Abstract
The neocortex contains multiple types of inhibitory neurons whose properties suggest they may play different roles within the cortical circuit. By recording from three cell types during two distinct network states (up and down states) in vitro, we were able to quantify differences in firing characteristics between these cells during different network regimes. We recorded from regular-spiking (RS) excitatory cells and two types of inhibitory neurons, the fast-spiking (FS) neurons and GFP- (and somatostatin-) expressing inhibitory neurons (GIN), in layer 2/3 of slices from mouse somatosensory neocortex. Comparisons of firing characteristics between these cells during up- and down-states showed several patterns. First, of these cell types, only GIN cells fired persistently during down-states, whereas all three cell types fired readily during up-states. Second, the onset of firing and distribution of action potentials throughout up-states differed by cell type, showing that FS cell up-state firing occurred preferentially near the beginning of the up-state, whereas the firing of RS cells was slower to develop at the start of the up-state, and GIN cell firing was sustained throughout the duration of the up-state. Finally, membrane potential and spike correlations between heterogeneous cell types were more pronounced during up-states and, in the case of RS synapses onto GIN cells, varied throughout the up-state. These results suggest that there is a division of labor between FS and GIN cells as the up-state progresses and suggest that GIN cells could be important in the termination of up-states.
INTRODUCTION
The neocortex has several subtypes of inhibitory neurons, but it has remained a challenge to understand how to differentiate these neurons from one another, whether they play different functional roles in the neocortical circuit, and how their firing relates to the activity of surrounding excitatory cells. To answer these types of questions, it is helpful to know the firing characteristics of each neuronal subtype during activated conditions and how the firing patterns of each cell type relate to one another as well as to surrounding excitatory cells (Gentet et al. 2010; Klausberger et al. 2003; Puig et al. 2008).
A starting point for studying inhibitory and excitatory neuron functions is to look at the firing of these cells during activated states in the slice preparation. One of the ways to get cells in the otherwise quiescent neocortical slice to fire spontaneously is to apply artificial cerebrospinal fluid (ACSF) containing low concentrations of divalent cations (Sanchez-Vives and McCormick 2000). Such conditions induce spontaneous fluctuations between two quasi-stable states, known as up- and down-states. up-states are characterized by spontaneous firing, a relatively positive resting membrane potential, high membrane potential variance, and high membrane conductance. During the contrasting down-states, little if any firing has previously been reported, neurons rest at a relatively negative resting membrane potential, there is little fluctuation in the membrane potential, and membrane conductance is low (Contreras et al. 1996; Destexhe and Pare 1999; Shu et al. 2003; Wilson and Kawaguchi 1996). Similar states have been observed in vivo during waking and slow-wave sleep (Petersen et al. 2003; Steriade et al. 1993, 2001), although their function during these conditions is unknown. Nonetheless, multiple cell types are activated during up-states, so they provide a substrate for comparing neuronal activity patterns and relationships between neuronal firing. It is possible that by studying the relationships between neurons under these conditions, we will begin to understand how such cell firing is related under other activated conditions as well.
For this study, we investigated the participation during up- and down-states of three cell types that are central to neocortical function. Specifically, we studied excitatory regular-spiking (RS) cells and two distinct subclasses of interneurons: the fast-spiking (FS) cells and a type of somatostatin-expressing interneuron defined by its GFP expression in a transgenic mouse (GIN cells) (Oliva et al. 2000). RS cells are thought to be the cells primarily responsible for sensory representations in the somatosensory cortex, whereas the role(s) of inhibitory neurons seems to be to regulate excitation.
Subtypes of inhibitory neurons have properties that suggest they play different roles within the circuit. FS neurons are so called because they have narrow action potentials (McCormick et al. 1985; Mountcastle et al. 1969; Simons 1978) compared with RS and GIN cells (Fanselow et al. 2008), and they are often associated with the calcium binding protein, parvalbumin (PV), but not the neuroactive peptide somatostatin. FS cells receive strong, depressing excitatory input from their upstream excitatory targets (Beierlein et al. 2003) and tend to synapse on the perisomal regions and proximal dendrites (Tamas et al. 1997; Thomson et al. 1996). Their firing rate during suprathreshold current injection can be high, and they do not typically display spike rate adaptation. In contrast, GIN interneurons express somatostatin (Oliva et al. 2000) but not PV and receive facilitating input from upstream cells (Beierlein et al. 2003; Fanselow et al. 2008; Gibson et al. 1999). Similar somatostatin-expressing cells synapse on the distal dendrites of their targets (Wang et al. 2004). Their firing during suprathreshold current steps is moderate in frequency and displays spike rate adaptation (Beierlein et al. 2003; Gibson et al. 1999). Another important difference between FS and GIN cells is that the resting membrane potential of GIN cells is typically ∼8 mV more depolarized than that of FS cells (Beierlein et al. 2003; Fanselow et al. 2008). The differences in physiological characteristics between FS and GIN cells, especially differences in resting membrane potential, synaptic dynamics, and firing rates, suggest that, whereas these two neuron subtypes are both inhibitory, they would respond differently to incoming input and provide unique outputs to their targets. We sought in this study to further differentiate these cell types based on their firing properties during up- and down-states.
The goal of this study was to compare firing characteristics of three unique cell types during two different activity states. This includes not only characterizing how a given cell fired during each state, but also how cells fired relative to one another in cases when they were concurrently active. To do this, we recorded simultaneously from heterogeneous pairs of cells (RS-FS, RS-GIN, FS-GIN) during application of low-divalent ACSF in a slice of mouse neocortex. Firing activity was sorted according to activity state (up or down), and firing characteristics were assessed and compared for each cell type and each state. We showed that, of our three target cell types, only GIN cells were active during down states, whereas all three types were active during up-states. In addition, GIN cells fired the most rhythmically of all three cell types and fired more rhythmically during down- than up-states. Relationships between the sub- and suprathreshold activity of different neuron types during up-states showed that FS-GIN neuronal pairs exhibited the greatest degree of correlated subthreshold and spiking activity. Finally, spike-triggered averages showed that the relationships between firing in a presynaptic cell and membrane potential in a postsynaptic cell were more pronounced during up- than down-states.
METHODS
Slice preparation
Thalamocortical slices were prepared as previously described (Agmon and Connors 1991) using tissue from the GIN strain of mice (Jackson Labs, Bar Harbor, ME) (Oliva et al. 2000). Animals were aged postnatal days 12–17 and included both sexes. All procedures were conducted with the approval of, and in accordance with, the animal use regulations and the IACUC of Brown University. Tissue was sliced in ACSF containing (in mM) 126 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 26 NaHCO3, 10 dextrose, and 2 CaCl2, saturated with 95% O2-5% CO2. Slices were stored in ACSF of the same composition at 32°C for 30–45 min and maintained at room temperature until used for recording. Slices were 400 μm thick.
Recordings
We performed whole-cell current-clamp recordings from pairs of neurons using micropipettes (4- to 7-MΩ resistance) filled with internal solution containing (in mM) 135 K-gluconate, 4 KCl, 2 NaCl, 10 HEPES, 0.2 EGTA, 4 ATP-Mg, 0.3 GTP-Tris, and 14 phosphocreatine-Tris (pH 7.25, 280–290 mOsm). Recordings were made with Axoclamp 2B amplifiers (Molecular Devices, Sunnyvale, CA). Membrane potentials reported here were not corrected for liquid junction potential, which is estimated as 14 mV (cf. Cruikshank et al. 2007). During all recordings, after cell characterization and baseline recording, slices were perfused with “low-divalent ACSF,” which had the same composition as the normal ACSF described above except that it contained 1 mM Ca2+ and 1 mM Mg2+. It should be noted that low divalent is relative to the ACSF compositions traditionally used for slice experiments. The 1 mM Ca2+ and 1 mM Mg2+ concentrations used here are, in fact, close to the composition of natural CSF (Somjen 2004).
Cell visualization and identification
Cells were viewed under infrared-differential interference contrast (IR-DIC) illumination using a Nikon E-600FN microscope and a Dage IR-1000 CCD-camera. GIN cells were identified by visualization of GFP under epifluorescence illumination. In addition, when injected with 600-ms suprathreshold current steps, these cells showed spike-rate adaptation, and the first afterhyperpolarization (AHP) in a train of spikes was the largest in the train (Beierlein et al. 2003). In some, but not all, cases, GIN cells showed an Ih current–induced sag in the voltage trajectory during hyperpolarizing current steps (87%, n = 100 cells). It should be noted here that GIN cells do not necessarily form a uniform subgroup of inhibitory cells. Halabisky et al. (2006) showed that fluorescent cells in the GIN mouse strain display a range of physiological characteristics (e.g., varying in their spiking responses to current steps). Nevertheless, GIN cells form a closely similar subgroup of inhibitory interneurons identified specifically by their expression of GFP in the GIN mouse line. In contrast, FS cells did not express GFP in these mice and did not show spike-rate adaptation during suprathreshold current steps. RS cells did not express GFP, showed adapting spiking rates during suprathreshold current steps, and, during a train of action potentials, the first AHP was smaller than subsequent AHPs (Beierlein et al. 2003). These criteria for classifying neurons are the same as those described in previous studies from our laboratory (Beierlein et al. 2003, Fanselow et al. 2008).
Identification of up-states
The times of the beginnings and ends of up-states were identified using a custom algorithm, written in Matlab (Natick, MA) by E.E.F. The algorithm located up-states in RS and FS cells in the following way. First, for a given trace containing up-states, action potentials for the entire recording were truncated at −45 mV, and the mean of the trace was subtracted from all values in the trace (Supplemental Fig. S1, A and B, top).1 The mean of the resulting trace was found, and a “mean criterion” was calculated, which was mean value of the zeroed trace × 100 (horizontal dotted lines in Supplemental Fig. S1, A and B, top). In addition, the zeroed trace was also differentiated and smoothed using a moving average window with a width of 20 ms, and the absolute value of the resulting trace was calculated (Supplemental Fig. S1, A and B, bottom). A variance criterion was calculated, which was variance of the trace × 100 (horizontal dotted lines in Supplemental Fig. S1, A and B, bottom). A 100-ms window was moved across the two generated traces in 10-ms steps. If, during a given window, the undifferentiated trace was greater than the mean membrane potential criterion, and the differentiated trace was greater than the variance criterion, the window was marked as a qualifying window. The beginning of the up-state (2 left vertical dotted lines in Supplemental Fig. S1, A and B) was defined as the beginning of the first qualifying window that was part of a group of windows that lasted for 500 ms or more (i.e., 5 consecutive 100-ms windows). The end of the up-state was defined as the end of the last window in such a group (2 right vertical dotted lines in Supplemental Fig. S1, A and B). Because we slid the moving window in 10-ms steps, the resolution of our state transition estimates is 10 ms.
Use of this algorithm allowed us to identify the beginning and end times of the up-states in a consistent manner across RS and FS cells. GIN cells were not used to identify up-state start and end times because their state transition times were often ambiguous (see results). Instead, the up-states in GIN cells were defined as the start and end times of up-states in simultaneously recorded neighboring RS or FS cells.
Data analysis
To calculate the average membrane voltage levels for up- and down-states, spikes were eliminated from the traces by finding the peak of each action potential and taking out the 3 ms before and 3 ms after each spike. The median of the resulting voltage trace was calculated. To calculate firing frequencies and interspike intervals during up- and down-states, the times of the peaks of action potentials were identified, and the distance between consecutive spikes was calculated. Average firing rates during down-states for GIN cells were calculated for the 10 s before and the 10 s after the up-states (periods from these 10-s epochs during which another up-state occurred were omitted from the analyses). The firing rates of GIN cells before and after the up-states were not found to be significantly different from one another (P = 0.6), so values from the 10-s preup-state epochs were reported here as the down-state values.
Three analyses were conducted to show the distributions of and relationships between interstimulus intervals in RS, FS, and GIN cell up-state firing and GIN cell down-state firing. First, histograms of interspike interval distributions were generated for each cell. For population data, these histograms were normalized to the highest peak in each histogram. Histograms were averaged across cells. The second analysis was conducted by plotting the duration of a given interspike interval against the duration of the subsequent interspike interval. This method indicates how similar sequential interspike intervals are. To quantify the degree of neighboring-interspike interval similarity, we calculated the distance along the y-axis by which a given point differed from the unity line and normalized this value by the corresponding x-axis value for that point. The values for each cell condition combination were averaged. Finally, a modified CV known as CV2 (Bacci and Huguenard 2006; Holt et al. 1996) was calculated. This statistic is given by the following equation, where Δti is the time interval between a given spike and the preceding one, and Δti+1 is the time between a given spike and the succeeding one
CV2 quantifies the regularity of firing by comparing adjacent interspike intervals. It is relatively insensitive to slow variations in mean spike firing rates. CV2 ranges from 0 for perfect regularity to 1 for entirely random spike intervals.
To characterize the distribution of spikes throughout up-states in all three cell types, we calculated a number of measures relating spike times to one another. First, we calculated the time from the up-state onset (as defined by the previously described detection algorithm) to the first spike generated by a given neuron. In addition to comparing times to first spike by averaging across cells, we also calculated these spike times for individual up-states and compared neurons of different types.
Second, we quantified the spiking rates during up- and down-states. For these measures, histograms of firing frequency were created for each cell and averaged across cells. The 95% CIs were calculated for these average traces.
Third, to determine whether there were relationships between the subthreshold activity of neurons during up-states, action potential times were identified for both cells in a simultaneously recorded pair. The 1 ms before and 3 ms after each action potential was excised from each trace, and the values between these points was interpolated. A cross-correlation was run on each up-state, and cross-correlations were averaged across cells.
Fourth, we calculated the correlations between action potentials from different types of neurons during up-states. In this case, vectors were created for each up-state for each simultaneously recorded cell pair. Each bin in a given vector contained zeros where no spikes occurred or an integer value indicating the number of spikes in the bin if spikes were present. Vectors were collected into 10-ms bins, and cross-correlations were calculated between these vectors and averaged across all up-states in a cell. CIs were calculated for nonshuffled data and data shuffled across up-states for a given pair of cells. Shuffling was performed 100 times with replacement.
To determine whether cells had a chemical synaptic connection, we injected brief pulses of current into the presynaptic cell of sufficient current to get it to spike on each pulse. Trains of eight pulses at 40 Hz were provided, and the postsynatpic potentials (PSPs) in the postsynaptic cell were recorded, if present. Connections were identified when PSPs differed from the baseline by ≥3 SD of the pre-PSP value.
Statistical tests
Many of the data sets collected and analyzed for this paper were not normally distributed as assessed by the Kolmogorov-Smirnov test. In such cases, nonparametric statistical tests were used to determine whether median values were statistically different from one another. First, the Kruskal-Wallis test was used to determine whether there were differences between median values of sets of variables. If there were, Bonferroni post hoc tests were performed to determine which groups differed significantly from one another. If data for a given comparison were normally distributed, a one-way ANOVA was performed, and Bonferroni post hoc tests were applied where appropriate. Groups were considered significantly different if P < 0.05. All statistics were performed using statistical functions in Matlab.
RESULTS
Identification of up-states and distribution across ages
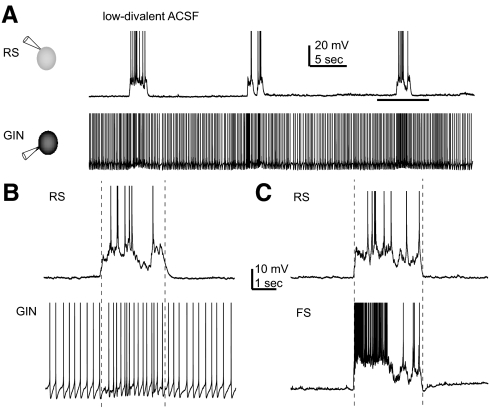
During application of low-divalent ACSF, the RS, FS, and GIN cells all showed up- and down-states (Fig. 1, A–C). The average up-state rate under our experimental conditions was 0.9 ± 0.1 up-states/min. The median up-state durations for RS and FS cells are shown in Table 1 and were not significantly different from one another (because GIN cells were not used to define up-state start and end times, there is no up-state duration value for GIN cells). up-states were identified in RS and FS cells using a custom algorithm as described in detail in methods. Briefly, the membrane voltage and the variance of the membrane voltage were each required to cross a threshold value as measured using a sliding window. An example of the algorithm can be seen in Supplemental Fig. S1, A and B. Furthermore, it can be seen in Supplemental Fig. S1, C and D that there was close agreement on up-state beginning and end times when simultaneously recorded RS and FS cells were independently used to identify the up-states. This algorithm allowed for consistent and unbiased detection of up-state beginning and end times.
Fig. 1.
up- and down-states in regular spiking (RS), fast spiking (FS), and GFP- (and somatostatin-) expressing inhibitory neurons (GIN) cells. A: simultaneously recorded RS and GIN cells during application of low-divalent artificial cerebrospinal fluid (ACSF). RS cells fired during up-states only, whereas GIN cells fired during both up- and down-states. B: period during horizontal line in A enlarged to show a single up-state in both cell types. C: up-state recorded in an FS cell and a different RS cell from that shown in A and B. In all 3 panels, action potentials are truncated for display purposes. Vertical dotted lines in B and C indicate start and end times of the up-states, as defined using the detection algorithm described in methods. In both cases, the RS cell was used to determine the up-state beginning and end.
Table 1.
Characteristics of up- and down-states in RS, FS, and GIN cells
| RS | FS | GIN | |
|---|---|---|---|
| Number of cells | 20 | 12 | 21 |
| Input resistance, MΩ* | 190 ± 17 | 114 ± 7 | 225 ± 25 |
| Number of up-states analyzed | 436 | 254 | 333 |
| up-state duration (ms) | 3354 ± 225 | 3772 ± 370 | — |
| down-state membrane potential, mV† | −68.1 ± 1.1 | −65.8 ± 1.6 | −58.7 ± 1.2 |
| up-state membrane potential, mV† | −56.2 ± 0.8 | −60.2 ± 0.6 | −54.3 ± 0.7 |
| down-state firing frequency, Hz‡ | — | — | 6.4 ± 1.7 |
| up-state firing frequency, Hz‡ | 10.3 ± 1.2 | 30.6 ± 2.6 | 11.0 ± 1.6 |
All data are medians ± SE.
FS vs. all others P < 0.01; RS and GIN not significantly different from one another, P > 0.6.
RS-down vs. RS-up: P < 1 × 10−5: FS-down vs. FS-up: P < 0.01; GIN-down vs. GIN-up: P < 0.05.
All values in these two rows different from one another (P < 0.02), except RS up and GIN up (P = 0.6). RS, regular spiking; FS, fast spiking; GIN, GFP-(and somatostatin-) expressing inhibitory neurons.
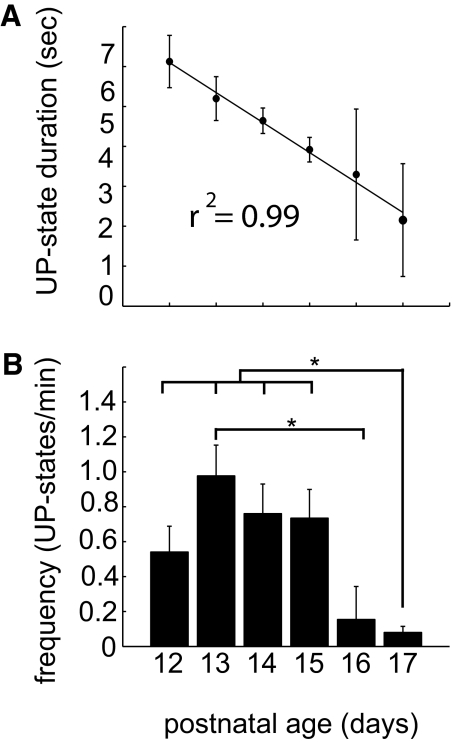
Both up-state duration and frequency varied as a function of the age of the animals, as shown in Fig. 2 for age ranges P12–P17. up-states were longest at P12 (Fig. 2A), and most prevalent at age P13 (Fig. 2B). Subsequent results were not sorted according to age.
Fig. 2.
up-state frequencies and duration by age. A: mean up-state duration decreased linearly with age from P12 to P17. Error bars represent ±SE. B: up-state frequencies in up-states per minute. Significant differences indicated with asterisks (P < 0.05). Number of cells analyzed: P12, 3; P13, 11; P14, 8; P15, 11; P16, 3; P17, 6.
RS and FS cell activity during up-states
RS and FS cells alternated between up- and down-states (Fig. 1, A–C), as previously described (Puig et al. 2008; Sanchez-Vives and McCormick 2000; Shu et al. 2003). During down-states, RS and FS cells typically did not fire, although the occasional isolated action potential was observed. In contrast, during the up-states, firing occurred in both cell types. RS cells fired at a median rate of 10.3 ± 1.2 Hz, and FS cells fired at a median rate of 30.6 ± 2.6 Hz. As shown in Supplemental Fig. S2, B and D, the distribution of membrane potential values was bimodal in RS and FS cells during low-divalent ACSF application, but unimodal during baseline recordings in normal ACSF (Supplemental Fig. S2, A and C). For both of these cell types, the median membrane potentials in up- versus down-states were significantly different from one another (see Table 1; P < 0.01).
It should be noted that recording temperature can affect the firing rates of neurons. However, the net effect of changes in temperature vary, with some authors reporting an increase in excitability of non-GIN cells (e.g., pyramidal cells) with cooling below normal body temperature (Reig et al. 2010; Thompson et al. 1985; Volgushev et al. 2000), and others reporting an increase in excitability of GIN neurons in the hippocampus with heating as temperature rises above 32°C (Kim et al. 2009). Thus, although our low recording temperature (32°C, compared with core body temperature of ∼37°C) might have affected the firing of cells during up-states, it is not clear what the net effect on each cell type would be, relative to its activity at temperatures in vivo. Furthermore, the effects of temperature may vary by cell type depending on the mix of ion channels active near rest.
GIN cell activity during up- and down-states
GIN cells also alternated between up- and down-states, but the differences between these states in GIN cells were more subtle than for RS and FS cells. For this reason, GIN cells were not used to define up-states; instead, up-states were identified in recordings from simultaneously recorded neighboring RS or FS cells (see methods for description of up-state identification algorithm). GIN cells fired during both up- and down-states (Fig. 1, A and B). The difference in membrane potential between up- and down-states in GIN cells (Supplemental Fig. S2, E and F) was statistically significant (Table 1). Note, however, that unlike RS and FS cells, the distribution of up-state membrane potentials in GIN cells during low-divalent ACSF application was unimodal, although it was wider than during baseline conditions. The median firing rate of GIN cells during down-states was 6.4 ± 1.7 Hz, whereas it increased significantly to 11.0 ± 1.6 Hz during up-states (P < 1 × 10−5).
Interspike interval characteristics
To compare firing characteristics between cell types during up-state firing, as well as with GIN cell firing during down-states, we calculated the interspike intervals during these states. It was of interest to compare interspike interval characteristics between cell types and states because distributions of interspike intervals show several important neuronal firing characteristics. First, they indicate maximum, median, and minimum firing rates. Second, the shape of their distribution is one indication of how regular or irregular their firing is. Firing regularity can also be examined by comparing neighboring interspike intervals (Fig. 3, A and B) and with the CV2 measure (Fig. 3C; see methods). The reason firing rates and regularity are important to quantify is that they indicate what influence a given neuron may have on its downstream targets. Synapses exhibit different synaptic dynamics, which are frequency-dependent and mediate how cells signal to downstream neurons.
Fig. 3.
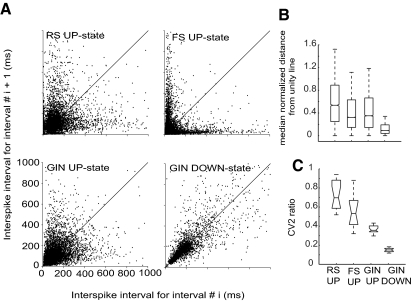
Relationships between sequential interspike intervals indicate the degree of rhythmicity of firing patterns. A: duration of interspike interval i plotted against duration of interspike interval i + 1 for each cell type and state during which firing was observed. Plots incorporate all observed interspike intervals across all recorded cells. Solid lines indicate unity. B: median normalized distance from unity line for interspike interval pairs. All median values were significantly different from one another (P < 0.001). C: median CV2 ratios across cells for each type and state for which firing occurred. Median values were all significantly different from one another (P < 0.05). Whiskers on box-and-whisker plots indicate upper and lower quartile ranges (highest and lowest 25%); boxes indicate the interquartile range (the middle 50%), and the notches on the boxes indicate 95% CIs for the median. Number of cells used in each analysis: RS, 20; FS, 12; GIN, 21.
Interspike intervals for representative individual cells are shown in Supplemental Fig. S3 for all three cell types studied and for up- and down-state firing for GIN cells. It can be seen that interspike interval distributions were widest in RS cells. Furthermore, the interspike intervals were shorter for GIN cells during up-states compared with down-states. FS cells displayed the shortest interspike intervals. Supplemental Fig. S4 shows average interspike interval distributions, which differed among all cell types and between states for GIN cells. Median interspike intervals for RS, FS, and GIN cells during up-states and GIN cells during down-states were all significantly different from one another (P < 1 × 10−5). In addition, the shapes of the distributions differed, with the FS distribution being heavily skewed toward short interspike intervals but with a long tail of larger interspike intervals. The average distributions of RS and GIN cells during up-states and GIN cells during down-states were similar, although the medians differed, as discussed above. However, the interspike interval distribution for GIN cells during the down-state decayed rapidly at ∼50 ms, indicating a fairly firm lower interspike interval limit (Supplemental Fig. S4, GIN down-state). Furthermore, the distributions themselves for each cell type were all significantly different from one another (Kolmogorv-Smirnov test: P < 1 × 10−4). It should be noted that, whereas the average histograms for the GIN cell up- and down-states are quite broad, this is in part because of the fact that the population of cells fired across a range of frequencies (cf. Fanselow et al. 2008). In some cases, the interspike interval histogram for an individual cell was much narrower than the mean for all GIN cells (cf. Supplemental Figs. S3 and S4, GIN cells).
Uniformity of firing: relationships between sequential interspike intervals
In addition to the interspike interval distributions, we also studied the relationships between sequential interspike intervals as a way to assess the degree of firing regularity during up-states for all three cell types and for GIN cells during down-states. The first of these methods involves plotting the duration of a given interspike interval against the duration of the subsequent interspike interval (Fig. 3, A and B). The farther a point was from the unity line, the less similar two sequential interspike intervals were. It can be seen in Fig. 3A that GIN cell firing during the down state was more regular (i.e., points were closer to the unity line) than during up-state firing for all three cell types. This effect was quantified in Fig. 3B by calculating the absolute values of the difference between the y-axis values and the unity line and normalizing these by dividing by the x-axis value for a given point. The median values for the distance from the unity line were all significantly different from one another (P < 1 × 10−3).
Another measure of firing regularity is CV2 (see methods; cf. Bacci and Huguenard 2006; Holt et al. 1996). CV2 varies from 0 (perfectly rhythmic firing) to 1 (random firing intervals). All median CV2 values (i.e., up-state firing for all 3 cell types and down-state firing for GIN cells) differed significantly from one another (P < 0.01; Fig. 3C). It can be seen in Fig. 3, A–C, that firing of GIN cells during the down-state was the most regular (smallest median distance from unity line, smallest CV2 ratio). In contrast, RS cell spiking during up-states was the least regular (highest median distance from the unity line, largest CV2 ratios).
Onset of up-states and time to first spike
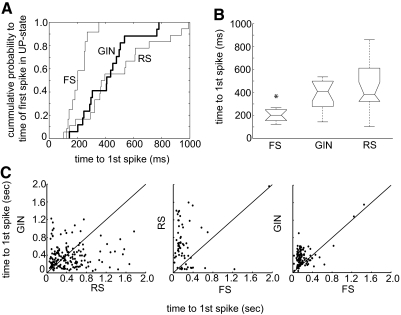
The onset of up-states was often marked most dramatically by firing of FS cells (cf. Fig. 1C). To quantify this, we measured the latency of the first spike in an up-state. A cumulative distribution of the average time to first spike shows that all cell types were capable of firing within ∼150 ms of up-state onset; however, the majority of GIN and RS times to first spike were longer than those for FS cells (Fig. 4A). The median time to first spike for each cell type is shown in Fig. 4B. FS cells fired significantly sooner than GIN and RS cells. RS cells showed the greatest variability of first spike latency.
Fig. 4.
Time to 1st spike during a given up-state differs according to cell type. A: cumulative distribution plots of times in milliseconds to the 1st spike in an up-state averaged for each cell. B: median times to 1st spike for each cell type. FS median is significantly different from RS and GIN (P < 0.001). Conventions for box-and-whisker plots as in Fig. 3. C: times to 1st spike in up-states for the neuronal pair types indicated (RS-GIN, n = 14 pairs, 166 up-states; RS-FS, n = 3 pairs, 62 up-states; FS-GIN, n = 5 pairs, 111 up-states). Note that times to 1st spike that were >2 s were not included in graphs in C. Solid lines indicate unity.
Although median values indicated population tendencies, it was also important to ask whether times to first spikes differed on an individual up-state basis. Thus in Fig. 4C, we plotted the time to the first spike for different pairs of simultaneously recorded cells. It can be seen that, whereas GIN and RS cell points fell uniformly on either side of the unity line, those for RS-FS and FS-GIN pairs did not. In both cases, the initial spike of the FS cell preceded that of the RS or GIN cell. Thus FS cells often led up-state firing, relative to RS and GIN neurons.
Distribution of firing during up-states
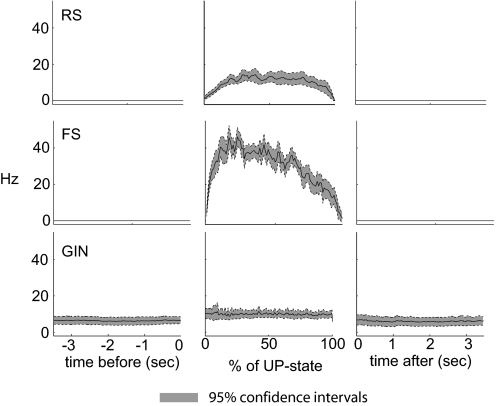
To compare the distribution of firing for all three cell types during up-states, we computed spike times as a percentage of total up-state duration. The resulting firing distributions are shown in the middle panels of Fig. 5. FS cells exhibited the bulk of their spikes during the first half of the up-state and then firing tapered off dramatically. In contrast, the firing rate of GIN cells was slower but more consistent throughout the up-states. RS cell firing built gradually during the first ∼20% of the up-state and then remained stable until falling during the last ∼5% of the up-state. Firing during the 3.5 s preceding and after up-states is shown in the left and right panels of Fig. 5 for comparison to firing levels during the up-states (no firing was present in RS or FS cells during down-states). We also analyzed these data by quantifying the firing rates during the first and last seconds of up-states that were ≥2 s in duration (Supplemental Fig. S5). That is, we analyzed the data without normalizing to up-state duration as in the middle panels of Fig. 5. The results were similar to those using normalized durations.
Fig. 5.
Distribution of firing before, during, and after up-states. Firing rates are shown for the 5 s before and after up-states (left and right, respectively) and for up-states themselves (middle). up-states were normalized in time by up-state durations, i.e., spike times were calculated as a percent of the total up-state duration. Solid lines indicate means and dotted lines/shading indicate 95% CIs. Number of cells analyzed: RS, 20; FS, 12; GIN, 21.
Spike and membrane potential correlations among cell types
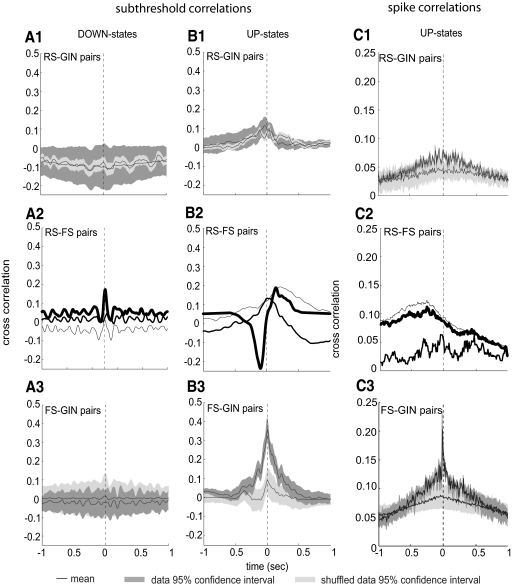
Because we recorded from pairs of cells simultaneously, we were able to determine the relationships between the activity of the different cell types during up- and down-states. These analyses were done by calculating the cross-correlations for either subthreshold membrane potentials (down-states: Fig. 6, A1–A3; up-states, Fig. 6, B1–B3) or for action potential times (up-states only, Fig. 6, C1–C3) for all three combinations of neuron types (RS-GIN, RS-FS, FS-GIN). As shown in Fig. 6, A1–B3, the strongest subthreshold correlation was observed between FS and GIN cells during up-states (Fig. 6B3). Note that for the three synaptically connected RS-FS pairs recorded, two had a monosynaptic RS to FS connection and one had a reciprocal connection. Because of this heterogeneity, we did not average the subthreshold traces (Fig. 6, A2 and B2). It can be seen in Fig. 6B2 that, during up-states, the two pairs with monosynaptic RS to FS connections (thinnest 2 traces) showed a positive correlation (RS cell as reference; see segments of trace to the right of the dotted line), whereas the pair with the reciprocal connection (boldest line in Fig. 6, B1 and B2) showed both a negative correlation preceding a given point in the RS cell membrane potential (i.e., to the left of the dotted line in Fig. 6B2) and a positive correlation following a given point in the RS cell membrane potential (i.e., to the right of the dotted line in Fig. 6B2). In addition to this, there were correlations between FS and GIN membrane potentials during up-states (Fig. 6B3), but these occurred at time 0, showing that these cells tended to depolarize simultaneously. Other subthreshold relationships were not different from the shuffled data.
Fig. 6.
Sub- and suprathreshold cross-correlations for each type of cell pair. A1–A3: subthreshold cross-correlations for RS-GIN (number of pairs = 14; number of up-states = 239), RS-FS (number of pairs = 3; number of up-states = 77), and FS-GIN (number of pairs = 5; number of up-states = 121) pairs, respectively, during down-states. Black lines indicate means of data and shuffled data. Dark and light shading indicate 95% CIs for nonshuffled data and shuffled data, respectively. B1–B3: action potential cross-correlations for the same 3 cell pairings as in A [number of spikes for RS-GIN pairs = 1,764 (RS) and 1,421 (GIN); number of spikes for RS-FS pairs: 1,079 (RS) and 10,471 (FS); number of spikes for FS-GIN pairs = 7,787 (FS) and 2,704 (GIN)]. C1–C3: spike cross-correlations for each heterogeneous cell pairing. CIs were not calculated for RS-FS pairs because n = 3 for this pairing. Number of pairs for RS-GIN = 14; number of pairs for FS-GIN = 5.
Similarly, when correlations between action potentials were calculated, it was clear that the highest degree of significant correlation was between FS and GIN cells (Fig. 6C3), whereas there was virtually none for the other cell pairs (Fig. 6, C1 and C2). Note, however, that the broad increase in RS-FS correlation preceding time 0 in Fig. 6C2 indicates that RS cell firing tended to follow FS firing, which correlates well with data in Fig. 5 showing that the bulk of FS firing tended to occur near the beginning of the up-state, whereas RS firing was distributed throughout.
Spike triggered averages among cell types
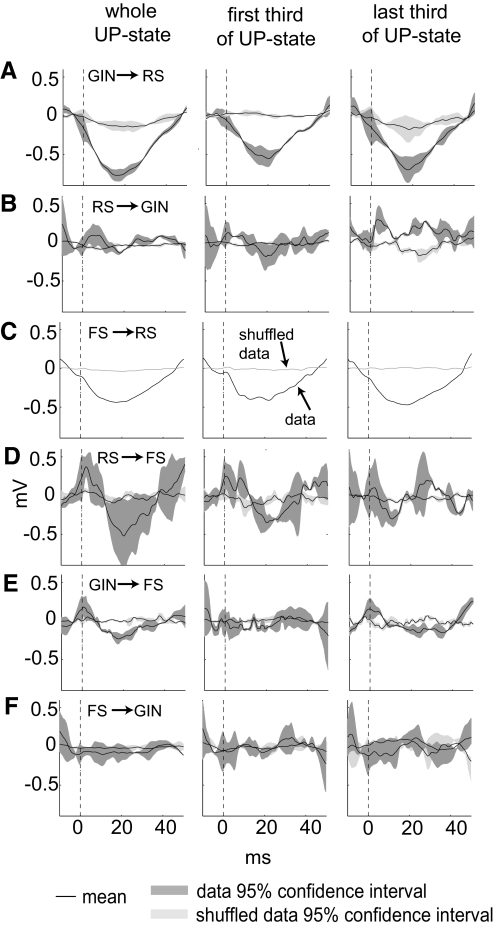
Finally, we estimated how the firing of one cell type influenced the subthreshold activity of other cell types during both up- and down-states by generating spike-triggered membrane potential averages for all the connected cell pairs we recorded (Fig. 7). In these analyses, we only used postsynaptic traces that did not contain an action potential within ±30 ms of the presynaptic spike. It can be seen in Fig. 7A that RS cells hyperpolarized after GIN spikes. This relationship did not hold for RS responses to GIN spikes during down-states, when no discernible depolarization or hyperpolarization was evident (data not shown). This could be because during down states RS membrane potentials are closer to the Cl− reversal potential than they are during up-states, and GIN cell-generated IPSPs would be larger during up-states. RS spikes caused a slight increase in GIN cell membrane potentials (Fig. 7B) during up-states, but this was not a dramatic effect, suggesting these were relatively weak synapses. A fairly predictable response was the tendency of an RS cell to be hyperpolarized after FS spikes (Fig. 7C; note that we only found 1 FS to RS connection, so no CIs were calculated). FS membrane potentials after RS spikes were highly variable (Fig. 7D), so it is difficult to determine whether there was any relationship here. During up-states, FS cells depolarized near the time of GIN spikes (dotted line in Fig. 7E), suggesting that FS and GIN cells receive simultaneous excitatory drive during the up-states. However, unlike the GIN-to-RS pairs, the FS cells were not inhibited after GIN spikes, suggesting ineffective synapses between these cell types during up-states. During down-states, there was no discernible correlation between GIN firing and FS membrane potential (data not shown).
Fig. 7.
Spike-triggered averages for heterogeneous cell pairings during up-states. A–F indicate spike-triggered average membrane potentials for each cell pairing during up-states. First column is spike triggered averages for the entire up-state; 2nd column is for presynaptic action potentials that occurred during the 1st 3rd of the up-state; 3rd column is for presynaptic action potentials that occurred during the last 3rd of the up-states. Lines and shading as in Fig. 6. Numbers of pairs analyzed (number of spikes in whole up-states, 1st 3rd and last 3rd): GIN → RS: 4 (889, 336, 215); RS → GIN: 3 (322, 107, 34); FS → RS: 1 (2,169, 738, 324); RS → FS: 3 (503, 204, 67); GIN → FS: 5 (5,393, 1,629, 2,286); FS → GIN: 5 (6,056, 2,581, 1,089).
Spike-triggered averages across the up-state
We next asked whether there were differences in the strength of these synapses at the beginning and ends of the up-states. To do this, we divided the up-states into thirds and compared the magnitudes of the PSPs during the first and last thirds (Fig. 7). It can be seen that the RS → GIN synapse facilitated slightly as the up-state progressed (Fig. 7B, middle and right). Other synapses did not change greatly across these periods.
DISCUSSION
During many activated conditions, both in vivo and in vitro, cortical cells operate in two distinct, alternating modes known as up- and down-states. In this study, we recorded simultaneously from pairs of excitatory RS and inhibitory FS cells, as well as an additional inhibitory cell type—the GIN cells (Oliva et al. 2000)—during up- and down-states. We found that, although all three types of cells fired readily during up-states, only the GIN cells fired during both states. In addition, whereas FS cells fired first and robustly at the beginning of the up-state and then tapered their firing, RS cells' activity built up gradually at the beginning and remained mostly steady throughout. GIN cells, in contrast, increased their average firing near the beginning of the up-state and maintained that rate throughout. Finally, we showed that synaptic strength was state dependent and that the strength of the RS-to-GIN synapse varied throughout the up-state in a manner predicted by the short-term dynamics of these synapses. Our results suggest that as a barrage of network activity arrives and evolves in the neocortex there may be a division of labor between GIN and FS inhibitory cells, and they imply that GIN cells could contribute to the termination of up-states, whereas FS cells are less likely to do so. It should be noted that this mechanism for up-state termination does not exclude other proposed mechanisms such as a build-up of a Na+-dependent K+ current (Compte et al. 2003). However, our results suggest that if inhibitory neurons do contribute to up-state termination, GIN cells are more likely to do so than FS cells.
Age dependence of up-state duration and frequency
We observed dramatic differences in both up-state duration, which decreased linearly with age (Fig. 2A), and up-state frequency, which peaked at P13 and declined thereafter (Fig. 2B). The mechanism by which these effects take place in developing mouse neocortex is unknown but may be related to the maturation of excitatory or inhibitory cell populations during this time (Long et al. 2005; McCormick and Prince 1987; Reyes et al. 1998). Given that the GIN cell network is maturing during this time period, it seems that up-state prevalence and duration are inversely related to GIN network maturation.
In a recent study, Reig et al. (2010) showed that the duration of up-states and the frequency of the oscillation between up- and down-states are affected by temperature. Therefore the absolute values of our up-state durations and frequencies might be different in vivo, where the body temperature is ∼37°C, compared with our in vitro data, which were recorded at 32°C. However, given that all of our recordings were done at 32°C, it is likely that the relative changes in up-state duration and frequency across age would still hold in vivo.
up-states as a model of spontaneous network activity
During up-states, RS, GIN, and FS cells were all active. The up-states observed here resemble endogenous brain activity in several ways. First, up-states are observed in vivo and are similar to those observed in vitro (Sanchez-Vives and McCormick 2000). Second, there was spontaneous activity of all cell types studied, and FS cells fired at a much faster rate (∼31 Hz) than did excitatory cells (∼10 Hz), a qualitative relationship that is also observed in vivo in both anesthetized and awake rodents (Simons and Carvell 1989; Vijayan et al. 2010). Finally, although down-states correlate with relatively low neuronal input conductance, up-states in vivo and in vitro constitute a high-conductance state, reminiscent of that observed in intact animals (Destexhe and Pare 1999; Destexhe et al. 2003; Rudolph et al. 2005). Therefore we consider up-states to be a potential window onto the normal firing relationships between different types of neurons.
During down-states, however, only GIN cells fired, suggesting these states are not likely to be representative of endogenous brain activity, as other cell types are generally spontaneously active in the intact neocortex (Simons and Carvell 1989; Vijayan et al. 2010). down-states may instead represent an incipient condition during which only GIN cells have been sufficiently activated to fire. This state could occur in part because GIN cells sit at a more positive resting membrane potential and have a lower action potential threshold than do RS or FS cells, causing them to be more readily driven to fire by low-level activation methods (Fanselow et al. 2008). Interestingly, GIN cells do not seem to need input from other cells to keep them active during the down-state. Instead, during this state they are intrinsically active, as are certain other neurons in the brain (Bean 2007).
State-dependent circuit properties
Multiple aspects of neuronal input, firing, and output are dictated by the state of the circuit at a given point in time. For example, GIN cells synapse on downstream RS cells with reasonably high frequency (33–90%; E. E. Fanselow, K. A. Richardson, and B. W. Connors, unpublished observations; cf. Beierlein et al., 2003 for statistics on similar low-threshold spiking cells), but the hyperpolarizing influence they exert on these RS cells is state-dependent. This was shown here by recording from synaptically connected GIN-to-RS pairs during both up- and down-states. RS cells showed robust hyperpolarization in response to GIN spikes during up-states (Fig. 7A) but not during down-states (data not shown). Interestingly, GIN-to-FS inhibition was weak during both states (see Fig. 7E for up-states). It is possible that hyperpolarizing RS responses to GIN-mediated IPSPs were larger during up-states because RS cells were more depolarized during up-states. However, this was not observed for GIN-to-FS connections, despite the depolarization of FS cells during up-states.
In addition, we found that synaptic strength was modulated across the up-state for RS-to-GIN synapses. These synapses showed little, if any, postsynaptic response at the beginning of an up-state (Fig. 7B, middle), but showed a postsynaptic response by the end of the up-state (Fig. 7B, right). This result corresponds well with previous data on these synapses showing that they are initially weak but facilitate during a train of presynaptic action potentials (Beierlein et al. 2003; Gibson et al. 1999). Our results show that the presynaptic RS activity evoked by up-states is sufficient to cause facilitation of RS-to-GIN synapses. This suggests GIN cells could be recruited by endogenous levels of neocortical activity and that, although GIN cells may not receive strong excitatory input at the onset of a barrage of excitatory activity, they may do so after some time and continual firing have elapsed.
Dynamic sources of inhibition
We showed that FS cells in layer 2/3 preferentially fire at the beginning of up-states and tend to begin firing earlier than do RS or GIN cells. These data reinforce those of Puig et al. (2008), which showed that FS cells in L2/3, but not L5, are activated preferentially during the first half of the up-states. This robust early activation and subsequent tapering of activity could be caused by at least three nonexclusive phenomena. First, FS cells have been shown to be highly excited by incoming synaptic activity, more so than RS cells (Cruikshank et al. 2007, 2010; Gibson et al. 1999). Cruikshank et al. (2007) showed that this effect in thalamocortical synapses is caused by the strength of thalamic input onto FS cells as well as circuit dynamics that resulted in greater suppression of RS responses. Because of these phenomena, the initial barrage of excitatory activity accompanying an up-state may robustly activate FS cells before other cell types. Second, it has been shown that FS cells receive dramatically depressing synapses from presynaptic RS cells (Beierlein et al. 2003), and thus their decline in activation throughout the up-state may reflect depressing input from upstream excitatory cells. Finally, FS cells may inhibit RS and GIN cells more near the start of the up-states (because of the robust of activity of FS cells during this time), so that, despite receiving similar input from the passing barrage of up-state activity, RS and GIN cells' activity would be relatively stronger later in the up-states when FS activity has declined.
Studies by McCormick and colleagues have shown that, throughout an up-state, there is a balance of excitatory and inhibitory conductances (Haider et al. 2006; Shu et al. 2003). That is, although the total conductance of a given neuron decreases slightly throughout the up-state, the excitatory and inhibitory conductances track one another closely. Other studies have suggested there is an abundance of inhibitory conductance during up-states, such that the inhibitory conductance is on the order of five times larger than the excitatory conductance (Destexhe et al. 2003). Either scenario may be critical for maintaining nonpathological conditions such as up-states. However, the type(s) of inhibitory cells contributing to the inhibitory conductances during the up-state has not been clear. This is especially relevant when one considers that RS-to-FS and FS-to-RS synapses both depress in response to presynaptic action potentials. Thus during an up-state, if the FS cells are less involved in the circuit because of ongoing synaptic depression, could there be another source of inhibition that substitutes to maintain the proper excitatory-inhibitory ratio?
A likely candidate for such a role in the neocortical circuit is the GIN cells (or, more broadly, the somatostatin-expressing interneurons). These cells receive strongly facilitating excitatory inputs from presynaptic RS cells, do not tend to inhibit one another, and have a high rate of projections to RS and FS cells. The facilitating excitatory input GIN cells receive suggests that, whereas these cells would not be activated at the beginning of a barrage of incoming activity, such as occurs during an up-state, they may be more readily activated after some time has passed—enough time for the RS-to-GIN synapses to facilitate. In this way, GIN cells would come “on-line” in the circuit later than would FS cells during an up-state.
State-dependent circuit function
Collectively, these results on the state-dependence of neuronal input and output properties suggest that circuit-level neuronal phenomena must be interpreted in the context (state) a circuit is in at a given point in time. This is because characteristics such as synapse dynamics (facilitation and depression) and postsynaptic response magnitude can differ between and throughout different states. This could be because of such factors as differences in firing rate and differences in membrane potential or membrane conductance between states. It is critical to understand how these factors influence which cells are available to the circuit under a given condition because this dictates how the neocortical circuit might respond to thalamocortical or intracortical input. (Table 2)
Table 2.
Numbers of cell pairs and synaptic connections
| Total Pairs | Reciprocal Connections | |
|---|---|---|
| RS-GIN pairs recorded | 14 | 5 |
| RS to GIN connections | 8 | |
| GIN to RS connections | 5 | |
| RS-FS pairs recorded | 3 | 1 |
| RS to FS connections | 2 | |
| FS to RS connections | 1 | |
| FS-GIN pairs recorded | 5 | 0 |
| FS to GIN connections | 0 | |
| GIN to FS connections | 5 |
See Table 1 for abbreviations.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-025983 and NS-050434 to B. W. Connors and NS-046163 to E. E. Fanselow and the Epilepsy Foundation through the generous support of the American Epilepsy Society and the Milken Family Foundation to E. E. Fanselow.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Patrick for general technical support and Dr. Brent Doiron for consultation about analysis methods.
Present address of E. E. Fanselow: Department of Neurobiology, University of Pittsburgh School of Medicine, W1458 Thomas E. Starzl Biomedical Science Tower, 200 Lothrop St., Pittsburgh, PA 15261.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41: 365–379, 1991 [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR. Enhancement of spike-timing precision by autaptic transmission in neocortical inhibitory interneurons. Neuron 49: 119–130, 2006 [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007 [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90: 2987–3000, 2003 [DOI] [PubMed] [Google Scholar]
- Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol 89: 2707–2725, 2003 [DOI] [PubMed] [Google Scholar]
- Contreras D, Timofeev I, Steriade M. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol 494: 251–264, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10: 462–468, 2007 [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65: 230–245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Pare D. Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol 81: 1531–1547, 1999 [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci 4: 739–751, 2003 [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol 100: 2640–2652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron 65: 422–435, 2010 [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999 [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci 26: 4535–4545, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabisky B, Shen F, Huguenard JR, Prince DA. Electrophysiological classification of somatostatin-positive interneurons in mouse sensorimotor cortex. J Neurophysiol 96: 834–845, 2006 [DOI] [PubMed] [Google Scholar]
- Holt GR, Softky WR, Koch C, Douglas RJ. Comparison of discharge variability in vitro and in vivo in cat visual cortex neurons. J Neurophysiol 75: 1806–1814, 1996 [DOI] [PubMed] [Google Scholar]
- Kim JA, Kauer JA, Connors BW. Hyperthermia increases intrinsic excitability and spontaneous synaptic activity of hippocampal CA3 pyramidal cells and interneurons. Soc Neurosci Abst 24116, 2009 [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421: 844–848, 2003 [DOI] [PubMed] [Google Scholar]
- Long MA, Cruikshank SJ, Jutras MJ, Connors BW. Abrupt maturation of a spike-synchronizing mechanism in neocortex. J Neurosci 25: 7309–7316, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–806, 1985 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J Physiol 393: 743–762, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol 32: 452–484, 1969 [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20: 3354–3368, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA 100: 13638–13643, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Ushimaru M, Kawaguchi Y. Two distinct activity patterns of fast-spiking interneurons during neocortical up states. Proc Natl Acad Sci USA 105: 8428–8433, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig R, Mattia M, Compte A, Belmonte C, Sanchez-Vives MV. Temperature modulation of slow and fast cortical rhythms. J Neurophysiol 103: 1253–1261, 2010 [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci 1: 279–285, 1998 [DOI] [PubMed] [Google Scholar]
- Rudolph M, Pelletier JG, Pare D, Destexhe A. Characterization of synaptic conductances and integrative properties during electrically induced EEG-activated states in neocortical neurons in vivo. J Neurophysiol 94: 2805–2821, 2005 [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034, 2000 [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature 423: 288–293, 2003 [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol 41: 798–820, 1978 [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol 61: 311–330, 1989 [DOI] [PubMed] [Google Scholar]
- Somjen GG. Ions in the Brain. Oxford: Oxford University Press, 2004 [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85: 1969–1985, 2001 [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol 500: 715–738, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Masukawa LM, Prince DA. Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci 5: 817–824, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC, Hahn J, Deuchars J. Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J Physiol 496: 81–102, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan S, Hale GJ, Moore CI, Brown EN, Wilson MA. Activity in the barrel cortex during active behavior and sleep. J Neurophysiol 103: 2074–2084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, Eysel UT. Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J Physiol 522: 59–76, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol 561: 65–90, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci 16: 2397–2410, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.