Abstract
Purpose
To determine the in vivo effect of doxycycline (doxy) on choroidal angiogenesis and pterygium growth by using a choroidal neovascular murine model (CNV), a directed in vivo angiogenesis assay (DIVAA) and a pterygium murine model.
Design
Experimental Study
Participants
3 murine models were investigated with 4 mice minimum per group and 22 maximum per group.
Methods
Mice received water with or without doxycycline (Leiter's Pharmacy, San Jose, CA). For the CNV, the neovascular lesion volume was determined in choroid-retinal pigment epithelial (RPE) flat mounts using confocal microscopy seven days after laser induction. For DIVAA, silicone capsules containing 10,000 human pterygium epithelial cells were implanted in the flanks of mice subcutaneously. After eleven days, neovascularization (NV) was quantified using spectrofluorimetry after murine tail-vein injection of fluorescein isothiocyanate (FITC)-labeled dextran. A pterygium epithelial cell model was developed by injecting 10,000 human pterygium epithelial cells in the nasal subconjunctival space in athymic nude mice. Doxy was started on day six at 50 mg/kg/day; corneal lesions that resulted from the injections were compared at days six and fifteen.
Main outcome measures
Student's t-test was used to evaluate the data for the CNV and DIVAA models and histologic preparations were used to evaluate pterygia lesions.
Results
There was significantly less NV and lesion volume with doxy taken in drinking water versus plain water. With doxy treatment, the laser-induced CNV showed a maximal 66% decrease in choroidal blood vessel volume (p≤0.008) and the DIVAA showed a 30% reduction of blood vessel growth and migration (p<0.004). Histologic preparations demonstrated that pterygium cell lesions regressed when mice were administered doxy for 9 days.
Conclusions
Doxycycline significantly inhibited angiogenesis in three murine models. The most dramatic effect was found in the choroidal neovascularization model followed by the pterygia epithelial cell DIVAA model. The anterior segment pterygium model also showed regression histologically. This suggests that doxycycline may be successful as an adjunctive treatment for choroidal neovascularization and pterygia in humans; clinical trials would be necessary to determine if there is a benefit.
Angiogenesis is a double-edged sword in that it is essential for life yet often contributes to disease and disability. The neovascularization (NV) associated with many ocular diseases including diabetic retinopathy, macular degeneration, pterygium and corneal transplant rejection is responsible for much morbidity, including blindness. Vascular endothelial growth factor (VEGF) and matrix metalloproteinases are elevated in exudative age-related macular degeneration (AMD).1–3 This disease contributes to a significant decline in quality of life, secondary to choroidal neovascularization and submacular hemorrhage. Experimental models of CNV such as the laser injury of RPE-Bruch’s membrane-choroid complex permit visualization of the pathological processes and quantitative measurement of angiogenesis inhibitors. Ocular investigations suggest that the immune response may play a pivotal role during CNV development.4 In the laser-induced CNV mouse model, there is an early influx of neutrophils, macrophages, NK cells and microglia cells within 72 hours with associated edema. By 7 days post-laser, the edema is much reduced with fewer immune cells, except for a persistence of microglial cells and an organized infiltrating membrane lesion.
Pterygia are common, benign, winged-shaped growths on the cornea. The condition is characterized by an advancing edge of epithelial cell overgrowth with squamous metaplasia, goblet cell hyperplasia, and abnormal p53 expression and with an underlying stroma of fibroblasts and blood vessels.5–8 These characteristic, wing-shaped lesions have a propensity to migrate into Bowman’s layer toward the central cornea and distort or obscure vision. In the initial phases of this disorder, it is believed that altered limbal basal epithelial cells invade onto normal corneal basement membrane over a dissolving Bowman’s layer.5 Elevated levels of matrix metalloproteinases such as MMP-1, MMP-2 and MMP-9 have been localized in cells at the base of the cornea (pterygium leading edge) and are most likely responsible for the destruction of Bowman’s layer and the local infiltration of pterygium cells within adjacent epithelial tissue. Human pterygia express, among other factors, fibronectin, which is associated with cell adhesion and migration, as well as pro-inflammatory cytokines, angiogenic growth factors and fibrogenic factors (e.g., vascular endothelial growth factor [VEGF], versican, CD31 antigen, transforming growth factor β, and interleukin-6 and -8).6,9
Doxycycline (doxy) is a low-cost bacteriostatic antibiotic taken orally and has been used safely for decades in humans. It has been shown to suppress the catalytic activity of many matrix metalloproteinases (MMP), including gelatinases and collagenases, independent of its antimicrobial effect.10 Cox, et al demonstrated a reduction in the migration of pterygium epithelial cells exposed to doxycycline grown in culture plates lined with fibronectin. The inhibitory effect of doxycycline on the activity of MMP-9 in the cell culture supernatant of these cells was also demonstrated (Invest Ophthalmol Vis Sci 2004; 45: E-Abstract 2939). In a chemical injury rat cornea model of neovascularization, Riazi-Esfahani, et al showed that doxycycline drops successfully prevented neovascularization when compared to normal saline drops, although the most effective agent used in this study was triamcinolone.11 Another rat corneal model for inhibition of angiogenesis compares oral doxy to oral and topical dexamethasone using an alkali burn method to produce angiogenesis. This showed a 37.4% reduction in neovascularization after 14 days of treatment using doxy, compared to a 70.7% and 67.4 % reduction using topical and oral dexamethasone (respectively).12 This again shows a steroid as the superior anti-angiogenic agent, but the potential complications and side effects from steroids can be prohibitive. The low side-effect profile of doxycycline motivated us to focus on doxy for our treatment modality.
In a study of human endothelial cells, doxy was shown to inhibit MMP activity, protein synthesis and mRNA expression.13 A reduction in the neovascularization induced by pterygium epithelial cells was shown in experiments utilizing doxycycline in the Directed In Vivo Angiogenesis Assay (Cox, et al. Invest Ophthalmol Vis Sci 2005; 46: E-Abstract 959). Chhipa described tumors that were significantly reduced in size when doxy was used in combination with cyclophosphamide in an athymic mouse breast cancer model.14 Corneal neovascularization can be inhibited with topical use of doxy in combination with other agents11 and alone given orally to rats after injury by alkali burn.12 Lee used a mouse model with enhanced cerebral angiogenesis induced by focal hyperstimulation with VEGF and showed that doxy inhibited NV through inhibition of MMP-9 activity and protein expression.15 We tested doxy’s ability to inhibit ocular-related angiogenesis when taken in drinking water using three separate in vivo mouse models and found a significant reduction in angiogenesis in each model: the CNV model, the DIVAA model and the ocular pterygium model.
METHODS
All animals were treated in accordance with the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research. Each experiment was approved by the Committee for Animal Research at the National Institutes of Health in Bethesda, Maryland. Before all surgical procedures, each animal was anesthetized with intraperitoneal ketamine and xylazine as previously published for the CNV model16 and in a 1:4 ratio of ketamine:xylazine for the pterygium model and DIVAA.
Choroidal Neovascularization Model
Orally administered doxy was given to C57/BL6 mice (Jackson Laboratories, Bar Harbor, ME) approximately 4 hours prior to laser disruption of Bruch’s membrane. In this murine model, angiogenesis was induced by breaking Bruch's membrane with a 50 micron spot size of laser.16 Four shots per eye surrounding the optic nerve were placed with a ND:YAG 532-nm laser (Alcon, Fort Worth, TX), power between 80 and 90 mW, and with 0.100 seconds of exposure time. The end point “bubble formation” assures breakage of Bruch’s membrane. Doxy was added daily to drinking water for 7 days at doses of 0.5 (n=15), 5.0 (n=12), and 50.0 (n=22) mg/kg/day; plain drinking water was used for control (n=22). After 7 days, the animals were euthanized with CO2 exposure, and retinal flatmounts were prepared. Optical sections were collected with confocal microscopy and lesion volume was analyzed using Volocity software (Volocity Classification Module; Improvision, Coventry, UK) as previously described .16 Student’s t-test was used to evaluate the data.
Directed In Vivo Angiogenesis Model
In the DIVAA assay17, silicone tubes (angioreactors) containing Matrigel (growth factor reduced or GFR), (BD Biosciences, San Jose, CA), with Phosphate Buffered Saline (PBS), 7 nM basic fibroblastic growth factor (bFGF) (Collaborative Research, Becton Dickinson, Bedford, MA) or 10,000 human pterygium epithelial cells were implanted under the skin of anesthetized nude mice. After 11 days, fluorescein isothiocyanate-dextran (FITC-d) (MW 2 × 106; Sigma-Aldrich, St.Louis, MO) was injected into the tail veins of the mice, and ten minutes later they were euthanized and the extent of neovascularization was quantified using a spectrofluorimeter (excitation 485 nm, emission 510 nm). For subsequent experiments, doxycycline was added to the drinking water at doses of 5.0 (n=6) or 30.0 mg/kg/day (n=7), or plain water (n=7) after implanting silicone tubes containing 10,000 human pterygium epithelial cells in Matrigel under the skin of anesthetized nude mice. Drinking water with or without doxy was changed daily. The same procedure as described above was performed after 11 days to quantify the extent of neovascularization. Student’s t-test was used to evaluate the data.
Pterygium Model
In the pterygium nude mouse model, 10,000 human pterygium epithelial cells mixed with 5 microliters of Matrigel (GFR) were injected under the nasal para-limbal subconjunctival space of athymic, nude mice on day 1. Mice injected in the same area with five microliters of Matrigel (GFR) alone were used as controls. Lesions were photographed at day six when mice were started treatment on either plain water or doxycycline (50 mg/kg/day) in their drinking water, which was changed daily. The dose was chosen based on earlier pilot experiments indicating that a higher dose would be needed (data not shown). We postulated that this difference may be due to less bioavailability of doxy to the cornea versus the subcutaneous space used in the DIVAA. Also, we initially tested topical doxy, but got no response, so we redesigned the experiment to include doxy in the drinking water. Photographs of ocular lesions at days six and fifteen were compared. Anterior segments were prepared for histology after mice were euthanized with CO2 exposure. Mouse anterior segments were fixed in 10% neutral buffered formalin and embedded in paraffin. For comparison, human anterior segments from cadaver eyes (Southern Eye Bank, New Orleans, LA) were removed and cryoprotected with an ascending gradient of sucrose (10%, 20%, and 30%) and then embedded in OCT (Tissue-Tek, Sakura Finetek, Torrance, CA). Sections were prepared and stained with hematoxylin and eosin.
Culture of Primary Human Pterygium Epithelial Cells
Use of pterygium specimens was approved by the Institutional Review Board of Texas Tech School of Medicine, Lubbock, TX. Specimens were handled in accordance with the Declaration of Helsinki. Pterygium were collected at surgery and the epithelium was separated from the underlying stroma. The epithelial tissue was held in place with a cover slip for 3 days in DMEM/F12 medium supplemented with 10% Fetal Calf Serum (FCS), 200 mM L-glutamine, 0.5% DMSO, and 1% antibiotic/antimycotic, during which time the cells migrated from the explant. Then, the explant was removed and the medium changed to kerotinocyte-Serum Free Medium with 5% FCS (Invitrogen, Carlsbad, CA), and 1% antibiotic/antimycotic to further promote epithelial cell growth. When the cells were 60–80% confluent, they were passaged several times with 0.25% trypsin. The cells were then placed in D-MEM/F-12 medium supplemented with 5% FBS, 1% L-glutamine, 0.5% DMSO, and 1% antibiotic/antimycotic. Medium was renewed every 2 to 3 days and replaced with serum-free medium 18–24 hr prior to performing subsequent experiments on cells from passage 3–5.
Cell Viability
The viability of human pterygium epithelial cells and HDMEC in serum-free medium containing 100, 500 and 1000 microgram/ml doxycycline was tested using trypan blue and cell quantification with a hemocytometer.
Gelatin Zymography
Gelatinase activity was compared from the serum-free medium of primary human pterygium epithelial cells grown for 24 hr in the presence or absence of different concentrations of doxy (10, 100, and 1000 microgram/ml) in two separate culture experiments. Ten microliters of medium were electrophoresed on Novex Zymogram gels (Invitrogen, Carlsbad, CA) containing 0.1% gelatin. MMP-9 and MMP-2 (Oncogene Research Products, San Diego, CA) were used as positive controls. After electrophoresis, the gels were incubated in activating buffer and stained following manufacturer’s instructions (Invitrogen, Carlsbad, CA). The zone of enzymatic activity was identified as a clear band on a blue background, and the gels were photographed by National Institutes of Health (NIH) Medical Arts.
Boyden Chamber Migration Assay Using Fibronectin
Primary human dermal microvascular endothelial cells (HDMEC) (Cambrex Bio Science Walkersville, Inc., Walkersville, MD) and pterygium epithelial cells were incubated in serum-free medium for 18 hours in standard plastic petri plates prior to harvesting for the Boyden Chamber cell migration assay (Chemicon/Millipore, Billerica, MA). With serum free medium (SFM) below the chamber, 250,000 cells with or without doxycycline (Leiter's Pharmacy, San Jose, CA) were placed into each of the upper chambers. Doxy concentrations ranged from: 20–500 micrograms/ml. The plates were incubated for 4–7 hr in 5% carbon dioxide (CO2) at 37°C. Non-migrating cells were removed from the inside of each chamber as per manufacturer’s instructions (Chemicon/Millipore). Migrating cells were placed in stain for 30 minutes and then rinsed in distilled water. Extraction buffer was added to solubilize the stain, and after shaking for 10 min, 150 microliters of the resulting solution was placed in a microtiter plate and read colorimetrically at 540 nm. More than three separate assays were performed for each dose of Doxy.
RESULTS
Doxycycline Inhibition of Experimental Choroidal Neovascularization
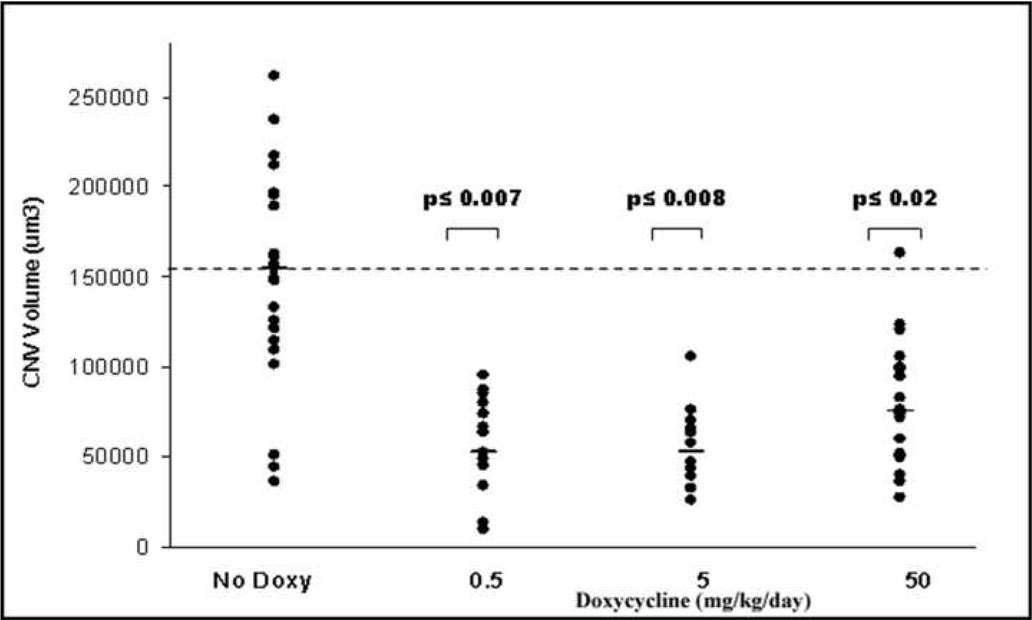
Since doxycycline inhibited endothelial cell migration (Fig 1), the efficacy of this drug was tested in a choroidal neovascularization assay. Choridal neovascularization (CNV) can be induced experimentally in rats and mice.16,18–20 Mice were administered doxycycline-enhanced water for approximately 4 hours, and then Bruch’s membranes were injured with laser to induce CNV. The animals were given plain water or doxy for seven days, at which time, the volumes of the lesions were determined. A striking decrease in choroidal lesion volume was observed in mice receiving Doxy (Fig 2). The neovascular lesion volume was reduced by 50% in mice receiving 50 mg/kg/day doxy in their drinking water versus plain water (Fig 3). The greatest reduction (approximately 60%) in lesion volume was observed at the 5mg/kg/day dose (p<0.008) of doxy (Fig 3). This reinforces the finding that Doxy can inhibit neovascularization.
Figure 1.
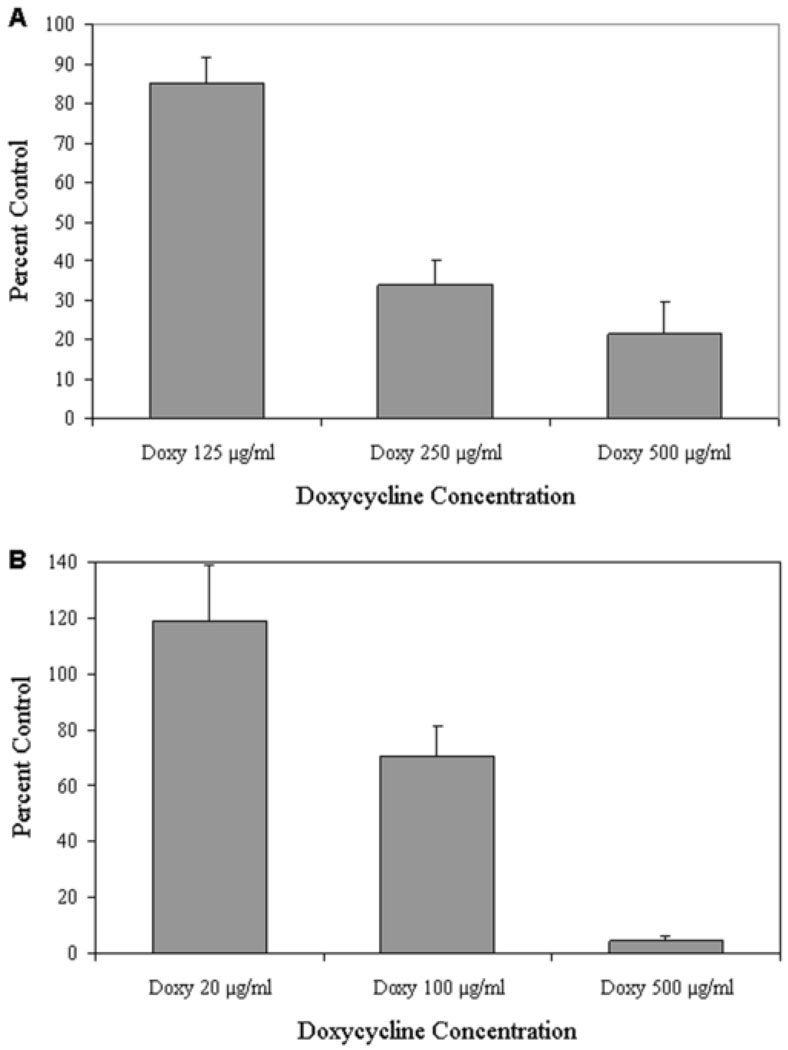
Doxycycline (doxy) reduced pterygium epithelial cell and human dermal microvascular endothelial cell (HDMEC) migration. Boyden migration assays of pterygium cells (A) and HDMEC (B). Cells were incubated in serum-free medium with or without doxy at concentrations ranging from 20–500 micrograms/ml.
Figure 2.
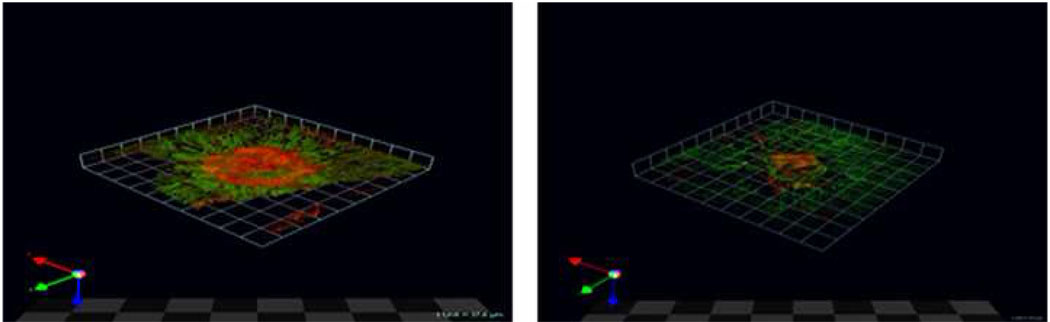
Doxycycline (doxy) inhibits choroidalneovascularization (CNV). Laser model of CNV in mice given plain water (left panel) or 50 mg/kg/day doxy added daily (rightpanel) for 7 days. Representative confocal microscope Z-series from neovessel projections (red) of choroid-retinal pigment epithelial (RPE) flat mounts at 7 days post-laser are shown. In green phalloidin outlines the RPE cytoskeleton. The arrows at the bottom left represent the X (green), Y (red) and Z (blue) axes.
Figure 3.
Doxycycline (doxy) reduces neovascular lesion volume by 66%. Laser model of choroidalneovascularization (CNV) in mice given doxy at doses of 0.5 (n=15), 5 (n=12), and 50 mg/kg (n=22) added daily to drinking water for 7 days. Tap water (n=22) was used as control. Plot shows volume of CNV lesions after daily administrations of effectors as indicated in the X-axis. Each point corresponds to one CNV lesion. The horizontal bars correspond to median values. The hatched horizontal line is a projection of the median value of the control mice.
Pterygium Cell induced Neovascularization in a Directed In Vivo Angiogenesis Model
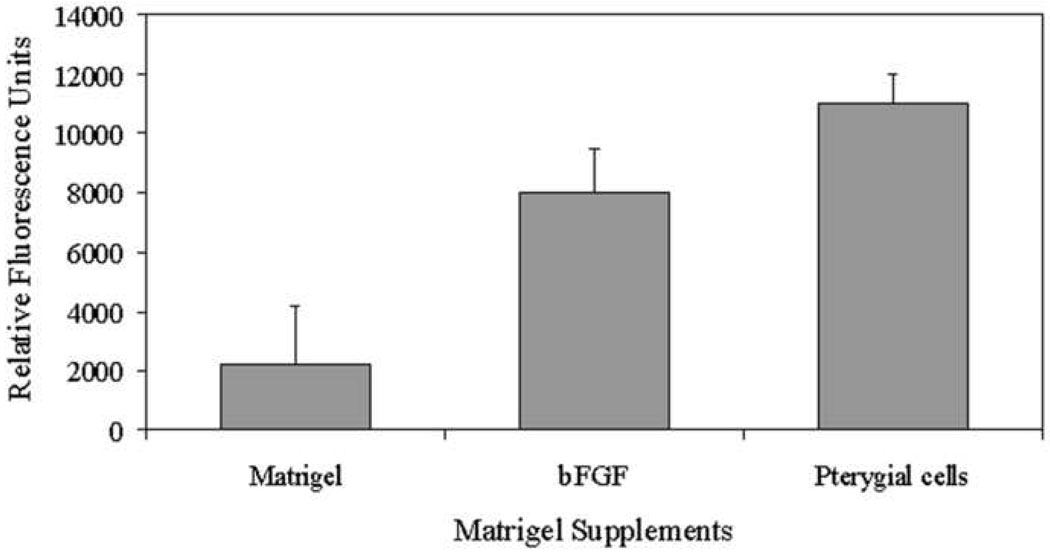
Given the previous reports that pterygia produce angiogenic factors, the angiogenic potential of passage four human pterygium epithelial cells was determined. Using DIVAA, silicone tubes (angioreactors) containing 10,000 pterygium cells, basic fibroblast growth factor (bFGF) in Matrigel as a positive control, or PBS in Matrigel as a negative control were implanted under the skin of anesthetized nude mice. After 11 days, quantification of endothelial cell invasion and vessel growth in the angioreactors demonstrated that human pterygium epithelial cells were highly angiogenic when compared with Matrigel alone and Matrigel with bFGF (p< 0.01 and < 0.05 respectively) (Fig 4). The mean relative fluorescence for six replicates per group was determined. The results demonstrate that pterygia epithelial cells induce neovascularization.
Figure 4.
Pterygium epithelial cells are angiogenic in Directed In Vivo Angiogenesis Assay (DIVAA). Mice were implanted with silicone tubes containing human pterygium epithelial cells, Matrigel with phosphate buffered saline (negative control) or Matrigel with basic fibroblastic growth factor (positive control) for 11 days. n=6 replicates/group. Neovascularization in the angioreactors was quantified using a spectrofluorimeter.
Doxycycline Inhibition of Pterygium Cell-mediated Angiogenesis
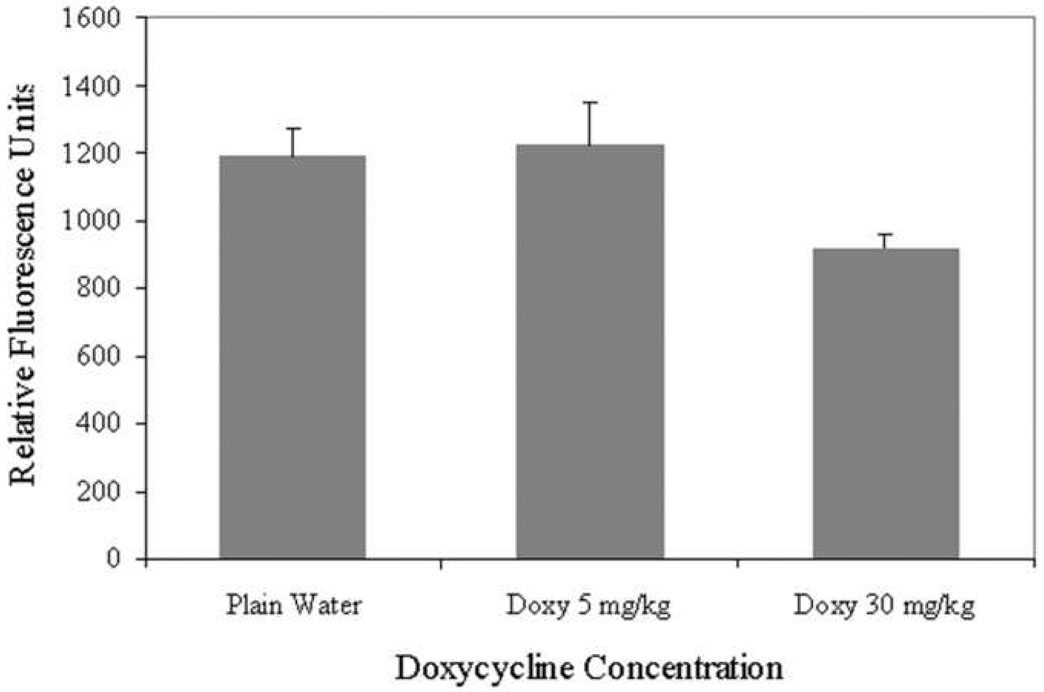
The effects of doxy on pterygium epithelial cell-mediated angiogenesis with DIVAA were investigated. Doxy at 30 mg/kg/day via drinking water decreased neovascularization by 30% (p< 0.004) (n=7) when compared to plain water (n=7) or Doxy at 5 mg/kg/day (n=6) (Fig 5). This result demonstrates the ability of doxy to inhibit angiogenesis in the DIVAA model.
Figure 5.
Doxycycline (doxy) inhibits angiogenesis in the Directed In Vivo Angiogenesis Assay (DIVAA) model. DIVAA of mice implanted with silicone tubes containing human pterygium epithelial cells and given plain water or doxy for 11 days. n=7 for plain water, n=7 for 30 mg/kg/day doxy, and n=6 for 5 mg/kg/day doxy. Neovascularization in the angioreactors was quantified using a spectrofluorimeter.
Experimental Pterygium Lesion Growth In Vivo
A pterygium epithelial cell model was generated by injecting 10,000 human pterygium epithelial cells mixed with Matrigel, or Matrigel alone (controls), under the nasal paralimbal subconjunctiva of athymic, nude mice. Over the course of 6 days, a mass could be seen on the corneal surface, although there was variability in the size of the lesions. Eyes were photographed 6 days after injection (Fig 6). Of the 26 eyes injected, 18 eyes (approximately 70%), had noticeable lesions. There were no other observed changes in the eye or in the general phenotype of the animal. Some of the lesions had visible blood vessel involvement and mice injected with Matrigel alone showed no noticeable lesions.
Figure 6.
An experimental nude mouse model of human pterygium epithelial cells. Photograph of mice six days after nasal para-limbalsubconjunctival injection of human pterygium epithelial cells. Eye (left panel) has noticeable lesion, while eye (right panel) has little if any pterygium cell involvement.
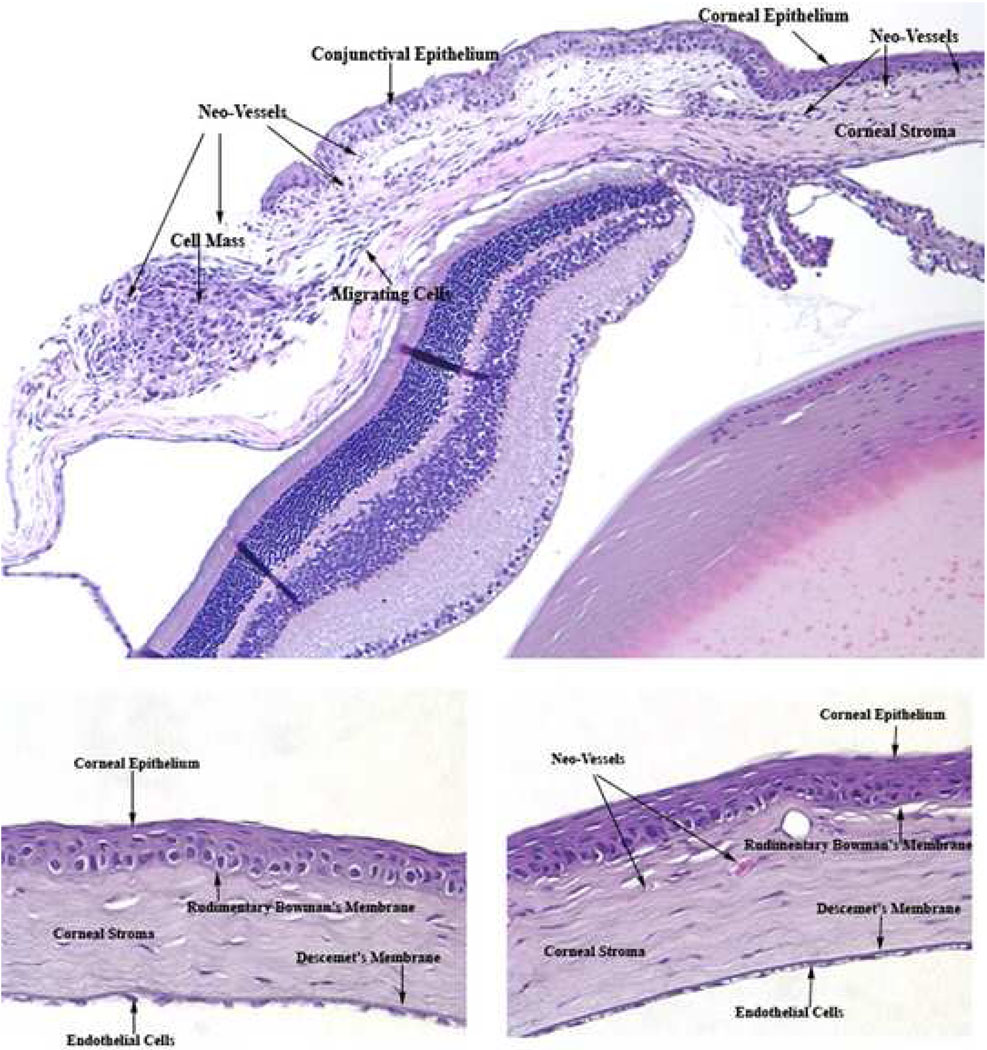
Histological examination of the anterior segment of mice six days after nasal para-limbal subconjunctival injection of human pterygium epithelial cells showed features similar to human pterygium (Fig 7, Fig 8). Cells that are fusiform in nature appear to be migrating from the cellular mass at the site of injection. Neovascularization and red blood cells are present near the injection site and distal to it in the corneal stroma. No vessels are present in the control (Matrigel only) cornea.
Figure 7.
Histology of pterygium mouse model shows features of human pterygium pathogenesis, with migrating cells and neovascularization (neo-vessels). Top panel: anterior segment of mouse six days after nasal para-limbalsubconjunctival injection of human pterygium epithelial cells. Bottom left panel: cornea of mouse six days after nasal para-limbalsubconjunctival injection of Matrigel only. Bottom right panel: cornea of mouse six days after nasal para-limbalsubconjunctival injection of human pterygium epithelial cells. Magnification= 10× top panel; 40× bottom panels.
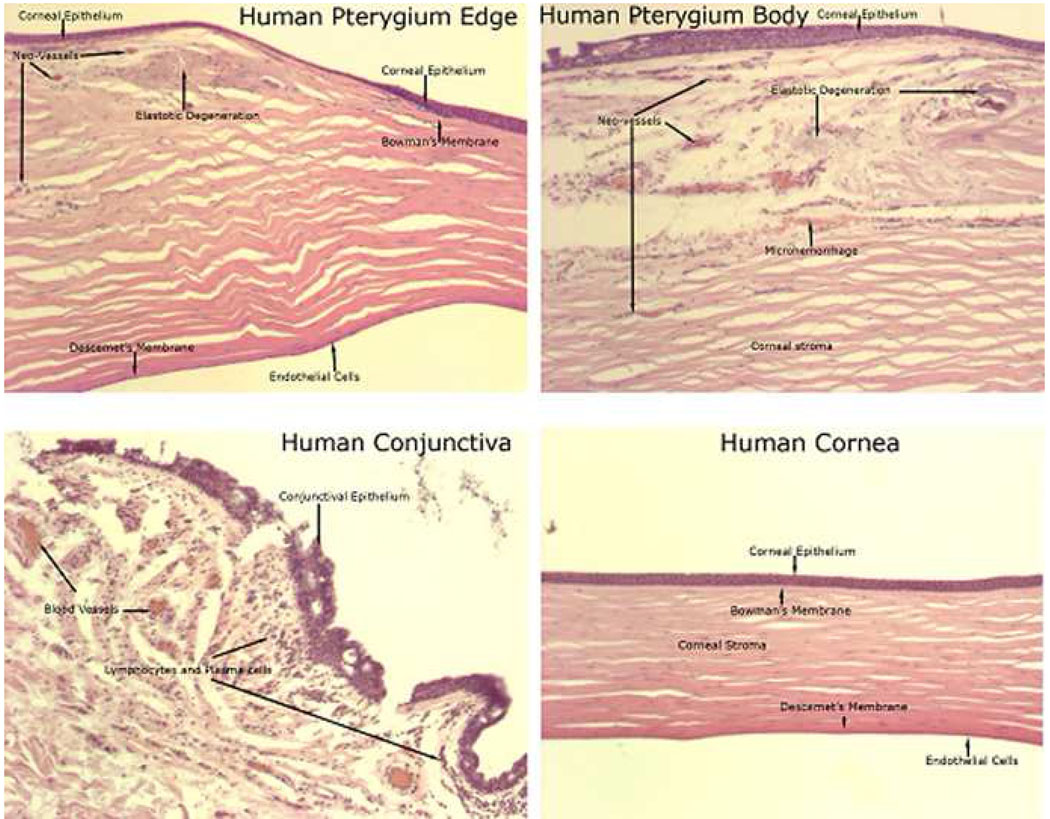
Figure 8.
Histology of anterior segment of human pterygium (upper panels) and normal human conjunctiva and cornea (lower panels).
Doxycycline Inhibition of Experimental Pterygium Lesion Growth
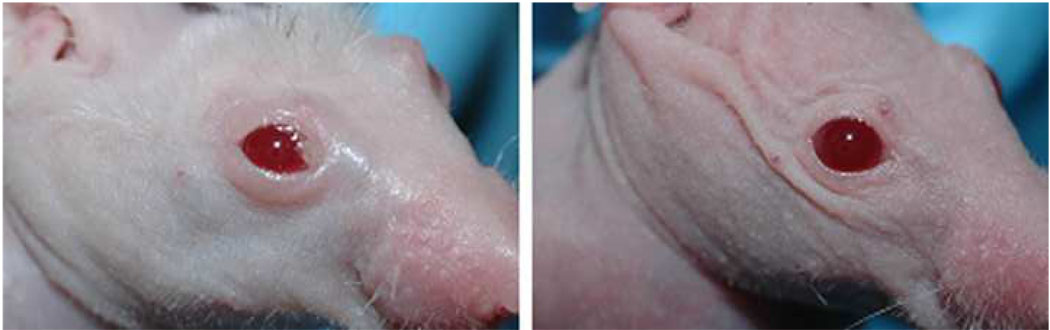
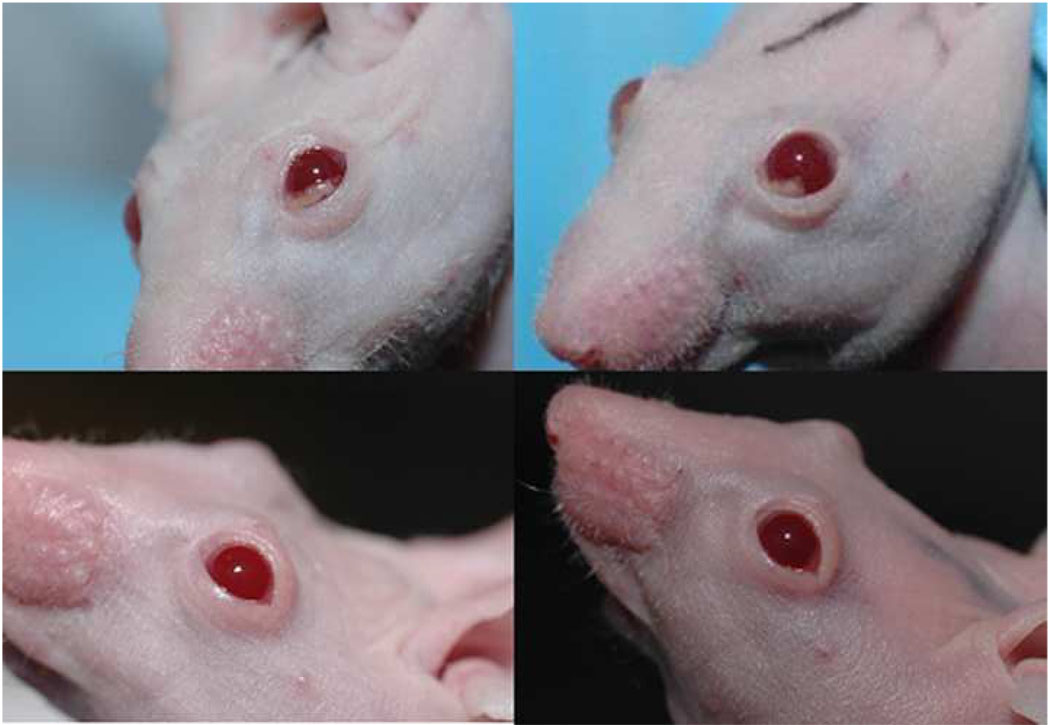
Given that doxycycline inhibited pterygium cell-derived gelatinolytic activities, migration of pterygium epithelial cells, and pterygium cell-mediated angiogenesis, the efficacy of this matrix metalloproteinase inhibitor was tested in our pterygium mouse model. Nasal para-limbal subconjunctival injections of 10,000 human pterygium epithelial cells resulted in a noticeable lesion by 6 days post-injection. At this time-point, mice were continued on plain water or water with doxy. Over the next 9 days, the mice receiving doxy (50 mg/kg/day) showed essentially complete regression of the lesions (n=4) (Fig 9). Mice given plain water did not show regression of lesions. These results demonstrate the ability of Doxy to reduce pterygium growth on the ocular surface.
Figure 9.
Doxycycline (doxy) causes regression of experimental pterygium epithelial cell lesions in a nude mouse model. Upper panels show mice 6 days post injection of pterygium cells; doxy (50 mg/kg/day) was started on this day in drinking water. Bottom panels show mice 15 days post injection of pterygium cells and after receiving doxycycline for 9 days. Mice receiving plain water did not show regression of lesions (data not shown).
Gelatinolytic Activities Secreted by Pterygium Cells
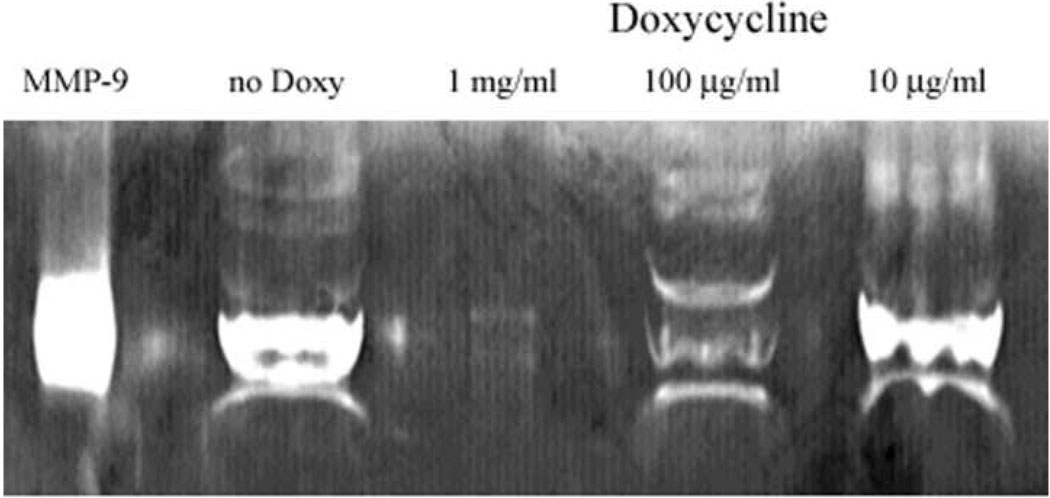
Given the elevated levels of MMP-1, -2, and -9 previously localized in pterygium5, MMP’s produced by the cultured pterygium cells (used above) were investigated. Cultured human pterygium epithelial cells were grown for 24 hr in serum free medium, and the culture medium was subjected to gelatin Zymography. The main zymogen migrated like purified recombinant human MMP-9, a ~84 kDa protein (Fig 10).
Figure 10.
Doxycycline (doxy) inhibits pterygium cell-generated gelatinolytic activity in vitro. Zymogram of media from cultured human pterygium cells grown in the presence or absence of doxy. Three concentrations of doxy were used. No doxy: medium from cells not treated with doxy. MMP-9 was used as a standard.
Effects of Doxycycline on Pterygium-derived Gelatinolytic Activities
The effect of Doxy on gelatinase activity was also investigated by Zymography. Cultured human pterygium epithelial cells were grown for 24 hr in serum-free medium with or without Doxy. The presence of doxy in the medium of the cultured cells effectively reduced the ~ 84 kDa gelatinolytic activity in a dose-dependent manner (Fig 10). No activity was detected in serum-free medium alone, and no other metalloproteinase activity was detected in the medium from pterygium cell cultures with or without doxy (data not shown).
Cell Viability
The viability of human pterygium epithelial cells and HDMEC in serum-free medium containing 100, 500 and 1000 microgram/ml doxycycline was unchanged compared to untreated cells in serum-free medium.
Migration of Human Dermal Microvascular Endothelial Cells
Migration experiments utilizing human pterygium epithelial cells and human dermal microvascular endothelial cells (HDMEC) demonstrated that 500 microgram/ml doxy reduced migration of these cells by 79% (p<0.0002) (Fig 1A) and 95% (p<0.002) (Fig 1B), respectively, when compared to HDMEC or pterygium cells grown without doxy in serum-free medium (100% control).
DISCUSSION
Pathological ocular angiogenesis is responsible for a significant amount of blindness. Angiogenesis is inhibited by doxycycline in vivo in other organ systems and during corneal neovascularization. Our data demonstrate that doxy causes a significant reduction in angiogenesis in a laser-induced choroidal neovascular model, DIVAA model, as well as regression of angiogenic pterygium lesions on the ocular surface. Choroidal neovascularization is associated with a variety of ocular diseases, including macular degeneration. Because of the strong anti-angiogenic properties of doxy, we tested the efficacy of doxy in a laser-induced CNV model that provides morphologic detail of early phases of new blood vessel formation. Disruption of Bruch’s membrane by laser energy produced RPE cell and endothelial cell proliferation and migration. By day 7, a well-defined CNV lesion was present. In mice given doxy by water daily, there was a 60% reduction in lesion volume. Oral doxycycline, even at a low dose of 0.5 mg/kg/day, significantly reduced blood vessel growth and migration. Our results have mechanistic implications. On one hand, in other systems, doxycycline has been shown to posess anti-inflammatory properties. Houri-Haddad et al. reported that doxycycline significantly reduces levels of TNF-alpha, IL-10 and IFNgamma induced by bacterial challenge with heat-killed Porphyromonas gingivalis in an in vivo mouse chamber model.21 In a recent 2-year clinical trial of postmenopausal women, a subantimicrobial oral dose of doxycycline reduced the severity of gingival inflammation through the inhibition of collagenase activity and reduction in protein levels, especially matrix metalloproneinase-8 (leukocyte-type collagenase), which accounts for approximately 80% of the total collagenase in gingival crevicular fluid.22
As mentioned earlier, CNV development studies suggest that the immune response may play a pivotal role during CNV development.4 In the laser-induced CNV mouse model, there is an early influx of neutrophils, macrophages, NK cells and microglia cells within 72 hours with associated edema. By 7 days post-laser, the edema is much reduced with fewer immune cells, except for a persistence of microglial cells and an organized infiltrating membrane lesion. On the other hand, the ability of doxycycline to inhibit MMPs suggests that blocking the catalytic effects of these enzymes might play a role in its biological properties. These metalloproteinases are upregulated in angiogenic lesions, and their inhibition or genetic ablation diminishes angiogenic switching, tumor number, and growth.23–26 In addition, oral administration of doxycycline in rats caused an elevation of serum levels of the antiangiogenic pigment epithelium-derived factor (PEDF), and a concomitant decrease in (MMP 9) activity. 27 Thus, we hypothesize that doxy inhibits matrix metalloproteinase activity and concomitant immune cell infiltration in the mouse CNV model. A future investigation focusing more specifically on the inflammatory mechanisms may prove to be beneficial.
The epithelial cells of the pterygium leading edge produce elevated levels of MMPs and VEGF.6,7 The DIVAA experiments showed that pterygium epithelial cells have the capacity to induce neovascularization and that doxy is an effective inhibitor of this process. Doxycycline also inhibited the migration of pterygium cell and microvascular endothelial cells in Boyden Chamber assays. The gelatinases, members of the matrix metalloproteinase family, play an important role in cell migration and angiogenesis. Matrix metalloproteinases such as MMP-9 have been shown to play a role in tumor and ischemic angiogenesis,28–30 and are inhibited by doxy.13, 15 The capacity of cultured human pterygium epithelial cells to secrete gelatinases was tested by growing cells for 24 hours in serum-free medium. The cells secreted a zymogen that co-migrated with recombinant MMP-9. This proteinase was inhibited in a dose-dependent manner in the presence of doxy. Our studies indicate that doxycycline is a potent inhibitor of pterygium epithelial cell induced angiogenesis and cell migration. It also causes regression of pterygium lesions on the ocular surface of nude mice. Doxy is a safe, inexpensive, and easily obtained around the globe. It may be a useful therapy for primary pterygium or as an adjunct to prevent recurrence after surgery. Pterygia are prevalent in populations near the equator and in persons with a large degree of ultraviolet light (UV) exposure.31, 32 Surgical removal of pterygium leads to recurrence reported as high as 88% unless coupled with therapies such as conjunctival autografts, mitomycin C, or radiation, which can reduce the recurrence rate significantly.33, 34 Previous studies of pteryium have relied on whole tissue or cultured cells, since there is no established animal model, although sporadic pterygium-like lesions have been reported in cattle and dogs.6 The pterygium-lesion murine model presented here is the first example of an animal model produced by human pterygial cells. The lesions increased in size after injection and regressed after oral doxy treatment. As in human pterygium, fibrovascular components are present in the mouse model. Neovessels, a prominent feature of pterygium, were observed in the cornea distant from the injection site. The fusiform cells seen migrating from the original mass of pterygium epithelial cells in Matrigel injected into the subconjunctival space appear to be migrating toward the central cornea, which suggests that the corneal cells may be secreting a chemotactic factor. Stromal areas of collagen degeneration were not observed in our model, but this may depend on longer experimental times, prolonged UV exposure or be specific to human pterygium. The fusiform cells are likely to be pterygium in origin, however it is possible that the injected cells activated native mouse fibroblasts. Dushku et al. suggested that pterygium cells may cause activation of fibroblasts at the head of the pterygium.5 Solomon et al. reported that pterygium fibroblasts overexpress insulin-like growth factor-binding protein-2, further evidence to support increased growth of fibrovascular tissue.35 The pathogenesis and pathologic characteristics of pterygium have emerged through prior histological and immunochemical studies of the pterygium leading edge. Elevated levels of matrix metalloproteinases have been localized in what are believed to be altered limbal basal cells (pterygium cells), which are adjacent to normal corneal basal cells and in the subepithelial plane above Bowman’s layer.5,6 This key junction is where the altered limbal basal cells locally infiltrate onto corneal basement membrane over a disappearing Bowman’s layer.5 Features of this migration are seen in the pterygium mouse model, including directionality of migrating cells (toward the central cornea) and neo-vessels. The invasion of the human pterygium cells may be less impeded in the mouse, since rodents’ bowman’s membrane is not clearly defined and cannot be detected with a light microscope.36 In mice, the subepithelial fibrils are randomly distributed and are not bundled.37 Higher mammals such as humans and cattle have a clearly defined well-developed bowman’s membrane. This difference may make comparison of human and murine pterygia more difficult. The secretory and functional aspects of these lesions warrant future investigation. The presence of neovascularization and fusiform cells distal to the injection site without doxy, and a decline in these factors when doxy was used, suggests that doxy taken by mouth in drinking water successfully reduced neovascularization in the anterior segment of the eye. This, in addition to the response of the posterior segment CNV to doxy taken the same way (by mouth) suggests that doxy taken orally may be successful in reducing neovascularization regardless of the location of the pathology. Each disease entity would require testing separately to determine doxy’s extent of effectiveness.
The use of doxy in the setting of ocular neovascular disease may serve as an adjunct to other anti-angiogenic treatments, although prospective human clinical trials have not been completed. Doxy may be a cost-effective way to enable a prolongation of time between VEGF-inhibitor treatment injections in patients with exudative AMD, and may prevent pterygia recurrence, especially for those persons living close to the equator with a high UV exposure. The economy of Doxy as well as the safety profile makes it potentially very beneficial. These factors, as well as doxy’s ability to reduce neovascularization and fibrovascular growth in three mouse models as shown in this paper, suggest that doxy may be a promising candidate for human clinical trial, which would be necessary to determine the benefit to humans.
ACKNOWLEDGEMENTS
The authors thank A. Garrick and N. Agarwalla for research assistance, manuscript review and discussions.
This work was supported by the National Eye Institute, National Institutes of Health (intramural), in Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at: Association for Research in Vision and Ophthalmology Annual Meeting May 2006, Fort Lauderdale, Florida.
REFERENCES
- 1.Campochiaro PA. Seeing the light: New insights into the molecular pathogenesis of retinal diseases. J Cell Physiol. 2007;213:348–354. doi: 10.1002/jcp.21213. [DOI] [PubMed] [Google Scholar]
- 2.Steen B, Sejersen S, Berglin L, et al. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1998;39:2194–2200. [PubMed] [Google Scholar]
- 3.Tatar O, Adam A, Shinoda K, et al. Matrix metalloproteinases in human choroidal neovascular membranes excised following verteporfin photodynamic therapy. Br J Ophthalmol. 2007;91:1183–1189. doi: 10.1136/bjo.2007.114769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nussenblatt RB, Ferris F., III Age-related macular degeneration and the immune response: implications for therapy. Am J Ophthalmol. 2007;144:618–626. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001;119:695–706. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- 6.Di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23:195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Dushku N, Reid TW. P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors. Curr Eye Res. 1997;16:1179–1192. doi: 10.1076/ceyr.16.12.1179.5036. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein O, Rosenthal G, Zirkin H, et al. Overexpression of p53 tumor suppressor gene in pterygia. Eye. 2002;16:619–621. doi: 10.1038/sj.eye.6700150. [DOI] [PubMed] [Google Scholar]
- 9.John-Aryankalayil M, Dushku N, Jaworski CJ, et al. Microarray and protein analysis of human pterygium. [Accessed December 28, 2009];Mol Vis [serial online] 2006 12:55–64. Available at: http://www.molvis.org/molvis/v12/a6/ [PubMed] [Google Scholar]
- 10.Golub LM, Lee HM, Ryan ME, et al. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 11.Riazi-Esfahani M, Peyman GA, Aydin E, et al. Prevention of corneal neovascularization: evaluation of various commercially available compounds in an experimental rat model. Cornea. 2006;25:801–805. doi: 10.1097/01.ico.0000220768.11778.60. [DOI] [PubMed] [Google Scholar]
- 12.Dan L, Shi-long Y, Miao-li L, et al. Inhibitory effect of oral doxycycline on neovascularization in a rat corneal alkali burn model of angiogenesis. Curr Eye Res. 2008;33:653–660. doi: 10.1080/02713680802245772. [DOI] [PubMed] [Google Scholar]
- 13.Hanemaaijer R, Visser H, Koolwijk P, et al. Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. Adv Dent Res. 1998;12:114–118. doi: 10.1177/08959374980120010301. [DOI] [PubMed] [Google Scholar]
- 14.Chhipa RR, Singh S, Surve SV, et al. Doxycycline potentiates antitumor effect of cyclophosphamide in mice. Toxicol Appl Pharmacol. 2004;202:268–277. doi: 10.1016/j.taap.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Lee CZ, Xu B, Hashimoto T, et al. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke. 2004;35:1715–1719. doi: 10.1161/01.STR.0000129334.05181.b6. [DOI] [PubMed] [Google Scholar]
- 16.Campos M, Amaral J, Becerra SP, Fariss RN. A novel imaging technique for experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:5163–5170. doi: 10.1167/iovs.06-0156. [DOI] [PubMed] [Google Scholar]
- 17.Guedez L, Rivera AM, Salloum R, et al. Quantitative assessment of angiogenic responses by the directed in vivo angiogenesis assay. Am J Path. 2003;162:1431–1439. doi: 10.1016/S0002-9440(10)64276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobi ET, Puliafito CA, Destro M. A new model for experimental choroidal neovascularization in the rat. Arch Ophthalmol. 1989;107:264–269. doi: 10.1001/archopht.1989.01070010270035. [DOI] [PubMed] [Google Scholar]
- 19.Frank RN, Das A, Weber ML. A model of subretinal neovascularization in the pigmented rat. Curr Eye Res. 1989;8:239–247. doi: 10.3109/02713688908997565. [DOI] [PubMed] [Google Scholar]
- 20.Economou MA, Wu J, Vasilcanu D, et al. Inhibition of VEGF secretion and experimental choroidal neovascularization by picropodophyllin (PPP), an inhibitor of the insulin-like growth factor-1 receptor. Invest Ophthalmol Vis Sci. 2008;49:2620–2626. doi: 10.1167/iovs.07-0742. [DOI] [PubMed] [Google Scholar]
- 21.Houri-Haddad Y, Halabi A, Soskolne WA. Inflammatory response to cholorhexidine, minocycline HCl, and doxycycline HCl in an in vivo mouse model. J Clin Periodontol. 2008;35:783–788. doi: 10.1111/j.1600-051X.2008.01290.x. [DOI] [PubMed] [Google Scholar]
- 22.Golub LM, Lee HM, Stoner JA, et al. Subantimicrobial-dose doxycycline modulates gingival crevicular fluid biomarkers of periodontitis in postmenopausal osteopenic women. J Periodontol. 2008;79:1409–1418. doi: 10.1902/jop.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das A, McGuire PG. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog Retin Eye Res. 2003;22:721–748. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Noda K, Ishida S, Inoue M, et al. Production and activation of matrix metalloproteinase-2 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2003;44:2163–2170. doi: 10.1167/iovs.02-0662. [DOI] [PubMed] [Google Scholar]
- 25.Plantner JJ, Jiang C, Smine A. Increase in interphotoreceptor matrix gelatinase A (MMP-2) associated with age-related macular degeneration. Exp Eye Res. 1998;67:637–645. doi: 10.1006/exer.1998.0552. [DOI] [PubMed] [Google Scholar]
- 26.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samtani S, Amaral J, Campos MM, et al. Doxycycline-mediated inhibition of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:5098–5106. doi: 10.1167/iovs.08-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert V, Wielockx B, Munaut C, et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003;17:2290–2292. doi: 10.1096/fj.03-0113fje. [DOI] [PubMed] [Google Scholar]
- 29.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 30.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Taylor HR, West SK, Rosenthal FS, et al. Corneal changes associated with chronic UV irradiation. Arch Ophthalmol. 1989;107:1481–1484. doi: 10.1001/archopht.1989.01070020555039. [DOI] [PubMed] [Google Scholar]
- 32.Wong TY, Foster PJ, Johnson GJ, et al. The prevalence and risk factors for pterygium in an adult Chinese population in Singapore: the Tanjong Pagar survey. Am J Ophthalmol. 2001;13:176–183. doi: 10.1016/s0002-9394(00)00703-0. [DOI] [PubMed] [Google Scholar]
- 33.Ozdamar Y, Metevelli S, Han U, et al. A comparative study of tissue blue and vicryl suture for closing limbal-conjunctival autografts and histologic evaluation alter pterygium excision. Cornea. 2008;27:552–558. doi: 10.1097/ICO.0b013e318165b16d. [DOI] [PubMed] [Google Scholar]
- 34.Hirst LW. Prospective study of primary pterygium surgery using pterygium extended removal followed by extended conjunctival transplantation. Ophthalmology. 2008;115:1663–1672. doi: 10.1016/j.ophtha.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Solomon A, Grueterich M, Li DQ, et al. Overexpression of insulin-like growth factor-binding protein-2 in pterygium body fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:573–580. doi: 10.1167/iovs.01-1185. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi S, Osawa T, Tohyama K. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J Morphol. 2002;254:247–258. doi: 10.1002/jmor.10030. [DOI] [PubMed] [Google Scholar]
- 37.Haustein J. On the ultrastructure of the developing and adult mouse corneal stroma. Anat Embryol (Berl) 1983;168:291–305. doi: 10.1007/BF00315823. [DOI] [PubMed] [Google Scholar]