Abstract
The ability to modulate digit forces during grasping relies on the coordination of multiple hand muscles. Because many muscles innervate each digit, the CNS can potentially choose from a large number of muscle coordination patterns to generate a given digit force. Studies of single-digit force production tasks have revealed that the electromyographic (EMG) activity scales uniformly across all muscles as a function of digit force. However, the extent to which this finding applies to the coordination of forces across multiple digits is unknown. We addressed this question by asking subjects (n = 8) to exert isometric forces using a three-digit grip (thumb, index, and middle fingers) that allowed for the quantification of hand muscle coordination within and across digits as a function of grasp force (5, 20, 40, 60, and 80% maximal voluntary force). We recorded EMG from 12 muscles (6 extrinsic and 6 intrinsic) of the three digits. Hand muscle coordination patterns were quantified in the amplitude and frequency domains (EMG–EMG coherence). EMG amplitude scaled uniformly across all hand muscles as a function of grasp force (muscle × force interaction: P = 0.997; cosines of angle between muscle activation pattern vector pairs: 0.897–0.997). Similarly, EMG–EMG coherence was not significantly affected by force (P = 0.324). However, coherence was stronger across extrinsic than that across intrinsic muscle pairs (P = 0.0039). These findings indicate that the distribution of neural drive to multiple hand muscles is force independent and may reflect the anatomical properties or functional roles of hand muscle groups.
INTRODUCTION
The unique repertoire of hand movements exhibited by humans relies on complex interactions between central and peripheral mechanisms (Schieber and Santello 2004). The complex biomechanical architecture and sensorimotor system of the hand allow it to assume a wide variety of postures through which forces and torques can be transmitted to objects during manipulative actions. However, the neural mechanisms underlying the coordination of multiple hand muscles for the modulation of digit forces remain poorly understood.
The relation between digit force and coordination patterns of hand muscles has been studied by recording the electromyographic (EMG) activity of simultaneously active finger muscles during force production tasks (Huesler et al. 2000; Johanson et al. 2001; Maier and Hepp-Reymond 1995a,b; Milner and Dhaliwal 2002; Pearlman et al. 2004; Valero-Cuevas 2000; Valero-Cuevas et al. 1998). Earlier studies reported a large inter- and between-subject variability in EMG–force relations (Maier and Hepp-Reymond 1995a,b). However, later studies (Valero-Cuevas 2000; Valero-Cuevas et al. 1998) pointed out that this large variability might have been caused by lack of experimental controls, such as constraining the digit force direction and posture. When both of these variables were controlled across force levels, Valero-Cuevas (2000) reported that, despite an infinite number of coordination patterns available to the CNS, the EMG activity of multiple hand muscles scales linearly with force during force production tasks performed with one digit. This finding provided significant insight into how the CNS deals with the redundancy of finger musculature within a digit. However, tasks requiring the coordinated action of several digits, such as grasping, introduce an additional control problem: the neural excitation of a given muscle innervating a digit has to be coordinated not only with muscles innervating the same digit but also with those innervating other digits. Therefore the question arises as to the extent to which the results obtained from single-digit force production tasks also apply to multidigit tasks.
Although the above-cited EMG studies have revealed covariations constraining the modulation of neural excitation of multiple hand muscles, they do not provide information regarding the mechanisms responsible for the covariation in the modulation of EMG activity across muscles. It has been proposed that correlated neural input leading to nearly synchronous or periodic modulation of the discharge of motor units may be a mechanism that could constrain the modulation of activity across multiple hand muscles and to coordinate digit forces (De Luca and Erim 2002; Santello and Fuglevand 2004; Santello and Soechting 2000; Winges and Santello 2004; Winges et al. 2008). Experimental evidence indicates that there are correlated neural inputs across hand muscles during force production (Butler et al. 2005; Hockensmith et al. 2005; Kilner et al. 2002; McIsaac and Fuglevand 2006, 2007, 2008; Reilly et al. 2004; Semmler et al. 2000; Yu et al. 2007) and object hold tasks (Winges and Santello 2004; Winges et al. 2006, 2008); however, in some cases the strength of correlated input can be weak (e.g., finger extensors; Keen and Fuglevand 2004). The strength and periodicity of correlated neural input can be quantified by EMG–EMG coherence analysis, through which common frequency characteristics of the neural drive to two muscles can be identified (Grosse et al. 2002; Johnston et al. 2005; Rosenberg et al. 1989; Winges et al. 2006).
Correlations of neural activity during force production tasks have also been studied between central and peripheral sites based on corticomuscular coherence (Grosse et al. 2002). Corticomuscular coherence is typically quantified as coherence between signals recorded from the scalp over specific brain regions and EMG that can capture important relations between cortical drive and the neural excitation of muscles. This is indicated by corticomuscular coherence's sensitivity to task parameters such as precision (Kristeva et al. 2007), force magnitude (Brown et al. 1998; Chakarov et al. 2009; Mima et al. 1999), and displacement (Riddle and Baker 2006). However, an important difference between EMG–EMG coherence and corticomuscular coherence is that the former approach can provide information regarding subcortical common inputs to muscles that may not be revealed by corticomuscular coherence (Grosse et al. 2002). Surprisingly, however, little attention has been given to EMG–EMG coherence as a means to gain insight into the coordination of hand muscles as a function of force as well as to the factors underlying the distribution of neural drive to muscles with different functional roles.
In the present study we addressed the question of how multiple intrinsic and extrinsic hand muscles are coordinated as a function of force. To investigate the patterns of activation in multiple muscles with actions on several digits while controlling force direction and posture, we designed a three-digit grasp task that allowed the quantification of hand muscle coordination within and across digits. Furthermore, we quantified the relation between hand muscle coordination patterns and total grasp force by examining the modulation of EMG amplitude across all muscles as well as EMG–EMG coherence.
Although it is theoretically possible that subjects might use different muscle coordination patterns for different force amplitudes, based on the results of Valero-Cuevas (2000), cited earlier, such a scenario seems unlikely. Therefore we hypothesized that the EMG amplitude of multiple, simultaneously active muscles would scale uniformly as force magnitude increased. We assumed that correlated neural input across motor nuclei of hand muscles contributes to the uniform scaling of EMG amplitude. Therefore we also hypothesized that the EMG–EMG coherence would be similar regardless of force amplitude. Last, we tested a third hypothesis that the EMG–EMG coherence across extrinsic pairs of hand muscles would be significantly stronger than that across intrinsic pairs of muscles. This hypothesis is based on observations of stronger common neural input across motor units of extrinsic thumb and index finger muscles than motor units of intrinsic adductor and abductor muscles of the index finger (Winges and Santello 2004; Winges et al. 2006, 2008). Weak common neural input has also been reported for intrinsic thumb and index finger muscles (McIsaac and Fuglevand 2008). Because we did not expect EMG–EMG coherence to vary with increasing levels of force, we expected that extrinsic–extrinsic hand muscle pairs would exhibit the strongest coherence compared with intrinsic–intrinsic muscle pairs regardless of the total grasp force.
METHODS
Subjects
Eight right-handed young adults (four men and four women; mean age and SD: 27.1 ± 5.3 yr) volunteered to participate in the study. Hand dominance was determined based on self-reported hand preference for writing and eating. All subjects were healthy and reported no history of hand injury, surgery, dysfunction, known neurological disorders, or musculoskeletal injuries of the hand. Subjects provided written informed consent before participating in the study and the Institutional Review Board at Arizona State University approved the procedures of the study.
Experimental procedures
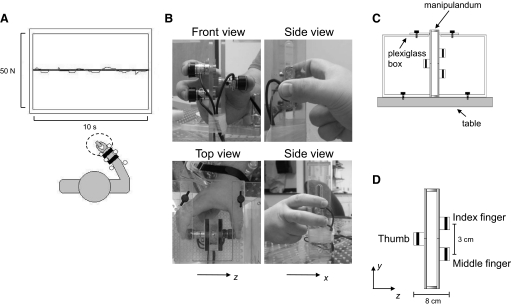
Subjects were asked to produce forces of different magnitudes with a three-digit grip (thumb, index, and middle fingers) on a rigid custom-made grip device equipped with force/torque sensors (see following text for details; Fig. 1B). Only the right hand was submitted to the experimental procedures. Subjects sat in an adjustable chair with a computer monitor located 1 m in front of them at eye level. The subject's forearm rested on a flat and rigid platform while immobilized and secured by adjustable plastic braces and Velcro straps fastened to the platform. To prevent any discomfort, a soft pad was placed under the forearm. In addition, a series of rigid dowels fixed on the platform were placed on both sides of the wrist, forearm, and in contact with the elbow (Fig. 1A). The hand was kept in a semisupinated posture (i.e., intermediate between fully pronated and supinated) whereas the wrist was maintained in a neutral posture. The fingertips of the index and middle fingers were separated 3 cm vertically and 8 cm horizontally from the thumb (Fig. 1, B and D). The spacing between the finger sensors ensured that the index and middle fingers did not rest against each other. The left hand was kept comfortable and resting on the table aside the experimental apparatus.
Fig. 1.
Experimental setup. Subjects sat in front of a monitor that displayed the target and actual force (dark and red lines, respectively; A) equivalent to the sum of thumb, index, and middle finger normal forces exerted on a grip manipulandum (B and D). Each subject's forearm was immobilized on the table during the force production task. Pictures on B show front, top, and side views of the grip manipulandum together with their anteroposterior and lateral frame of reference (x and z, respectively). A Plexiglas box anchored to the table (C) was used to secure the manipulandum in place during the execution of the task. Note that for the side views of the manipulandum (B), the Plexiglas box is not shown for clarity. D shows the grip manipulandum with 3 force/torque sensors, their relative distances, and the sensor-based frame of reference.
The distance between the wrist and the grip device was adjusted to allow subjects to place the distal pads of the three digits on their respective force/torque sensors while gripping the device using a comfortable hand posture. Specifically, the thumb metacarpal–phalangeal (mcp) joint was abducted and flexed (∼50 and 35°, respectively), whereas the interphalangeal joint angle was set at a neutral posture (∼0°); the index finger mcp joint was abducted and extended (∼10 and 30°, respectively), whereas the proximal and distal interphalangeal (pip and dip, respectively) joints were flexed and in a neutral adduction/abduction posture (∼40 and ∼0°, respectively); the middle finger mcp joint was in a neutral adduction/abduction posture and extended (∼0 and 30°, respectively), flexed at the pip and dip joints (∼50 and 30°, respectively). Because our task required force production by the thumb, index, and middle fingers only, we asked subjects to keep the ring and little fingers flexed (curled against the palm of the hand) during each experimental trial. The distance between the wrist and the grip device (see following text) was adjusted for each subject to ensure similar digit joint angles across all subjects. To prevent movement of the grip device during force production, the device was secured at its top and bottom to a Plexiglas box whose base was anchored to the table (Fig. 1C). Subjects reached for the grip device from the open front side of the box.
An important aspect of the experimental design was to ensure that digit posture (joint angles) relative to the force sensors remained constant across trials within and across experimental conditions. Therefore several controls were implemented to ensure and verify repeatability of digit posture within and across subjects. Specifically, placement of rigid dowels on either side of the forearm, around the wrist, and in between the thumb and index finger prevented forearm lateral and anterior–posterior translation relative to the fixed position of the grip device. Keeping the distance between the grip device and the wrist invariant across trials ensured that the hand position and digit posture relative to the grip device remained constant across trials and experimental conditions. Additional controls were 1) finger pads of all three digits were traced with a black felt tip pen to facilitate proper repositioning of the finger pads on the force transducers at the beginning of each trial; 2) pen marks were placed on the arm and plastic platform to aid in the replication of the position of the forearm in the sagittal plane; 3) subjects were instructed to minimize finger movement during the rest intervals while allowing slight abduction of the thumb, index, and middle fingers; and 4) postures of the hand and arm were monitored throughout the experiment by a second experimenter.
Despite all of the above-cited controls, variability in finger posture may have occurred due to small trial-to-trial differences in the position of the pad of the thumb and/or fingers relative to the respective force/torque sensor. To quantify the variability of the fingertip position relative to the sensor, we performed off-line analysis of the center of pressure (CoP) of each digit relative to the center of its sensor (the CoP was computed using the sensor's force and torque measurements; see following text for details). Trials in which the distance of the subjects' digit CoP from the center of the sensor was >3 mm were excluded from the analysis.
Subjects performed two force production tasks: 1) maximal voluntary force (MVC) and 2) submaximal voluntary forces (sMVC) of different magnitudes. The MVC task was always performed before the submaximal force production task. For both tasks, we asked subjects to generate isometric normal forces (perpendicular to the surface of the sensor) simultaneously with the thumb, index, and middle fingers on the grip manipulandum for a period of 6 s. MVC was defined as the sum of the normal forces exerted by the three digits (see following text for details). For the sMVC task, subjects were required to generate a total isometric normal force, defined as the sum of normal forces exerted by the three digits, of 5, 20, 40, 60, and 80% of MVC.
MAXIMUM VOLUNTARY FORCE PRODUCTION.
Subjects were instructed to 1) increase the isometric force exerted by the three digits (in the flexion direction) from baseline (∼1 N following the initial contact with all sensors) to maximum over a 3-s period while gripping the device and 2) hold the force for 6 s. Subjects received no visual feedback of digit forces. All subjects were verbally encouraged to maximize their force production normal to the force/torque sensors and minimize the tangential components of the digit forces (anteroposterior and vertical, x- and y-components, respectively; Fig. 1, B and D). Subjects performed three trials for the MVC task and the trial with the largest total normal force was used as the reference value to compute the five target forces for the submaximal force production task. A rest period of 3 min between trials was given to each subject.
SUBMAXIMAL VOLUNTARY FORCE PRODUCTION.
Subjects performed one block of three trials for each of the five target forces. The order of the target forces was randomized within and across subjects. Visual feedback of the target and actual forces was provided on a computer monitor throughout each trial (visual gain 19.6 pixels/N). A black horizontal line in the center of the monitor denoted the target force, whereas a red line denoted the sum of the three digits' normal forces (Fig. 1A, top). Subjects were required to gradually increase the total normal force exerted by the three digits from baseline (∼1 N) to the target force. Data collection (6 s) started when the subject reached the target force and maintained the total force close within the target. For a trial to be considered valid, subjects had to maintain the total normal force within ±10% of the target force (the force tolerance boundaries were not displayed on the monitor). When this criterion was not met, data collection stopped and the trial was repeated after a rest period. Across all subjects, roughly 1% of the trials had to be repeated. Rest periods of 30 s between trials and 3 min between blocks of trials were provided to minimize fatigue.
Apparatus
DIGIT FORCES.
Three Nano17/SI-25-250 force/torque sensors (nominal resolution: 0.0015 N; ATI Industrial Automation, Apex, NC) were used to measure forces and torques in three dimensions. The diameter of the sensors was 17 mm. The sensors were mounted on a customized manipulandum (height: 20 cm; width: 2.2 cm; depth: 4.4 cm) such that the center of the thumb sensor was aligned with the midpoint of the vertical distance between the index and middle finger sensors (Fig. 1, B–D). The contact surface of the sensor was covered with a thin circular plate covered with sandpaper (80-grit silicon–carbide D-weight Resign sandpaper; diameter: 17 mm; thickness: 7 mm, coefficient of static friction: ∼1.08; Alligator).
ELECTROMYOGRAPHY.
Intramuscular electromyographic (EMG) recordings were obtained from 12 muscles of the right hand. We inserted fine-wire electrodes (diameter: 25 μm; California Fine Wire, Grover Beach, CA) using hypodermic needles into the muscle bellies of six intrinsic and six extrinsic muscles innervating the thumb, index, and middle fingers. The intrinsic hand muscles were: first and second dorsal interosseus (FDI, 2DI), abductors of the index and middle fingers, respectively; first and second palmar interosseus (FPI, 2PI), adductors of the index and middle fingers, respectively; abductor pollicis brevis (ABPB), abductor of the thumb; and flexor pollicis brevis (FPB), a flexor of the thumb. The extrinsic hand muscles were flexor pollicis longus (FPL), a flexor of the thumb; extensor pollicis longus (EPL), extensor of the thumb; index and middle finger compartments of flexor digitorum superficialis (FDS2 and FDS3), flexors of the index and middle fingers, respectively; and index and middle finger compartments of extensor digitorum (ED2, ED3), extensors of the index and middle finger, respectively.
Prior to electrode insertion, about 2 mm of insulation was removed from the tip of the fine wire to increase the recording volume of the electrode. The electrodes were then inserted into the belly of each muscle or muscle compartment using either a [½]- or 1[½]-in. 27-gauge hypodermic needle. Approximate coordinates of the insertion sites of each muscle were estimated based on published anatomical landmarks (Agur et al. 1991) and coordinates obtained through previous intramuscular EMG electrode insertions (Winges and Santello 2004; Winges et al. 2006, 2008). The target muscle was also palpated to further locate the thickest portion of the muscle while it was voluntarily activated.
After the electrodes were inserted in the target muscle, the quality of the electrical signal was visually inspected during voluntary isometric contractions. When necessary, the depth and/or the angle of insertion of the electrode were adjusted until the signal-to-noise ratio of the EMG signal was maximal in the target muscle and cross talk was negligible in response to activation of any of the neighboring muscles across the entire voluntary force range. Last, electrode placement was further verified by using electrical stimulation (50–400 μA, 1-ms duration, square pulse, 1 Hz; S48 Stimulator, Grass Instruments, West Warwick, RI). Isolated movements about a target joint and/or phalanx induced by electrical stimulation were used to further assess correct placement of the electrode in the desired target muscle or muscle compartment. After completion of these procedures, the needle was removed and the wire remained in the muscle belly for the remainder of the experiment. Electrode insertion and verification of correct placement took about 2 h.
Two surface electrodes (10-mm-diameter gold-plated silver disc, Model F-E5GH; Grass Instruments) were placed on the radial styloid and served as a reference electrode for all intramuscular electrodes. During data collection, EMG signals from all muscles were displayed on a second computer monitor using a data acquisition interface (Micro 1401, Cambridge Electronic Design, Cambridge, UK) to monitor the quality of the recordings. The EMG signals were amplified (×2,000) and band-pass filtered (3–3,000 Hz; Neurodata Acquisition System model 12, Grass Instruments). Off-line analyses revealed that 95% of the power spectral density of our EMG data was <500 Hz.
Data acquisition
Force and EMG data were acquired through 12-bit A/D converter boards (sampling frequency: 2 kHz; PCI-6225, National Instruments, Austin, TX) through a custom data acquisition interface (LabVIEW version 8.0, National Instruments) and stored on a personal computer for further analysis.
Data analysis
Data were analyzed off-line with custom-written software (Matlab, The MathWorks, Natick, MA).
DIGIT FORCES.
The output of each force/torque sensor (normal force) was filtered (20 Hz low-pass, second-order, zero-lag Butterworth filter) before further processing. The force and torque outputs of each sensor were used to verify repeatability of digit position and force direction. The CoP of each digit was computed and analyzed to verify repeatability of digit posture within and across experimental conditions. The coordinates of each digit's CoP were computed using the sensor's force and torque output, i.e., CoPx = −(My + h · Fx)/Fz and CoPy = (Mx − h · Fy)/Fz, where x, y, and z are the anteroposterior, vertical, and horizontal axes in the sensor frame of reference (Fig. 1, B–D); h is the distance between the center of the outermost surface of the insulating plate (see preceding text) and the center of the sensor (h = 7 mm); Fx, Fy, and Fz denote the x-, y-, and z-force components, respectively, whereas Mx and My denote the x- and y-torque components, respectively, measured by the force/torque sensor relative to its reference frame. To further quantify across-trial variability in forces, we computed the two-dimensional digit force direction in the y–z plane (Fig. 1D). Digit force direction was calculated as the angle of the resultant vector formed by the tangential (vertical) and normal force components relative to the horizontal axis of a coordinate system with its origin at the center of the force/torque sensor. The force in the x (anterior–posterior) direction was not used for the calculation because digit forces in this direction were minimal (range for all digits and forces: 0.2–0.8 N). Therefore analysis of force direction was limited to the y–z plane (Fig. 1D). For each trial and experimental condition, the following variables were computed on the normal force exerted by each digit as well as on the sum of normal forces exerted by the thumb, index, and middle fingers throughout the entire trial: 1) average force, 2) SD of average force, and 3) coefficient of variation (CV) of average force.
HAND MUSCLE COORDINATION.
EMG amplitude domain analysis. Before processing, the EMG recorded from each muscle for each trial was visually inspected to verify the absence of artifacts. EMG signals were then rectified, averaged over the 6-s duration of each trial, and normalized to the mean amplitude of the signal obtained from the MVC trial. We will refer to these EMG amplitudes as aEMG. The magnitude of aEMG could vary between 0 and 1. For each subject, aEMG values were averaged across all trials within each force condition and for each muscle and used to create a vectorial representation of the EMG pattern of all muscles, i.e., a 12-dimensional (12D) vector (Valero-Cuevas et al. 1998). We will refer to this vector as a muscle activation pattern vector. The magnitude of each muscle activation pattern vector was computed as the square root of the sum of the squares of its components. To determine whether subjects used either the same or different hand muscle activation pattern across target forces, we determined the cosine of the angle between pairs of muscle activation pattern vectors, computed as the dot product obtained from pairwise comparisons of force levels. The cosine of the angle between muscle activation pattern vector pairs, equivalent to the Pearson's correlation coefficient (r), was used to quantify the degree of similarity in their orientation, the maximum or minimum value of 1 or 0, respectively, indicating that the vectors are collinear or perpendicular to each other. Due to the limited range (0 to 1) of possible values for the cosines of the angle (α) between muscle activation pattern vector pairs, for statistical purposes the absolute cosine values were transformed using the Fisher's z-transformation: z = 0.5 · 〈log {[1 + cos (α)]/[1 − cos (α)]}〉.
Frequency domain analysis (coherence).
To quantify correlations of muscle activations in the frequency domain, we computed EMG–EMG coherence. Before computing coherence (see following text), we processed the EMG data as follows.
1) For each muscle, the EMG signals recorded on each trial were concatenated to create a long trial (18 s; 36,000 data points). This procedure was used to increase the number of disjoint segments (windows) and thus the reliability of the coherence estimation (Maris et al. 2007).
2) The assumption of EMG signal stationarity was tested for each concatenated trial using the procedures described by Halliday and colleagues (2009). Briefly, each signal is broken down into disjoint segments and a periodogram for each of these segments is generated. The test consists of comparisons of the spread of values from these periodograms at each frequency with values expected for an exponentially distributed variate. The signal is considered stationary if the spread of values obtained from the disjoint segments are not significally different from those obtained through an exponential distribution. Stationarity was tested across the 0- to 55-Hz frequency range on 18 nonoverlapping segments (1 s each) and confirmed for most of our EMG data (454 of 480 concatenated trials; ∼95%). Coherence analysis was performed only on the stationary EMG data associated with the five submaximal target forces. With regard to the EMG signals recorded during MVCs, >30% of the data were nonstationary. Therefore we did not perform coherence analysis of EMG recorded during MVC trials.
EMG–EMG coherence was performed separately on 66 pairs of EMG signals, which accounts for all possible pair combinations of the 12 muscles we recorded from (see Table 1 for the list and labels of muscle pairs). EMG–EMG coherence was performed on the interference EMG because rectification alters the power spectrum of the EMG and impairs the ability to detect coherence between two EMG signals (Neto and Christou 2010). EMG–EMG coherence was computed from data for each muscle pair using the cross-spectrum of two EMG signals (fxy) squared and normalized by the product of the autospectrum of each signal (fxx and fyy) at each frequency (λ), that is
| (1) |
Table 1.
Muscle pair groupings and labels used in Fig. 7
| Muscle Pair | Intrinsic–Intrinsic | Muscle Pair | Intrinsic–Extrinsic |
|---|---|---|---|
| 1 | FDI–FPI | 31 | FDI–ED2 |
| 2 | FDI–2DI | 32 | FDI–ED3 |
| 3 | FDI–2PI | 33 | FDI–EPL |
| 4 | FDI–ABPB | 34 | FDI–FDS2 |
| 5 | FDI–FPB | 35 | FDI–FDS3 |
| 6 | FPI–2DI | 36 | FDI–FPL |
| 7 | FPI–2PI | 37 | FPI–ED2 |
| 8 | FPI–ABPB | 38 | FPI–ED3 |
| 9 | FPI–FPB | 39 | FPI–EPL |
| 10 | 2DI–2PI | 40 | FPI–FDS2 |
| 11 | 2DI–APBP | 41 | FPI–FDS3 |
| 12 | 2DI–FPB | 42 | FPI–FPL |
| 13 | 2PI–ABPB | 43 | 2DI–ED2 |
| 14 | 2PI–FPB | 44 | 2DI–ED3 |
| 15 | ABPB–FPB | 45 | 2DI–EPL |
| 46 | 2DI–FDS2 | ||
| Muscle Pair | Extrinsic–Extrinsic | 47 | 2DI–FDS3 |
| 16 | ED2–ED3 | 48 | 2DI–FPL |
| 17 | ED2–EPL | 49 | 2PI–ED2 |
| 18 | ED2–FDS2 | 50 | 2PI–ED3 |
| 19 | ED2–FDS3 | 51 | 2PI–EPL |
| 20 | ED2–EPL | 52 | 2PI–FDS2 |
| 21 | ED3–EPL | 53 | 2PI–FDS3 |
| 22 | ED3–FDS2 | 54 | 2PI–FPL |
| 23 | ED3–FDS3 | 55 | ABPB–ED2 |
| 24 | ED3–EPL | 56 | ABPB–ED3 |
| 25 | EPL–FDS2 | 57 | ABPB–EPL |
| 26 | EPL–FDS3 | 58 | ABPB–FDS2 |
| 27 | EPL–FPL | 59 | ABPB–FDS3 |
| 28 | FDS2–FDS3 | 60 | ABPB–FPL |
| 29 | FDS2–FPL | 61 | FPB–ED2 |
| 30 | FDS3–FPL | 62 | FPB–ED3 |
| 63 | FPB–EPL | ||
| 64 | FPB–FDS2 | ||
| 65 | FPB–FDS3 | ||
| 66 | FPB–FPL |
Coherence was estimated from nonoverlapping data segments of 1-s duration (i.e., 2,000 samples per segment), thus resulting in a frequency bin resolution of 1 Hz. We analyzed coherence over a frequency range between 0 and 55 Hz.
Individual estimates of coherence obtained from each muscle pair were used to pool coherence from muscle pairs (Amjad et al. 1997). Pooled coherence was used to assess the overall effect(s) of force on correlated neural input to all motor nuclei or to functional groups of motor nuclei. Pooled coherence can be considered as a weighted average of individual coherence estimates and is calculated as follows
| (2) |
where i is the trial number, k is the total number of coherence estimates (k = 66, one per muscle pair) used to compute the average, and L is the number of disjoint segments used in the spectra calculations for coherence estimate i. We computed pooled coherence on individual subjects and force condition separately to determine the effect of force magnitude on coherence 1) across all muscle pairs combined and within the 0- to 55-Hz frequency range, 2) within four separate frequency ranges and all muscle pairs combined, and 3) across the 0- to 55-Hz frequency range within each of three muscle groups.
For analysis 2, we computed pooled coherence within frequency bands (in Hz) 0–5 (common drive), 6–15 (alpha), 16–35 (beta), and 36–55 (gamma), identified based on the presumed source and task dependence of coherent oscillations according to Brown (2000). Briefly, coherence in the 0- to 5-Hz frequency band is found during isometric contractions and is thought to be of central origin (De Luca et al. 1982a), whereas the 5- to 15-Hz frequency band appears to be associated with physiological tremor and common modulation of motor-unit discharge during slow movements (Vallbo and Wessberg 1993). Finally, substantial evidence indicates that the source of EMG–EMG coherence and corticomuscular coherence in the 15- to 35- and 35- to 55-Hz frequency bands is due to oscillations in the motor cortex during low and high isometric force production tasks, respectively (Andrykiewicz et al. 2007; Brown 2000; Kilner et al. 2000; Kristeva et al. 2007; Mima et al. 1999; Omlor et al. 2007). For analysis 3, we separated muscle pairs into functional groups based on whether two muscles were both extrinsic, both intrinsic, or one intrinsic and one extrinsic.
For statistical purposes, individual and pooled coherence estimates were z-transformed by computing the arc hyperbolic tangent transformation (Fisher transformation) of coherency (Rxy)
| (3) |
where x = Rxy This transform acts to normalize the variance of the coherence estimates, which is necessary due to the limited range (0–1) of possible coherence values (Mima et al. 1999). Two variables were extracted from z-transformed coherence: 1) peak coherence and 2) the integral of coherence. Note that peak coherence was computed on coherence computed from each muscle pair, force level, and subject and determined as the largest value across the entire frequency range above significance level. The integral of coherence was computed only for statistically significant values. The significance level of the coherence estimate was computed using the test provided by Rosenberg et al. (1989).
We computed the integral of z-transformed coherence estimates pooled across subjects over the entire frequency range (0–55 Hz; analyses 1 and 3) and within four frequency bands (analysis 2). The effect of target force on the magnitude of these integrals of coherence was analyzed using nonparametric statistics (see following text).
Statistical analysis
Separate one-way ANOVAs with repeated measures were used to test the effect of target force sMVC (5 levels) on the following variables: 1) total normal force, 2) SD of average total force, 3) CV of average total force, 4) magnitude of muscle activation pattern vectors, and 5) z-transformed cosine of the angle between muscle activation pattern vector pairs. Two-way ANOVAs with repeated measures were performed to test the effects of the within-subject factors sMVC and Digit (3 levels: thumb, index, middle) on the following variables: 1) normal component of force, 2) SD of force, 3) CV of force produced by each digit, 4) digit CoP, and 5) z-transformed percentage force contributions of each digit to the total normal force. Two-way ANOVA with repeated measures was also used to test the effect of the factors sMVC and Muscle (12 levels) on the normalized EMG amplitude (aEMG). When appropriate, post hoc comparisons using Tukey's pairwise comparisons with Bonferroni adjustments were performed. The effect of sMVC on digit force direction in the y–z plane and the x-component of digit force were tested on each finger separately using Kruskal–Wallis nonparametric tests. This test was preferred to a parametric test because the force direction for one of the fingers (index finger) was not normally distributed across subjects. Significance level for all tests was set at P ≤ 0.05.
We performed nonparametric statistical analysis (Nichols and Holmes 2002) to determine the effect of grip force magnitude on the integral of z-transformed EMG–EMG coherence estimates. The null hypothesis was that no significant difference exists when comparing two experimental conditions (i.e., two force levels). The nonparametric approach tests this hypothesis by randomly assigning the observations from all subjects (n = 8) across two force conditions. For each permutation, the total number of observations for each force condition remains the same (i.e., 8 for 5% and 8 for 80% MVC) and the mean difference between the integrals of the two experimental conditions is calculated. This procedure is repeated for all 256 possible combinations (16C8 = 256) to generate a distribution of all mean differences. The proportion of mean differences obtained from all permutations that are larger than the absolute value of the experimentally observed mean difference is used to calculate the Monte Carlo P value (Maris et al. 2007). The null hypothesis is refuted if this P value is equal to or less than the significance level of 0.05. These procedures were used for the coherence analyses 1–3 described earlier. The preceding nonparametric statistical approach was preferred to parametric tests (e.g., test of difference; Amjad et al. 1997) because of its insensitivity to the “multiple comparisons problem” and control of type I errors (see Maris et al. 2007 for more details).
RESULTS
The main goal of the present study was to determine the effect of force during grasping on the coordination of simultaneously active hand muscles. We first describe the results of the tests used for validating the experimental protocol, followed by analyses of digit forces. We then describe the relation between target force (sMVC) and hand muscle activation pattern both in the amplitude and frequency domain (analyses of muscle activation pattern vectors and EMG–EMG coherence, respectively). Mean values are presented with SEs.
Validation of the experimental protocol
DIGIT PLACEMENT.
We used several controls to ensure repeatability of hand posture across trials and experimental conditions. We found that all subjects performed the task by consistently exerting normal forces within negligible distances (<2 mm) between the CoP of each digit and the center of its sensor across all force conditions (no significant main effect, or interaction for, sMVC and Digit; P = 0.821, P = 0.175, P = 0.884, respectively).
DIGIT FORCE DIRECTION.
The effect of sMVC on digit force direction in the y–z plane was analyzed to further verify that subjects exerted forces in similar directions across experimental conditions (see methods). On average, the thumb, index, and middle fingers exerted forces that were nearly perpendicular to the surface of the sensor (between ∼2 and 10° relative to the horizontal axis of the sensor) across all sMVCs. Force magnitude had no significant effect on force direction for any of the three digits [Kruskal–Wallis test: H(4) = 6.66, 0.98, and 1.07 for thumb, index, and middle fingers; P = 0.155, P = 0.913, P = 0.9, respectively).
Digit forces
TOTAL NORMAL FORCE.
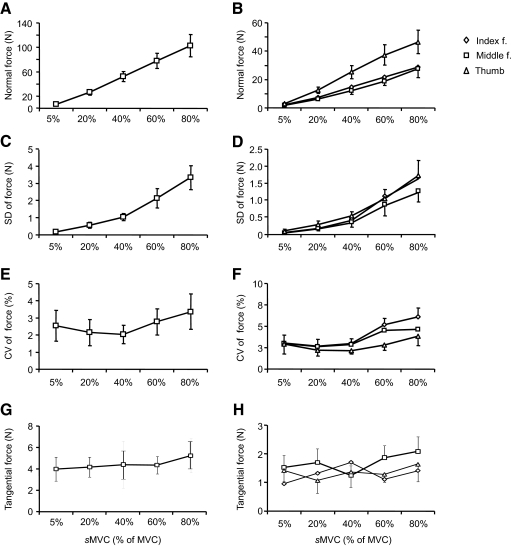
Figure 2A shows the total normal force produced by thumb, index, and middle fingers as a function of target force. Subjects generated total normal forces (in N) of 6.83 (±1.09), 26.20 (±4.29), 52.12 (±8.35), 77.96 (±12.32), and 102.72 (±18.09) when asked to exert 5, 20, 40, 60, and 80% of their MVC, respectively. Total force increased significantly with target force [F(4,35) = 104.5, P < 0.001], all five sMVC forces being significantly different from each other (P < 0.001 for all comparisons). Similarly, the SD of the total force increased significantly with target sMVC magnitudes [Fig. 2C; F(4,35) = 73.92, P < 0.001], SDs being significantly different for all pairwise force comparisons (P < 0.001 for all comparisons). The coefficient of variation (CV) of the total force was significantly affected by sMVC [Fig. 2E; F(4,35) = 3.39, P = 0.019], but significant differences were found only for the comparisons between 20 and 80% and between 40 and 80% of MVC (P = 0.04 and P = 0.02, respectively).
Fig. 2.
Magnitude of total and individual digit forces. A, C, and E show the sum of normal forces exerted by each digit as a function of target force, SD of total normal force, and coefficient of variation (CV) of total normal force, respectively. B, D, and F show the data for individual digits (normal forces, SD, and CV, respectively). G and H show the sum of tangential forces exerted by each digit and by individual digits as a function of target force, respectively. Data are averages of all subjects (±SE).
INDIVIDUAL DIGIT FORCES AND FORCE SHARING PATTERNS.
Even though all digits exerted larger forces with increasing target sMVC, the thumb always exerted the highest normal force [Fig. 2B; main effect of sMVC: F(4,105) = 203.80, P < 0.001; main effect of Digit: F(2,105) = 82.00, P < 0.001; significant interaction sMVC × Digit: F(8,105) = 7.57; P < 0.001]. Index and middle fingers exerted similar normal forces (P = 0.234). Significant differences were found among all five force levels as well as between thumb versus index and middle finger normal forces (both P < 0.001). The relative force contribution of each digit to the total force (i.e., force sharing pattern) remained relatively constant across the target sMVCs. Specifically, the thumb, index, and middle fingers produced, on average, 45–48, 28–30, and 23–28% of the total force, respectively, across all target sMVCs. ANOVA performed on the z-transformed percentage force contributions of each digit revealed that the force sharing pattern was not significantly affected by sMVC (P = 0.915). We found that SD of digit forces significantly increased with sMVC [Fig. 2D; F(4,105) = 201.32, P < 0.001]. Post hoc tests on SDs from pairwise force comparisons revealed that all but one comparison (5 vs. 20% MVC, P = 0.303) were significantly different from each other (P < 0.001). SD was statistically similar across digits (P = 0.198). Larger target forces were associated with larger force CV [Fig. 2F; main effect of sMVC: F(4,105) = 4.5; P = 0.002]. Pairwise sMVC comparisons revealed significant differences in CV of digit forces between 60 and 80% versus 5, 20, and 40% sMVC forces (P < 0.001 for all comparisons). The effect of sMVC was uniform across digits (P = 0.384).
Hand muscle activation patterns
AMPLITUDE DOMAIN ANALYSIS.
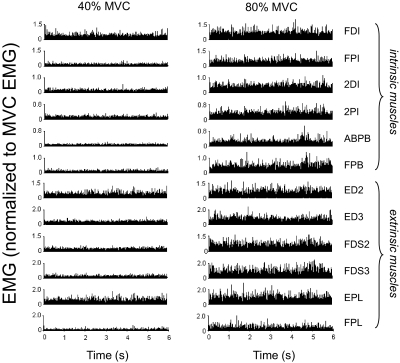
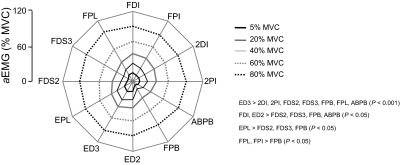
Figure 3 shows rectified EMG from 12 muscles of a representative subject during force production of 40 and 80% of MVC (left and right columns, respectively). EMG amplitude is shown in normalized form as a percentage of the EMG measured during the MVC. Note that all muscles tended to exhibit larger EMG amplitude for the larger sMVC. For each muscle, EMG amplitude was averaged across the entire trial duration (6 s) and normalized to the EMG amplitude recorded during the MVC (aEMG; see methods). The relation between aEMG and sMVC is shown in Fig. 4. The aEMG increased in all muscles in a fairly uniform fashion, as indicated by the nearly parallel displacement of each line as a function of sMVC (Fig. 4). Note, however, that the degree of similarity in the aEMG patterns tends to be higher for neighboring target forces. Note also that ED2, ED3, and EPL are characterized by the largest aEMG.
Fig. 3.
EMG amplitude at 40 and 80% of maximal voluntary force. Rectified electromyogram (EMG) from one subject is shown for each of the 12 muscles that were simultaneously active during one trial performed at 40 and 80% of maximal voluntary force (MVC; left and right columns, respectively). EMG amplitude is shown in normalized form as percentage of EMG recorded during MVC. See text for muscle abbreviations.
Fig. 4.
EMG amplitude as a function of target force and muscle. The EMG amplitude of each muscle (normalized to the EMG recorded during maximal voluntary contraction; aEMG) and target force magnitude. Data are plotted separately for each muscle and are averaged across all subjects. Significant differences (P < 0.05) between aEMG values are summarized on the right of the panel.
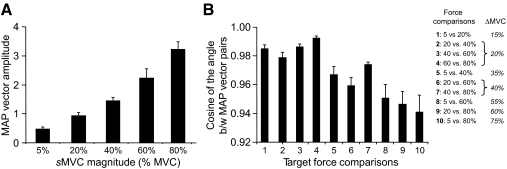
The preceding observations were confirmed by ANOVA, revealing significant main effects of sMVC and Muscle on aEMG [F(4,420) = 1,105.21 and F(11,420) = 9.59, respectively; both P < 0.001], but no significant interaction between these factors [F(44,420) = 0.50, P = 0.997]. All pairwise comparisons between force magnitudes revealed significant differences among aEMG values (P < 0.001 for all comparisons). Based on the results shown in Fig. 4, we expected the magnitude but not the orientation of the muscle activation pattern vector (see methods) to change significantly with sMVC. Linear regression analysis revealed that the muscle activation pattern vector magnitude increased linearly with sMVC for each subject (range: 0.88–0.98; median: 0.95; all P < 0.001). Furthermore, the muscle activation pattern vector magnitude was significantly different at each sMVC force [F(4,35) = 222.15, P = 0.001; Fig. 5A]. All pairwise comparisons of sMVCs (n = 10) revealed significant differences between muscle activation pattern vector magnitudes (P = 0.001 for all comparisons). Importantly, we found a high degree of similarity in orientation between pairs of muscle activation pattern vectors for all sMVC pairwise comparisons (Fig. 5B), as indicated by large values in the cosines of the angle between vector pairs (range across subjects: 0.897–0.997; mean of all subjects and target force comparisons: 0.9680 ± 0.003). Further examination of the pairwise comparisons revealed that muscle activation pattern vectors associated with neighboring target forces (i.e., 5 vs. 20%, 20 vs. 40%, etc.) were characterized by a higher degree of similarity than those associated with target forces that were further apart (i.e., 5 vs. 40%, 20 vs. 50%, etc.). This can be seen as a trend for lower cosines of the angle between muscle activation pattern vector pairs with increasing differences between target forces (labeled as ΔsMVC in the table in Fig. 5B). This was confirmed by ANOVA performed on the z-transformed cosines of the angles between pairs of muscle activation pattern vectors [significant main effect of pairwise force comparison: F(9,70) = 11.25, P = 0.001]. Despite this trend, however, the degree of similarity in muscle activation pattern vectors was overall very high throughout all target force comparisons.
Fig. 5.
Magnitude and length of muscle activation pattern vectors. A shows the magnitude of muscle activation pattern vectors as a function of target force. B shows the cosines of the angle between pairs of muscle activation patterns vectors for each pairwise force comparison. The table shows the notation used for comparisons 1 through 10 denoting increasingly larger differences (ΔsMVC) between target forces. Data in both panels are averages of all subjects (±SE).
FREQUENCY DOMAIN ANALYSIS.
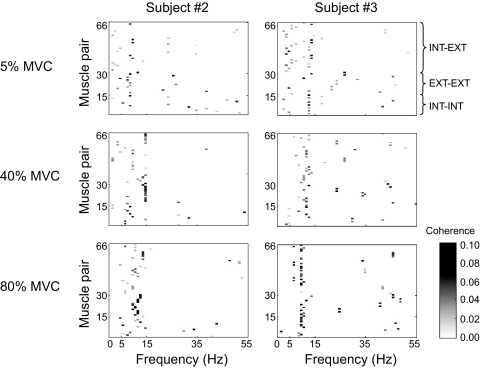
We computed EMG–EMG coherence to quantify the strength and frequency of correlated neural input across the 12 muscles studied (see methods). Figure 6 shows the distribution of significant coherence peaks across muscle pairs and frequencies for three target forces (5, 40, and 80% MVC) for two representative subjects. Large coherence peaks primarily occurred within the low-frequency range (<15 Hz), regardless of muscle pair and force level. Significant coherence peaks across all subjects, muscle pairs, and force levels ranged from 0.02 to 0.10 (median: 0.05) and occurred at a median frequency of 12 Hz.
Fig. 6.
Distribution of significant peak coherence. The occurrence of statistically significant peak coherence is shown for each muscle pair (n = 66; see Table 1 for labels) across the entire frequency range (0–55 Hz). Muscle pairs are ordered based on their anatomical/functional characteristics (extrinsic–extrinsic, EXT–EXT; intrinsic–intrinsic, INT–INT; intrinsic–extrinsic, INT–EXT). Data from 2 representative subjects are shown for 3 of the 5 target force levels.
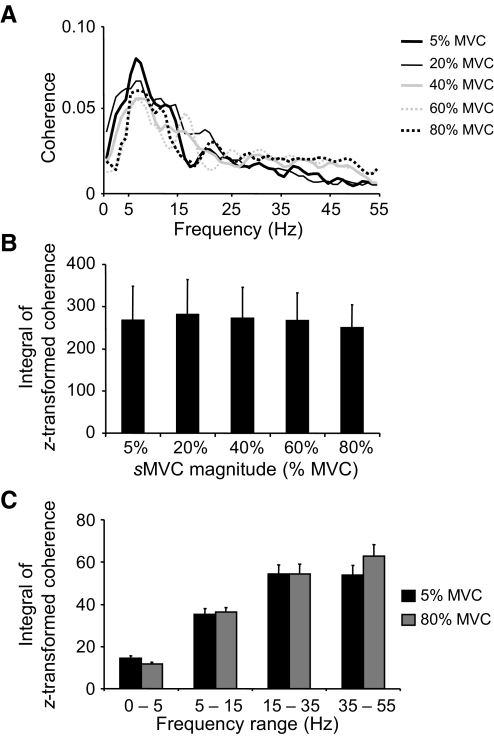
Figure 7A shows pooled coherence estimations computed across all 66 muscle pairs over the 0- to 55-Hz frequency range and averaged across all subjects for each sMVC. The overall profile of pooled coherence remained fairly constant across all target forces, with the strongest coherence occurring within the common drive and alpha frequency range (<15 Hz). As expected from the data shown in Fig. 7A, the integrals of the z-transformed pooled coherence were very similar across sMVC magnitudes.
Fig. 7.
EMG–EMG pooled coherence across all muscle pairs. A shows EMG–EMG pooled coherence computed across all muscle pairs (n = 66) for each target force and averaged across subjects. B shows the integral of the z-transformed coherence from 0 to 55 Hz pooled across all muscles for each target force. C shows the integral of the z-transformed coherence pooled across all muscles and computed within the 0- to 5-, 6- to 15-, 16- to 35-, and 35- to 55-Hz frequency ranges for 5 and 80% MVC. Note that the integrals shown in B and C were computed on coherence above the significance level. Data are averages of all subjects (±SE values are shown in B and C).
We found that the integrals of z-transformed pooled coherence estimates were not significantly different for any of the pairwise target force comparisons (Fig. 7B; P = 0.324). Therefore further statistical analyses of pooled coherence focused on the comparison between the smallest and largest target force (5 vs. 80% MVC; Fig. 7C). This comparison revealed no significant differences in the integral of z-transformed coherence within any of the frequency bands (0–5, 6–15, 16–35, and 36–55 Hz; P = 0.144).
The preceding analyses revealed that coherence was not significantly modulated as a function of force across the entire 0- to 55-Hz frequency range or within smaller frequency ranges. However, pooling coherence across all 66 muscle pairs might have obscured possible differences in the extent to which target force might have affected coherence within given muscle groups. Therefore we computed pooled coherence within three muscle groups: extrinsic–extrinsic, intrinsic–intrinsic, and intrinsic–extrinsic muscle pairs.
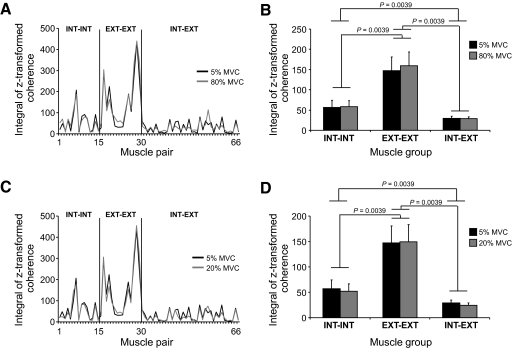
Figure 8A shows the integrals of z-transformed coherence computed on individual muscle pairs over the frequency range of 0–55 Hz and averaged across all subjects for the 5 and 80% MVCs. Data are plotted as a function of the three above-mentioned muscle groups (see Table 1 for muscle pair labels). Two main observations can be made: 1) coherence was overall very similar for the two target forces for each muscle pair, regardless of muscle group; and 2) coherence was typically stronger for extrinsic–extrinsic muscle pairs than that for intrinsic–intrinsic muscle pairs, the weakest coherence occurring in intrinsic–extrinsic muscle pairs. Both observations can also be made for the integrals of z-transformed pooled coherence computed within each of the muscle groups for the two target forces (Fig. 8B).
Fig. 8.
EMG–EMG pooled coherence: individual muscle pairs and muscle groups. A and C show the integral of z-transformed coherence computed over the 0- to 55-Hz frequency range for each muscle pair for 2 force comparisons (5 vs. 80% and 5 vs. 20% MVC, respectively). Data are ordered based on the muscle pair anatomical/functional characteristics: extrinsic–extrinsic, intrinsic–intrinsic, and intrinsic–extrinsic muscle pairs (EXT–EXT, INT–INT, and INT–EXT, respectively). Note the similarity in coherence across the 3 force levels. B and D show the integral of z-transformed pooled coherence computed over the frequency range of 0–55 Hz within EXT–EXT, INT–INT, and INT–EXT muscle groups for 2 force comparisons (5 vs. 80% and 5 vs. 20% MVC, respectively). Pooled coherence was computed on 15 EXT–EXT, 15 INT–INT, and 36 INT–EXT muscle pairs, respectively. Significant differences and P values are shown for muscle group comparisons. Note that the integrals shown in A–D were computed on coherence above the significance level. Data are averages of all subjects (±SE values are shown in B and D).
Nonparametric statistical analysis revealed that the strength of coherence was not significantly affected by target force within any of the three muscle groups (P values ranged from 0.58 to 0.78). However, coherence pooled across the two target forces within each muscle group revealed a significantly stronger coherence for 1) the extrinsic–extrinsic muscle group than that for the intrinsic–intrinsic and intrinsic–extrinsic muscle groups and 2) intrinsic–intrinsic than that for intrinsic–extrinsic muscle groups (P = 0.004 for all comparisons; Fig. 8B). To determine whether these differences between muscle groups were confined to the largest target force difference (5 vs. 80% MVC), we also performed the above analyses for the smallest target force difference (5 vs. 20% MVC). Figure 8, C and D shows the integrals of the z-transformed coherence for each muscle pair and pooled within each muscle group, respectively. This analysis showed the same results as that performed on the 5 versus 80% force comparison (P = 0.004 for all comparisons; Fig. 8D).
DISCUSSION
We quantified the effect of force during a three-digit grasp on hand muscle coordination defined as relations between EMG amplitudes and EMG–EMG coherence. We report three main findings: 1) subjects modulated the magnitude of the same EMG coordination pattern across a wide range of grasp forces; 2) the strength and distribution of correlated neural input to hand muscles remained invariant across grasp forces; and 3) coherence analysis revealed grouping of muscle pairs, with extrinsic and intrinsic muscle pairs exhibiting the strongest and weakest coherence, respectively. These findings are discussed within the theoretical framework of muscle synergies and muscle-pair-specific distribution of common neural input.
Coordination of hand muscles for multidigit grasping
As expected, subjects accurately produced total normal forces of increasing magnitude (Fig. 2, A and B). Consistent with numerous previous studies, the SD of the total normal force and the individual digit force increased in a roughly linear manner as a function of force (Fig. 2, C and D; Christou et al. 2002; Moritz et al. 2005; Slifkin and Newell 1999, 2000; Taylor et al. 2003). The CV of total normal force was similar at the lower target forces (5, 20, and 40% of MVC force; Fig. 2E), but was greater at the larger target forces (60 and 80% of MVC force). The higher CV of force for the highest target forces differs from findings from previous studies on the index finger (Christou et al. 2002; Moritz et al. 2005) and grasp force (Sprague et al. 2009), but is similar to results obtained by others (Taylor et al. 2003; Tracy et al. 2002). These divergent findings are likely a result of differences in the muscle group involved, the range of forces studied, and differences between single and multijoint contractions associated with these tasks.
Consistent with previous observations (Kinoshita et al. 1995), in the present three-digit grasp, subjects used the same digit force sharing pattern at all target forces (Fig. 2B).This behavior was accompanied by a modulation in the magnitude of the 12D EMG muscle activation pattern vector resulting from a similar rate of increase in EMG amplitude across all muscles with increasing force (lack of significant interaction sMVC × Muscle). This observation was further confirmed by the fact that the orientation of muscle activation pattern vectors was highly correlated for all force comparisons, also denoting a uniform scaling of EMG amplitude of each muscle with total grasp force. The trend of larger differences in the EMG muscle activation pattern vectors for larger target force comparisons is likely due to nonlinearities of EMG–force relations that characterize low force production tasks (Valero-Cuevas 2000). In turn, these might affect the number of muscle activation patterns that can generate low versus large forces. The scaling of EMG muscle activation pattern vector magnitude with forces and a relatively invariant vector orientation are consistent with results obtained from a study of a single-digit force production task (Valero-Cuevas 2000). In that study, the magnitude of the seven dimensional EMG muscle activation pattern vector of all index finger muscles scaled with static seven and dynamic fingertip force, whereas its orientation remained relatively invariant (similar to our study, pairwise comparisons of average EMG muscle activation pattern vectors yielded correlation coefficients >0.96; palmar force direction, Table 1; Valero-Cuevas 2000). Given the redundancy of finger musculature and the availability of a large number of feasible muscle coordination patterns, Valero-Cuevas (2000) proposed that the CNS may simplify the modulation of fingertip force by modulating the magnitude, but not the structure, of the same coordination pattern of muscles within a digit. Even though several methodological differences exist between the present study and that of Valero-Cuevas (2000), our results are an important extension of the same theoretical framework developed for one-digit tasks to tasks involving multiple digits. Another important contribution of our study is the application of coherence analysis to gain further insight into the mechanisms binding the above-described correlation in the modulation of multiple hand muscles (see following text).
Note that the larger EMG amplitude exhibited by some of the digit extensor and intrinsic muscles is likely explained by the fact that these muscles, being antagonists to the digit flexor muscles that are more directly responsible for grip force, might not have been maximally activated during the MVC task (see methods). Therefore across-muscle differences in MVC-normalized EMG amplitude should not be interpreted as differences in the force exerted by extensor and intrinsic versus flexor muscles. This caveat does not affect the key finding that EMG amplitude scaled uniformly across all muscles with force.
The implications of the present results are that the CNS constrains the simultaneous modulation of neural drive not only to muscles innervating one digit, but also to those innervating different digits. As such, the simplifying strategy revealed by the study of Valero-Cuevas (2000) could also potentially be involved for grasping tasks requiring the coordination of multiple digit forces. It should be emphasized, however, that both the study by Valero-Cuevas (2000) and ours were performed in a highly constrained scenario whereby the posture of the digits was kept constant across all force levels. Although the modulation of EMG coordination patterns has been described for motion-to-force transition tasks performed with one digit (Venkadesan and Valero-Cuevas 2008), the coordination of hand muscles responsible for dynamic interdigit coordination remains to be addressed.
Neural mechanisms underlying hand muscle coordination
We computed EMG–EMG coherence across all muscle pair combinations to determine the relation between digit forces and correlated neural input to hand muscles. We found that the strength of coherence (quantified as the integral of the z-transformed coherence) pooled across all muscles pairs was insensitive to the total grasp force across the range 5–80% of MVC. Because EMG–EMG coherence captures the net effect of all inputs across motor neurons, this result can be interpreted as evidence for a force-independent, “default” distribution of excitatory drive to the hand muscles we studied. This interpretation is supported by the fact that small hand muscles have been shown to have an upper motor-unit recruitment limit of roughly 50–60% of MVC (De Luca et al. 1982b; Moritz et al. 2005). Therefore in the present study increases in force production ≤50–60% of MVC were presumably accomplished by motor-unit recruitment and changes in discharge rate, whereas further force increases must have been solely due to increases in discharge rate. Note that the notion of a force-independent distribution of neural drive to hand muscles is consistent with the above-cited results of the EMG amplitude analyses revealing very similar coordination patterns across a wide range of forces.
Although differences exist in the analysis of coherence between this study and others (e.g., integral of z-transformed coherence vs. peak coherence value), the present evidence for force-independent neural drive to hand muscles is similar to that provided by corticomuscular coherence studies, which reported no differences in corticomuscular coherence across a range of target forces (e.g., 60–80% of MVC; Brown et al. 1998; Mima et al. 1999). However, these studies found a tendency for a shift in the corticomuscular coherence spectrum from the beta to the gamma frequency band when force increased to 80–100% of MVC, although this occurred in less than half of all subjects (Brown et al. 1998; Mima et al. 1999). A direct comparison with the present study, however, cannot be made because over one third of the EMG data from MVC trials was nonstationary, thus preventing the computation of EMG–EMG coherence.
The finding that corticomuscular coherence is independent of muscle force is not consistent (Chakarov et al. 2009; Kilner et al. 2000). For example, Chakarov et al. (2009) found that corticomuscular coherence increased when subjects isometrically flexed the index finger at forces of 8–24% of MVC. These differences across studies, however, are most likely due to task differences. Specifically, Chakarov et al. (2009) asked subjects to perform sinusoidal force-matching tasks, which may require greater levels of attention and visuomotor processing than steady isometric contractions. Therefore it remains to be determined whether, similar to corticomuscular coherence, EMG–EMG coherence would be modulated in the beta and/or gamma frequency bands were subjects to perform tasks that involve greater attention, visuomotor processing, and cortical involvement (Kristeva et al. 2007; Kristeva-Feige et al. 2002). Another factor that prevents a direct comparison between corticomuscular coherence and EMG–EMG coherence studies is that they provide complementary, but different, information. Specifically, corticomuscular coherence identifies correlations between cortical and muscular activity, whereas EMG–EMG coherence reveals correlations in the neural drive from cortical and subcortical inputs (Grosse et al. 2002).
Examination of the frequency band(s) at which coherent muscle activity occurs can provide information about the sources of correlated neural inputs (see methods). We found that coherence was stronger in the alpha band (≤15 Hz) compared with that in all other frequency bands (Figs. 6 and 7A). This is consistent with studies that have quantified EMG–EMG coherence within and across muscles using single motor-unit recordings reporting coherence peaks in the 1- to 12-Hz frequency ranges (Farmer et al. 1993; Johnston et al. 2005; Semmler 2002; Semmler et al. 2003, 2004). Insight into the sources of correlated neural input within and across hand muscles has been provided by Farmer and colleagues (1993). These authors reported that healthy subjects exhibited significant coherence in both the 1- to 12- and 16- to 32-Hz frequency bands, whereas patients suffering from cortical (internal capsule or cerebral peduncle) stroke exhibited a greatly reduced coherence in the 16- to 32-Hz band. These results indicate that these frequency bands reflect different sources of common input, the 1- to 12-Hz band likely reflecting subcortical common drives to hand muscles (Farmer et al. 1993). Although these observations may suggest the involvement of subcortical inputs for the alpha band EMG–EMG coherence found by the present study, further work is required to test this interpretation.
Organization of correlated neural inputs to motor nuclei of hand muscles
Winges et al. (2008) reported stronger motor-unit synchrony (a time-domain measure of common neural input) across motor units of extrinsic than of intrinsic hand muscles. Specifically, motor-unit synchrony across two extrinsic muscles (FPL and FDP2) was more than twice the magnitude of motor-unit synchrony across two intrinsic muscles (FDI and FPI). These authors pointed out that this result together with findings from previous studies of the same object hold task (Fig. 4; Winges et al. 2008) suggest the existence of a muscle-pair-specific distribution of common neural input to hand muscles. A recent study of a two-digit force production task confirmed the above-cited observations by revealing weaker common neural input across motor nuclei of intrinsic muscles of the thumb and index finger than that across extrinsic muscles (McIsaac and Fuglevand 2008). Such distribution of common neural inputs would appear to favor a higher independence of intrinsic than of extrinsic muscle pairs. Based on this finding, we hypothesized that EMG–EMG coherence would be distributed in a heterogeneous fashion across muscle pairs, the expectation being that extrinsic muscle pairs would exhibit stronger coherence than that of intrinsic muscle pairs. Even though the present task differs from that used by Winges et al. (2008), our results support our hypothesis (Fig. 8).
It should also be emphasized that the same muscle-pair-specific distribution of EMG–EMG coherence was found across a wide range of grasp forces. Although it is clear that extrinsic and intrinsic muscles contribute to the generation of fingertip force, the present data, which are consistent with previous observations (Winges et al. 2008), point to a muscle-pair-specific distribution of neural drive. A possible explanation for such an organization may be found in one or more properties that distinguish intrinsic and extrinsic hand muscles, including differences in how much force they can generate and their innervations, as well as potential differences in their functional roles (Winges et al. 2008). Specifically, intrinsic muscles are particularly important for fine control of the direction of fingertip forces, a function that might be better served by separate, rather than common, neural inputs (see also the discussion in Winges et al. 2008). This interpretation is consistent with the experimental observations of common cortical circuits underlying the control of shoulder, elbow, and wrist muscles, as opposed to separate cortical circuits associated with an intrinsic hand muscle (FDI; Devanne et al. 2002). Although force magnitude did not elicit a change in the distribution of common neural input (stronger for extrinsic than intrinsic muscle pairs), the extent to which such an organization is insensitive to other task characteristics is the subject of ongoing investigations.
Conclusions
We found that the coordination of multiple hand muscles, defined as correlated modulation of the EMG amplitude of each muscle as well as EMG–EMG coherence, was invariant across a wide range of grasp forces. Furthermore, correlated neural input to hand muscles was heterogeneously distributed, such that the EMG–EMG occurring across extrinsic hand muscle pairs was significantly stronger than that across intrinsic hand muscle pairs. These findings indicate that digit force modulation during grasping occurs through neural mechanisms that bind the distribution of neural inputs to hand muscles in a relatively fixed fashion. We propose that the organization of correlated inputs described by the present study might reflect the functional role(s) of hand muscle groups.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMSD) Grant 2R01 AR-47301. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIAMSD or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of B. Poston: Human Motor Control Section, Medical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Building 10, Room 7D37, 10 Center Drive, MSC 1428, Bethesda, MD 20892-1428.
REFERENCES
- Agur AMR, Lee MJ, Anderson JE. Grant's Atlas of Anatomy (9th ed.). Baltimore, MD: Williams & Wilkins, 1991 [Google Scholar]
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor.J Neurosci Methods 73: 69–79, 1997 [DOI] [PubMed] [Google Scholar]
- Andrykiewicz A, Patino L, Naranjo JR, Witte M, Hepp-Reymond MC, Kristeva R. Corticomuscular synchronization with small and large dynamic force output.BMC Neurosci 8: 101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol 60: 97–108, 2000 [DOI] [PubMed] [Google Scholar]
- Brown P, Salenius S, Rothwell JC, Hari R. Cortical correlate of the Piper rhythm in humans.J Neurophysiol 80: 2911–2917, 1998 [DOI] [PubMed] [Google Scholar]
- Butler TJ, Kilbreath SL, Gorman RB, Gandevia SC. Selective recruitment of single motor units in human flexor digitorum superficialis muscle during flexion of individual fingers.J Physiol 567: 301–309, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakarov V, Naranjo JR, Schulte-Monting J, Omlor W, Huethe F, Kristeva R. Beta-range EEG–EMG coherence with isometric compensation for increasing modulated low-level forces.J Neurophysiol 102: 1115–1120, 2009 [DOI] [PubMed] [Google Scholar]
- Christou EA, Grossman M, Carlton LG. Modeling variability of force during isometric contractions of the quadriceps femoris.J Mot Behav 34: 67–81, 2002 [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive in motor units of a synergistic muscle pair.J Neurophysiol 87: 2200–2204, 2002 [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions.J Physiol 329: 113–128, 1982a [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Control scheme governing concurrently active human motor units during voluntary contractions.J Physiol 329: 129–142, 1982b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanne H, Cohen LG, Kouchtir-Devanne N, Capaday C. Integrated motor cortical control of task-related muscles during pointing in humans.J Neurophysiol 87: 3006–3017, 2002 [DOI] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man.J Physiol 470: 127–155, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse P, Cassidy MJ, Brown P. EEG–EMG, MEG–EMG and EMG–EMG frequency analysis: physiological principles and clinical applications.Clin Neurophysiol 113: 1523–1531, 2002 [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Rigas A, Conway BA. A periodogram-based test for weak stationarity and consistency between sections in time series.J Neurosci Methods 180: 138–146, 2009 [DOI] [PubMed] [Google Scholar]
- Hockensmith GB, Lowell SY, Fuglevand AJ. Common input across motor nuclei mediating precision grip in humans.J Neurosci 25: 4560–4564, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesler EJ, Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. III. Synchronisation of single motor units.Exp Brain Res 134: 441–455, 2000 [DOI] [PubMed] [Google Scholar]
- Johanson ME, Valero-Cuevas FJ, Hentz VR. Activation patterns of the thumb muscles during stable and unstable pinch tasks.J Hand Surg Am 26: 698–705, 2001 [DOI] [PubMed] [Google Scholar]
- Johnston JA, Winges SA, Santello M. Periodic modulation of motor-unit activity in extrinsic hand muscles during multidigit grasping.J Neurophysiol 94: 206–218, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle.J Neurophysiol 91: 57–62, 2004 [DOI] [PubMed] [Google Scholar]
- Kilner JM, Alonso-Alonso M, Fisher R, Lemon RN. Modulation of synchrony between single motor units during precision grip tasks in humans.J Physiol 541: 937–948, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters.J Neurosci 20: 8838–8845, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Kawai S, Ikuta K. Contributions and co-ordination of individual fingers in multiple finger prehension.Ergonomics 38: 1212–1230, 1995 [DOI] [PubMed] [Google Scholar]
- Kristeva R, Patino L, Omlor W. Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output.NeuroImage 36: 785–792, 2007 [DOI] [PubMed] [Google Scholar]
- Kristeva-Feige R, Fritsch C, Timmer J, Lucking CH. Effects of attention and precision of exerted force on beta range EEG–EMG synchronization during a maintained motor contraction task.Clin Neurophysiol 113: 124–131, 2002 [DOI] [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. I. Contribution of 15 finger muscles to isometric force.Exp Brain Res 103: 108–122, 1995a [DOI] [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. II. Muscular synergies in the spatial and temporal domain.Exp Brain Res 103: 123–136, 1995b [DOI] [PubMed] [Google Scholar]
- Maris E, Schoffelen JM, Fries P. Nonparametric statistical testing of coherence differences.J Neurosci Methods 163: 161–175, 2007 [DOI] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Influence of tactile afferents on the coordination of muscles during a simulated precision grip.Exp Brain Res 174: 769–774, 2006 [DOI] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Motor-unit synchrony within and across compartments of the human flexor digitorum superficialis.J Neurophysiol 97: 550–556, 2007 [DOI] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Common synaptic input across motor nuclei supplying intrinsic muscles involved in the precision grip.Exp Brain Res 188: 159–164, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TE, Dhaliwal SS. Activation of intrinsic and extrinsic finger muscles in relation to the fingertip force vector.Exp Brain Res 146: 197–204, 2002 [DOI] [PubMed] [Google Scholar]
- Mima T, Simpkins N, Oluwatimilehin T, Hallett M. Force level modulates human cortical oscillatory activities.Neurosci Lett 275: 77–80, 1999 [DOI] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle.J Neurophysiol 93: 2449–2459, 2005 [DOI] [PubMed] [Google Scholar]
- Neto OP, Christou EA. Rectification of the EMG signal impairs the identification of oscillatory input to the muscle.J Neurophysiol 103: 1093–1103, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples.Hum Brain Mapp 15: 1–25, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlor W, Patino L, Hepp-Reymond MC, Kristeva R. Gamma-range corticomuscular coherence during dynamic force output.NeuroImage 34: 1191–1198, 2007 [DOI] [PubMed] [Google Scholar]
- Pearlman JL, Roach SS, Valero-Cuevas FJ. The fundamental thumb-tip force vectors produced by the muscles of the thumb.J Orthop Res 22: 306–312, 2004 [DOI] [PubMed] [Google Scholar]
- Reilly KT, Nordstrom MA, Schieber MH. Short-term synchronization between motor units in different functional subdivisions of the human flexor digitorum profundus muscle.J Neurophysiol 92: 734–742, 2004 [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Digit displacement, not object compliance, underlies task dependent modulations in human corticomuscular coherence.NeuroImage 33: 618–627, 2006 [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains.Prog Biophys Mol Biol 53: 1–31, 1989 [DOI] [PubMed] [Google Scholar]
- Santello M, Fuglevand AJ. Role of across-muscle motor unit synchrony for the coordination of forces.Exp Brain Res 159: 501–508, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Soechting JF. Force synergies for multifingered grasping.Exp Brain Res 133: 457–467, 2000 [DOI] [PubMed] [Google Scholar]
- Schieber MH, Santello M. Hand function: peripheral and central constraints on performance.J Appl Physiol 96: 2293–2300, 2004 [DOI] [PubMed] [Google Scholar]
- Semmler JG. Motor unit synchronization and neuromuscular performance.Exerc Sport Sci Rev 30: 8–14, 2002 [DOI] [PubMed] [Google Scholar]
- Semmler JG, Kornatz KW, Enoka RM. Motor-unit coherence during isometric contractions is greater in a hand muscle of older adults.J Neurophysiol 90: 1346–1349, 2003 [DOI] [PubMed] [Google Scholar]
- Semmler JG, Sale MV, Meyer FG, Nordstrom MA. Motor-unit coherence and its relation with synchrony are influenced by training.J Neurophysiol 92: 3320–3331, 2004 [DOI] [PubMed] [Google Scholar]
- Semmler JG, Steege JW, Kornatz KW, Enoka RM. Motor-unit synchronization is not responsible for larger motor-unit forces in old adults.J Neurophysiol 84: 358–366, 2000 [DOI] [PubMed] [Google Scholar]
- Slifkin AB, Newell KM. Noise, information transmission, and force variability.J Exp Psychol Hum Percept Perform 25: 837–851, 1999 [DOI] [PubMed] [Google Scholar]
- Slifkin AB, Newell KM. Variability and noise in continuous force production.J Mot Behav 32: 141–150, 2000 [DOI] [PubMed] [Google Scholar]
- Sprague RL, Deutsch KM, Newell KN. Adaptive force control in grasping as a function of level of developmental disability.J Intellect Disabil Res 53: 797–806, 2009 [DOI] [PubMed] [Google Scholar]
- Taylor AM, Christou EA, Enoka RM. Multiple features of motor-unit activity influence force fluctuations during isometric contractions.J Neurophysiol 90: 1350–1361, 2003 [DOI] [PubMed] [Google Scholar]
- Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles.J Appl Physiol 92: 1004–1012, 2002 [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range.J Neurophysiol 83: 1469–1479, 2000 [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Zajac FE, Burgar CG. Large index-fingertip forces are produced by subject-independent patterns of muscle excitation.J Biomech 31: 693–703, 1998 [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Wessberg J. Organization of motor output in slow finger movements in man.J Physiol 469: 673–691, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkadesan M, Valero-Cuevas FJ. Neural control of motion-to-force transitions with the fingertip.J Neurosci 28: 1366–1373, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winges SA, Johnston JA, Santello M. Muscle-pair specific distribution and grip-type modulation of neural common input to extrinsic digit flexors.J Neurophysiol 96: 1258–1266, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winges SA, Kornatz KW, Santello M. Common input to motor units of intrinsic and extrinsic hand muscles during two-digit object hold.J Neurophysiol 99: 1119–1126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winges SA, Santello M. Common input to motor units of digit flexors during multidigit grasping.J Neurophysiol 92: 3210–3220, 2004 [DOI] [PubMed] [Google Scholar]
- Yu WS, Kilbreath SL, Fitzpatrick RC, Gandevia SC. Thumb and finger forces produced by motor units in the long flexor of the human thumb.J Physiol 583: 1145–1154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]