Abstract
Maternal licking in rats affects the development of the spinal nucleus of the bulbocavernosus (SNB), a sexually dimorphic motor nucleus that controls penile reflexes involved with copulation. Reduced maternal licking results in decreased motoneuron number, size, and dendritic length in the adult SNB, as well as deficits in adult male copulatory behavior. Our previous findings that licking-like tactile stimulation influences SNB dendritic development and upregulates Fos expression in the lumbosacral spinal cord suggest that afferent signaling is changed by differences in maternal stimulation. Oxytocin afferents from the hypothalamus are a possible candidate, given previous research that has shown oxytocin is released following sensory stimulation, oxytocin modulates excitability in the spinal cord, and is a pro-erectile modulator of male sex behavior. In this experiment, we used immunofluorescence and immediate early gene analysis to assess whether licking-like tactile stimulation of the perineum activated parvocellular oxytocinergic neurons in the hypothalamus in neonates. We also used enzyme immunoassay to determine whether this same stroking stimulation produced an increase in spinal oxytocin levels. We found that stroking increased Fos immunolabeling in small oxytocin-positive cells in the paraventricular nucleus of the hypothalamus, in comparison to unstroked or handled control pups. In addition, sixty seconds of licking-like perineal stimulation produced a transient 89% increase in oxytocin levels in the lumbosacral spinal cord. Together, these results suggest that oxytocin afferent activity may contribute to the effects of early maternal care on the masculinization of the SNB and resultant male copulatory behavior.
Keywords: maternal care, motoneuron, sexual behavior, development, oxytocin, dendrite, experience, hormone, masculinization
INTRODUCTION
Early social contact between mother and offspring shapes the neural and behavioral development of offspring (Hofer, 1978; Hofer, 1994). In humans, parental care is a crucial factor in the development of offspring, with parental deprivation or loss predicting future mental health problems (Agid et al., 1999; Carter et al., 1999) and individual differences in maternal care influencing the physiological and psychological development of children (Hane and Fox, 2006). Rats and non-human primates also show substantial sensitivity to early maternal influences (e.g., Pryce et al., 2005), and as such serve as excellent model systems to manipulate maternal care. Natural variations in rodent maternal behavior produce offspring that differ on many neural and behavioral dimensions. Pups that receive higher levels of maternal licking, grooming, and arched-back nursing, for example, show greater hippocampal neuron density (Bredy et al., 2003), greater levels of neurotrophin expression throughout the brain (Liu et al., 2000), altered dopamine levels in the prefrontal cortex (Zhang et al., 2005), altered GABA receptor subunit expression in the amygdala (Caldji et al., 1998; Caldji et al., 2000), altered hypothalamic-pituitary-adrenal axis development (Liu et al., 1997), altered oxytocin receptor expression (Francis et al., 2000), and consequent changes in the many behaviors mediated by these structures. Cross-fostering shows that these maternal effects are epigenetic, with the level of maternal care received predicting the offspring’s phenotype.
Adult sexual behavior is also shaped by early maternal care. In female rats, natural variations in maternal licking contribute to partner preference, receptivity, and paced mating behavior (Cameron et al., 2008a, b). In males, experimental reductions in maternal licking produce behavioral deficits in adult copulatory behavior. These deficits include increased latency to ejaculation, increased latency to post-ejaculatory intromission, and increased inter-intromission intervals (Moore, 1984).
Reductions in licking influence neural development relevant to male sexual behavior as well. One of the neural structures that controls male copulatory behavior is the spinal nucleus of the bulbocavernosus (SNB). The SNB (also known as the dorsomedial nucleus, Schroder, 1980) is a sexually dimorphic population of motoneurons in the lumbar spinal cord, which innervates the anal sphincter of both males and females and additionally in males, the bulbocavernosus (BC) and levator ani (LA) muscles of the perineum (Breedlove and Arnold, 1980; Schroder, 1980; McKenna and Nadelhaft, 1986). The BC and LA muscles encircle the base of the penis and their fast, robust contractions produce an intense penile erection with flaring of the glans that allows seminal plug formation and removal (Sachs, 1982; Hart and Melese-D’Hospital, 1983). The development of SNB motoneurons extends well into the early postnatal period (Nordeen et al., 1985; Goldstein et al., 1990) and, interestingly, is sensitive to maternal care. Specifically, reductions in maternal licking produce decreased motoneuron number (Moore et al., 1992), motoneuron size, and dendritic length in the SNB (Lenz and Sengelaub, 2006).
The tactile stimulation conferred by maternal licking appears to be a critical component of the behavior, with supplemental stimulation offsetting the negative behavioral and physiological effects of maternal deprivation (Suchecki et al., 1993; Levy et al., 2003; Chatterjee et al., 2007). We have previously found licking-like tactile stimulation to be an important modulator of neural development in the SNB, with pups that received low levels of stimulation showing deficits in ex copula penile reflexes in adulthood as well as reduced dendritic length in the SNB relative to animals receiving high levels of stimulation (Lenz et al., 2008). We have also found that licking-like tactile stimulation of the perineum produces transient increases in spinal Fos expression in the area of the SNB dendritic field, suggesting that tactile stimulation may regulate SNB dendritic growth through an activity-dependent mechanism (Lenz and Sengelaub, 2009).
Supraspinal afferent input also regulates dendritic development in the SNB, with thoracic spinal transection causing localized redistributions of the dendritic arbor (Hebbeler and Sengelaub, 2003). The SNB receives input from many supraspinal afferent populations that have been shown to influence penile reflex behavior, including serotonergic, noradrenergic, dopaminergic, and oxytocinergic afferents (Giuliano and Rampin, 2000). Although any or several of these populations could be candidate mechanisms, we have chosen to first focus on the role of oxytocin inputs from the paraventricular nucleus (PVN) of the hypothalamus, for reasons elaborated below.
Oxytocinergic afferents from the PVN innervate the lumbosacral spinal cord. Interestingly, oxytocin is centrally released following sensory stimulation, and electrically stimulating the dorsal penile nerve or stroking the perineum activates oxytocin neurons in the PVN. Oxytocin has also been shown to be a proerectile modulator of male copulatory behavior, altering glutamatergic signaling to increase the excitability of dorsal horn neurons. In addition, the development of the oxytocinergic system is known to be sensitive to early life experience (Francis et al., 2000; Todeschin et al., 2009). These findings together suggest that oxytocin signaling in the spinal cord may mediate the effects of maternal licking or licking-like tactile stimulation on the morphology of SNB motoneurons and resultant penile reflexes. The goal of the current research was to determine whether early maternal licking-like stimulation regulates oxytocin signaling in the developing spinal cord.
METHODS
Animals
Untimed pregnant Sprague Dawley rat dams (Harlan, Indianapolis, IN) were maintained on a 12:12 h light/dark cycle, with unlimited access to food and water. On postnatal day (P) 1, pups were sexed and culled to litters of eight with even sex ratios where possible. All experimental manipulations were performed on P10, during the period when the maternal licking behavior is most robust. All experimental procedures were approved by the Institutional Care and Use Committee at Indiana University and in accordance with national animal care and use guidelines.
Immediate early gene analysis
Pups were removed from the dam and placed on a heating pad in a novel plastic container. Pups were handled individually and stimulated with a moist paintbrush in the anogenital region for 60 seconds. Control groups consisted of an undisturbed control group, which was left with the dam and not stroked, and a handled control group, which was held by the experimenter in the same manner as the stroked animals, but not stroked, for 60 seconds.
Pups were sacrificed 30 minutes following the stroking manipulation, a period that has been previously shown to achieve maximum expression of the immediate early gene, Fos, in the brain and spinal cord following maternal care or licking-like stimulation (Fenoglio et al., 2006; Lenz and Sengelaub, 2009). All animals were weighed, overdosed with urethane (approximately. 25g/100 of body weight), and transcardially perfused with saline followed by cold 4% paraformaldehyde. Brains were removed, postfixed in the same fixative overnight, and then transferred to sucrose phosphate buffer (10% w/v, pH 7.4) for cryoprotection until tissue sank. Brains were then frozen-sectioned horizontally at 30 microns into PBS into three alternate series. One series was counterstained with thionin to locate the PVN. The other two series were processed immunohistochemically to visualize the immediate early gene product, Fos, and the peptide hormone, oxytocin.
Immunohistochemistry
Immunohistochemistry series underwent a procedure similar to Bharati and Goodson (2006). Briefly, sections were rinsed in PBS in free-floating wells, incubated for 1 hr in PBS + 5% bovine serum albumin (BSA) + 0.3% Triton-X for blocking, and incubated for 24 hours in rabbit anti-Fos (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and guinea pig anti-oxytocin (1:1000; Bachem, Torrance, CA) in PBS + 2.5% BSA + 0.3% Triton-X + 0.05% sodium azide at 4°C. Following primary antibody incubation, sections were rinsed and incubated for 2 hr in goat anti-rabbit secondary antibody conjugated to Alexa Fluor 488 (3μl/ml; Invitrogen, Eugene, OR) and goat anti-guinea pig secondary antibody conjugated to Alexa Fluor 594 (5μl/ml; Invitrogen) in PBS + 2.5% BSA + 0.3% Triton-X + 0.05% sodium azide at RT. Sections were rinsed in PBS mounted onto gelatin-coated slides, and cover-slipped with Vectashield Hard Set (Vector Laboratories, Burlingame, CA).
Data collection
Cell counts were performed under epifluorescent illumination using a Nikon Eclipse 80i microscope (Nikon Instruments, Melville, NY) interfaced with a camera (Microfire; Optronics; Santa Barbara, CA). We have previously performed pilot studies using a DAPI counterstain, which showed that Fos labeling in neonates to be primarily nuclear. Some nonspecific vascular labeling of epithelial cells also occurs (see Fig. 1b), but this nonspecific labeling is morphologically distinguishable from the clear, spherical nuclear labeling in neurons. To ensure accuracy, cell counts were performed at higher magnification where the Fos label is more distinguishable from background (20×; see Fig 1c), and cells were only counted as double labeled when there was clear co-localization between the oxytocin immunopositive cell body and a spherical Fos-positive nucleus, often with a visible nucleolus.
Figure 1.
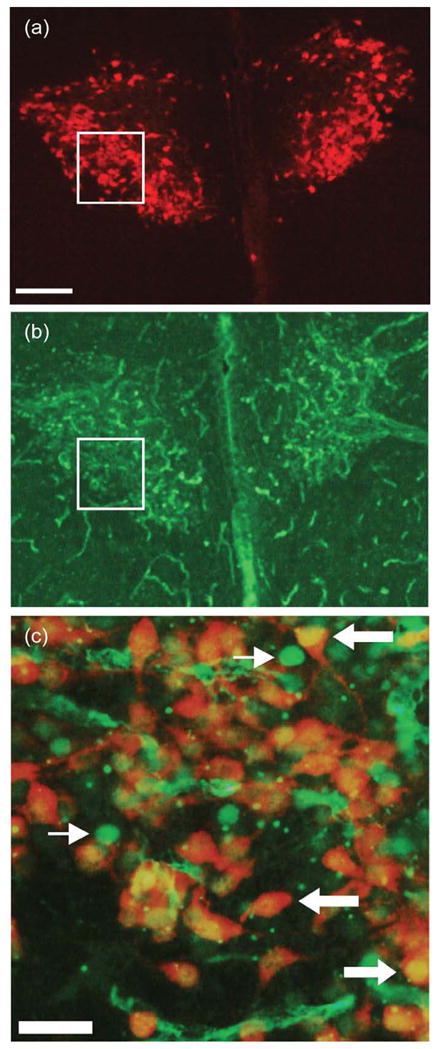
Digital light micrographs under epifluorescent illumination of a transverse section through the PVN of the hypothalamus in a stroked male. (a) Single-labeling for oxytocin; (b) Single-labeling for the immediate early gene product, Fos; (c) higher power (20×) merged image from location of boxes in (a) and (b). In all groups, there was substantial double-labeling for Fos and oxytocin. Scale bars = 250 μm in (a); 50 μm in (c). Thin arrows in (c) indicate Fos-positive nuclei, and thick arrows indicate cells double-labeled for Fos and oxytocin,
The number of Fos and oxytocin double-labeled cells was quantified unilaterally across the entire extent of the PVN, in one of three alternate series. The total number of oxytocin single-labeled cells was also quantified, and the overall percentage of oxytocin neurons in the PVN that were double labeled for Fos was determined. Final cell counts were corrected for sampling by multiplying the number of cells counted by the sampling ratio of the sections examined (e.g., ×2 for the unilateral sampling, and x3 for the alternate series). Cells counts were taken from one of three alternate series to avoid the possibility of double-counting cells. The area of double-labeled somata was assessed by measuring the cross-sectional area of every double-labeled cell across the rostrocaudal extent of that series of sections. Soma areas were measured using a video-based morphometry system (Stereo Investigator, MBF Bioscience, Williston, VT) at a final magnification of 1350X. This measurement was taken to subsequently determine whether double-labeled cells were parvocellular or magnocellular.
Previous research has shown that some of the parvocellular oxytocinergic PVN neurons (but not any magnocellular neurons) project to the spinal cord, specifically to the lumbosacral spinal cord. Therefore, we assessed whether perineal stimulation produced selective activation of parvocellular versus magnocellular cell types. Because the cytoarchitectonic boundaries often used to distinguish between the parvocellular and magnocellular portions of the PVN were difficult to determine in neonates and in fluorescently-labeled material, cell populations were distinguished on the basis of size. Double-labeled cells were characterized relative to the mean soma size of labeled cells in undisturbed animals as small (more than one standard deviation below the mean cell size), average (within one standard deviation of the mean), or large (more than one standard deviation above the mean). Once the number of double-labeled cells belonging to the different size categories was determined for each animal, this number was expressed as a percentage of the total number of double-labeled cells. The percentage of double-labeled cells within each size category was analyzed using a two-way repeated measures ANOVA (group by size, with size as the repeated factor). Appropriate planned comparisons were performed using Tukey’s highly significant difference (HSD).
Oxytocin Enzyme Immunoassay (EIA)
Male pups were removed from the dam and isolated from the dam and littermates on a heating pad for 60 minutes to produce a clear baseline in oxytocin signal. This isolation period was chosen based on previous literature showing that, following tactile stimulation of the skin, oxytocin levels return to baseline levels by 60 minutes. Following this separation period, a moist paintbrush was used to stimulate the perineal skin of the pups for 60 seconds. This stimulation period has previously been shown to activate oxytocin neurons in the PVN. Control pups were either left undisturbed except for the separation period, or handled, but not stroked, for 60 seconds following the separation period. A subset of stroked animals were sacrificed two, ten or 60 minutes following stroking to determine the time course over which spinal oxytocin levels are increased, with these timepoints chosen based on previous measures of central oxytocin following sensory stimulation (Stock and Uvnas-Moberg, 1988). Based on these data (see results below), ten minutes was chosen as the optimal time point at which to measure oxytocin following stroking.
Ten minutes following stimulation treatment, pups were rapidly decapitated and their spinal cords were visualized via laminectomy and rapidly removed. The SNB-containing L5-S1 spinal cord segments were subsequently isolated, weighed, frozen on dry ice, and stored at −70°C until all samples were collected and the assay performed.
To extract the peptide hormone, tissue was homogenized in PBS and the protease inhibitor, aprotonin and subsequently underwent a boiling extraction procedure modified from Vacher et al.. Briefly, tissue was boiled in acetic acid for 10 minutes, centrifuged at 4°C for 15 minutes, and the resultant supernatant removed and evaporated overnight. Extracted peptide was reconstituted in assay buffer (provided with assay kit), at a volume that placed sample concentrations in the middle of the standard curve. Following sample reconstitution, a competitive EIA for oxytocin (#900-153; Assay Designs, Ann Arbor, Michigan) was performed to obtain the concentration of oxytocin in the samples, using the manufacturer’s instructions. A seven-point standard curve was used to obtain sample concentration values, and all standards and samples were run in duplicate on the assay. The intra-assay coefficient of variation (CV) was 4.6%. Final oxytocin concentration was corrected for wet weight of tissue prior to data analysis, and expressed as pg/mg of tissue. Because multiple assay runs were necessary, all groups were included on each assay. Inter-assay variation was 7.7%. A one-way ANOVA was performed to statistically compare the concentration (pg/mg of tissue) of oxytocin in the spinal samples, and appropriate post-hoc comparisons made using Tukey’s HSD.
RESULTS
Fos and Oxytocin Double Labeling
All groups showed robust and equivalent labeling for oxytocin in both the PVN and supraoptic nucleus of the hypothalamus. The number of oxytocin immunopositive cells did not differ across groups (undisturbed = 3941.00 ± 304.27 cells; handled = 3554.50 ± 764.55 cells; stroked = 3475.33 ± 262.19 cells; F (2, 8) = 0.22, ns). All animals, regardless of stroking condition, showed substantial single labeling for Fos in the PVN as well as double labeling for oxytocin and Fos (Fig. 1). Stroking condition did not produce significant group differences in the percentage of oxytocin-labeled PVN neurons which were also labeled for Fos (undisturbed = 65.34 ± 2.91%; handled = 54.85 ± 4.71%; stroked = 53.75 ± 3.70%; F (2, 8) = 2.80; ns).
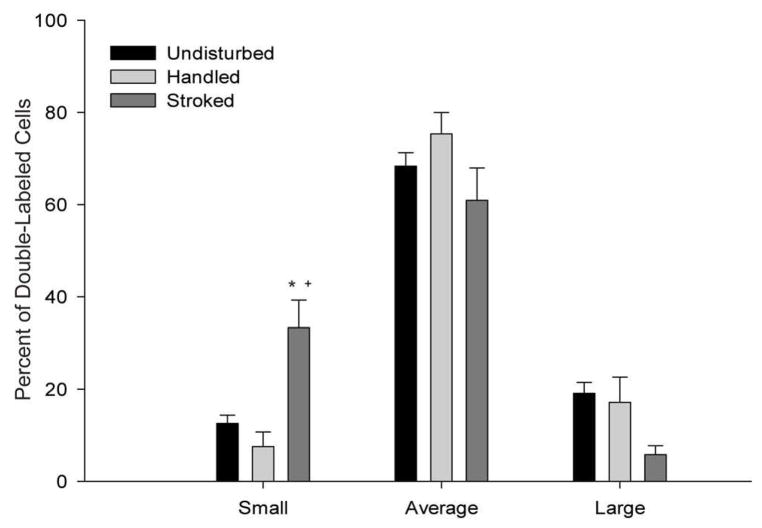
When cells were sorted on the basis of size, however, two-way ANOVA showed Fos and oxytocin double labeling was influenced by stroking condition (F (4, 16) = 5.24, p < .01). Subsequent one-way ANOVA showed that there was a main effect of stroking condition on the percentage of Fos and oxytocin double-labeled cells that were characterized as small (F (2, 8) = 13.33, p < .01; Fig. 2). Post-hoc comparisons showed that stroking produced a 165% increase in the percentage of double-labeled small cells relative to undisturbed pups (undisturbed = 12.56 ± 1.80; stroked = 33.32 ± 5.92; p < . 05). Stroked animals also had a significant increase in the percentage of double-labeled small cells relative to handled pups (handled = 7.54 ± 3.17 p < .01). There was no difference across stroking condition in the number of double-labeled cells characterized as average (F (2, 8) = 2.19, ns) or large (F (2, 8) = 3.03, ns).
Figure 2.
The percentage of total PVN neurons double labeled for oxytocin and Fos that were classified as small (more than 1 SD below the mean cell size in undisturbed controls), average (within 1 SD of the mean), or large (more than 1 SD above the mean) in undisturbed pups, handled pups, and pups stroked for 60 seconds. Stroking significantly increased the percentage of double-labeled small cells. * denotes significantly different from undisturbed pups, p < 0.05; + denotes significantly different from handled pups, p < 0.05.
Oxytocin EIA
To determine the optimal latency for measuring the peak in oxytocin signal, the concentration of oxytocin in the L5-S1 spinal cord segments was determined for several latencies following 60 seconds of perineal stroking. Following 60 minutes of isolation, pups were stroked and sacrificed either two minutes (n = 2), ten minutes (n = 11), or 60 minutes later (n = 2). Two minutes following stroking, mean oxytocin levels were 14.77 ± 4.58 pg/mg. Ten minutes following stroking, mean oxytocin levels increased to 25.07 ± 2.06 pg/mg, and by 60 minutes following stroking, levels had decreased again, to a mean concentration of 10.60 ± 4.80 pg/mg. Therefore, 10 minutes was chosen as the optimal period at which to measure oxytocin following stroking.
A one-way ANOVA showed that stroking condition produced significant differences in spinal oxytocin levels (F (2, 24) = 6.34, p < .01; Fig. 3). Relative to undisturbed pups (n = 10; 13.26 ± 2.4 pg/mg) stroked pups (n = 11) showed a significant 89% increase in spinal oxytocin levels (25.07 ± 2.06 pg/mg; p < .01). Handled pups (n = 6) had a mean oxytocin concentration of 17.99 ± 3.7 pg/mg, which was not significantly different from either undisturbed (p = .25) or stroked males (p = .08).
Figure 3.
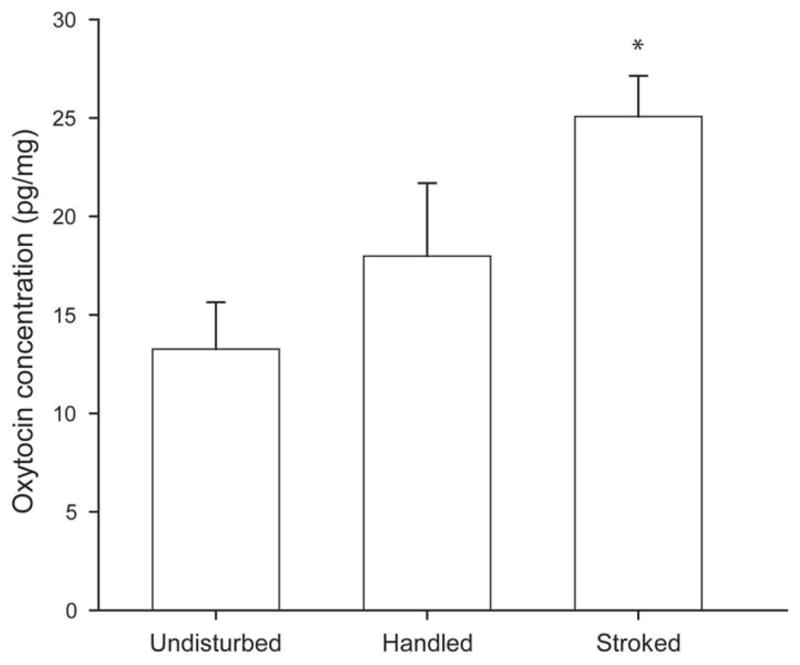
Mean oxytocin concentration (pg/mg tissue) in the L5-S1 spinal segments of undisturbed pups, pups handled for 60 seconds, or pups that received perineal stroking for 60 seconds. Spinal cords were extracted 10 minutes following the stimulation condition. Oxytocin concentrations were significantly elevated in the stroked animals relative to unstimulated controls. * denotes significantly different from undisturbed, p < 0.05.
DISCUSSION
Previous research has shown that adult male copulatory behavior and SNB motoneuron number are both shaped by early maternal care (Moore, 1984; Moore et al., 1992). Our previous work has shown that SNB motoneuron size and dendritic length are also decreased by reductions in early maternal licking of the perineum, and that tactile stimulation may mediate these effects. In these experiments, we examined the role that supraspinal oxytocin afferents from the hypothalamus might play in mediating these effects. We found that licking-like tactile stimulation increased the percentage of small oxytocinergic cells in the PVN that were activated relative to unstroked and handled controls, some of which likely project to the lumbosacral spinal cord. We also found that this stroking manipulation produced a transient significant increase in oxytocin levels in the lumbosacral spinal cord relative to baseline levels or levels in handled pups. Together, these data suggest that spinal oxytocin may contribute to the effects of maternal care on SNB motoneuron development.
Fos and Oxytocin Double Labeling in the PVN
We measured the activation of oxytocinergic neurons in the PVN of the hypothalamus following licking-like tactile stimulation of the perineum, using double immunohistochemistry for oxytocin and the immediate early gene product, Fos, and specifically assessed the activation of small versus large oxytocinergic neurons.
When oxytocin and Fos double-labeled cells were distinguished on the basis of size, we found that that stroking stimulation activated both parvocellular and magnocellular cells. Since peripheral oxytocin levels were not measured, we cannot conclude whether or not the particular stimulation manipulations used in this experiment altered oxytocin levels in the bloodstream. However, the activation of both parvocellular and magnocellular oxytocin neurons is consistent with previous research showing that oxytocinergic neurons in all subregions of the rabbit PVN are activated by anogenital stroking (Caba et al., 2003). Such a result may also explain previous research which has shown both central and peripheral oxytocin levels to be increased by various sensory stimulations. Moreover, both maternal separation (as seen by all pups, even the undisturbed group) and handling are considered neonatal stress manipulations and because oxytocin signaling is known to be influenced by stress, it could well be that the activation of both parvocellular and magnocellular oxytocinergic neurons in the PVN reflect a general stress effect.
While all groups showed some activation in both parvocellular and magnocellular populations, we found that stroking specifically increased the percentage of small double-labeled cells relative to unstimulated or handled controls. This result suggests that the parvocellular cell population was specifically activated by licking-like tactile stimulation, and that this activation may be responsible for the increased oxytocin levels we measured in the spinal cord following stroking stimulation (see below).
In this experiment, parvocellular neurons were distinguished from magnocellular neurons on the basis of size, and not based on previously published cytoarchitectonic boundaries, because said cytoarchitectonic features were difficult to distinguish in fluorescently-labeled material and in neonates. No previous research has documented the average cell size of parvocellular versus magnocellular oxytocinergic cells in neonates, though recent research has used a size criterion to distinguish between these cell types in adults, with parvocellular neurons characterized as having a diameter of between 6–12 μm (area of 28–113 μm2) and magnocellular neurons characterized as having a diameter of greater than 12 μm (area of 114 μm2 or greater; Todeschin et al., 2009). The classification scheme used in this experiment may have resulted in classifying some parvocellular cells as ‘average’ instead of ‘small’, thereby making our criterion potentially conservative. However, based on previously published research documenting mean cell size in easily recognizable subregions of the adult PVN, such as the periventricular PVN (pPVN; Sawchenko and Swanson, 1982), and our own measurements of mean cell size in those same subregions in neonates, we estimate the mean cell size in 10 day old pups to be approximately 62% that of adults. Our cutoff for small cells used in this experiment (77 μm2) corresponds well to the criterion used in Todeschin et al. when corrected for the age of the animals examined. We therefore feel confident that the cells classified as small were parvocellular oxytocinergic neurons.
Oxytocin EIA
Sixty seconds of stroking stimulation to the perineum produced an increase in oxytocin levels in the L5-S1 spinal cord segments relative to unstimulated control animals. This finding suggests that stroking stimulation, which has previously been shown to increase physiological activity of oxytocin neurons in the PVN, may specifically activate the parvocellular PVN neurons that project to the lumbosacral spinal cord. Previous research has also shown that similar sensory stimulations increase CSF levels of oxytocin, suggesting that central oxytocin signaling is regulated by tactile stimulation. Our double-labeling for Fos and oxytocin in the PVN suggest that this is the case (see above).
Handled, but unstroked, animals had oxytocin levels that were not significantly different either from unstimulated control or stroked animals. As seen in Figure 3, handled animals had oxytocin levels moderately between undisturbed and stroked animals. This result is consistent with the role of tactile stimulation in upregulating oxytocin levels, given that holding a pup for 60 seconds constitutes a sensory stimulation. Since handling without stroking is a milder sensory stimulation than handling and stroking combined, it follows that handling would produce less substantial increases in spinal oxytocin than stroking during handling.
It should be noted that, while significant changes in spinal oxytocin above baseline were measured following stroking stimulation, the assay used in the present experiment cannot distinguish between oxytocin in nerve terminals and oxytocin actually released in the synapse, because it is performed on homogenized tissue. It is possible that stroking stimulation increases the trafficking of oxytocin-containing vesicles to the nerve terminal, but does not actually increase the release of oxytocin into the synapse. If this is the case, then oxytocin signaling in the spinal cord would not be changed by the tactile stimulation provided by maternal care. Nonetheless, given previous research that has shown both central and peripheral oxytocin levels are increased by a variety of sensory stimulations, it seems unlikely that the stroking manipulation used in this experiment produced oxytocin accumulation in the synapse without synaptic release.
Enzyme immunoassays are often used to measure real concentrations of hormone in the blood or other tissues. In order to interpret the values obtained in an immunoassay as the actual concentrations found in the tissue, the extraction recovery must also be measured. In the current experiment, we were interested in levels of spinal oxytocin relative to baseline, therefore extraction recovery was not measured. As such, it is highly likely that the actual concentrations of spinal oxytocin are higher than were measured in this experiment. However, it seems unlikely that the acute (10 minute) manipulation used in this experiment could produce different hormone extraction efficiencies across groups. Therefore the relative increase in spinal oxytocin levels measured in this experiment likely resulted from the stroking manipulation itself, and not changes in extraction efficiency.
Mechanisms of oxytocin action
The development of dendrites is shaped by afferent input (Morest, 1969; Rakic, 1975), therefore alterations in afferent signaling could alter SNB development. SNB dendritic development is activity-dependent, with blockade of NMDA receptors producing decreases in SNB dendritic length (Hebbeler et al., 2002). Developmental deafferentation is known to negatively affect dendritic arborization, resulting in decreased dendrite length and arbor complexity (Smith, 1974; Bradley and Berry, 1976; Parks, 1981; Mizrahi and Libersat, 2002), and eliminating supraspinal input to the SNB, via thoracic spinal transection, causes localized redistributions of the dendritic arbor (Hebbeler and Sengelaub, 2003). Oxytocin has been shown in culture to alter the physiological properties of glutamatergic dorsal horn neurons from neonatal animals. Specifically, application of oxytocin increased the amplitude of EPSCs and the frequency of spontaneous AMPA-mediated EPSCs of dorsal horn neurons, and an oxytocin receptor antagonist prevented these physiological effects (Jo et al., 1998). This study in combination with our previous results demonstrating the possible mediating role of primary sensory afferent activity suggest that oxytocin released in the spinal cord following licking-like tactile stimulation may enhance the effects of this stimulation on local spinal cord activity. Therefore, maternally-induced changes in oxytocinergic afferent and primary sensory afferent activity may be acting in concert to shape the dendritic development of SNB motoneurons.
Conclusions
Previous research has shown that the development of the SNB is sensitive to variations in maternal licking or licking-like tactile stimulation, and that sensory afferent signaling may mediate these effects. Here, we have shown that stroking the perineum produced an increase in the activation of parvocellular oxytocin neurons in the PVN (some of which project to the lumbosacral spinal cord), as well as producing transient increases in spinal oxytocin levels. The current results demonstrate that supraspinal oxytocin afferent signaling is altered by the tactile stimulation provided by maternal care and may therefore regulate the development of the SNB and resultant copulatory behavior. This research coupled with our previous work shows that maternal effects on the developing SNB are likely achieved via changes both in the signaling of primary sensory and supraspinal oxytocinergic afferents in the lumbosacral spinal cord. These results suggest that multiple converging mechanisms together mediate maternal effects on the developing nervous system, and implicate early life experience as a potent modulator of masculinization.
Acknowledgments
This work was supported by NIH grants 5T32HD049336, 5R01NS047264 (DRS) and F31NS064793 (KML). We are grateful to Dr. James Goodson for instruction on the immunolabeling of Fos and oxytocin, and to Dr. Rose Stewart and Dr. Gregory Demas for their guidance in developing oxytocin extraction and assay protocols, and to our anonymous reviewers for their helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR, Gessa GL. Oxytocin: an extremely potent inducer of penile erection and yawning in male rats. Eur J Pharmacol. 1986;130:265–272. doi: 10.1016/0014-2999(86)90277-3. [DOI] [PubMed] [Google Scholar]
- Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P, Berry M. The effects of reduced climbing and parallel fibre input on Purkinje cell dendritic growth. Brain Res. 1976;109:133–151. doi: 10.1016/0006-8993(76)90384-x. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Grant RJ, Champagne DL, Meaney MJ. Maternal care influences neuronal survival in the hippocampus of the rat. Eur J Neurosci. 2003;18:2903–2909. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Caba M, Rovirosa MJ, Silver R. Suckling and genital stroking induces Fos expression in hypothalamic oxytocinergic neurons of rabbit pups. Brain Res Dev Brain Res. 2003;143:119–128. doi: 10.1016/s0165-3806(03)00064-6. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS ONE. 2008a;3:e2210. doi: 10.1371/journal.pone.0002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Fish EW, Meaney MJ. Maternal influences on the sexual behavior and reproductive success of the female rat. Horm Behav. 2008b;54:178–184. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Carter JD, Joyce PR, Mulder RT, Luty SE, Sullivan PF. Early deficient parenting in depressed outpatients is associated with personality dysfunction and not with depression subtypes. J Affect Disord. 1999;54:29–37. doi: 10.1016/s0165-0327(98)00132-3. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol. 1988;272:579–604. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Chatterjee-Chakraborty M, Rees S, Cauchi J, de Medeiros CB, Fleming AS. Maternal isolation alters the expression of neural proteins during development: ‘Stroking’ stimulation reverses these effects. Brain Res. 2007;1158:11–27. doi: 10.1016/j.brainres.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Di S, Tasker JG. Rapid synapse-specific regulation of hypothalamic magnocellular neurons by glucocorticoids. Prog Brain Res. 2008;170:379–388. doi: 10.1016/S0079-6123(08)00431-7. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J Neurosci. 2006;26:2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Bernabe J, McKenna K, Longueville F, Rampin O. Spinal proerectile effect of oxytocin in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1870–1877. doi: 10.1152/ajpregu.2001.280.6.R1870. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Rampin O. Central neural regulation of penile erection. Neurosci Biobehav Rev. 2000;24:517–533. doi: 10.1016/s0149-7634(00)00020-8. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990;10:935–946. doi: 10.1523/JNEUROSCI.10-03-00935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol. 2001;433:222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychol Sci. 2006;17:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Hart BL, Melese-D’Hospital PY. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav. 1983;31:807–813. doi: 10.1016/0031-9384(83)90277-9. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Sengelaub DR. Development of a sexually dimorphic neuromuscular system in male rats after spinal transection: Morphologic changes and implications for estrogen sites of action. J Comp Neurol. 2003;467:80–96. doi: 10.1002/cne.10911. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. N-methyl-D-aspartate receptor blockade inhibits estrogenic support of dendritic growth in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 2002;451:142–152. doi: 10.1002/cne.10347. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Hidden regulatory processes in early social relationships. In: Bateson PPG, Klopfer PH, editors. Perspectives in Ethology. Plenum Press; New York: 1978. pp. 135–166. [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Jo YH, Stoeckel ME, Freund-Mercier MJ, Schlichter R. Oxytocin modulates glutamatergic synaptic transmission between cultured neonatal spinal cord dorsal horn neurons. J Neurosci. 1998;18:2377–2386. doi: 10.1523/JNEUROSCI.18-07-02377.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Graham MD, Parada M, Fleming AS, Sengelaub DR, Monks DA. Tactile stimulation during artificial rearing influences adult function and morphology in a sexually dimorphic neuromuscular system. Dev Neurobiol. 2008;68:542–557. doi: 10.1002/dneu.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Sengelaub DR. Maternal licking influences dendritic development of motoneurons in a sexually dimorphic neuromuscular system. Brain Res. 2006;1092:87–99. doi: 10.1016/j.brainres.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Sengelaub DR. Maternal care effects on SNB motoneuron development: The mediating role of sensory afferent distribution and activity. Dev Neurobiol. 2009;69:603–615. doi: 10.1002/dneu.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F, Melo AI, Galef BG, Jr, Madden M, Fleming AS. Complete maternal deprivation affects social, but not spatial, learning in adult rats. Dev Psychobiol. 2003;43:177–191. doi: 10.1002/dev.10131. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Libersat F. Afferent input regulates the formation of distal dendritic branches. J Comp Neurol. 2002;452:1–10. doi: 10.1002/cne.10275. [DOI] [PubMed] [Google Scholar]
- Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev Psychobiol. 1984;17:347–356. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- Moore CL, Dou H, Juraska JM. Maternal stimulation affects the number of motor neurons in a sexually dimorphic nucleus of the lumbar spinal cord. Brain Res. 1992;572:52–56. doi: 10.1016/0006-8993(92)90449-j. [DOI] [PubMed] [Google Scholar]
- Morest DK. The growth of dendrites in the mammalian brain. Z Anat Entwicklungsgesch. 1969;128:290–317. doi: 10.1007/BF00522529. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Parks TN. Changes in the length and organization of nucleus laminaris dendrites after unilateral otocyst ablation in chick embryos. J Comp Neurol. 1981;202:47–57. doi: 10.1002/cne.902020105. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Rakic P. Role of cell interaction in development of dendritic patterns. Adv Neurol. 1975;12:117–134. [PubMed] [Google Scholar]
- Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Schroder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- Smith DE. The effect of deafferentation on the postnatal development of Clarke’s nucleus in the kitten--a Golgi study. Brain Res. 1974;74:119–130. doi: 10.1016/0006-8993(74)90115-2. [DOI] [PubMed] [Google Scholar]
- Stock S, Uvnas-Moberg K. Increased plasma levels of oxytocin in response to afferent electrical stimulation of the sciatic and vagal nerves and in response to touch and pinch in anaesthetized rats. Acta Physiol Scand. 1988;132:29–34. doi: 10.1111/j.1748-1716.1988.tb08294.x. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: the roles of feeding and stroking. Brain Res Dev Brain Res. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, McKellar S. The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J Comp Neurol. 1979;188:87–106. doi: 10.1002/cne.901880108. [DOI] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Calas A, Facchinetti P, Giuliano F. Oxytocinergic and serotonergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neuroscience. 1998;82:241–254. doi: 10.1016/s0306-4522(97)00290-x. [DOI] [PubMed] [Google Scholar]
- Todeschin AS, Winkelmann-Duarte EC, Jacob MH, Aranda BC, Jacobs S, Fernandes MC, Ribeiro MF, Sanvitto GL, Lucion AB. Effects of neonatal handling on social memory, social interaction, and number of oxytocin and vasopressin neurons in rats. Horm Behav. 2009;56:93–100. doi: 10.1016/j.yhbeh.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Bruzelius G, Alster P, Lundeberg T. The antinociceptive effect of non-noxious sensory stimulation is mediated partly through oxytocinergic mechanisms. Acta Physiol Scand. 1993;149:199–204. doi: 10.1111/j.1748-1716.1993.tb09612.x. [DOI] [PubMed] [Google Scholar]
- Vacher CM, Fretier P, Creminon C, Calas A, Hardin-Pouzet H. Activation by serotonin and noradrenaline of vasopressin and oxytocin expression in the mouse paraventricular and supraoptic nuclei. J Neurosci. 2002;22:1513–1522. doi: 10.1523/JNEUROSCI.22-05-01513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronneau-Longueville F, Rampin O, Freund-Mercier MJ, Tang Y, Calas A, Marson L, McKenna KE, Stoeckel ME, Benoit G, Giuliano F. Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience. 1999;93:1437–1447. doi: 10.1016/s0306-4522(99)00262-6. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Clemens LG. Neurophysin-containing pathway from the paraventricular nucleus of the hypothalamus to a sexually dimorphic motor nucleus in lumbar spinal cord. J Comp Neurol. 1993;336:106–116. doi: 10.1002/cne.903360109. [DOI] [PubMed] [Google Scholar]
- Yanagimoto M, Honda K, Goto Y, Negoro H. Afferents originating from the dorsal penile nerve excite oxytocin cells in the hypothalamic paraventricular nucleus of the rat. Brain Res. 1996;733:292–296. doi: 10.1016/0006-8993(96)00800-1. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Chretien P, Meaney MJ, Gratton A. Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. J Neurosci. 2005;25:1493–1502. doi: 10.1523/JNEUROSCI.3293-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]