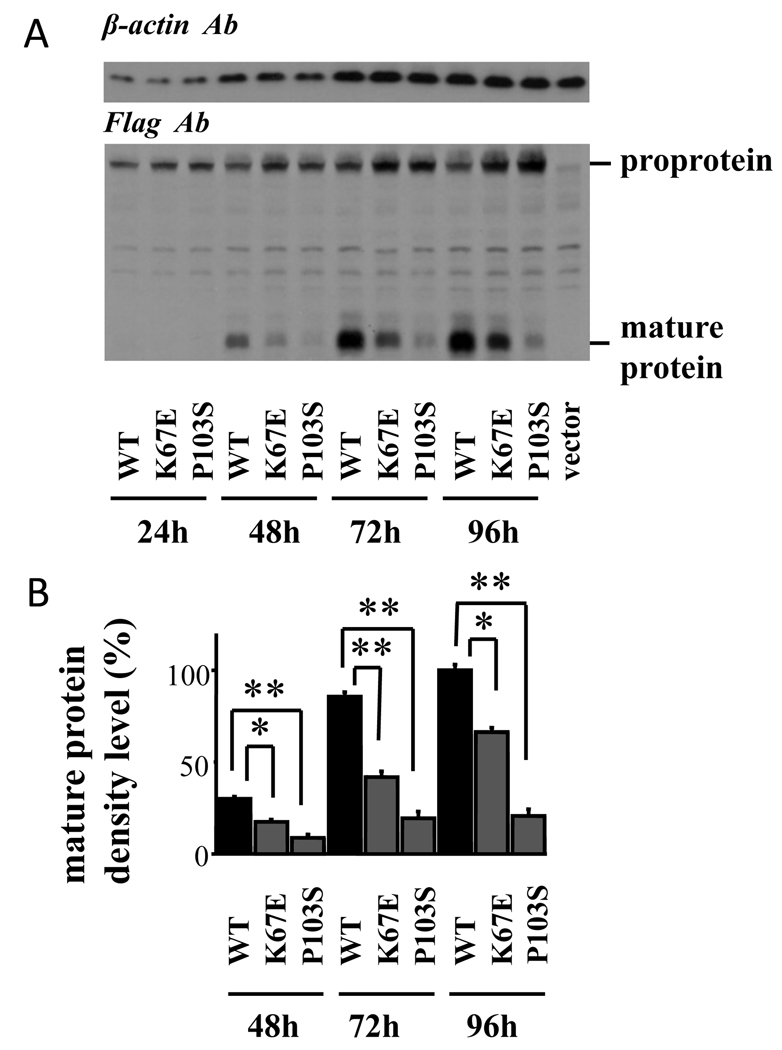
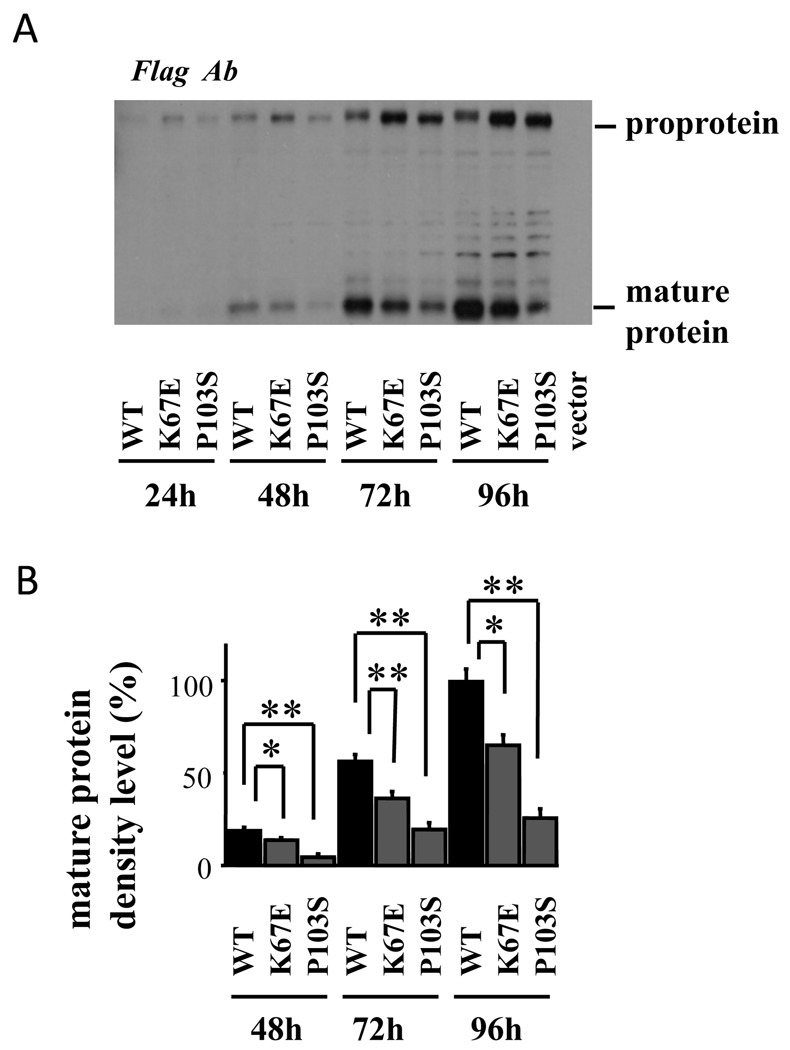
The cell lysates and conditioned media of 293F cells transfected with the wild-type and mutant GDF-9 demonstrated similar results, with the mature protein levels of both GDF-9 mutants, especially GDF-9P103S, markedly reduced compared with the wild-type GDF-9 (Fig. 6 and Fig. 7).
Fig. 6.
Production of GDF-9 by 293F cells transfected with wild-type and mutant plasmids. The 293F cells transfected with the indicated plasmids were cultured for the indicated hours, and cell lysates were subjected to SDS/PAGE immunoblotting analysis using anti-FLAG Ab and anti-β-actin Ab. CTRL: control cells transfected with the empty vector. Results are shown as representative of those obtained from three independent experiments in panel A. The band intensity of scanned images was statistically analyzed, and the cumulative data from three independent experiments are presented with mean ± SEM in panel B. Each experiment was performed with triplicate samples. *, P <0.05; **, P < 0.01 between the indicated groups.
Fig. 7.
Production of GDF-9 by 293F cells transfected with wild-type and mutant plasmids. The 293F cells transfected with the indicated plasmids were cultured for the indicated hours, and conditioned media were treated with acetone to precipitate proteins followed by the SDS/PAGE immunoblotting analysis using anti-FLAG Ab. CTRL: control cells transfected with the empty vector. Results are shown as representative of those obtained from three independent experiments in panel A. The band intensity of scanned images was statistically analyzed (panel B). All results are shown as mean ± SEM of data from at least three separate experiments, each performed with triplicate samples. *, P <0.05; **, P < 0.01 between the indicated groups.
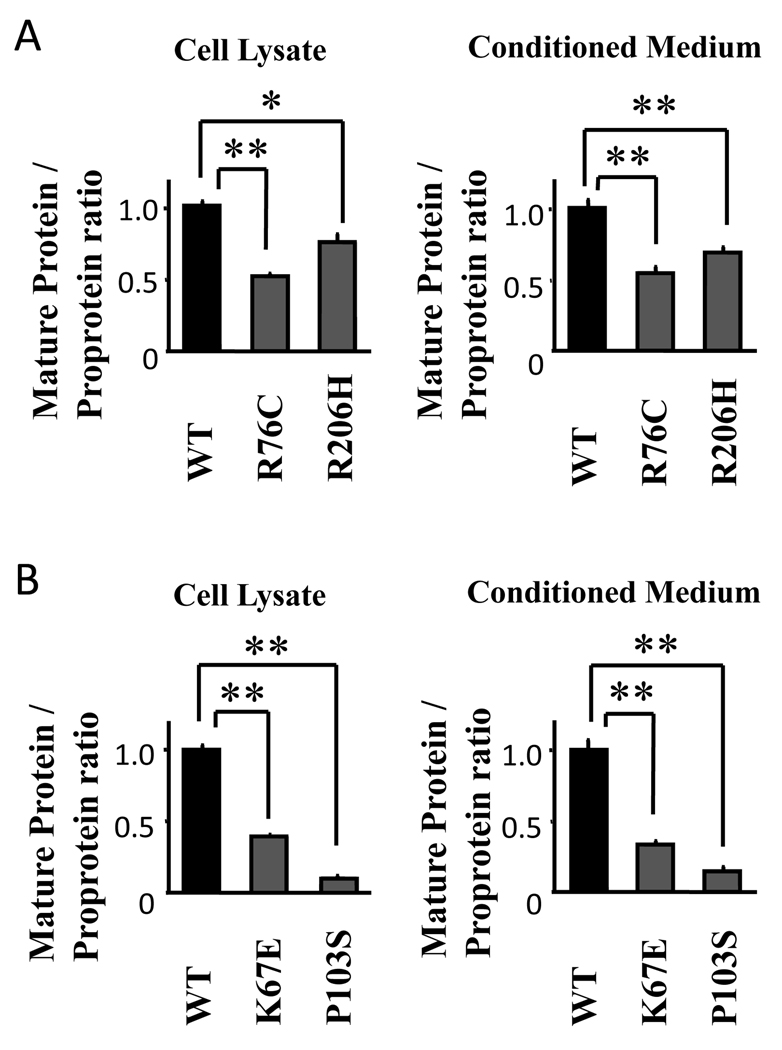
To determine the processing efficiency of wild-type and mutant BMP-15 and GDF-9 proproteins, we calculated the ratio of mature protein to proprotein in the cell lysates and conditioned media presented in Fig. 4–Fig. 7. The ratios of all BMP-15 and GDF-9 mutants were significantly lower than wild-type BMP-15 and GDF-9, respectively (Fig. 8), suggesting that the reduced mature protein production by the BMP-15 and GDF-9 mutants is attributed to impaired processing of the proprotein. Alternatively, it is also possible that proproteins with mutations are less stable than the wild type proproteins.
Fig. 8.
Ratios in the expression levels of the mature proteins to the corresponding proproteins produced by 293F cells transfected with wild-type and mutant BMP-15 (A) and GDF-9 (B). The 293F cells transfected with the indicated plasmids were cultured for 96 h. Cell lysates and conditioned media were then subjected to immunoblotting analysis using anti-FLAG Ab. The ratios of the mature proteins to the corresponding proproteins were determined using densitometric analysis of the SDS/PAGE immunoblotting results. All results are shown as mean ± SEM of data from at least three separate experiments, each performed with triplicate samples. *, P <0.05; **, P < 0.01 between the indicated groups.
4. Discussion
Mutations in the BMP-15 and GDF-9 genes have been identified to occur with a high incidence in POI patients. Interestingly, the mutation sites in most BMP-15 and GDF-9 mutations identified to date are located in the proregion, not in the functional mature region. Thus, if the processing of these mutant proproteins occurred normally, the resulting mature proteins should be indistinguishable from the wild-type, and no functional defects in these mutants would be expected. In the current study, we have demonstrated impaired production of the BMP-15 and GDF-9 mature proteins, derived from proproteins with mutations in the proregion that were identified in POI patients, which lead to a significant reduction in mature protein production.
All TGF-β superfamily members require the proregion domain to form a dimeric proprotein, which can then be processed by specific cleavage enzymes to separate the proregion dimer from the functional mature protein dimer. The consequence of the amino acid substitution in the proregion of BMP-15R76C and BMP-15R206H mutations on the formation of the three-dimensional structure is unknown. However, it is predicted at physiological pH that the replacement of a positively charged arginine with an uncharged cysteine or histidine may be a steric hindrance to forming the proper structure of the proprotein dimers, resulting in a reduced production of the functional dimeric mature protein. Further, the additional cysteine in BMP-15R76C may form a disulfide bond with one of the pre-existing cysteine residues, which may lead to the production of a misfolded proprotein. Replacement of the positively charged lysine with a negatively charged glutamic acid in GDF-9K67E is also likely to impair proprotein dimer formation due to improper ionic linkage of the tertiary structure. The amino acid substitution of proline with serine in GDF-9P103S is potentially critical for the formation of the proper secondary structure because proline has a distinctive cyclic structure in the molecule, thereby providing an exceptional conformational rigidity compared with other amino acids. Also, proline substitution often interrupts the α-helix configuration in the tertiary structure of proteins. In this context, it is noteworthy that the proline at position 103 is conserved in all mammals examined including mouse, rat, sheep, goat, cow, pig, chimpanzee and human, providing further support that a proline substitution is likely to have functional consequences (Kovanci et al., 2007; Palmer et al., 2006).
Ewes homozygous for a BMP-15 mutation have a block in follicle development at the primary stage, whereas heterozygous ewes exhibit an increased ovulation rate and smaller corpora lutea compared with wild-type ewes (Davis et al., 1992; Galloway et al., 2000; Hanrahan et al., 2004; McNatty et al., 2005; Montgomery et al., 1992). Based on the biological functions of rhBMP-15 we proposed a mechanism by which a mutation may result in the paradoxical effects seen in the homozygous and heterozygous ewes (Shimasaki et al., 2004). In homozygous mutant ewes, cessation of folliculogenesis at the primary stage corresponds to impaired granulosa cell proliferation. As BMP-15 is a mitogen for granulosa cells (Otsuka et al., 2000) and stimulates the expression of KL (Otsuka et al., 2002) that is essential for normal folliculogenesis (Driancourt et al., 2000), the primary cause of infertility in the homozygous ewes could be attributed to the lack of bioactive forms of this protein. In contrast, reduced levels of BMP-15 mature protein in the heterozygous ewes may be sufficient to allow growing follicles to progress through the FSH-independent stage of early folliculogenesis. However, during subsequent FSH-dependent stages a reduction in BMP-15 production may increase follicle sensitivity to FSH by reducing the BMP-15 inhibition of FSH receptor production (Otsuka et al., 2001), resulting in precocious follicle maturation. A recent study by McNatty et al., however, showed a higher level of cAMP production in response to hCG, but not FSH, by granulosa cells from heterozygous BMP-15 mutant ewes (Inverdale) compared with the wild-type (McNatty et al., 2009). Nevertheless, an earlier acquisition of gonadtropin responsiveness may promote more dominant follicle selection in the heterozygous ewes than the wild-type leading to precocious follicle maturation resulting in an increased ovulation rate. This is consistent with findings of smaller mean corpora lutea weights in the heterozygous compared with wild-type ewes (Shackell et al., 1993). As GDF-9 and BMP-15 share many biological activities in addition to the paradoxical effects of homozygous versus heterozygous mutations on ovulation rate and litter size in ewes, the findings of reduced production/secretion of functional BMP-15 and GDF-9 mature proteins in the current study suggests that this can also be anticipated in women with POI and mothers of dizygotic twins. It is worthy to note that genetic studies have identified specific loci at Xq22, Xq26-q28 and Xp11.2-p22.1 that are associated with POI (Marozzi et al., 2000; Simpson et al., 1999; Zinn et al., 1998), and thus the BMP-15 gene that is mapped at Xp11.2 (Dube et al., 1998) is a candidate gene for the pathogenesis of POI.
As described above, mutations in the BMP-15 and GDF-9 genes in ewes cause infertility or superfertility in a dosage-sensitive manner (Galloway et al., 2000; Hanrahan et al., 2004; McNatty et al., 2005). In addition, McNatty and colleagues were able to mimic both the infertile and superfertile phenotypes of the BMP-15 and GDF-9 mutant ewes by immunizing wild-type ewes against BMP-15 or GDF-9 (Juengel et al., 2002; Juengel et al., 2004). Collectively, these findings suggest that reduced levels of either BMP-15 or GDF-9 may cause higher ovulation rate and litter size whereas absence of either BMP-15 or GDF-9 leads to anovulation. In women, a large number of mutations in the BMP-15 and GDF-9 genes have been identified in POI patients and mothers of dizygotic twins (Di Pasquale et al., 2004; Dixit et al., 2005; Dixit et al., 2006; Kovanci et al., 2007; Laissue et al., 2006; Otsuka et al., 2000). All of these carriers are heterozygous and no homozygous carriers have been reported. Interestingly, most of the affected women have mutations in the proregion of BMP-15 or GDF-9. Given that all four mutations studied in the current study caused a significant reduction in the levels of the BMP-15 or GDF-9 mature protein production, the affected heterozygous women carrying these mutations may experience multiple ovulations that is undetectable in the absence of successful conception, resulting in an increased likelihood of bearing dizygotic twins. However, with the trend for women in developed countries to delay childbearing until their 30s combined with the possibility that the ovarian reserve in these women could be exhausted earlier than normal women, this may lead to the earlier onset of amenorrhea resulting from POI. In this context, it is known that mothers of dizygotic twins reach menopause significantly earlier than mothers of monozygotic twins (Martin et al., 1997). It is notable that a mutation at position 103 of GDF-9 (GDF-9P103S) has been identified in both POF patients and mothers of dizygotic twins, supporting the concept described above (Kovanci et al., 2007; Palmer et al., 2006).
In summary, the current study examined the biological impact of BMP-15R76C, BMP-15R206, GDF-9K67E and GDF-9P103S identified in women with POI and/or mothers of dizygotic twins. Consistent with the location of all four mutations in the proregion, each of these mutations resulted in impaired posttranslational processing of the proproteins which caused a significant reduction in production of the mature proteins. It is well established that reduced levels of BMP-15 and/or GDF-9 in ewes is associated with increased ovulation rate and litter size. Taken together, it is not unreasonable to speculate that women with BMP-15 or GDF-9 mutations may have an early period of enhanced fertility, resulting in an increased likelihood of bearing dizygotic twins, prior to a progression to premature infertility due to a more rapid exhaustion of the ovarian reserve and subsequent diagnosis with POI.
Acknowledgements
The authors thank Dr. Kirsten McTavish and other members of the Shimasaki lab for helpful suggestions and critical reading of the manuscript. We also thank Ms. Andi Hartgrove for her secretarial assistance, and to Dr. Sylvia M. Evans for the P19 cell line and Dr. Peter I. Schrier for the COV-434 cell line. This work was supported by NIH Grant RO1 HD41494 and by NICHD/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. The authors have nothing to disclose.
The abbreviations used are
- BMP-15
bone morphogenetic protein-15
- GDF-9
growth and differentiation factor-9
- POI
primary ovarian insufficiency
- POF
premature ovarian failure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albright F, Smith PH, Fraser R. A syndrome characterized by primay ovarian insufficieny and decreased stature. Am. J. Med. Sci. 1942;204:625–648. [Google Scholar]
- Bakalov VK, Anasti JN, Calis KA, Vanderhoof VH, Premkumar A, Chen S, Furmaniak J, Smith BR, Merino MJ, Nelson LM. Autoimmune oophoritis as a mechanism of follicular dysfunction in women with 46,XX spontaneous premature ovarian failure. Fertil. Steril. 2005;84:958–965. doi: 10.1016/j.fertnstert.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, Robison LL, Sklar CA. Acute ovarian failure in the childhood cancer survivor study. J. Clin. Endocrinol. Metab. 2006;91:1723–1728. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- Conway GS. Premature ovarian failure. Br. Med. Bull. 2000;56:643–649. doi: 10.1258/0007142001903445. [DOI] [PubMed] [Google Scholar]
- Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet. Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- Davis GH, McEwan JC, Fennessy PF, Dodds KG, McNatty KP, O WS. Infertility due to bilateral ovarian hypoplasia in sheep homozygous (FecXI FecXI) for the Inverdale prolificacy gene located on the X chromosome. Biol. Reprod. 1992;46:636–640. doi: 10.1095/biolreprod46.4.636. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am. J. Hum. Genet. 2004;75:106–111. doi: 10.1086/422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, Einaudi S, Radetti G, Russo G, Sacco M, Wasniewska M, Cole T, Beck-Peccoz P, Nelson LM, Persani L. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovaruan failure. J. Clin. Endocrinol. Metab. 2006;91:1976–1979. doi: 10.1210/jc.2005-2650. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, Chakravarty B, Singh L. Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause. 2005;12:749–754. doi: 10.1097/01.gme.0000184424.96437.7a. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, Chakrabarty B, Singh L. Missense mutations in the BMP15 gene are associated with ovarian failure. Hum. Genet. 2006;119:408–415. doi: 10.1007/s00439-006-0150-0. [DOI] [PubMed] [Google Scholar]
- Driancourt MA, Reynaud K, Cortvrindt R, Smitz J. Roles of KIT and KIT LIGAND in ovarian function. Rev. Reprod. 2000;5:143–152. doi: 10.1530/ror.0.0050143. [DOI] [PubMed] [Google Scholar]
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol. Endocrinol. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MPE, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat. Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries) Biol. Reprod. 2004;70:900–909. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- Hashimoto O, Moore RK, Shimasaki S. Posttranslational processing of mouse and human BMP-15: Potential implication in the determination of ovulation quota. Proc. Natl. Acad. Sci. USA. 2005;102:5426–5431. doi: 10.1073/pnas.0409533102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr. Rev. 1997;18:107–134. doi: 10.1210/edrv.18.1.0291. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Jick H, Porter J, Morrison AS. Relation between smoking and age of natural menopause. Lancet. 1977;1:1354–1355. doi: 10.1016/s0140-6736(77)92562-4. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Hudson NL, Heath DA, Smith P, Reader KL, Lawrence SB, O'Connell AR, Laitinen MPE, Cranfield M, Groome NP, Ritvos O, McNatty KP. Growth differentiation factor-9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep. Biol. Reprod. 2002;67:1777–1789. doi: 10.1095/biolreprod.102.007146. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Hudson NL, Whiting L, McNatty KP. Effects of immunization against bone morphogenetic protein 15 and growth differentiation factor 9 on ovulation rate, fertilization, and pregnancy in ewes. Biol. Reprod. 2004;70:557–561. doi: 10.1095/biolreprod.103.023333. [DOI] [PubMed] [Google Scholar]
- Kovanci E, Rohozinski J, Simpson JL, Heard MJ, Bishop CE, Carson SA. Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil. Steril. 2007;87:143–146. doi: 10.1016/j.fertnstert.2006.05.079. [DOI] [PubMed] [Google Scholar]
- Laissue P, Christin-Maitre S, Touraine P, Kuttenn F, Ritvos O, Aittomaki K, Bourcigaux N, Jacquesson L, Bouchard P, Frydman R, Dewailly D, Reyss A-C, Jeffery L, Bachelot A, Massin N, Fellous M, Veitia RA. Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur. J Endocrinol. 2006;154:739–744. doi: 10.1530/eje.1.02135. [DOI] [PubMed] [Google Scholar]
- Laissue P, Vinci G, Veitia RA, Fellous M. Recent advances in the study of genes involved in non-syndromic premature ovarian failure. Mol. Cell Endocrinol. 2008;282:101–111. doi: 10.1016/j.mce.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Vuojolainen K, Jaatinen R, Ketola I, Aaltonen J, Lehtonen E, Heikinheimo M, Ritvos O. A novel growth differentiation factor-9 (GDF-9) related factor is co-expressed with GDF-9 in mouse oocytes during folliculogenesis. Mech. Dev. 1998;78:135–140. doi: 10.1016/s0925-4773(98)00161-0. [DOI] [PubMed] [Google Scholar]
- Lass A. The fertility potential of women with a single ovary. Hum. Reprod. Update. 1999;5:546–550. doi: 10.1093/humupd/5.5.546. [DOI] [PubMed] [Google Scholar]
- Ledig S, Ropke A, Haeusler G, Hinney B, Wieacker P. BMP15 mutations in XX gonadal dysgenesis and premature ovarian failure. Am. J. Obstet. Gynecol. 2008;198(84):e81–e85. doi: 10.1016/j.ajog.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Li Q, Rajanahally S, Edson MA, Matzuk MM. Stable expression and characterization of N-terminal tagged recombinant human bone morphogenetic protein 15. Mol. Hum. Reprod. 2009;15:779–788. doi: 10.1093/molehr/gap062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WX, Moore RK, Otsuka F, Shimasaki S. Effect of intracellular interactions on the processing and secretion of bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9: Implication of the aberrant ovarian phenotype of BMP-15 mutant sheep. J. Biol. Chem. 2003;278:3713–3719. doi: 10.1074/jbc.M210598200. [DOI] [PubMed] [Google Scholar]
- Liao WX, Moore RK, Shimasaki S. Functional and molecular characterization of naturally occurring mutations in the oocyte-secreted factors BMP-15 and GDF-9. J. Biol. Chem. 2004;279:17391–17396. doi: 10.1074/jbc.M401050200. [DOI] [PubMed] [Google Scholar]
- Marozzi A, Manfredini E, Tibiletti MG, Furlan D, Villa N, Vegetti W, Crosignani PG, Ginelli E, Meneveri R, Dalpra L. Molecular definition of Xq common-deleted region in patients affected by premature ovarian failure. Hum. Genet. 2000;107:304–311. doi: 10.1007/s004390000364. [DOI] [PubMed] [Google Scholar]
- Martin NG, Healey SC, Pangan TS, Heath AC, Turner G. Do mothers of dizygotic twins have earlier menopause? A role for fragile X? Am.J. Med. Genet. 1997;69:114–116. doi: 10.1002/(sici)1096-8628(19970303)69:1<114::aid-ajmg23>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Massagué J, Attisano L, Wrana JL. The TGF-β family and its composite receptors. Trends Cell. Biol. 1994;4:172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- McMahon HE, Sharma S, Shimasaki S. Phosphorylation of bone morphogenetic protein-15 and growth and differentiation factor-9 plays a critical role in determining agonistic or antagonistic functions. Endocrinology. 2008;149:812–817. doi: 10.1210/en.2007-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatty KP, Heath DA, Hudson NL, Lun S, Juengel JL, Moore LG. Gonadotrophin-responsiveness of granulosa cells from bone morphogenetic protein 15 heterozygous mutant sheep. Reproduction. 2009;138:545–551. doi: 10.1530/REP-09-0154. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Smith P, Moore LG, Reader K, Lun S, Hanrahan JP, Groome NP, Laitinen M, Ritvos O, Juengel JL. Oocyte-expressed genes affecting ovulation rate. Mol. Cell. Endocrinol. 2005;234:57–66. doi: 10.1016/j.mce.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol. Cell. Endocrinol. 2000;169:123–131. doi: 10.1016/s0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Montgomery GW, McNatty KP, Davis GH. Physiology and molecular genetics of mutations that increase ovulation rate in sheep. Endocr. Rev. 1992;13:309–328. doi: 10.1210/edrv-13-2-309. [DOI] [PubMed] [Google Scholar]
- Mottershead DG, Pulkki MM, Muggalla P, Pasternack A, Tolonen M, Myllymaa S, Korchynskyi O, Nishi Y, Yanase T, Lun S, Juengel JL, Laitinen M, Ritvos O. Characterization of recombinant human growth differentiation factor-9 signaling in ovarian granulosa cells. Mol. Cell. Endocrinol. 2008;283:58–67. doi: 10.1016/j.mce.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Nelson LM, Anasti JN, Kimzey LM, Defensor RA, Lipetz KJ, White BJ, Shawker TH, Merino MJ. Development of luteinized graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J. Clin. Endocrinol. Metab. 1994;79:1470–1475. doi: 10.1210/jcem.79.5.7962345. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S. A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc. Natl. Acad. Sci. USA. 2002;99:8060–8065. doi: 10.1073/pnas.122066899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J. Biol. Chem. 2001;276:11387–11392. doi: 10.1074/jbc.M010043200. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yao Z, Lee TH, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15: Identification of target cells and biological functions. J. Biol. Chem. 2000;275:39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- Palmer JS, Zhao ZZ, Hoekstra C, Hayward NK, Webb PM, Whiteman DC, Martin NG, Boomsma DI, Duffy DL, Montgomery GW. Novel variants in growth differentiation factor 9 in mothers of dizygotic twins. J. Clin. Endocrinol. Metab. 2006;91:4713–4716. doi: 10.1210/jc.2006-0970. [DOI] [PubMed] [Google Scholar]
- Rebar RW, Connolly HV. Clinical features of young women with hypergonadotropic amenorrhea. Fertil. Steril. 1990;53:804–810. [PubMed] [Google Scholar]
- Rebar RW, Erickson GF, Yen SS. Idiopathic premature ovarian failure: clinical and endocrine characteristics. Fertil. Steril. 1982;37:35–41. [PubMed] [Google Scholar]
- Rossetti R, Di Pasquale E, Marozzi A, Bione S, Toniolo D, Grammatico P, Nelson LM, Beck-Peccoz P, Persani L. BMP15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum. Mutat. 2009;30:804–810. doi: 10.1002/humu.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackell GH, Hudson NL, Heath DA, Lun S, Shaw L, Condell L, Blay LR, McNatty KP. Plasma gonadotropin concentrations and ovarian characteristics in Inverdale ewes that are heterozygous for a major gene (FecX1) on the X chromosome that influences ovulation rate. Biol. Reprod. 1993;48:1150–1156. doi: 10.1095/biolreprod48.5.1150. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- Simpson JL. Genetic and phenotypic heterogeneity in ovarian failure: overview of selected candidate genes. Ann. NY Acad. Sci. 2008;1135:146–154. doi: 10.1196/annals.1429.019. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Rajkovic A. Ovarian differentiation and gonadal failure. Am. J. Med. Genet. 1999;89:186–200. doi: 10.1002/(sici)1096-8628(19991229)89:4<186::aid-ajmg3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Skillern A, Rajkovic A. Recent developments in identifying genetic determinants of premature ovarian failure. Sex. Dev. 2008;2:228–243. doi: 10.1159/000152039. [DOI] [PubMed] [Google Scholar]
- Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, Mulder J, Green D, Nicholson HS, Yasui Y, Robison LL. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2006;98:890–896. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- Takebayashi K, Takakura K, Wang HQ, Kimura F, Kasahara K, Noda Y. Mutation analysis of the growth differentiation factor-9 and -9B genes in patients with premature ovarian failure and polycystic ovary syndrome. Fertil. Steril. 2000;74:976–979. doi: 10.1016/s0015-0282(00)01539-9. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Adams JM, Mulder JE, Martin KA, Sluss PM, Crowley WF., Jr A randomized, controlled trial of estradiol replacement therapy in women with hypergonadotropic amenorrhea. J. Clin. Endocrinol. Metab. 1996;81:3615–3621. doi: 10.1210/jcem.81.10.8855811. [DOI] [PubMed] [Google Scholar]
- Zhao H, Qin Y, Kovanci E, Simpson JL, Chen Z-J, Rajkovic A. Analyses of GDF9 mutation in 100 Chinese women with premature ovarian failure. Fertil. Steril. 2007;88:1474–1476. doi: 10.1016/j.fertnstert.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZZ, Painter JN, Palmer JS, Webb PM, Hayward NK, Whiteman DC, Boomsma DI, Martin NG, Duffy DL, Montgomery GW. Variation in bone morphogenetic protein 15 is not associated with spontaneous human dizygotic twinning. Hum. Reprod. 2008;23:2372–2379. doi: 10.1093/humrep/den268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn AR, Tonk VS, Chen Z, Flejter WL, Gardner HA, Guerra R, Kushner H, Schwartz S, Sybert VP, Van Dyke DL, Ross JL. Evidence for a Turner syndrome locus or loci at Xp11.2-p22.1. Am. J. Hum. Genet. 1998;63:1757–1766. doi: 10.1086/302152. [DOI] [PMC free article] [PubMed] [Google Scholar]