Abstract
Fragile X Syndrome (FXS), the most common inherited form of intellectual disability, is caused by loss of the fragile X mental retardation protein (FMRP). FMRP is a negative regulator of local mRNA translation downstream of group 1 metabotropic glutamate receptor (Gp1 mGluR) activation. In the absence of FMRP there is excessive mGluR-dependent protein synthesis, resulting in exaggerated mGluR-dependent long-term synaptic depression (LTD) in area CA1 of the hippocampus. Understanding disease pathophysiology is critical for development of therapies for FXS and the question arises of whether it is more appropriate to target excessive LTD or excessive mGluR-dependent protein synthesis. Priming of long-term potentiation (LTP) is a qualitatively different functional consequence of Gp1 mGluR-stimulated protein synthesis at the same population of CA1 synapses where LTD can be induced. Therefore we determined if LTP priming, like LTD, is also disrupted in the Fmr1 knockout (KO) mouse. We found that mGluR-dependent priming of LTP is of comparable magnitude in wild-type (WT) and Fmr1 KO mice. However, whereas LTP priming requires acute stimulation of protein synthesis in WT mice, it is no longer protein synthesis dependent in the Fmr1 KO. These experiments show that the dysregulation of mGluR-mediated protein synthesis seen in Fmr1 KO mice has multiple consequences on synaptic plasticity, even within the same population of synapses. Furthermore, it suggests that there is a bifurcation in the Gp1 mGluR signaling pathway, with one arm triggering synaptic modifications such as LTP priming and LTD and the other stimulating protein synthesis that is permissive for these modifications.
INTRODUCTION
Fragile X Syndrome (FXS) is the most common inherited form of intellectual disability. The disease is usually caused by expansion of a CGG triplet repeat sequence upstream of the FMR1 gene that results in transcriptional silencing and consequent loss of FMRP, the fragile X mental retardation protein (Garber et al. 2008; Pieretti et al. 1991). FMRP is an RNA-binding protein (Ashley Jr et al. 1993; Siomi et al. 1993) that has been shown to regulate, among other things, synaptic protein synthesis (Brown et al. 2001; Feng et al. 1997; Khandjian et al. 2004; Laggerbauer et al. 2001; Lu et al. 2004; Todd et al. 2003; Weiler et al. 2004; Zalfa et al. 2003). In the Fmr1 knockout (KO) mouse, basal protein synthesis in the hippocampus is significantly elevated over wild-type (WT) levels (Dolen et al. 2007; Qin et al. 2005).
Group 1 metabotropic glutamate receptors (Gp1 mGluRs) are expressed in neurons throughout the brain (Masu et al. 1991). Activation of Gp1 mGluRs has been shown to trigger dendritic mRNA translation, including the synthesis of FMRP (Weiler and Greenough 1993, 1999). In the hippocampus, one functional consequence of activating Gp1 mGluRs is induction of long-term synaptic depression (LTD) at the Shaffer collateral–CA1 synapse (Huber et al. 2001; Oliet et al. 1997; Palmer et al. 1997), expressed in part by a loss of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)–type glutamate receptors (Snyder et al. 2001; Waung et al. 2008). In WT rats and mice, mGluR-stimulated protein synthesis is obligatory for stable expression of LTD (Huber et al. 2000, 2001). LTD magnitude is enhanced in the Fmr1 KO mouse, possibly due to exaggerated protein synthesis (Huber et al. 2002). Consistent with this interpretation, mGluR-LTD in the KO also no longer requires acute stimulation of protein synthesis, presumably due to constitutive overexpression of “LTD proteins” (Hou et al. 2006; Nosyreva and Huber 2006). Because LTD mechanisms are believed to be important for sculpting synaptic connections during postnatal development, a reasonable conjecture is that exaggerated LTD could be pathogenic in FXS (Huber et al. 2002). Moreover, increased LTD in hippocampal area CA1 could contribute specifically to the cognitive impairment that is characteristic of this disease.
Understanding disease pathophysiology is critical for development of therapies for FXS and the question arises of whether it is more appropriate to target excessive LTD and impaired AMPA receptor function (Lynch et al. 2008) or excessive mGluR-dependent protein synthesis (Bear 2005; Bear et al. 2004). The phenomenon of LTP priming, first described by Abraham and colleagues in rats (Cohen and Abraham 1996; Cohen et al. 1998; Raymond et al. 2000), offers an interesting opportunity to distinguish among these alternatives. Normally, weak high-frequency stimulation (HFS) elicits modest long-term synaptic potentiation (LTP) at the Shaffer collateral–CA1 synapse. However, if Gp1 mGluRs are first stimulated briefly with a low concentration of the selective agonist DHPG (R,S-dihydroxyphenylglycine; 10 μM), then the LTP caused by subsequent HFS is substantially augmented. Like mGluR-LTD induced by higher DHPG concentrations, LTP priming in WT rats is abolished by inhibitors of mRNA translation, but not by inhibitors of transcription. Thus LTD and LTP priming are qualitatively different functional consequences of Gp1 mGluR-stimulated protein synthesis at the Shaffer collateral–CA1 synapse. In the current study we ask whether LTP priming, like LTD, is also disrupted in the Fmr1 KO mouse.
We find, first, that priming of LTP results from weak activation of Gp1 mGluRs with DHPG in mouse CA1, as previously reported in rats. Second, although priming is quantitatively similar in Fmr1 KO and WT mice, it is blocked by a protein synthesis inhibitor only in the WT. These findings suggest, first, that proteins overexpressed in FXS are not restricted to “LTD proteins” because they apparently include those required for LTP priming as well. Second, the findings indicate that there is a posttranslational component of mGluR-dependent LTP priming. Instead of serving as a trigger for LTP priming (or LTD), dendritic protein synthesis may rather serve as a gate for synaptic plasticity that normally opens only in response to an mGluR signaling event. In fragile X, this gate is perpetually open due to excessive basal protein synthesis and overexpression of proteins that are normally rate-limiting for these forms of synaptic modification.
METHODS
Animals
Fmr1 mutant mice (Jackson Labs) were bred on the C57Bl/6J clonal background. In an effort to reduce variability due to rearing conditions, all experimental animals were bred from Fmr1 heterozygote mothers, group housed (animals weaned to solitary housing were excluded), and maintained on a 12:12-h light:dark cycle. The Institutional Animal Care and Use Committee at MIT approved all experimental techniques.
Slice preparation
Transverse hippocampal slices (350 μm thick) were prepared from 6- to 10-wk-old mice in ice-cold dissection buffer containing (in mM): NaCl 87, sucrose 75, KCl 2.5, NaH2PO4 1.25, NaHCO3 25, CaCl2 0.5, MgCl2 7, ascorbic acid 1.3, and d-glucose 10 (saturated with 95% O2-5% CO2). Immediately following slicing, the CA3 region was removed. Slices were recovered in artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 124, KCl 3.5, NaH2PO4 1.23, NaHCO3 26, CaCl2 2, MgCl2 1, and d-glucose 10 (saturated with 95% O2-5% CO2) at room temperature for ≥3 h prior to recording.
Electrophysiology
Field recordings were performed in a submersion chamber, perfused with ACSF (2–3 ml/min) at 30°C. Field EPSPs (fEPSPs) were recorded in CA1 stratum radiatum with extracellular recording electrodes filled with ACSF. Baseline responses were evoked by stimulation of the Schaffer collaterals at 0.033 Hz with a two-contact cluster electrode (FHC) using a 0.2-ms stimulus yielding 40–60% of the maximal response. Priming was induced by applying 10 μM DHPG for 10 min (Mellentin et al. 2007). Pairs of primed and unprimed slices were recorded simultaneously. LTP was induced with a 1-s 100-Hz tetanus. Protein synthesis was inhibited by applying 60 μM cycloheximide (CHX) for 30 min as follows: 15 min of pretreatment during baseline recording, 10 min during DHPG application, and 5 min post-DHPG application; or during the equivalent time of baseline recording in unprimed slices.
fEPSP recordings were filtered at 0.1 Hz to 1 kHz, digitized at 10 kHz, and analyzed using pClamp9 (Axon Instruments). The initial slope of the response was used to assess changes in synaptic strength. Data were normalized to the baseline response and are presented as group means ± SE. LTP was measured by comparing the average response 55–60 min posttetanus to the average of the last 5 min of baseline. ANOVA and unpaired t-test were used to determine statistically significant differences. Experiments used aged-matched and interleaved WT and Fmr1 KO mice. For all experiments the experimenter was blind to genotype.
Reagents
R,S-DHPG was purchased from Tocris Biosciences (Ellisville, MO). All other reagents were purchased from Sigma (St. Louis, MO). Fresh bottles of DHPG were prepared as a 100× stock in H2O, aliquoted, and stored at −80°C. Fresh stocks were made once a week. CHX was prepared at 100× stock in H2O daily. These stocks were diluted in ACSF to achieve final concentration.
RESULTS
To confirm that there is facilitation of LTP by prior Gp1 mGluR activation in mice, we first established a tetanization protocol that produced a subsaturable level of LTP and has been shown to be amenable to priming (Cohen et al. 1998; Mellentin et al. 2007). Brief HFS (1-s 100-Hz) produced a modest but reliable level of LTP 1 h posttetanus in slices from both WT and Fmr1 KO mice (WT: 111.2 ± 2.1%, n = 9; KO: 113.8 ± 3.1%, n = 9; Fig. 1). As has been reported previously reported, there was no significant difference in the basal level of hippocampal LTP in Fmr1 KO mice compared with WT mice (P = 0.51) (Godfraind et al. 1996; Paradee et al. 1999).
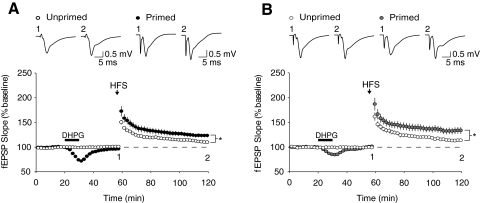
Fig. 1.
R,S-Dihydroxyphenylglycine (DHPG) application facilitates subsequently induced long-term synaptic potentiation (LTP). Field excitatory postsynaptic potentials (fEPSPs) were recorded from the CA1 region of hippocampal slices from either (A) wildtype (WT) or (B) Fmr1 knockout mice (KO). After 1 h of baseline recording, unprimed slices were administered a 1-s 100-Hz tetanus (indicated by arrow), which induced a modest level of LTP in both WT and KO slices (WT: 111.2 ± 2.1%, n = 9 slices from 9 animals, open black circles; KO: 113.8 ± 3.1%, n = 9 slices from 8 animals, open gray circles). In both genotypes, a 10-min priming application of the group 1 metabotropic glutamate receptor (Gp1 mGluR) agonist DHPG (10 μM, black bar) significantly enhanced the magnitude of subsequent LTP induced using this same 100-Hz tetanus (WT: 123.9 ± 3.8%, n = 11 slices from 11 animals, closed black circles, P < 0.02; KO: 133.7 ± 6.7%, n = 11 slices from 10 animals, closed gray circles, P < 0.02). Representative field potential traces (average of 10 sweeps) were taken at times indicated by numerals.
We then replicated the previously reported mGluR-dependent priming of LTP in WT slices (Cohen et al. 1998; Mellentin et al. 2007). The Gp1 mGluR agonist DHPG (10 μM) was bath applied to slices for 10 min after a stable 20-min baseline recording period. DHPG application produced a transient depression of synaptic responses that recovered to baseline levels after a 30-min washout. The same tetanus protocol as above (1-s, 100-Hz) now produced a significantly larger magnitude of LTP compared with unprimed slices (unprimed: 111.2 ± 2.1%, n = 9; primed: 123.9 ± 3.8%, n = 10; P = 0.012; Fig. 1A). These findings in mice are consistent with those previously reported in rats (Cohen et al. 1998; Mellentin et al. 2007).
We next characterized the effect of DHPG application on subsequent LTP in Fmr1 KO mice. As was the case in WT animals, the DHPG priming protocol also enhanced LTP in slices from Fmr1 KO mice (unprimed: 113.8 ± 3.1%, n = 9; primed: 133.7 ± 6.7%, n = 11; P = 0.016; Fig. 1B). However, there was no significant difference in the magnitude of facilitation seen in primed Fmr1 KO slices as compared with primed WT slices (P = 0.22).
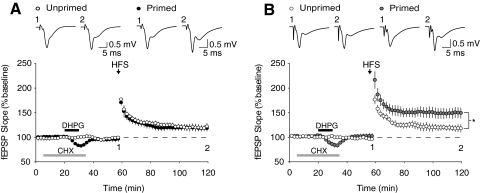
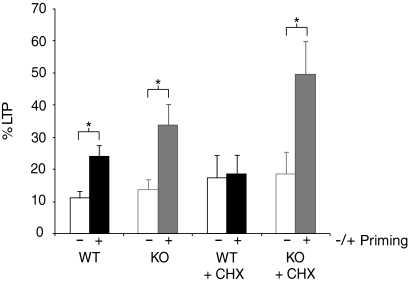
Finally, we examined the role of protein synthesis in DHPG-induced priming in both WT and Fmr1 KO mice. As expected (Raymond et al. 2000), a brief application of the protein synthesis inhibitor CHX (60 μM, 30 min) completely abolished DHPG-induced priming in WT slices (unprimed: 117.5 ± 7.0%, n = 7; primed: 118.6 ± 6.0%, n = 9; P = 0.94; Fig. 2A). However, this same treatment had no effect on DHPG-induced priming in slices from Fmr1 KO mice (unprimed: 118.5 ± 7.0%, n = 7; primed: 149.6 ± 11.0%, n = 8; P = 0.035; Fig. 2B). These results show that although the magnitude of LTP enhancement induced by DHPG priming is not quantitatively different in Fmr1 KO mice, induction of priming is qualitatively different in that it no longer requires the synthesis of new proteins (Fig. 3; Supplemental Fig. S1).1
Fig. 2.
DHPG-induced priming of LTP does not require protein synthesis in Fmr1 KO mice. Delivery of the protein synthesis inhibitor cycloheximide (60 μM CHX, 30 min; gray bar) before and during DHPG priming prevented facilitation of LTP in slices from WT mice (A; unprimed: 117.5 ± 7.0%, n = 7 slices from 6 animals, open black circles; primed: 118.6 ± 6.0%, n = 8 slices from 6 animals, closed black circles; P = 0.94); however, this treatment had no effect on DHPG-induced priming in slices from Fmr1 KO mice (B; unprimed: 118.5 ± 6.1%, n = 7 slices from 6 animals, open gray circles; primed: 149.6 ± 11.0%, n = 8 slices from 7 animals, closed gray circles; P < 0.05). Representative field potential traces (average of 10 sweeps) were taken at times indicated by numerals.
Fig. 3.
Summary of DHPG-induced priming of LTP and its protein synthesis dependence in wildtype and Fmr1 KO mice. Bar graphs represent the average percentage LTP observed 55–60 min posttetanus. Wildtype unprimed: open black; wildtype primed: closed black; Fmr1 KO unprimed: open gray; Fmr1 KO primed: closed gray. Asterisks denote significant differences (unpaired Student's t-test, P < 0.05).
DISCUSSION
In this study we characterized mGluR-dependent priming of LTP in the fragile X background. In WT mice we confirmed previous reports that application of the Gp1 mGluR agonist DHPG at a low dose enhances the magnitude of subsequent LTP and that this priming of LTP is protein synthesis dependent. In the Fmr1 KO we determined that although mGluR dependent priming of LTP is not significantly enhanced, it no longer requires acute protein synthesis at the time of induction. These experiments show that the dysregulation of mGluR-mediated protein synthesis seen in Fmr1 KO mice has multiple consequences on synaptic plasticity, even within the same population of CA1 synapses.
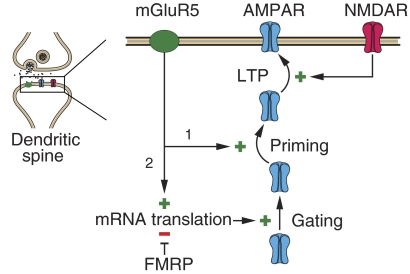
Although our goal was to determine whether the impact of excessive protein synthesis in area CA1 of Fmr1 KO mice is exclusive to mGluR-LTD, the results also have implications for the mechanisms of LTP priming. The simplest model for priming is that Gp1 mGluR signaling facilitates subsequent LTP induction directly by stimulating protein synthesis. However, priming and protein synthesis are decoupled in the Fmr1 KO. Priming still results from mGluR activation in the KO (Fig. 1), but via a mechanism that operates without acute stimulation of protein synthesis above basal levels (Fig. 2). This finding suggests a model, illustrated in Fig. 4, in which signaling from Gp1 mGluRs bifurcates, with one arm triggering priming and the other stimulating protein synthesis that is permissive for synaptic modifications, including LTP priming and LTD. In WT mice and rats, the protein synthesis “gate” is closed under basal conditions so no priming (or LTD) is possible without concurrent mGluR stimulation of mRNA translation. In the KO, however, increased basal protein synthesis leaves the gate open so that the varied consequences of mGluR activation are determined solely by posttranslational modifications. The fact that the priming trigger can be dissociated from the protein synthesis gate in Fmr1 KO mice could be exploited in future studies to distinguish these bifurcating pathways, as only interventions that disrupt the trigger pathway would be effective at blocking LTP priming in these mice. Thus in addition to serving as a valuable disease model, Fmr1 KO mice are also useful for dissecting the diverse mechanisms of Gp1 mGluR signaling and for understanding the role of mGluRs in normal brain function.
Fig. 4.
Model. The finding that LTP priming by mGluR activation occurs in the Fmr1 KO without a need for acute protein synthesis suggests a bifurcation in the signaling pathway. The priming step (1) occurs in response to mGluR activation via a mechanism involving posttranslational modification of synaptic proteins (possibly the AMPA receptor itself). In WT animals, priming is not possible without (2) concurrent mGluR activation of mRNA translation and synthesis of protein(s) that gate plasticity. In the absence of the translational repressor FMPP, the gating proteins are constitutively overexpressed, rendering priming no longer sensitive to protein synthesis inhibitors. The identity of the hypothetical gating proteins remains to be determined. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.
There have been several studies exploring the induction mechanisms of mGluR-induced priming (Cohen et al. 1998, 1999; Mellentin et al. 2007), yet little is known about how the facilitation is achieved. Although priming depends on the availability of newly synthesized proteins, the exact identity of the proteins required has not been explored. Fmr1 KO mice may also prove to be a valuable tool in this regard. The fact that priming no longer requires protein synthesis in the Fmr1 KO mouse suggests that the proteins required for the priming effect are already present. It may be possible to identify candidate proteins that are necessary for priming by comparing their basal expression levels in Fmr1 KO mice with basal and primed protein levels in WT controls. Although speculative at this point, the protein Arc is an interesting candidate gating molecule. It is normally expressed at low levels, but can be rapidly synthesized in response to Gp1 mGluR activation (Park et al. 2008; Waung et al. 2008) and has been reported to be overexpressed in the Fmr1 KO (Zalfa et al. 2003). It is known to interact with the molecular machinery responsible for AMPA receptor cycling through the synaptic membrane (Chowdhury et al. 2006; Shepherd et al. 2006) and has been implicated in both LTP and LTD (Park et al. 2008; Plath et al. 2006; Waung et al. 2008). Finally, the absence of Arc renders synapses virtually immutable by experience or deprivation, at least in visual cortex (McCurry et al. 2010).
Altered regulation of mGluR-dependent priming of LTP may contribute to the cognitive impairment seen in FXS, which is one of the defining characteristics of this syndrome. Recent studies have shown that competition for translation machinery or newly synthesized proteins may be a limiting factor for the number of synapses that can undergo long-lasting changes in synaptic efficacy (Fonseca et al. 2004). This competition may serve as an important checkpoint, so that only a subset of synapses undergo stabilization. Although the ability to have long-lasting changes in synaptic efficacy is undoubtedly beneficial, it may be possible to have too much of a good thing. FXS could be a case in point, where extraneous synapses are maintained, contributing to the cognitive deficits seen in the disorder.
The mGluR theory of fragile X posits that exaggerated responses to Gp1 mGluR activation are responsible for multiple aspects of the disease phenotype (Bear et al. 2004). A key assumption is that FMRP negatively regulates varied responses triggered by mGluR-stimulated protein synthesis. Findings that mGluR-dependent LTP priming in the hippocampal area CA1, epileptogenesis in CA3 (Chuang et al. 2005), and LTD in both CA1 (Huber et al. 2002; Nosyreva and Huber 2006) and cerebellum (Koekkoek et al. 2005) are all dysregulated in the Fmr1 KO provide considerable support for this proposal.
GRANTS
This work was partly supported by National Institute of Mental Health Training Grant T32-MH-082718 to B. D. Auerbach. National Institute of Child Health & Human Development Grant 2R01HD046943 to M. F. Bear.
DISCLOSURES
M. Bear is a founder and equity holder of Seaside Therapeutics, Inc., Cambridge, MA.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Arnold Heynen for valuable comments on the manuscript and K. Oram, E. Sklar, and S. Meagher for technical and administrative assistance.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding.Science 262: 563–566, 1993 [DOI] [PubMed] [Google Scholar]
- Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation.Genes Brain Behav 4: 393–398, 2005 [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation.Trends Neurosci 27: 370–377, 2004 [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome.Cell 107: 477–487, 2001 [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking.Neuron 52: 445–459, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model.J Neurosci 25: 8048–8055, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Abraham WC. Facilitation of long-term potentiation by prior activation of metabotropic glutamate receptors.J Neurophysiol 76: 953–962, 1996 [DOI] [PubMed] [Google Scholar]
- Cohen AS, Coussens CM, Raymond CR, Abraham WC. Long-lasting increase in cellular excitability associated with the priming of LTP induction in rat hippocampus.J Neurophysiol 82: 3139–3148, 1999 [DOI] [PubMed] [Google Scholar]
- Cohen AS, Raymond CR, Abraham WC. Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase C.Hippocampus 8: 160–170, 1998 [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice.Neuron 56: 955–962, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association.Mol Cell 1: 109–118, 1997 [DOI] [PubMed] [Google Scholar]
- Fonseca R, Nagerl UV, Morris RG, Bonhoeffer T. Competing for memory: hippocampal LTP under regimes of reduced protein synthesis.Neuron 44: 1011–1020, 2004 [DOI] [PubMed] [Google Scholar]
- Garber KB, Visootsak J, Warren ST. Fragile X syndrome.Eur J Hum Genet 16: 666–672, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind JM, Reyniers E, De Boulle K, D'Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. Long-term potentiation in the hippocampus of fragile X knockout mice.Am J Med Genet 64: 246–251, 1996 [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression.Neuron 51: 441–454, 2006 [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation.Proc Natl Acad Sci USA 99: 7746–7750, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression.Science 288: 1254–1257, 2000 [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis–dependent long-term depression in hippocampal area CA1.J Neurophysiol 86: 321–325, 2001 [DOI] [PubMed] [Google Scholar]
- Khandjian EW, Huot ME, Tremblay S, Davidovic L, Mazroui R, Bardoni B. Biochemical evidence for the association of fragile X mental retardation protein with brain polyribosomal ribonucleoparticles.Proc Natl Acad Sci USA 101: 13357–13362, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, De Schutter E, Oostra BA, Ito M, De Zeeuw CI. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in fragile X syndrome.Neuron 47: 339–352, 2005 [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation.Hum Mol Genet 10: 329–338, 2001 [DOI] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O'Donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development.Proc Natl Acad Sci USA 101: 15201–15206, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement.Eur J Pharmacol 585: 2–13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor.Nature 349: 760–765, 1991 [DOI] [PubMed] [Google Scholar]
- McCurry CL, Shepherd JD, Tropea D, Wang KH, Bear MF, Sur M. Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation.Nat Neurosci 13: 450–457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellentin C, Jahnsen H, Abraham WC. Priming of long-term potentiation mediated by ryanodine receptor activation in rat hippocampal slices.Neuropharmacology 52: 118–125, 2007 [DOI] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome.J Neurophysiol 95: 3291–3295, 2006 [DOI] [PubMed] [Google Scholar]
- Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells.Neuron 18: 969–982, 1997 [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus.Neuropharmacology 36: 1517–1532, 1997 [DOI] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function.Neuroscience 94: 185–192, 1999 [DOI] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD.Neuron 59: 70–83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome.Cell 66: 817–822, 1991 [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories.Neuron 52: 437–444, 2006 [DOI] [PubMed] [Google Scholar]
- Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse.J Neurosci 25: 5087–5095, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation.J Neurosci 20: 969–976, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors.Neuron 52: 475–484, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein.Cell 74: 291–298, 1993 [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation.Nat Neurosci 4: 1079–1085, 2001 [DOI] [PubMed] [Google Scholar]
- Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95.Proc Natl Acad Sci USA 100: 14374–14378, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate.Neuron 59: 84–97, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. Metabotropic glutamate receptors trigger postsynaptic protein synthesis.Proc Natl Acad Sci USA 90: 7168–7171, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. Synaptic synthesis of the Fragile X protein: possible involvement in synapse maturation and elimination.Am J Med Genet 83: 248–252, 1999 [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Spangler CC, Klintsova AY, Grossman AW, Kim SH, Bertaina-Anglade V, Khaliq H, de Vries FE, Lambers FA, Hatia F, Base CK, Greenough WT. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses.Proc Natl Acad Sci USA 101: 17504–17509, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses.Cell 112: 317–327, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.