Abstract
Belgian Waterslager (BW) canaries have an inherited hearing loss due to missing and abnormal hair cells, but it is unclear whether the loss is congenital or developmental. We used auditory brainstem responses and scanning electron microscopy to describe the development of auditory sensitivity and hair cell abnormalities in BW and non-BW canary. In both strains, adult ABR thresholds were higher than behavioral thresholds, but BW canaries exhibited higher thresholds than non-BW canaries across all frequencies. Immediately post-hatch, ABR thresholds and hair cell numbers were similar in both strains. Two weeks later, thresholds were significantly higher in BW canaries and hair cell number progressively decreased as the birds aged. These data show that in BW canaries: the peripheral auditory system is functionally similar to non-BW canary from hatch to 2 weeks, ABR thresholds improve during this developmental period, actually becoming better than those of adults, but then worsen as the bird continues to age, and hair cell number and appearance is similar to non-BW canaries at hatch but progressively declines after 30 days of age. These data show that the hearing loss characteristic of BW canaries is, at least in part, developmental and is established by the time song learning begins.
Keywords: sensorineural hearing loss, auditory brainstem response, development, canary
1. Introduction
Canary breeders have selectively bred birds for specific song traits (song canaries) and plumage (type canaries) for centuries (Güttinger, 1985). This has led to a large number of canary strains but no single prototypical strain. Type canaries, such as border and Norwich, retain song characteristics that are similar to that of wild canaries. On the other hand, song canaries are extremely popular and four are bred for their song characterstics: American Singers (cross between border type and Roller), Spanish Timbrados (developed around the 1940’s, see Lohr et al., 2004; Walker and Avon, 1993), Harz Rollers (established in the 1700’s) and Belgian Waterslagers (strain almost became extinct during World War I, see Gleich et al., 1995a for review). Song studies show domestication has affected song characteristics in both Harz Rollers and Belgian Waterslagers (BW) canaries such that their songs are distinguishable from both wild and other type canary songs (Güttinger, 1985; Mundinger, 1999).
The BW canary, the focus on this study, has been the subject of many song learning studies (e.g., Gardner et al., 2005; Marler and Waser, 1977; Nottebohm and Nottebohm, 1978) and is best known for its characteristic low-pitched, loud song (Güttinger, 1985). Interestingly, this strain of canary has an auditory threshold shift of 20–40 dB at frequencies above 1 kHz (Okanoya and Dooling, 1985; 1987), exhibits poor frequency discrimination above 1 kHz and has reduced temporal integration (Lauer et al., 2007). However, they are better on some temporal discrimination tasks than non-BW canaries (e.g., gap detection and temporally reversed stimuli; Lauer et al., 2007). Cross-breeding experiments have confirmed a hereditary basis for this hearing loss (Okanoya et al., 1990). Wright et al. (2004) found that birds with at least one non-BW Z chromosome have ‘normal’ hearing at high frequencies; whereas, females with a single BW Z or males with two BW Z chromosomes show a threshold shift at high frequencies.
Adult BW canaries have on average 30% fewer hair cells and abnormal stereovillar bundles (Gleich et al., 1994a; Weisleder and Park, 1994; Weisleder et al., 1996), 12% fewer VIIIth nerve fibers (Gleich et al., 2001) and smaller volume of nucleus magnocellularis (NM) and nucleus laminaris (NL) (Kubke et al., 2002) compared to non-BW canaries. These birds also show continuous low levels of hair cell death (apoptosis) and spontaneous supporting cell proliferation and hair cell differentiation that can be upregulated after damage due to noise exposure (Gleich et al., 1997; Wilkins et al., 2001). Interestingly, this upregulation, as well as the continuous hair cell proliferation, does not repair the papilla in BW canaries. Thus, the rate of hair cell death in the papilla of the adult BW canary must be the same as the rate of proliferation (Wilkins et al., 2001). Hearing loss in the BW canary has been measured behaviorally in birds as young as 4–8 months (Okanoya and Dooling, 1987; Okanoya et al., 1990), and the typical adult cochlear pathology is also present in BW canaries as young as 2 months old (Gleich et al., 1994a). In terms of peripheral function, despite elevated cochlear microphonic (CM) and compound action potential (CAP) thresholds, the slopes of the growth functions are the same in BW and non-BW canaries. The CAP and CM growth functions in BW canaries are shifted to higher sound pressure levels, especially at the higher frequencies (see Figures 2 & 4 in Gleich et al., 1995a).
Whether this hearing loss is congenital or developmental cannot be determined with behavioral techniques. In chickens and mammals, sensorineural hearing loss can lead to a reduction in cell number, cell size, and volume in the cochlear nuclei. However, this effect is restricted to a sensitive period during development (Moore, 1990; also see reviews in Gleich and Strutz, 1997; Kubke et al., 2002). Loss of cells is typically seen only after cochlear removal (Hashisaki and Rubel, 1989; Moore, 1990; Nordeen et al., 1983; Parks, 1979; Trune, 1982) or massive cochlear damage (e.g., aminoglycosides); however, more subtle types of hearing loss, like a conductive hearing loss during a sensitive period, can lead to a reduction of the cochlear nucleus volume and reduced cell size (rat: Coleman et al., 1982; gerbil: Gleich and Strutz, 1997; mouse: Webster, 1988). In BW and non-BW canaries, the overall anatomical structure, and the total number of cells in NM and NL were comparable, but the volumes of NM and NL were smaller in BW canaries (Kubke et al., 2002) suggesting that the onset of pathology in the BW canary occurs before final maturation of these nuclei (Gleich et al., 1998).
Taken together, these results show anatomical differences in the inner ear and behavioral hearing loss of BW canaries as young as 2–4 months. The current study was designed to determine whether these anatomical and functional deficits were present at hatch or occurred over a post-hatch developmental time period. We looked for functional differences using the auditory brainstem response (ABR) by measuring tone- and click-evoked thresholds as well as latencies and amplitudes for peaks in the ABR waveforms of both adult and nestling non-BW and BW canaries. We used scanning electron microscopy (SEM) to quantify hair cell number and stereovillar bundle abnormality over the same post-hatch developmental time period.
2. Materials and methods
2.1. Hearing Sensitivity
The methodology used is the same as that used for testing auditory sensitivity via the ABR in adult and nestling budgerigars (Brittan-Powell and Dooling, 2004; Brittan-Powell et al., 2002).
Nine adult (1–3 years old) non-BW (normal hearing) and nine adult (between 1–4 years old) BW canaries (hearing-impaired) were used for adult measures. The birds were housed in an aviary at the University of Maryland College Park and were either bred at the University or purchased from local breeders. The Animal Care and Use Committee at the University of Maryland approved all animal use. Eleven nestling non-BW canaries bred in our aviary (where both parents had normal ABR thresholds) were used in these experiments. Six of these birds were recorded approximately 6–10 times between 5–35 days post-hatching (DPH). An additional 5 non-BW canaries were recorded at 7–9 days and again at 60 and 90 DPH. Fifteen nestling BW canaries were also used. Some were bred in the UM aviary, but others were obtained from Rockefeller University Field Station. Seven of the 15 BW canaries were recorded 3–5 times between 5 and 35 DPH. The remaining 8 birds were recorded only once at ages ranging from 7 to 12 DPH.
For testing, the bird was sedated with either an intramuscular (IM) injection (for adults, fledglings and older nestlings) or a subcutaneous injection (younger nestlings) of ketamine (25–50 mg/kg) and diazepam (2 mg/kg). The speaker (JBL Professional Series speaker, Model 2105H, James B Lansing Sounds Inc) was 30 cm from the bird’s right ear (90° azimuth relative to the bird’s beak; 0° elevation relative to the bird’s right ear). A standard three electrode array was used (standard platinum alloy, subdermal needle electrodes; Grass F-E2; West Warwick, RI), non-inverting electrode just under the skin high at the vertex, inverting electrode directly behind the right ear canal, and the ground electrode behind the canal of the ear contralateral to stimulation. The stimulus presentation, ABR acquisition, equipment control, and data management were coordinated using a Tucker-Davis Technologies (TDT System 2, Gainesville, FL, USA) modular rack-mount system controlled by an optical cable-linked 350-MHz Pentium PC containing a TDT AP2 Digital Signal Process board and running TDT ‘BIOSIG’ software.
All canaries were presented with multiple stimulus trains that varied in frequency and intensity (e.g., Brittan-Powell and Dooling, 2004; Brittan-Powell et al., 2002; Brittan-Powell et al., 2005; Wright et al., 2004). Each individual tone burst was 5 ms in duration (1 ms rise/fall COS2) with 20 ms inter stimulus interval (ISI). Frequencies for the tones bursts were: 0.5, 1.0, 1.5, 2.0, 2.86, 4.0, 5.7 and 8.0 kHz. The click train consisted of a series of 0.1 ms (100 us) broadband clicks with 25 ms ISI. All trains were presented at a rate of 4/s and sampled at 20 kHz for 235 ms following onset of the stimulus train. Five hundred averages for each polarity/phase were added together to cancel the CM and each polarity/phase was replicated. The biological signal was amplified (× 100K) and notch filtered at 60 Hz with the DB4 during collection. The signal was bandpass filtered from 0.03 to 3 kHz after collection using the BIOSIG program (to reduce electrical noise; Hall, 2007) and are the settings used in our previous papers (Brittan-Powell and Dooling, 2004; Brittan-Powell et al., 2002; Wright et al., 2004).
Stimulus intensities were calibrated in the free field by placing the ½-in. microphone of a sound level meter (System 824; Larson Davis, Inc. Provo, UT) at the approximate position of the bird’s ear. Continuous tones were generated using the TDT BIOSIG program and measured using the fast weighting A scale on the sound level meter. To determine the intensity of the short duration click, we used the peak equivalent SPL of the click. This was determined using an oscilloscope and noting the peak-to-peak voltage of the click. A test tone, e.g., a 1000 Hz tone, was played and adjusted until the peak-to-peak voltage was the same as it was for the click. The SPL required to match the amplitude of the click, as indicated by the sound level meter, was the peak equivalent SPL (dB pSPL) of the click stimulus.
The ABR waveforms produced in response to high intensity sounds were visually examined to determine which peaks would be used to measure latencies, amplitudes, and thresholds. For nestlings, we defined response onset as follows: (1) there was at least one positive deflection within 1–15 ms after the sound reached the outer ear canal and (2) this response was replicable on successive trials (onset of response criteria from Brittan-Powell and Dooling, 2004). Data for the different response variables (e.g., threshold, latency, amplitude) were included in the analysis if at least two nestlings for that age met the criteria defined above. Individual data was used for all statistical tests. ABR threshold was defined as the intensity 2.5 dB (one-half step in intensity) below the lowest stimulus level at which a response could be visually detected on the trace between 1 and 10 ms (see Brittan-Powell and Dooling, 2004; Brittan-Powell et al., 2005).
2.2. Hair cell number and morphology
Fourteen (five 1 DPH (weight range = 1.99–2.6grams), four 12–13 DPH and five adult non-BW and thirty-two (four 1DPH (weight range = 1.4–2.19g), four 12–13 DPH, six 20 DPH, six 31 DPH, six 66 DPH and six adult) BW canaries were used in morphological analysis. For details of histological processing and dissection of the tissue see Lippe et al., (1991). Basilar papillae were fixed (3.5% glutaraldehyde followed by 1% osmium tetroxide) dehydrated to 70% ethanol, the tectorial membrane removed, dehydrated to 100% and critical point dried in CO2. Papillae were mounted on aluminum stubs, sputter coated with gold, and viewed at 15 kV using either an Amray 1820 or Joel 820 scanning electron microscope (Ryals et al., 1999).
Quantification of hair cell number, as well as morphological changes to the hair cell stereovillar bundles were performed in order to provide some correlation of morphological state to behavioral and electrophysiological measures of auditory sensitivity. The total number of hair cells was determined using SEM montages (700×) of the entire basilar papilla (BP). Each montage was divided into 4 intervals of 25% of length from the basal to apical tip. Hair cells were counted in each interval and summed to obtain total hair cell number. Higher magnification (4,000×) SEM photomicrographs were constructed at positions 20, 50 and 80% of length from the base of the BP for analysis of stereovillar bundle abnormalities. Each interval was approximately 60µm wide (along the basal to apical dimension) and extended from the neural to the abneural edge. Based on the predicted place-frequency map of the canary (Gleich et al., 2004), these areas correspond to the following frequency ranges: 20% of length from base ~ 3500–5100Hz; 50% from base ~ 1600–2100Hz; 80% of length from base ~ 600–800Hz. Hair cells with more than one stereovillar bundle were defined as having abnormal stereovillar bundles. Hair cells were counted in each interval as either normal (one bundle) or abnormal (multiple bundles). Experimentation on animals was approved by both the University of Maryland College Park and James Madison University’s Animal Care and Use Committee.
3. Results
3.1.a Physiological development: ABR thresholds in adult canaries
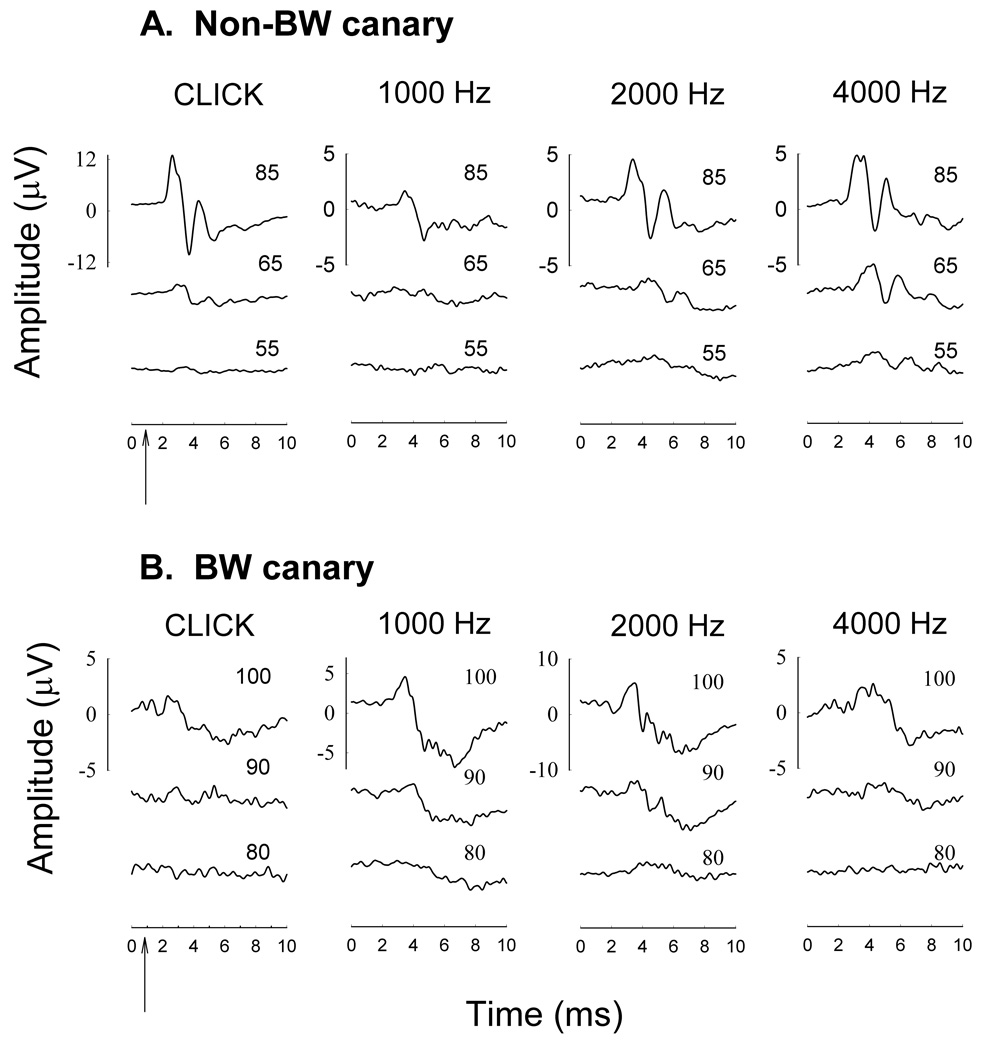
ABR waveforms for all adult canaries consisted of 1 large positive reflecting peak (auditory nerve, wave 1) and 1 or 2 smaller peaks (though not always present at all frequencies or intensity levels) occurring within the first 6 ms after sound stimulation. In non-BW and BW canaries, the ABR waveform peaks decreased in latency and increased in amplitude with increasing sound pressure level; however, higher sound pressure levels were necessary to elicit responses in BW canaries. The waveform morphology in response to clicks and pure tones differed among strains. At most frequencies, there was no ABR response in the BW canaries below 85–90 dB SPL while a large response was still evident at the same SPL in the non-BW canary (see Figure 1). Also, for most BW canaries, there was no discernable 2nd peak, and the response fell into the noise faster, which is indicative of less synchrony along the auditory pathway and resulted in higher thresholds. For example, click-evoked responses resulted in considerably different thresholds between the two strains, with BW canaries exhibiting thresholds almost 40 dB higher (83.1 +/− 2.12) than non-BW canaries (46.7 +/− 3.06 dB pSPL; avg +/− SE; (F(1,17)=95.6, p<.0001; one-way ANOVA; JMPv8 software).
Figure 1.
Typical ABR waveforms for non-BW (A) and BW canaries (B) in response to the click, 1.0 kHz, 2.0 kHz and 4.0 kHz stimuli. Arrow indicates when sound reaches outer ear. For the click and 4.0 kHz, the degradation in the ABR waveform morphology is apparent for the BW canary as compared to the non-BW canary.
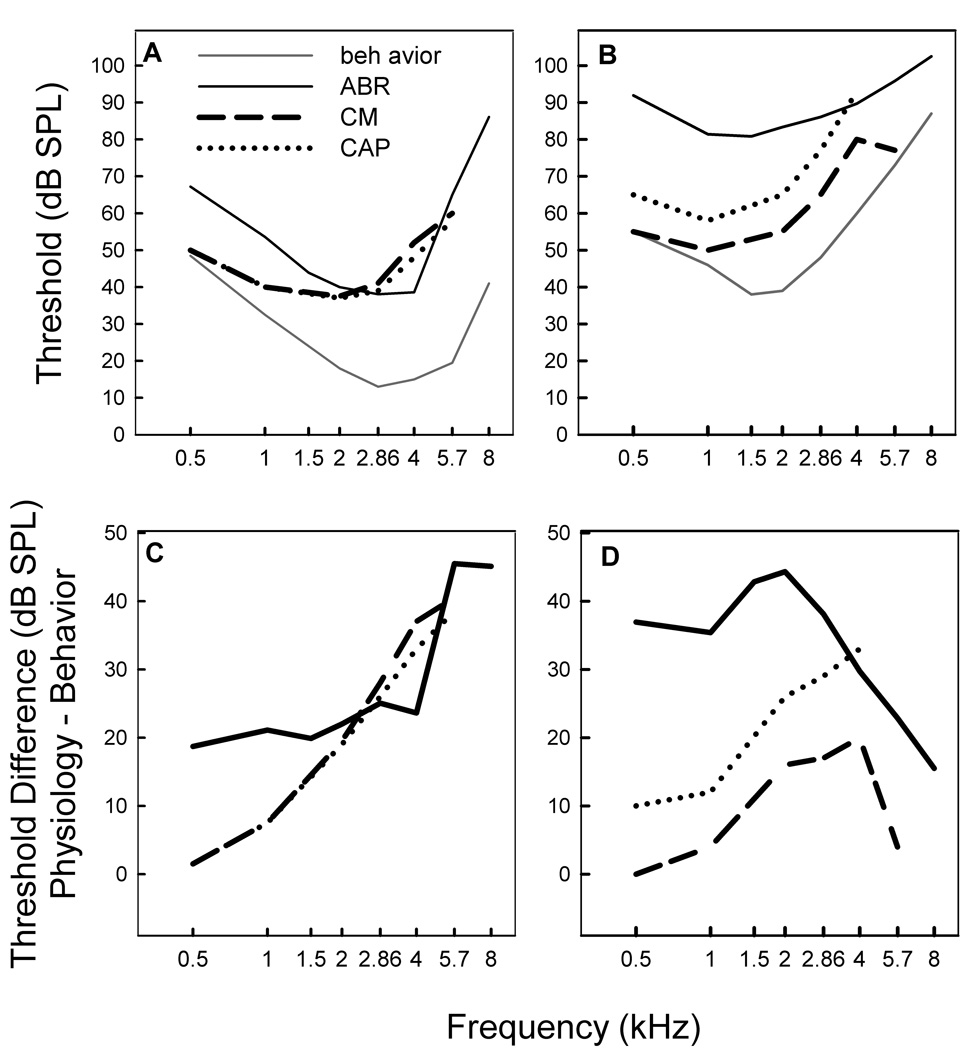
The ABR-derived audiograms (black line Figure 2 A&B) for the two strains differed significantly (two factor ANOVA: strain and frequency, F(15,128)=44.90, p<.0001), showing significant strain (F(1,128)=440.95, p<.0001), frequency (F(7,128)= 26.43, p<.0001), and interaction (freq × strain F(7, 128)=6.80, p <.0001) effects. Posthoc comparisons (LSMeans Contrast) showed higher thresholds for BW canaries than non-BW canaries across all frequencies tested (F(7,128)=52.3, p<.0001). ABR thresholds for BW canaries were at best 16 and at worst 50 dB higher than ABR thresholds for non-BW canaries. Figure 2 A&B also compares published behavioral and physiological measures for the two strains, along with the ABR-derived audiograms from this study. No matter the method, audibility curves measured physiologically in non-BW and BW canaries show consistently elevated thresholds compared to behavior.
Figure 2.
Behavioral (solid gray lines – average of data from Okanoya and Dooling, 1985; 1987 for non-BW and for BW canary) and ABR-derived (solid black lines) thresholds for adult non-BW (A) and BW canaries (B). CM (dashed line) and CAP (dotted line) data also plotted for comparison (Gleich et al., 1995a). Differences between physiological and behavioral measure for adult non-BW (C) and BW canaries (D).
While the ABR audiogram for the non-BW canary generally follows the shape of the behavioral audiogram, threshold, as measured by the ABR, is more elevated than the behavioral audiogram above 4 kHz. In BW canaries, the shape of the ABR audiogram is flatter and less similar to that of the behavioral audiogram. These threshold differences are shown in Figure 2 C & D. For non-BW canaries, ABR thresholds are 20 dB higher than behavior at frequencies < 4kHz and 40 dB higher at frequencies > 4 kHz, but CM and CAP thresholds increase as a function of increasing frequency. Above 2 kHz, all three physiological methods show roughly similar changes in non-BW canaries. For BW canaries, ABR thresholds are roughly 40 dB higher than behavior at frequencies < 2 kHz but the differences decrease (ABR thresholds become more similar to behavioral thresholds) as frequency increases. CM and CAP thresholds are lower than ABR thresholds in BW canaries and increase with increasing frequency until 4 kHz. CAP, like ABR, thresholds also become more similar to behavioral thresholds above 4 kHz in BW canaries.
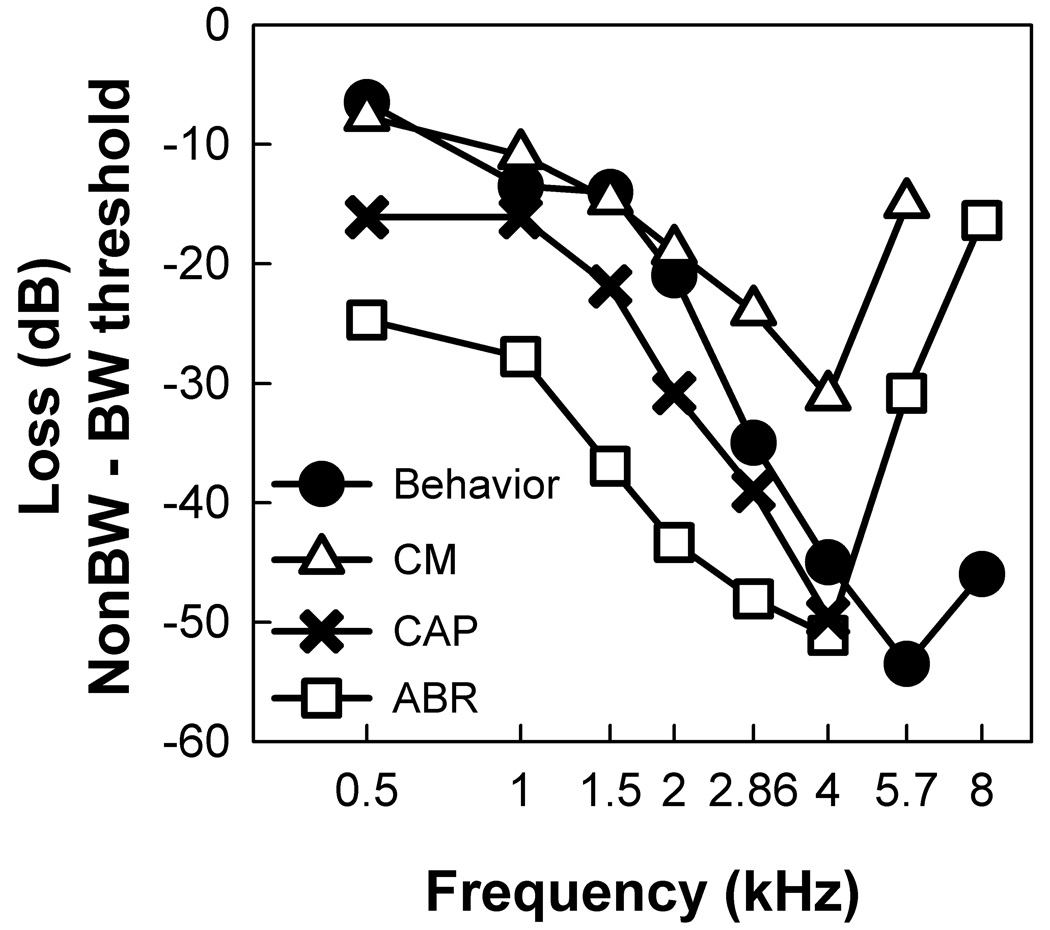
The estimate of threshold loss in BW canaries, relative to the non-BW canary, also differs substantially across different methods (Figure 3; CAP and CM data are re-plotted from Gleich et al., 1995a). Compared to the behavioral estimates of hearing loss, ABR estimates of hearing loss are 5–20 dB greater (predict more hearing loss) at low and mid frequencies (.5 – 4 kHz) but 20–30 dB lower (predict less hearing loss) at high frequencies (> 4 kHz). Yet, despite this, the behavioral and physiological measures are consistent in indicating an increasing hearing loss as frequency increased from 0.5 to 4 kHz. Interestingly, behavioral, CAP, and ABR methods estimate a 40–50 dB loss at 4 kHz.
Figure 3.
Differences between non-BW and BW canaries for behavioral and physiological threshold measures. For the CAP at 6 kHz, the loss could not be determined because the sound system had a maximum output of 100 dB, and there was no CAP response at 100 dB. Behavioral (filled circle: average of data from Okanoya and Dooling, 1985; 1987 for non-BW and for BW canary) and ABR-derived (open square) thresholds for adult non-BW and BW canaries. CM (open triangle) and CAP (x) data also plotted for comparison (Gleich et al., 1995a).
3.1.b Physiological development: ABR thresholds in nestling canaries
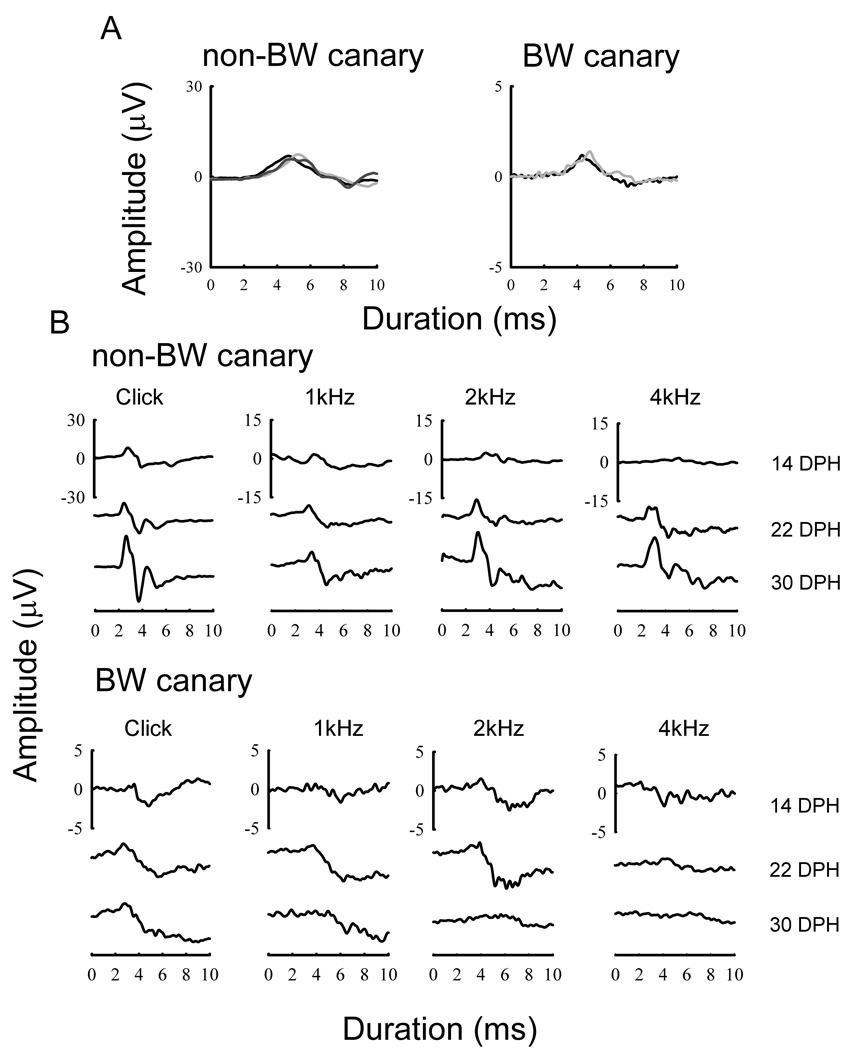
For nestling canaries, ABR responses were reliably obtained for both strains at the earliest day tested (4–6 DPH; Figure 4A). There was no difference in the wave morphology at this age, but the ABR amplitudes for BW canaries were much lower than non-BW canaries at high SPLs. ABR waveforms, for a single non-BW and a single BW canary, in response to the click (85 dB pSPL) and 3 different tones (85 dB SPL) at three times during development are shown in Figure 4B. Morphological differences in the waveform are apparent between strains: distinct peaks occurred in the non-BW canaries by 14 DPH, but these peaks were lower in amplitude and less defined at the same age in BW canaries, even at 85 dB SPL. The peaks in the waveform for the non-BW canaries became more distinctive and increase in amplitude as the birds aged, indicating increased neural synchrony; similar developmental changes were less obvious in the waveforms of BW canaries.
Figure 4.
Waveforms in response to different stimuli. (A) Responses to a 115 dB pSPL click for 3 non-BW (left) and 2 BW canaries (right) at 5–6 days post hatch; different gray lines represent individual birds. (B) Typical non-BW and BW canary ABR waveforms in response to a click and 3 tones played at 85 dB SPL at 14 days, 22 days and 30 DPH. BW canary waveform amplitude is reduced compared to non-BW canary waveform amplitudes in all examples. There are major differences in the waveform morphology between the two strains.
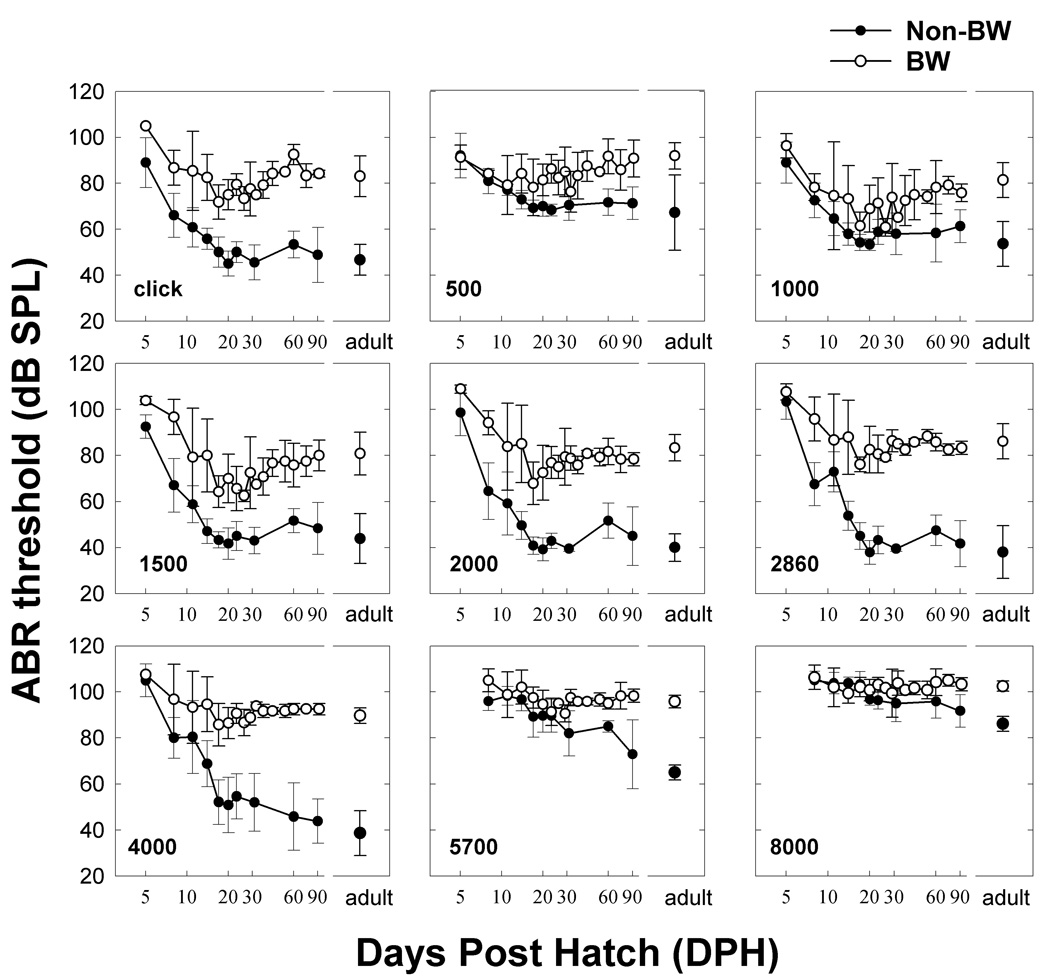
Figure 5 shows the development of thresholds for each strain in response to the click and the eight frequencies tested as a function of age. At the earliest ages tested, both strains showed ABR thresholds that were above 80–90 dB SPL at all frequencies. In non-BW canaries, click and tone-evoked thresholds decreased to adult values by 2–3 weeks of age. Thresholds in BW canaries were similar to those from non-BW canaries right after hatching and also decreased during the first 2–3 weeks of life.
Figure 5.
Threshold development as a function of age for the click and each frequency tested – non-BW (closed circles) and BW (open circles). Note: Morphological comparisons were made at 20% of length from base ~ 3500–5100Hz; 50% from base ~ 1600–2100Hz; 80% of length from base ~ 600–800Hz kHz.
Click-evoked thresholds were significantly different (F(21,75)=17.67, p<.0001; 2way ANOVA strain and age). BW canaries exhibited higher click thresholds (strain, F(1,75)=219.31, p<.0001) than non-BW canaries; there were also significant differences across ages (F(10,75)=10.48, p<.0001). Interestingly, the rate of threshold change was not different between the strains (slope: strain by age, F(10,75)=.98, p>.05); however, posthoc comparisons showed that BW canary click thresholds were higher than non-BW click thresholds at all ages (F(11,75) = 22.39, p< .0001, LSMeans Contrast).
The initial development of thresholds over the first 2–3 weeks was similar for both canary strains at 0.5 and 1 kHz; the difference between strains occurred only in older birds. For the frequency range of 1.5 to 4 kHz, ABR thresholds in the BW canary appeared elevated compared to non-BW canary at all ages, except at hatching. For 5.7 and 8 kHz the difference was much less and only appeared for older birds. For BW canaries, click and tone-evoked thresholds between 1 and 3 kHz reached a minimum around 3 weeks after hatching and deteriorated in older birds. These qualitative observations are supported by statistical analysis (3way ANOVA, age, frequency, strain: F(128,748)=21.02, p<.0001). Tone-evoked ABR thresholds were lower in non-BW than BW canaries (strain: F(1,748)=612.48, p<.0001). For both strains, thresholds improved with age (F(15,748)=30.113, p<.0001), and there was a significant effect of frequency on threshold (F(7, 748)=158.87, p<.0001). We also found a significant effect of strain × age × frequency (F(105,748)=1.98, p<.0001).
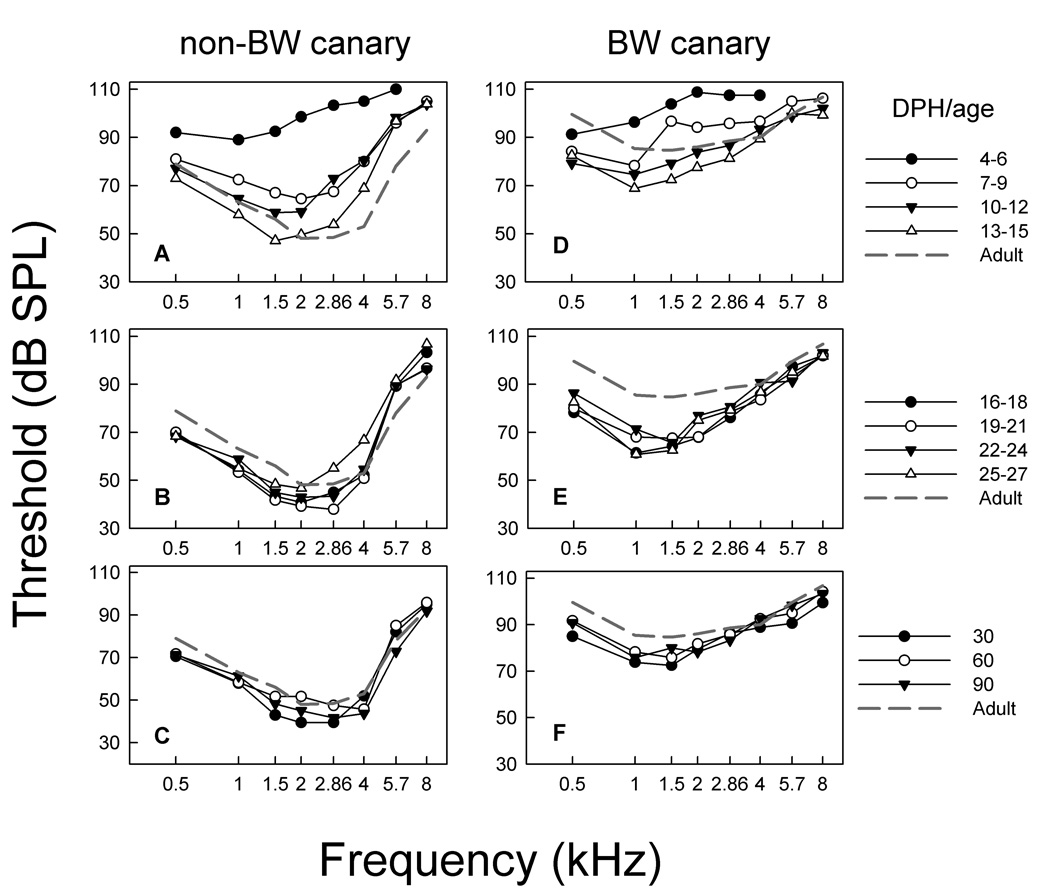
Figure 6 shows the threshold data from Figure 5 replotted as a function of frequency with age as a parameter. Thresholds at the earliest ages were high and could only be obtained over a restricted frequency range in both strains of canaries (Figure 6 A & D, closed circles). Thresholds improved dramatically over the next 10 days for all frequencies, decreasing by 30–40 dB in non-BW canaries. A 20 dB improvement in threshold was seen for BW canaries. Looking at the thresholds as a function of frequency showed that by about 16–18 DPH, ABR thresholds in non-BW canary were similar to those of adults. Interestingly, ABR thresholds in BW canaries at this same age were actually LOWER than adult BW canaries, by 10–20 dB at frequencies below 2.86 kHz and worsen as the animals’ age. By 2–3 months of age, ABR thresholds for BW canaries increased and stabilized around the adult mean.
Figure 6.
ABR audiogram development for both strains. A–C show development of audiogram in non-BW canary, with adult-like thresholds evident by ~ 16–18 DPH. D–F show audiogram development in the BW canary. Thresholds are lower than adults in young birds (2–3 weeks post hatch; E), but thresholds increased and were adult-like ~ 2–3 months of age.
Together, the data shown in Figures 5 and 6 suggests that brainstem responses to the click and mid-range frequencies (1.5–4 kHz) in BW canaries are always elevated compared to normal canaries, with the exception of perhaps the earliest days tested (5–10 DPH). But, more importantly, the data show that ABR thresholds in BW canaries improve to better than adult levels between 2 and 4 weeks of age and subsequently decline reaching adult thresholds by 2–3 months of age.
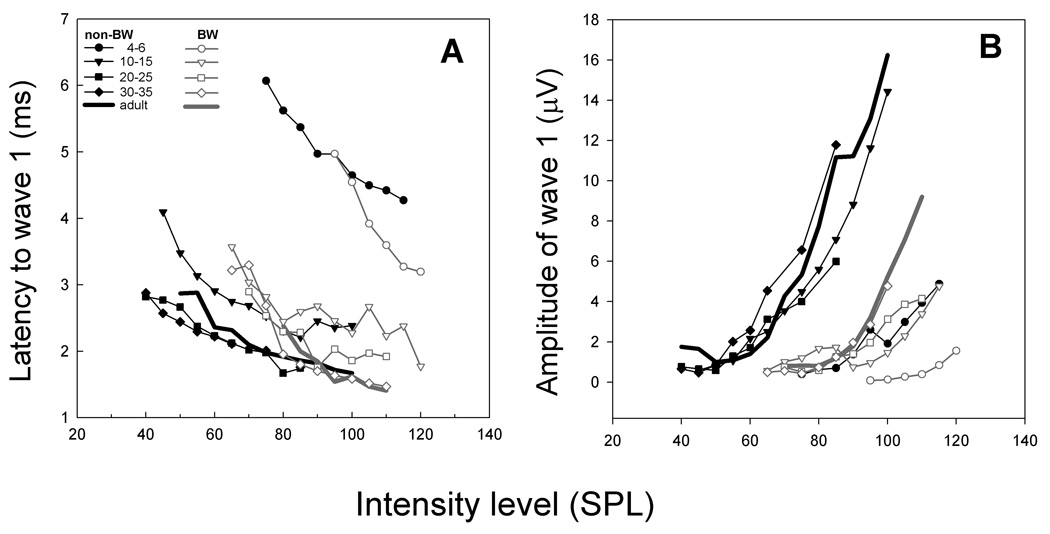
3.2 Physiological Development: Latency and Amplitude Functions in canaries
A comparison of the latency of wave 1 in response to clicks of different intensities showed that latency (Figure 7A) had stabilized and was similar for both strains by 30 days (squares). Wave 1 amplitude (Figure 7B) increased dramatically within the first two weeks. ABR growth functions in both strains were shallow at an age of 4–6 days, became steeper and were adult like by 30 DPH. The ABR growth functions were shifted towards higher sound pressure levels in BW canaries as compared to non-BW canaries.
Figure 7.
Latency and amplitude I/O curves in response to click stimuli. Black symbols/lines are for non-BW canaries and gray symbols/lines represent BW canary data. Latency decreases and amplitude increases in response to clicks as the birds’ age.
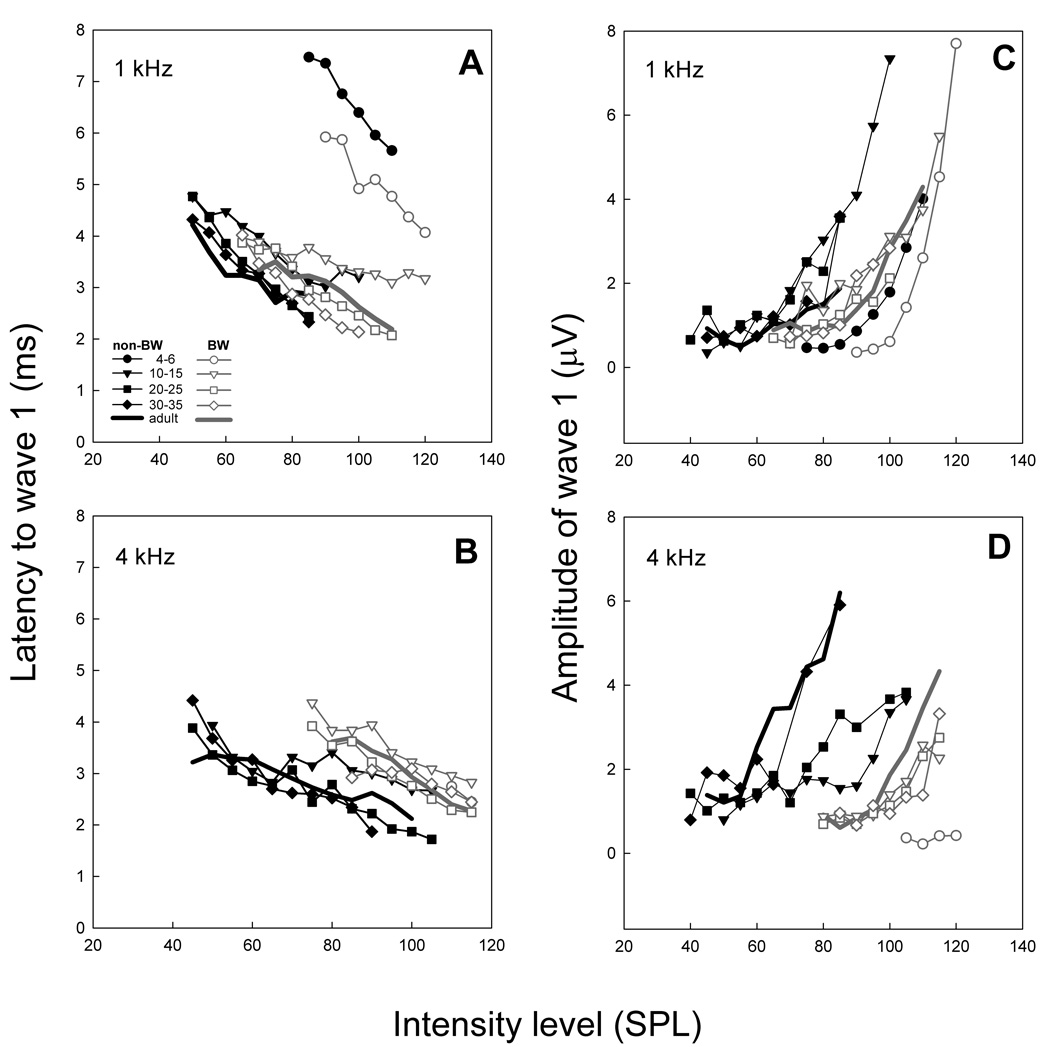
Level dependent latency and amplitude changes at 1 and 4 kHz for both strains (Figure 8) were near adult values by 20–25 DPH (time of fledging). Compared to the non-BW canary, the shift of the BW canary ABR growth function along the sound pressure level axis was more pronounced at 4 kHz and less pronounced at 1 kHz (similar to CAP amplitude responses found by Gleich et al., 1995a). There were, however, differences in the growth of ABR amplitude in the two frequencies. Growth curves at 4 kHz, compared to 1 kHz, were steeper in adult non-BW canaries, yet there was little difference in slope between the two frequencies for the BW canaries.
Figure 8.
Latency and amplitude I/O curves for non-BW (black symbols/lines) and BW canaries (gray symbols/lines) in response to 1 and 4 kHz tone bursts. Latency and amplitude in response to 1 kHz tones was similar in both strains, but BW canaries have longer latencies and lower amplitudes in response to 4 kHz tones.
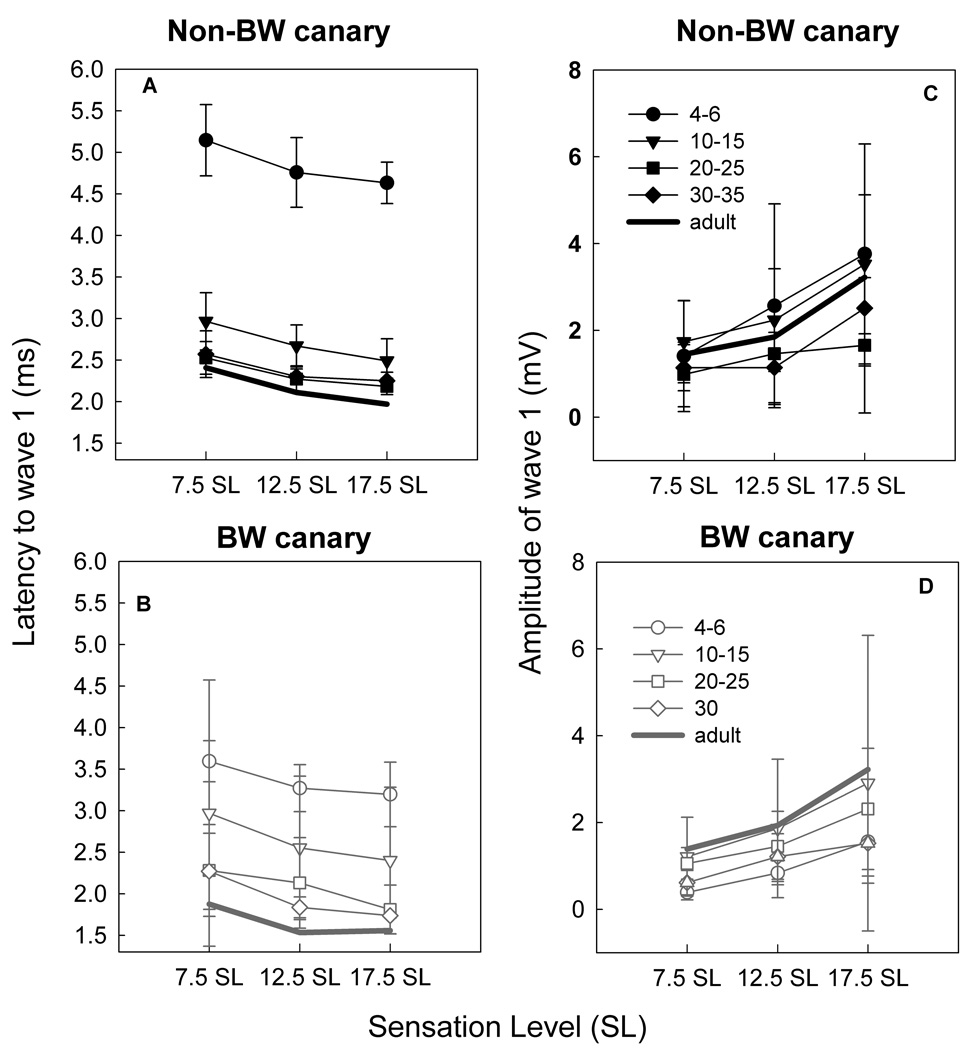
By plotting latency and amplitude as a function of SL (7.5, 12.5, and 17.5 dB above threshold) for the click, we removed threshold as a factor in latency or amplitude changes as a function of age. These data continued to show a decrease in latency to peak 1 in response to a click (Figure 9 A&B) as a function of age. As expected, there was a slight decrease in latency with increasing SL at all ages and in both strains. While there was considerable variability in amplitude (Figure 9 C&D; also seen in CAP responses, Gleich et al., 1995a; Figure 2), there were also systematic increases in amplitude as a function of increasing SL.
Figure 9.
Latency (A,B) and amplitude (C,D) changes in response to click stimulation at three different sensation levels (SL) for non-BW (A,C) and BW canaries (B,D). There are decreases in latency with age but also as sensation level increases. Amplitudes increase as a function of sensation level.
3.3 Morphological development
In the current study, the mean number of hair cells was 2856 ± 231 in five adult non-BW canaries and is consistent with previously reported numbers (2998 mean of six non-BW canaries; Gleich et al., 1994b). The mean hair cell number for six adult BW canaries (2266 ± 273) was significantly less than non-BW canaries (F(1,9)=14.53, p<.001) but resembled previous work showing that adult BW canaries have, on average, 30% fewer hair cells than non-BW canaries (2365 hair cells two BW canaries; Gleich et al., 1994a).
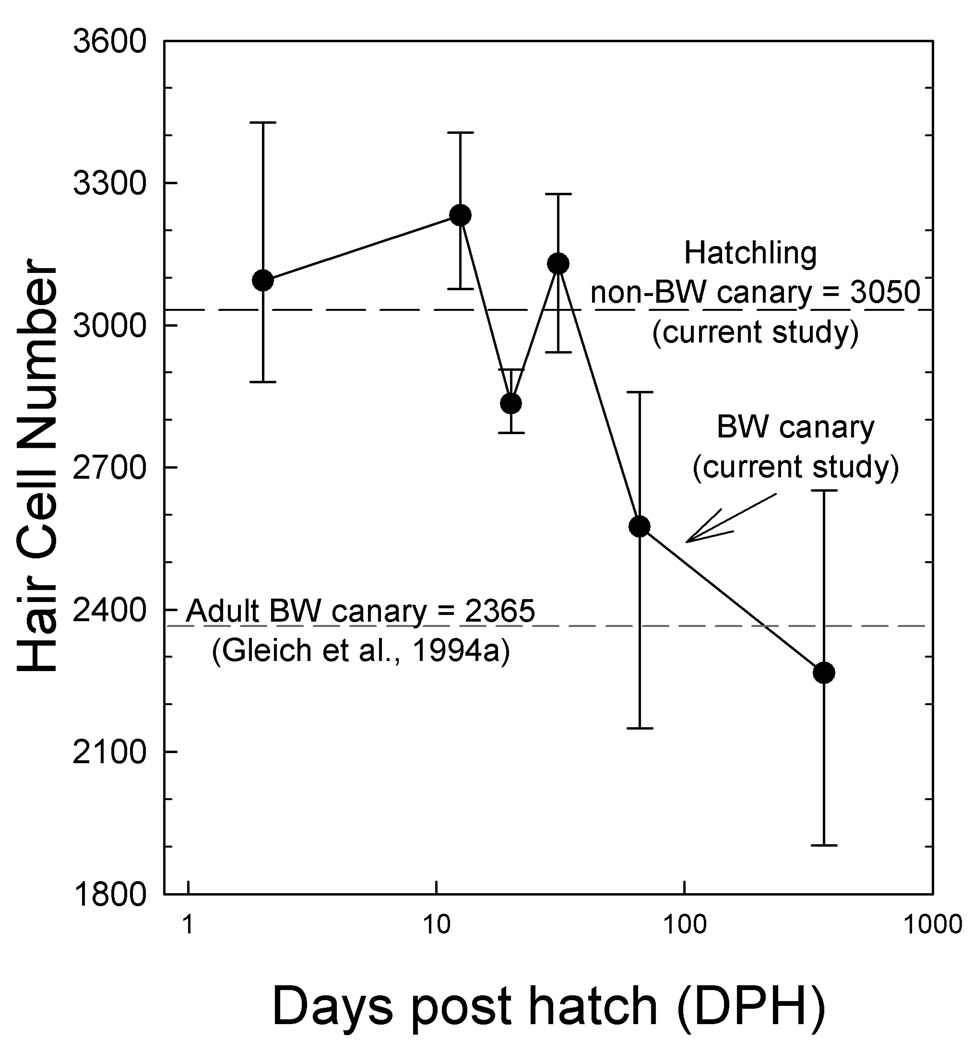
Figure 10 shows that the total hair cell number in BW canaries is roughly constant around 3000 between hatching and 30 DPH and declines only in older birds. A two way ANOVA (strain, age) showed that hair cell number was not significantly affected by strain (F(1,22)=2.87, p>.05) but was significantly affected by age (F(2,22)=23.98, p<.0001), with a significant interaction between strain and age (F(2,22)=9.045, P<.01). Also, there was no systematic difference of hair cell loss between basal and apical basilar papilla sections, which argues that there is no gradient of hair cell loss along the papilla, rather the whole papilla in BW canaries appears uniformly affected.
Figure 10.
Number of hair cells (median plus ranges) as a function of age for BW canaries compared to the total number of hair cells in non-BW canaries at hatch (black dashed line) and adult BW canary (gray dashed line) from Gleich et al. (1994a).
Post hoc comparisons (LSMeans Differences Tukey HSD) revealed no significant difference of hair cell number between different non-BW age groups (p>.05). However, adult BW canaries had significantly fewer hair cells than adult non-BW as well as BW canaries at 24hr and 12–13 DPH (p<.05). Hair cell numbers in developing BW canaries decrease significantly with age (F(5,26)=16.75, p<.0001). BW canaries two months and older have significantly fewer hair cells than birds one month and younger (posthoc, LSMeans Differences Tukey HSD, p<.05, Figure 10 line graph), except at 20 DPH. Hair cell totals for 20 day old birds were not significantly different from 66 day old birds (p>.05). In summary, these data show that BW canaries have the normal number of hair cells at hatching with hair cell numbers decreasing beyond 30 days. Loss of hair cells occurs throughout the BP in BW canaries. While the absolute number of hair cells decreases most in the apical half (wider, greater numbers of hair cells), the average loss is 16–17% throughout the papilla by 66 DPH.
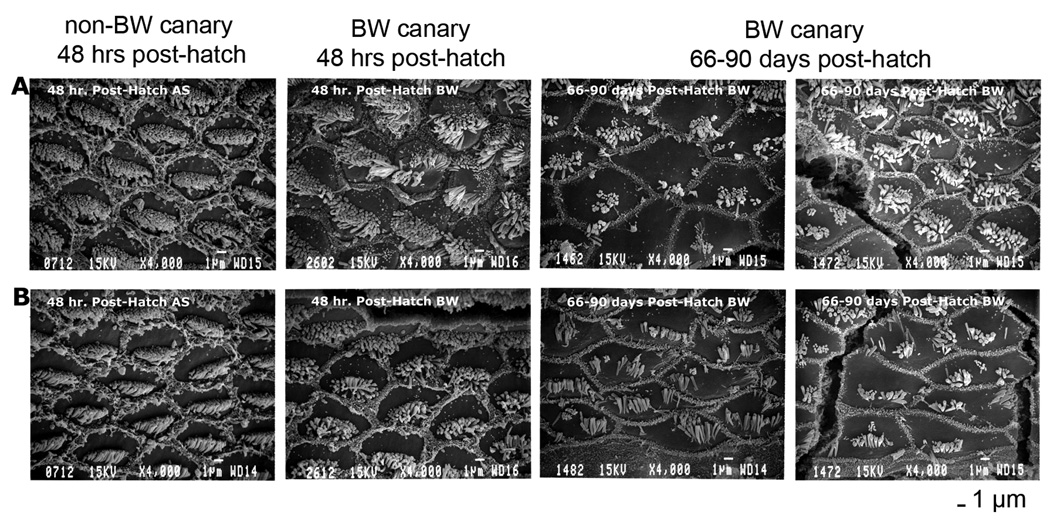
Figure 11 is a photomicrograph of hair cells in BW and non-BW (American singer, AS) canaries within 48 hours of hatch and at 3 months of age. Stereovilli on hair cells of non-BW canaries are arranged in regularly formed adult-like bundles within 48 hours of hatch while stereovillar bundles in some rare hair cells of BW canaries exhibit irregularities such as stereovillar bundle splaying and/or multiple bundles.
Figure 11.
SEM photomicrographs of hair cells in non-BW and BW canaries approximately 50% from the base. A) Hair cells on the neural edge of the papilla (tall hair cells) B) Hair cells on the abneural edge of the papilla (short hair cells). Some hair cells on the neural edge of BW basilar papilla show multiple stereovillar bundles within 48 hours post-hatch. By 2–3 months post-hatch multiple stereovillar bundles and other stereovillar abnormalities are similar to those seen in adult BW. Scale bar is 1µm; magnification = 4,000×
Hair cells with multiple stereovillar bundles were almost never (1.0% of cells) observed in non-BW nestling canaries (5 in 175 cells in one bird) and never seen in adult non-BW canaries. Kinocilia were present in all non-BW nestling canaries (139 cells in 496; 28% of total); these cells were almost exclusively located in the apical half of the BP. Kinocilia had decreased to only 0.2% of cells by 12–13 days post-hatch and none of the adult non-BW canary hair cells exhibited kinocilia.
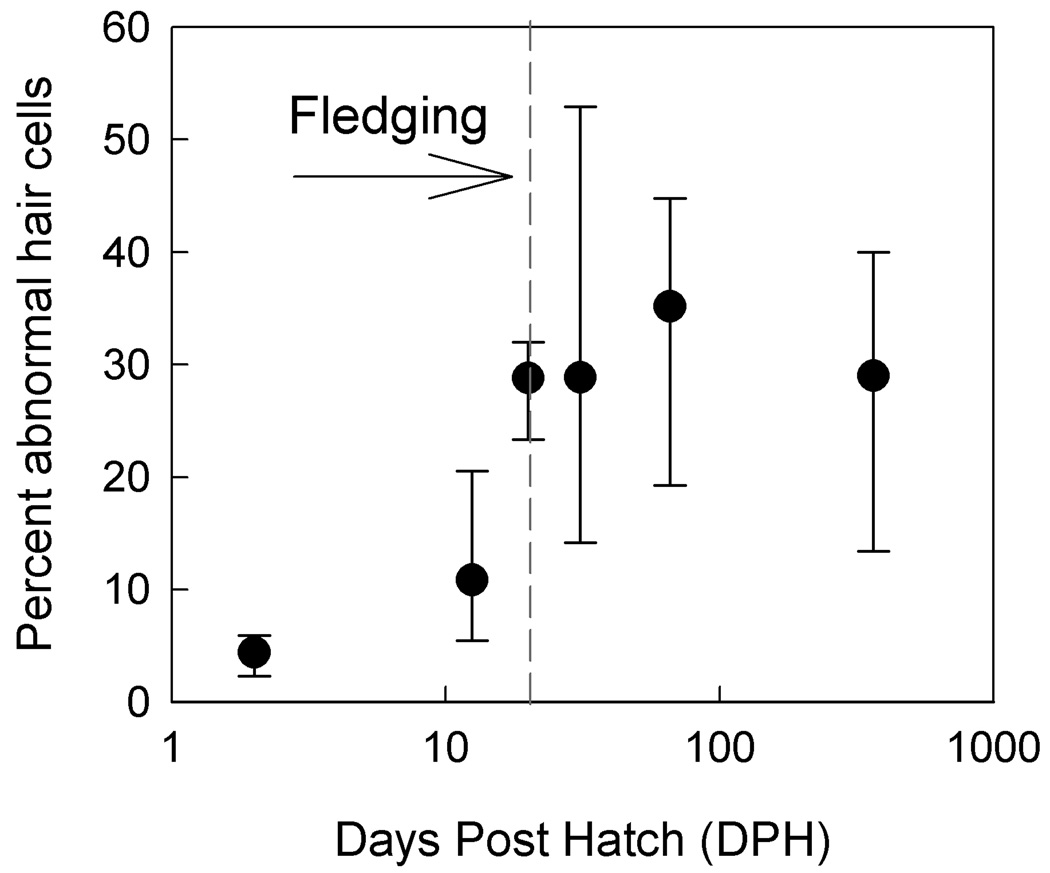
Subtle irregularities in hair bundle organization were present predominantly on hair cells along the neural edge of hatchling BW canaries (see Fig. 11 top middle). More severe distortion was quantified by counting hair cells with multiple stereovillar bundles. These were seen at low incidence in all nestling BW canaries (27 cells in 631; 4.3%). Kinocilia were also seen on hair cells in the apical half of the BP in all nestling BW canaries (15.2% of cells counted) but had decreased to 3.5% by 12–13 DPH. By 20 DPH, 28% of hair cells counted (179 in 625 in six out of 6 birds) had multiple stereovillar bundles. This degree of abnormal stereovillar bundles is similar to that of adult BW canaries (122 in 425 cells in 6 out of 6 birds). The transition from near normal hair cell arrangement in hatchlings to severe hair cell pathology is illustrated in Figure 11 and quantified in Figure 12. In general, when multiple stereovillar bundles were seen in nestling BW canaries, they were located on hair cells along the neural edge in the mid-basal region of the basilar papilla. The position 50% from the basal tip initially had the largest number of abnormal cells, but by 20 DPH abnormal cells were seen throughout the basilar papilla.
Figure 12.
The percent of abnormal hair cells increases significantly in BW canaries as the animals age (F(5,28)=10.92, p<.0001; medians and ranges shown). The largest change occurred between the 2nd and 3rd week post hatch. This is around the time of best sensitivity in BW canaries (see Figure 6, E).
4. Discussion
Previous anatomical and behavioral studies showed pathologies in BW canaries as young as 2 months (Gleich et al., 1994a; Lauer, 2006; Lauer et al., 2009; Lauer et al., 2007; Okanoya and Dooling, 1987; Okanoya et al., 1990). However, it was not clear whether the pathologies observed in the young adult birds are congenital or progress during post-hatch development. Massive sensorineural hearing loss, like that following cochlea removal, caused cell loss in second order auditory nuclei in mammals and the chicken (Hashisaki and Rubel, 1989; Moore, 1990; Mostafapour et al., 2000; Parks, 1979; Tierney et al., 1997; Trune, 1982) but only during a sensitive period. A study of BW canaries found a reduction in the size, but no loss of neurons, in nucleus magnocellularis (NM) and a reduced volume of NM and nucleus laminaris (NL) (Kubke et al., 2002). They provided two possible explanations for this observation: NM neurons may have received normal auditory input during an early stage of development, which established a normal complement of NM cells, or the reduced, but remaining, input provided by the auditory nerve in BW canaries was sufficient to prevent neuronal death in case of a congenital inner ear pathology.
We found that ABR waveform morphology, threshold, and inner ear morphology was very similar for hatchlings of both strains. The peripheral auditory system in altricial birds is generally inefficient at the time of hatching, showing high thresholds, long latencies and reduced amplitudes (Aleksandrov and Dmitrieva, 1992; Brittan-Powell and Dooling, 2004). We did not, however, find a reduced number of hair cells or large stereovillar bundle abnormalities in hatchlings. Hair cell abnormalities were rare but present in hatchling BW canaries, with hair cell loss and adult-like pathology developing within 4–6 weeks after hatching. Over the first week post hatch, the opening of the external ear canal and improvement in middle ear function (likely due to ossification of the columella and reduction of middle ear fluids) most likely aided in increasing frequency range and rapid improvement in threshold and is most likely responsible for the changes seen in both strains during this period in development (McFadden et al., 1996; Saunders et al., 1973; Walsh et al., 1986).
After this initial period of improving hearing, physiological measures of synchrony increased in the non-BW canary but appeared to breakdown in BW canaries. Higher SPLs were necessary to evoke ABR in all BW canaries, as was the case with earlier published CM and CAP measurements on this strain (Gleich et al., 1995a). Even at high SPLs, the waveform morphology of BW canaries never showed the synchronous alternation of positive-negative-positive peaks seen in non-BW canaries (Figure 1), suggesting pathological responses beyond the cochlear level (e.g., Kubke et al., 2002). This is also the time frame where ABR thresholds across strains began to diverge (2–3 weeks after hatching, Figures 5,6), the total number of hair cells declined (4 weeks, Figure 10) and the number of abnormal hair cells increased (3 weeks, Figure 12) in BW canaries. Together, these data showed that the degradation of the peripheral auditory system of the BW canary can be seen physiologically and morphologically around 14 DPH; by 3 weeks 30–40% of the hair cells showed multiple bundles while the number of hair cells continued to decrease at least up to 3 months of age. The net loss between 30 days and 60 days post-hatch was approximately 500 hair cells corresponding to about 15 hair cells per day. This rate of hair cell loss per day is more than two times higher than the hair cell turn over in adult BW canaries (Gleich et al., 1997).
We did not perform any measures of hair cell regeneration in the current study; however, the fact that hair cell number was similar to non-BW at hatch and declined to adult BW levels by 2 months suggests a net loss of hair cells over this period. Adult BW canaries can increase the rate of hair cell regeneration quite substantially following additional hair cell loss induced by sound overexposure (Dooling et al., 2008; Gleich et al., 1997). In adult BW canaries, the hair cell turnover was 6–7 cells per day (Gleich et al. 1997) with a 1:1 ratio between cells that were lost and cells that were newly generated (Wilkins et al., 2001). This 1:1 ratio is not established in nestling BW canaries.
There are two possible hypotheses that might explain this. First, it is possible that the ratio between cells that were lost and those that were newly generated was greater than 1:1 in the first weeks after hatching in BW canaries and reached a 1:1 ratio by 2 months of age. Alternatively, there may only be a loss of hair cells during the first 2 months after hatching and the generation of additional new hair cells begins subsequent to this initial loss and stabilized the number at a level of 70% compared to non-BW canaries. We do not know whether hair cell turnover is occurring right after hatching. If we assume hair cell turnover begins right after hatching, the balance of hair cell death and hair cell regeneration may only deviate from a 1:1 ratio between 30 days and 2 months of age. Alternatively, if there is no hair cell turnover in BW canaries before the age of 30 days, perhaps the hair cell loss beginning 4 weeks post hatch leads to a delayed and insufficient hair cell regeneration that only later (> 3 months of age) reaches a 1:1 ratio. It is possible that the scattered loss of hair cells that develops in the BW papilla 4–6 weeks after hatch is a suboptimal stimulus for triggering supporting cell proliferation and hair cell regeneration (but it appears sufficient to hold the balance in adult BW) compared to the massive hair cell wipe out caused by sound overexposure.
Using SEM, Gleich et al. (1994a; 1997; 1995b) found that on average 2.5% of the hair cells in adult BW canaries had a relatively small surface that was covered with microvilli resembling developing hair cells at various stages of maturity. They concluded that newly generated hair cells in the adult BW canary basilar papilla first develop normally and only subsequently degrade after the onset of their function. The current study found a near normal morphology of the basilar papilla right after hatching in BW canaries (Figures 10–12), which was followed by the early, rapid formation of multiple stereovillar bundles in the first 20 DPH and only later followed by hair cell loss. These data suggest that the mechanism inducing pathological multiple bundle formation will eventually lead to the death of the hair cell.
While the anatomical data of BW canaries show loss of hair cells and the formation of abnormal hair cells not before 30 and 20 DPH respectively (Fig. 10, 12), ABR thresholds for clicks and for frequencies between 1.5 and 4 kHz are clearly elevated before 20 DPH (Fig. 5, 6) suggesting a pathology along the auditory nerve originating beyond the hair cells. Evidence for such an additional neural pathology can also be derived from the comparison of CM and CAP thresholds of BW and non BW canaries reported by Gleich et al. (1995a) and replotted in Fig. 2. CAP and CM thresholds were roughly the same in non-BW canaries (compare CM [dashed line] and CAP [dotted line] in Fig. 2A), but CAP thresholds were on average 10 dB higher than CM thresholds in BW canaries (see Fig. 2B). This is remarkable given that BW canaries have, on average, 30% fewer hair cells but only 10% fewer auditory nerve fibers. Although we do not know the pattern of connection between hair cells and auditory nerve fibers in BW canaries and have no clear idea which type of hair cell (tall or short) might be more affected, some pathology appears to affect the nerve beyond, and/or in addition to, the hair cell pathology. Studies by Liberman and McFadden show evoked potentials thresholds are insensitive to diffuse neuronal loss as long as there is normal hair cell activity (El-Bradry and McFadden, 2007; Kujawa and Liberman, 2009; Liberman et al., 1997). Assuming that a normal looking hair cell epithelium (as we clearly see in young BW canaries; Fig. 10–12) is associated with normal hair cell function, the elevated thresholds of BW canaries before 20 DPH could mean that the nerve might already be affected in very young hatchlings/nestlings and could represent a congenital condition that was not assessed in our study.
ABRs as a predictor of sensorineural hearing loss
In general, ABR audiograms are higher but roughly follow the shape of behavioral thresholds (e.g., Borg and Engström, 1983; Brittan-Powell et al., 2002; Stapells and Oates, 1997; Wenstrup, 1984). Part of this discrepancy, of course, is due to temporal integration since ABR studies typically use very short stimuli (here 5 ms) while behavioral studies typically use longer stimuli (hundreds of ms) to determine threshold (Brittan-Powell et al., 2002; Florentine et al., 1988; Watson and Gengel, 1969). But, there are also clear differences in thresholds that vary in a frequency specific way between audiograms of both strains (Figure 2 and 3). For non-BW canaries, the mean difference between behavioral and ABR thresholds held constant at ~20 dB for frequencies < 4 kHz but increased > 4 kHz, which is typical of birds (Brittan-Powell et al., 2002). In BW canaries, the pattern of frequency dependent threshold difference between ABR and behavior deviates from that seen in non-BW canaries. The mean difference between behavioral and ABR thresholds (Figure 2d) in BW canaries was most pronounced <4 kHz; ABR thresholds were higher than (overestimate) behavioral thresholds by 40–45 dB. ABRs are a measure of neural synchrony and greater hair cell damage has been found in the apical than basal regions of the papilla (Gleich et al., 1994a; Weisleder and Park, 1994; Weisleder et al., 1996), which could contribute to reduced ABR amplitudes and elevated thresholds at low frequencies. At frequencies > 2 kHz, there is a decrease in mean difference between behavioral and ABR thresholds as a function of increased frequency; this is similar to what is seen in humans with mild to moderate sensorineural hearing loss (e.g., Beattie et al., 1996; Conijin et al., 1993; Stapells et al., 1990). In a meta-analysis review of 32 studies, Stapells (2000) reviewed threshold estimation via tone-evoked ABRs and found that ABR thresholds to non-masked tones in humans with very steep hearing losses result in an underestimate of behavioral thresholds (i.e., ABR thresholds were lower than behavioral thresholds). It is possible that the ABR evoked at high frequencies in BW canaries is due to a response from the entire papilla including a low frequency portion of the papilla, suggesting an explanation as to why the difference in measurements (Fig. 2d) become smaller with increasing frequency (e.g., ~15 dB at 8kHz in BW canaries compared to ~45 dB at 8 kHz in non-BW canaries). The brief tone bursts used in ABR testing, with splatter of acoustic energy to regions of better hearing sensitivity, may cause activation of a larger portion of the papilla in the higher frequency range in BW canaries (as in humans with steep losses; see Picton et al., 1979; Stapells et al., 1990). If this is the case, a notched-noise ABR paradigm may yield better estimates of hearing loss in BW canaries.
Comparison with other animal models of hearing loss
Given previous studies on age dependent hearing loss in gerbils (Boettcher et al., 1993; Hamann et al., 2002), we expected that the difference between behavioral and ABR thresholds would be small for ABR growth functions that were steep (1 kHz for BW and 4 kHz for both strains) and large for shallow growth functions (1 kHz non-BW canaries, see Fig. 8). This held true for 4 kHz where we saw a difference of about 20–30 dB (Figure 2 c&d solid lines; typical of birds, Brittan-Powell et al., 2002) but not for 1 kHz. Here, the opposite was true: non-BW canary thresholds were ~20 dB higher and BW canary thresholds were ~35 dB higher than behavioral thresholds. In BW canaries, the pattern of frequency dependent threshold difference between ABR and behavior deviates from that seen in non-BW canaries. The difference is most pronounced in the 1–2 kHz (steep slopes) region reaching 40–45 dB and decreases with increasing frequency to less than 20 dB at 8 kHz. In other words, the BW canary inner ear pathology differentially affects ABR and behavioral measures of hearing. This is consistent with age dependent hearing loss in gerbils, where the discrepancy between behavioral and ABR thresholds increases with age: ABR growth functions become more shallow and result in decreased amplitude and thus higher ABR thresholds in old gerbils.
Hamann et al. (2002) suggested that behavior and ABR may give similar results when hair cells are missing or pathological. Some studies have reported correlated changes in behavioral and ABR thresholds following cochlear damage by noise or aminoglycoside exposure (Borg, 1982; Borg and Engström, 1983). However, discrepancies between both measures have also been observed. Soliman et al. (1993) report that approximately 30% of the epileptic patients in their study population showed elevated ABR thresholds although thresholds determined by pure tone audiometry (behavioral thresholds) were normal. In gerbils, ABR studies show that age dependent changes develop in animals older than 2 years (Boettcher et al., 1993; Mills et al., 1990). In contrast, using behavioral methods thresholds remain stable in gerbils up to 3 years of life (Hamann et al., 2002). The discrepancy between ABR and behavioral thresholds increased with age and hearing loss determined by ABR appeared more severe than behaviorally determined hearing loss (Hamann et al., 2002) and is similar to what we see in the BW canary but at an earlier stage in development.
In canaries and gerbils, the type and degree of pathology appears to affect the relation between behavioral and ABR thresholds and hair cell loss appears as an important factor. In old gerbils, hair cell loss is observed at the apex but not towards the base (Tarnowski et al., 1991). In behavioral studies, initial age related hearing loss was found in gerbils at frequencies ≤ 2kHz (the region of hair cell loss) but not at 10 kHz (without hair cell loss) despite elevated ABR thresholds (Hamann et al., 2002; Sinnott et al., 1997). Although ABR and behavioral thresholds are typically correlated, the difference between both thresholds is affected by pathology. Ngan and May (2001) showed on average a difference of 20 – 35 dB between the thresholds of the most sensitive auditory nerve fibers (that most likely determine behavioral threshold) and ABR in normal hearing cats. In noise-exposed cats, the difference between thresholds of the most sensitive fibers and the ABR decreased with increasing hearing loss, with both thresholds converging for a hearing loss over 60 dB. Since the ABR is a measure of the synchronized response of nerve fibers to stimulus onset, response magnitude depends on the population of activated neurons (typically a considerable number of neurons need to be activated to obtain a response above background noise) and the degree of synchronization. Fewer responding neurons and/or a decreased degree of synchronization will reduce the ABR response and result in elevated ABR thresholds. If a behaving animal uses the (remaining) most sensitive neurons and bases its response on an increase in discharge rate rather than on the synchronized response of a whole population of neurons, behavioral threshold shifts could be different than those determined by ABR.
Differences found at the level of the auditory nerve may also contribute to the “overestimate” of hearing loss by ABR compared to behavior in the frequency range below 4 kHz in BW canaries. The number of auditory nerve fibers is reduced by 12% compared to non BW canaries and BW canaries have a higher proportion of thin (1 – 2 µm) and thick (3.5 – 6 µm) and fewer medium sized (2 – 3.5 µm) axons than non BW canaries (Gleich et al., 2001) which could affect synchronization.
At high frequencies, behavioral and ABR thresholds converge in BW canaries similar to what Ngan and May (2001) reported for the traumatized region of the cat cochlea. We do not know the tonotopy of the BW basilar papilla, but the convergence of behavioral and ABR thresholds might be due to a “corner” effect. The best frequencies of hair cells and auditory neurons represented on the basilar papilla might be limited to 4–6 kHz. The responses to higher frequencies (6 kHz and above) would be limited by the steep slopes of the high frequency tuning curve flanks. High level stimuli with frequencies above 6 kHz would elicit responses in the high frequency reponse area of auditory neurons with characteristic frequencies below 6 kHz.
The generator of the early positive ABR deflection is most likely the response of the auditory nerve fibers (Brittan-Powell et al., 2002). In contrast to the CAP, the amplitude of the ABR is much lower due to the distant recording site. At 4 kHz, CAP amplitudes are much lower in BW than non-BW canaries (Gleich et al., 1994a, Gleich et al., 1995a Figure 2). We see a similar shift towards higher SPL in ABR amplitudes in BW canaries (Figure 8). Boettcher et al. (1993) found the reductions in CAP amplitudes in gerbils was due to a decrease in the number of synchronized auditory nerve fibers contributing to the CAP, which then contributed to the reduction in ABR amplitude.
Summary
The BW canary provides a unique model for examining the interaction of developmental hearing loss and hair cell pathology but also for the implication for song learning. The present study clearly shows that important changes occur in the auditory periphery of BW canaries in the period 10–20 days post-hatch. By the time these birds are leaving the nest, their hearing sensitivity is diminished. The sensitive period for learning song typically occurs after about 14 days post hatch (see review in, Catchpole and Slater, 1995), which is coincident with when hearing sensitivity begins to improve in altricial birds but when hearing loss begins in BW canaries. Therefore, there is little time for exposure to a ‘normal’ acoustic environment in the nest for these developing birds. We suspect that the restricted acoustic environment during the song development period contributes significantly to the unique low-pitched vocalizations in BW canaries.
ACKNOWLEDGEMENTS
The authors would like to thank Amanda Lauer for comments on earlier drafts and Fernando Nottebohm for use of BW canaries from Rockefeller University Field Station. This work was supported in part by grant DC-001372 from the National Institute of Deafness and Communicative Disorders of the National Institutes of Health to RJD and BMR and P30DC004664.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleksandrov LI, Dmitrieva LP. Development of auditory sensitivity of altricial birds: absolute thresholds of the generation of evoked potentials. Neurosci. Behav. Physiol. 1992;22:132–137. doi: 10.1007/BF01192385. [DOI] [PubMed] [Google Scholar]
- Beattie RC, Garcia E, Johnson A. Frequency-specific auditory brainstem responses in adults with sensorineural hearing loss. Audiology. 1996;35:194–203. doi: 10.3109/00206099609071941. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Mills JH, Norton BL. Age-related changes in auditory evoked potentials of gerbils. I. Response amplitudes. Hearing Res. 1993;71:137–145. doi: 10.1016/0378-5955(93)90029-z. [DOI] [PubMed] [Google Scholar]
- Borg E. Susceptibility of the sympathectomized ear to noise-induced hearing loss. Acta Physiol. Scand. 1982;114 doi: 10.1111/j.1748-1716.1982.tb06999.x. [DOI] [PubMed] [Google Scholar]
- Borg E, Engström B. Hearing thresholds in the rabbit. Acta Oto-Laryngol. 1983;95:19–26. doi: 10.3109/00016488309130911. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Dooling RJ. Development of auditory sensitivity in budgerigars (Melopsittacus undulatus) J. Acoust. Soc. Am. 2004;115:3092–3102. doi: 10.1121/1.1739479. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Dooling RJ, Gleich O. Auditory brainstem responses in adult budgerigars (Melopsittacus undulatus) J. Acoust. Soc. Am. 2002;112:999–1008. doi: 10.1121/1.1494807. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Lohr B, Hahn DC, Dooling RJ. Auditory brainstem responses in the Eastern Screech Owl: An estimate of auditory thresholds. J Acoust Soc Am. 2005;118:314–321. doi: 10.1121/1.1928767. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Bird Song: Biological themes and variations. Great Britain: Cambridge University Press; 1995. [Google Scholar]
- Coleman J, Blatchley BJ, Williams JE. Development of the dorsal and ventral cochlear nuclei in rat and effects of acoustic deprivation. Dev. Brain Res. 1982;4:119–123. doi: 10.1016/0165-3806(82)90104-3. [DOI] [PubMed] [Google Scholar]
- Conijin EG, Brocaar MP, Van Zanten GA. Frequency-specific aspects of the auditory brainstem response threshold elicited by 1000-Hz filtered clicks in subjects with sloping cochlear hearing losses. Audiology. 1993;32:1–11. doi: 10.3109/00206099309072923. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Dent ML, Lauer AM, Ryals BM. Functional recovery after hair cell regeneration in birds. In: Salvi RJ, Popper AN, Fay RR, editors. Hair cell regeneration, repair, and protection. Vol. 33. New York, NY: Springer Handbook of Auditory Research; 2008. pp. 117–140. [Google Scholar]
- El-Bradry MM, McFadden SL. Electrophysiological Correlates of Progressive Sensorineural Pathology in Carboplatin-Treated Chinchillas. Brain Research. 2007;1134:122–130. doi: 10.1016/j.brainres.2006.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentine M, Fastl H, Buus S. Temporal integration in normal hearing, cochlear impairment, and impairment simulated by masking. J. Acoust. Soc. Am. 1988;84:195–203. doi: 10.1121/1.396964. [DOI] [PubMed] [Google Scholar]
- Gardner TJ, Naef F, Nottebohm F. Freedom and rules: the acquisition and reprogramming of a bird's learned song. Science (New York, N.Y. 2005;308:1046–1049. doi: 10.1126/science.1108214. [DOI] [PubMed] [Google Scholar]
- Gleich, Kadow C, Strutz J. The postnatal growth of cochlear nucleus subdivisions and neuronal somata of the anteroventral cochlear nucleus in the Mongolian gerbil (Meriones unguiculatus) Audiol Neurootol. 1998;3:1–20. doi: 10.1159/000013775. [DOI] [PubMed] [Google Scholar]
- Gleich O, Strutz J. Age-dependent effects of the onset of a conductive hearing loss on the volume of the cochlear nucleus subdivisions and the expression of c-fos in the mongolian gerbil (Meriones unguiculatus) Audiol. Neurootol. 1997;2:113–127. doi: 10.1159/000259235. [DOI] [PubMed] [Google Scholar]
- Gleich O, Dooling RJ, Manley GA. Inner-ear abnormalities and their functional consequences in Belgian Waterslager canaries (Serinus canarius) Hearing Res. 1994a;79:123–136. doi: 10.1016/0378-5955(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Gleich O, Klump GM, Dooling RJ. Peripheral basis for the auditory deficit in Belgian Waterslager canaries (Serinus canarius) Hearing Res. 1995a;82:100–108. doi: 10.1016/0378-5955(94)00166-n. [DOI] [PubMed] [Google Scholar]
- Gleich O, Dooling RJ, Presson JC. Evidence for supporting cell proliferation and hair cell differentiation in the basilar papilla of adult Belgian Waterslager canaries (Serinus canarius) J. Comp. Neurol. 1997;377:5–14. doi: 10.1002/(sici)1096-9861(19970106)377:1<5::aid-cne2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Gleich O, Dooling RJ, Ryals BM. A quantitative analysis of the nerve fibers in the VIIIth nerve of Belgian Waterslager canaries with a hereditary sensorineural hearing loss. Hearing Res. 2001;151:141–148. doi: 10.1016/s0378-5955(00)00221-5. [DOI] [PubMed] [Google Scholar]
- Gleich O, Manley GA, Mandl A, Dooling RJ. Basilar papilla of the canary and zebra finch: A quantitative scanning electron-microscopic description. Journal of Morphology. 1994b;220:1–24. doi: 10.1002/jmor.1052210102. [DOI] [PubMed] [Google Scholar]
- Gleich O, Fischer F, Köppl C, Manley G. Hearing organ evolution and specialization: Archosaurs. In: Manley G, Popper A, Fay R, editors. Evolution of the vertebrate auditory system. New York: Springer Verlag; 2004. pp. 224–255. [Google Scholar]
- Gleich O, Dooling RJ, Manley GA, Klump GM, Strutz J. Evidence for Continuous Hair Cell Regeneration in a Song Bird with Hereditary Cochlear Hearing-Loss. HNO. 1995b;43:287–293. [PubMed] [Google Scholar]
- Güttinger HR. Consequences of Domestication on the Song Structures in the Canary. Behaviour. 1985;94:254–278. [Google Scholar]
- Hall JW. New Handbook of Auditory Evoked Responses. Boston: Allyn and Bacon; 2007. [Google Scholar]
- Hamann I, Gleich O, Klump G, Kittel M, Boettcher F, Schmiedt R, Strutz J. Behavioral and evoked-potential thresholds in young and old Mongolian gerbils (Meriones unguiculatus) Hearing Res. 2002;171:82. doi: 10.1016/s0378-5955(02)00454-9. [DOI] [PubMed] [Google Scholar]
- Hashisaki GT, Rubel EW. Effects of unilateral cochlea removal on anteroventral cochlear nucleus neurons in developing gerbils. J Comp Neurol. 1989;283:5–73. doi: 10.1002/cne.902830402. [DOI] [PubMed] [Google Scholar]
- Kubke MF, Dent ML, Hodos W, Carr CE, Dooling RJ. Nucleus magnocellularis and nucleus laminaris in Belgian Waterslager and normal strain canaries. Hearing Res. 2002;164:19–28. doi: 10.1016/s0378-5955(01)00387-2. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding Insult to Injury: Cochlear Nerve Degeneration after "Temporary" Noise-Induced Hearing Loss. Journal of Neuroscience. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM. dissertation. College Park: University of Maryland; 2006. Perceptual consequences of hereditary inner ear abnormalities in Belgian Waterslager canaries. [Google Scholar]
- Lauer AM, Dooling RJ, Leek MR. Psychophysical evidence of damaged active processing mechanisms in Belgian Waterslager Canaries. Journal of comparative physiology. 2009;195:193–202. doi: 10.1007/s00359-008-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Dooling RJ, Leek MR, Poling K. Detection and discrimination of simple and complex sounds by hearing-impaired Belgian Waterslager canaries. J. Acoust. Soc. Am. 2007;122:3615–3627. doi: 10.1121/1.2799482. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Chesney CP, Kujawa SG. Effects of selective inner hair cell loss on DPOAE and CAP in carboplatin-treated chinchillas. Auditory Neuroscience. 1997;3:255–268. [Google Scholar]
- Lippe WR, Westbrook EW, Ryals BM. Hair cell regeneration in the chicken cochlea following aminoglycoside toxicity. Hearing Res. 1991;56:203–210. doi: 10.1016/0378-5955(91)90171-5. [DOI] [PubMed] [Google Scholar]
- Lohr B, Lauer A, Newman M, Dooling RJ. Hearing in the red-billed firefinch and the spanish Timbrado canary: The influence of natural and artificial selection on auditory abilities and vocal structure. Bioacoustics. 2004;14:83–98. [Google Scholar]
- Marler P, Waser MS. Role of auditory feedback in canary song development. J. Comp. Physiol. Psychol. 1977;91:8–16. doi: 10.1037/h0077303. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Walsh EJ, McGee J. Onset and development of auditory brainstem responses in the Mongolian gerbil (Meriones unguiculatus) Hearing Res. 1996;100:68–79. doi: 10.1016/0378-5955(96)00108-6. [DOI] [PubMed] [Google Scholar]
- Mills JJ, Schmiedt RA, Kulish LF. Age-related changes in auditory potentials of Mongolian gerbil. Hearing Res. 1990;46:210–210. doi: 10.1016/0378-5955(90)90002-7. [DOI] [PubMed] [Google Scholar]
- Moore DR. Auditory brainstem of the ferret: early cessation of developmental sensitivity of neurons in the cochlear nucleus to removal of the cochlea. Journal of Comparative Neurology. 1990;302:810–823. doi: 10.1002/cne.903020412. [DOI] [PubMed] [Google Scholar]
- Mostafapour SP, Cochran SL, Del Puerto NM, Rubel EW. Patterns of cell death in mouse anteroventral cochlear nucleus neurons after unilateral cochlea removal. J Comp Neurol. 2000;426:561–571. doi: 10.1002/1096-9861(20001030)426:4<561::aid-cne5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Mundinger PC. Genetics of Canary song learning: Innate Mechanisms and other neurobiological considerations. In: Hauser MD, Konishi M, editors. The Design of Animal Communication. Cambridge: The MIT Press; 1999. pp. 369–389. [Google Scholar]
- Ngan EM, May BJ. Relationship between the auditory brainstem response and auditory nerve thresholds in cats with hearing loss. Hearing Res. 2001;156:44–52. doi: 10.1016/s0378-5955(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Killackey HP, Kitzes LM. Ascending Auditory Projections to the Inferior Colliculus in the Adult Gerbil, Meriones unguiculatus. The Journal of Comparative Neurology. 1983;214:131–143. doi: 10.1002/cne.902140203. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME. Relationship between Song Repertoire and Age in Canary, Serinus canarius. Zeitschrift Fur Tierpsychologie-Journal of Comparative Ethology. 1978;46:298–305. [Google Scholar]
- Okanoya K, Dooling RJ. Colony differences in auditory thresholds in the canary (Serinus canarius) Acoust. Soc. Am. 1985;78:1170–1176. doi: 10.1121/1.392885. [DOI] [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ. Strain differences in auditory thresholds in the canary (Serinus canarius) J Comp Psychol. 1987;101:213–215. [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ, Downing JD. Hearing and vocalizations in hybrid Waterslager-Roller canaries (Serinus canarius) Hearing Res. 1990;46:271–275. doi: 10.1016/0378-5955(90)90008-d. [DOI] [PubMed] [Google Scholar]
- Parks TN. Afferent Influences on the Development of the Brain Stem Auditory Nuclei of the Chicken: Otocyst Ablation. J. Comp. Neurol. 1979;183:665–678. doi: 10.1002/cne.901830313. [DOI] [PubMed] [Google Scholar]
- Picton TW, Ouellette J, Hamel G, Smith AD. Brainstem evoked potentials to tonepips in notched noise. Journal of Otolaryngology. 1979;10:1–41. [PubMed] [Google Scholar]
- Ryals BM, Dooling RJ, Westbrook E, Dent ML, MacKenzie A, Larsen ON. Avian species differences in susceptibility to noise exposure. Hearing Res. 1999;131:71–88. doi: 10.1016/s0378-5955(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Coles RB, Gates GR. The development of auditory evoked responses in the cochlea and cochlear nuclei of the chick. Brain Res. 1973;63:59–74. doi: 10.1016/0006-8993(73)90076-0. [DOI] [PubMed] [Google Scholar]
- Sinnott JM, Street SL, Mosteller KW, Williamson TL. Behavioral measures of vowel sensitivity in Mongolian gerbils (Meriones unguiculatus): effects of age and genetic origin. Hearing Res. 1997;112:235–246. doi: 10.1016/s0378-5955(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Soliman S, Mostafa M, Kamal N, Raafat M, Hazzaa N. Auditory evoked potentials in epileptic patients. Ear Hear. 1993;14:235–241. doi: 10.1097/00003446-199308000-00002. [DOI] [PubMed] [Google Scholar]
- Stapells DK. Threshold estimation by the tone-evoked auditory brainstem response: A literature meta-analysis. Journal of Speech-Language Pathology and Audiology. 2000;24:74–83. [Google Scholar]
- Stapells DR, Oates P. Estimation of pure-tone audiogram by the auditory brainstem response: A review. Audiol. Neurootol. 1997;2:257–280. doi: 10.1159/000259252. [DOI] [PubMed] [Google Scholar]
- Stapells DR, Picton TW, Durieux-Smith A, Edwards CG, Moran LM. Thresholds for short-latency auditory-evoked potentials to tones in notched noise in normal-hearing and hearing-impaired subjects. Audiology. 1990;29:262–274. doi: 10.3109/00206099009072857. [DOI] [PubMed] [Google Scholar]
- Tarnowski BI, Schmiedt RA, Hellstrom LI, Lee FS, Adams JC. Age-related changes in cochleas of Mongolian gerbils. Hearing Res. 1991;54:124–134. doi: 10.1016/0378-5955(91)90142-v. [DOI] [PubMed] [Google Scholar]
- Tierney TS, Russell FA, Moore DR. Susceptibility of developing cochlear nucleus neurons to deafferentation-induced death abruptly ends just before the onset of hearing. J. Comp. Neurol. 1997;378:295–306. doi: 10.1002/(sici)1096-9861(19970210)378:2<295::aid-cne11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Trune DR. Influence of neonatal cochlear removal on the development of mouse cochlear nucleus: I. Number, size, and density of its neurons. J Comp Neurol. 1982;209:409–424. doi: 10.1002/cne.902090410. [DOI] [PubMed] [Google Scholar]
- Walker GBR, Avon D. Coloured, type and song canaries: A complete guide. 2nd Edition. London: Blandford Press; 1993. [Google Scholar]
- Walsh EJ, McGee J, Javel E. Development of auditory-evoked potentials in the cat. I. Onset of response and development of sensitivity. J. Acoust. Soc. Am. 1986;79:712–724. doi: 10.1121/1.393461. [DOI] [PubMed] [Google Scholar]
- Watson CS, Gengel RW. Signal Duration and Signal Frequency in Relation to Auditory Sensitivity. Journal Acoustic Society of America. 1969;46:989–997. doi: 10.1121/1.1911819. [DOI] [PubMed] [Google Scholar]
- Webster DB. Conductive hearing loss affects the growth of the cochlear nuclei over an extended period of time. Hearing Res. 1988;32:185–192. doi: 10.1016/0378-5955(88)90090-1. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Park TJ. Belgian Waterslager canaries are afflicted by Scheibe's-like dysplasia. Hearing Res. 1994;80:64–70. doi: 10.1016/0378-5955(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Lu Y, Park TJ. Anatomical basis of a congenital hearing impairment: basilar papilla dysplasia in the Belgian Waterslager canary. J Comp Neurol. 1996;369:292–301. doi: 10.1002/(SICI)1096-9861(19960527)369:2<292::AID-CNE9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Wenstrup J. Auditory sensitivity in the fish catching bat, Noctilo leporinus. J. Comp. Physiol. A. 1984;155:91–101. [Google Scholar]
- Wilkins HR, Presson JC, Popper AN, Ryals BM, Dooling RJ. Hair cell death in a hearing-deficient canary. J. Assoc. Res. Otolaryngol. 2001;2:79–86. doi: 10.1007/s101620010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TF, Brittan-Powell EF, Dooling RJ, Mundinger PC. Sex-linked inheritance of hearing and song in the Belgian Waterslager canary. Proc. Biol. Sci. 2004;271 Suppl 6:S409–S412. doi: 10.1098/rsbl.2004.0204. PMCID: PMC1810118. [DOI] [PMC free article] [PubMed] [Google Scholar]