Abstract
Trace eyelid conditioning is a form of associative learning that requires several forebrain structures and cerebellum. Previous work suggests that at least two conditioned stimulus (CS)-driven signals are available to the cerebellum via mossy fiber inputs during trace conditioning: one driven by and terminating with the tone and a second driven by medial prefrontal cortex (mPFC) that persists through the stimulus-free trace interval to overlap in time with the unconditioned stimulus (US). We used electric stimulation of mossy fibers to determine whether this pattern of dual inputs is necessary and sufficient for cerebellar learning to express normal trace eyelid responses. We find that presenting the cerebellum with one input that mimics persistent activity observed in mPFC and the lateral pontine nuclei during trace eyelid conditioning and another that mimics tone-elicited mossy fiber activity is sufficient to produce responses whose properties quantitatively match trace eyelid responses using a tone. Probe trials with each input delivered separately provide evidence that the cerebellum learns to respond to the mPFC-like input (that overlaps with the US) and learns to suppress responding to the tone-like input (that does not). This contributes to precisely timed responses and the well-documented influence of tone offset on the timing of trace responses. Computer simulations suggest that the underlying cerebellar mechanisms involve activation of different subsets of granule cells during the tone and during the stimulus-free trace interval. These results indicate that tone-driven and mPFC-like inputs are necessary and sufficient for the cerebellum to learn well-timed trace conditioned responses.
INTRODUCTION
Trace eyelid conditioning seems to provide an opportunity to apply an experimentally tractable behavior to the study of interactions between brain systems (Clark et al. 2002; Kalmbach et al. 2009; Weiss and Disterhoft 1996). Whereas delay eyelid conditioning involves the acquisition of eye closure using training trials in which a tone conditioned stimulus (CS) overlaps in time with a reinforcing unconditioned stimulus (US), trace eyelid conditioning involves paired presentations of the same stimuli separated by a stimulus-free trace interval (typically 500 ms). This small procedural difference has an important influence on the brain systems involved. Whereas delay eyelid conditioning involves learning in the cerebellum that can occur without the forebrain (Mauk and Thompson 1987; Thompson and Steinmetz 2009), trace eyelid conditioning requires the hippocampus, caudate nucleus, primary sensory cortex, and medial prefrontal cortex (mPFC) in addition to the cerebellum (Flores and Disterhoft 2009; Galvez et al. 2007; Kalmbach et al. 2009; Kim et al. 1995; Kronforst-Collins and Disterhoft 1998; McLaughlin et al. 2002; Moyer et al. 1990; Solomon et al. 1986; Takehara et al. 2003; Weible et al. 2000; Woodruff-Pak et al. 1985).
Recent studies suggest that trace eyelid conditioning involves cerebellar learning in response to mossy fiber input driven by the mPFC. Trace eyelid conditioning requires a region of mPFC that projects to the cerebellum via the lateral pontine nuclei (Kalmbach et al. 2009; Weible et al. 2000, 2007). Inactivation of the lateral pontine nuclei abolishes trace but not delay eyelid responses, suggesting that the mossy fibers that are necessary for trace conditioning are distinct from tone-driven mossy fibers that are necessary for delay eyelid conditioning (Kalmbach et al. 2009). Such mPFC-driven mossy fibers may be necessary for trace eyelid conditioning because 1) tone-driven mossy fiber input to the cerebellum does not outlast the tone CS (Aitkin and Boyd 1978; Boyd and Aitkin 1976; Freeman and Muckler 2003) and 2) the cerebellum does not learn when the reinforcing unconditioned stimulus (US) arrives more than ∼400 ms after a mossy fiber input—as occurs for tone-driven mossy fibers in trace conditioning (Kalmbach et al. 2009). Together with observations that mPFC neurons fire persistently through the trace interval (Takehara-Nishiuchi and McNaughton 2008), these observations suggest that a mPFC-driven mossy fiber input that persists through the trace interval enables the cerebellar learning that is necessary for trace eyelid conditioning (Fig. 1A). Explicitly stated, during trace eyelid conditioning, the forebrain provides a delay conditioning-like input to the cerebellum that enables learning of the observed eyelid responses.
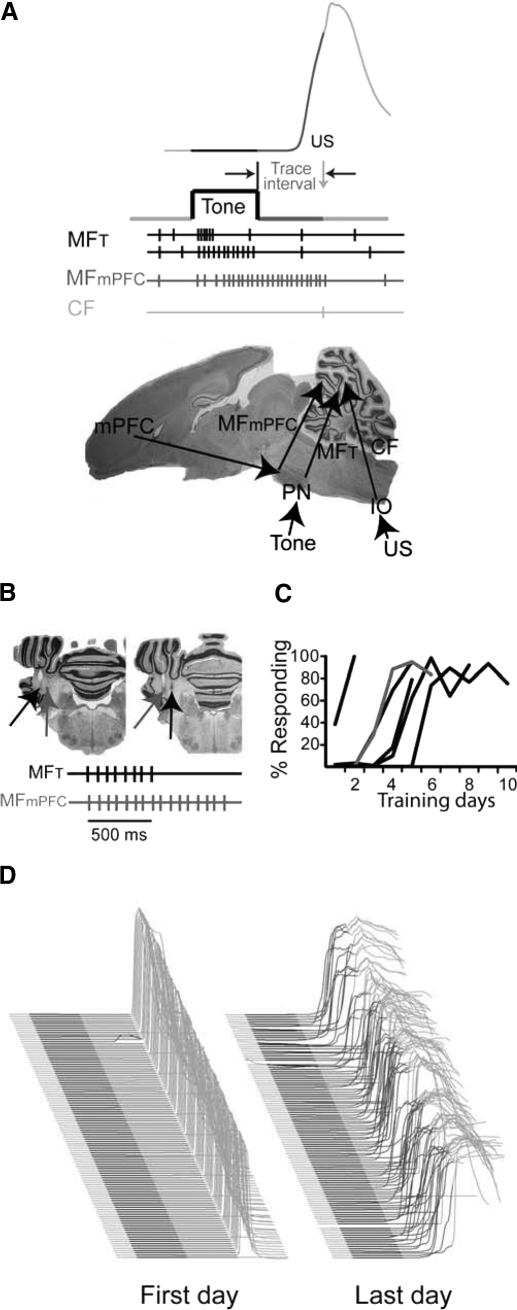
Fig. 1.
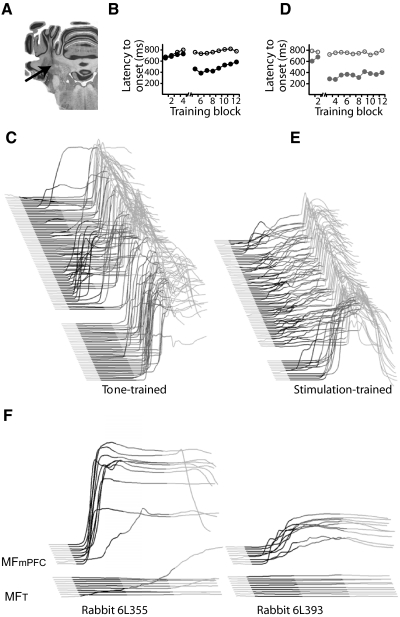
Stimulating mossy fibers to mimic medial prefrontal cortex (mPFC)-driven and tone-driven input supports learning. A: the procedures, neural pathways, and putative signals involved in trace eyelid conditioning. Top: trace eyelid conditioning involves the repeated presentation of a neutral stimulus, such as a tone, followed by a stimulus-free interval (trace interval) and a blink-evoking stimulus such as electrical stimulation around the eye [unconditioned stimulus (US)]. With repeated presentations, rabbits learn to close their eye in response to the tone. A sample sweep plotting eyelid position during a representative trial in a well-trained rabbit is shown at top. An upward deflection indicates eyelid closure, the black region marks the duration of the tone, and the dark gray region marks the trace interval. Responses during the light gray region are reflexive responses to the US. Middle: signals from cerebellar inputs during trace conditioning to a tone. Tones activate a subset of mossy fibers (MFT) that respond only to the beginning of the tone or others that respond for the duration of the tone. Other mossy fibers receive input from mPFC (MFmPFC) and putatively are activated at tone onset and respond persistently through the trace interval, consistent with the activity of mPFC neurons during trace eyelid conditioning. The US activates climbing fibers (CFs). Bottom: sagittal, whole brain section showing the pathways and structures involved in trace eyelid conditioning. PN, pontine nuclei; IO, inferior olive. B: stimulation protocol and electrode placements. Two electrodes were placed 1 mm lateral to each other in the middle cerebellar peduncle (2 sample placements are shown). One electrode was used to deliver stimulation to mimic how tones activate mossy fibers and the other to mimic mossy fibers putatively driven by persistent activity in mPFC. C: learning curves for 6 rabbits given mossy fiber stimulation during eyelid conditioning. The gray curve indicates the rabbit whose sample behavioral sweeps are shown in D. D: 108 trials from the first and last training days are stacked from first on bottom to last on top. The black region denotes the tone and the dark gray region denotes the trace interval. Responses during these intervals are learned.
We tested a component of this hypothesis by using electrical stimulation of mossy fibers during eyelid conditioning to mimic the temporal patterns of activity observed in tone driven mossy fibers and in mPFC (e.g., a compound input where 1 input overlaps in time with the US and another that ends when the CS would end). We found that combined presentation of these two inputs is necessary and sufficient for the cerebellum to learn behavioral responses that quantitatively match normal trace responses using a tone CS. Probe trials provided evidence that the cerebellum learns to respond to the mPFC-like input that overlaps with the US while learning to suppress responding to the tone-like input (that does not). These data seem to explain how the offset of the tone influences the timing of responses (Kehoe and Napier 1991; Kehoe and Weideman 1999), despite learning occurring to a mossy fiber input that is driven by CS onset (Kalmbach et al. 2010). Computer simulations suggest that these behavioral properties arise from the cerebellum learning to suppress responding to granule cells active during the CS while learning to initiate responding to granule cells active between CS offset and US onset (the trace interval).
These data support the general hypothesis that trace eyelid conditioning involves a collaboration between forebrain and cerebellum in which 1) the mPFC provides activity in response to the tone that persists into the trace interval, which 2) enables cerebellar learning and, along with tone-activated mossy fibers, provides a pattern of input that is necessary and sufficient for the cerebellum to learn and express the behavioral responses that are observed. The temporal properties of these responses arise in part because the cerebellum learns to respond to the input that persists through the trace interval while learning to suppress responding to the input that terminates with the tone CS. These data offer a concrete instance of the computational need for persistent activity in mPFC and suggest mechanisms for how this activity, together with activity driven more directly by the outside world, is decoded and processed by a downstream structure to solve a challenging behavioral task.
METHODS
Rabbits
Data were obtained from 33 male New Zealand albino rabbits (Oryctolagus cuniculus, Myrtle's rabbitry, Thompsons Station, TN). The animals weighed between 2.5 and 3 kg, were individually housed, were fed daily, and had free access to water. Treatment of animals and surgical procedures were in accordance with National Institutes of Health Guidelines and an institutionally approved animal welfare protocol.
Surgery
Animals were prepared for microstimulation, reversible inactivation, or behavioral experiments. Before surgery, a preanesthetic (40 mg/kg ketamine and 5 mg/kg acepromazine) was injected subdermally, and each rabbit was positioned in a stereotaxic restrainer such that lambda was 1.5 mm ventral to bregma. General anesthesia was maintained with isofluorene (2% mixed in oxygen), and sterile procedures were used throughout the surgery. In six rabbits, a 26-gauge stainless steel guide cannula (Plastics One, Roanoke, VA) was placed in the interpositus nucleus of the cerebellum ipsilateral to the training eye (5 mm lateral, 13.3 mm ventral, and 1 mm anterior from lambda), and two laterally spaced (1 mm) stimulating electrodes (A-M Systems, Carlsborg, WA; tip exposed to obtain impedance of ∼100–200 kΩ) were placed in the middle cerebellar peduncle ipsilateral to the training eye (5.5 mm lateral, 16 mm ventral, and 3 mm anterior from lambda). In five rabbits, a guide cannula was placed in the interpositus nucleus of the cerebellum. Six rabbits were implanted with a single stimulating electrode in the middle cerebellar peduncle. Sixteen rabbits were prepared with a head stage for behavioral experiments. These various implants, a bolt to attach the eyelid detector to the skull, anchor screws, and ground screws were secured in place with dental acrylic. Finally, two stainless steel stimulating electrodes were implanted in the periorbital muscles of the eye. Animals were given postoperative analgesics and antibiotics for 2 days after surgery and were allowed to recover for a week before experiments began.
Conditioning
Animals were trained in custom-designed, well-ventilated, and sound attenuating chambers measuring ∼90 × 60 × 60 cm (length, width, height). Each chamber was equipped with a speaker that was connected to an audio source module (model V85-05, Coulborn Instruments, Allentown, PA) used to generate tones and with electrical leads connecting isolated pulse stimulators (model 2100, A-M Systems, Carlsborg, WA) to the periorbital electrodes to deliver trains of electrical pulses used as the US. Separate stimulators were used to time the delivery of constant current pulses by stimulus isolators (model A360, World Precision Instruments, Sarasota, FL) connected to the electrodes implanted in the middle cerebellar peduncle. To measure eyelid position, an infrared emitter/detector was attached directly to the head stage of each rabbit to record movements of the left external eyelid by detecting changes in the amount of reflected light (Medina et al. 2000).
Stimulus presentation was controlled by custom-designed software for all experiments. Rabbits were given daily eyelid conditioning sessions comprised of 12 blocks of nine trials (1 CS alone trial and 8 paired trials per block). For 21 rabbits, training trials involved presentation of a tone CS (1 kHz, 85 dB sinusoidal tone) and the US, which was a 50 ms train of constant current pulses (100 Hz, 1 ms pulse width, 2–3 mA) delivered through the periorbital electrodes. Fifteen tone-trained rabbits underwent trace conditioning for 10–15 days (the number of days to reach asymptotic response rates). For this training, the CS was presented for 500 ms, and on paired trials was followed 500 ms later by the US. The remaining six tone-trained rabbits underwent delay conditioning for 10 days. On paired delay conditioning trials, the CS was presented for 1,050 ms and co-terminated with the US. Trials were separated by a mean intertrial interval of 30 ± 10 s.
Twelve rabbits were given daily eyelid conditioning sessions where the CS was constant current cathodal stimulation (50 Hz, 100 μs pulse width, 100 μA) through electrodes implanted in the middle cerebellar peduncle. We stimulated mossy fibers in the middle cerebellar peduncle rather than their cell bodies in the pontine nuclei to minimize stimulation of fibers of passage and antidromic activation of forebrain structures necessary for trace conditioning. Because the electrodes seem to have a limited life span, we trained rabbits at least until they reached 60% responding during one session rather than for a set number of days. For six rabbits, training involved stimulation through two separate mossy fiber electrodes: one to mimic activity of auditory-driven mossy fibers and one to mimic activity of mPFC neurons during a trace conditioning trial with a 500 ms tone and 500 ms trace interval. To mimic auditory-driven mossy fiber activity—which does not outlast the presentation of a tone (Aitkin and Boyd 1978; Boyd and Aitkin 1976; Freeman and Muckler 2003)—a 500 ms train of pulses was delivered through one electrode. To mimic persistent activity in mPFC, the second electrode was used to deliver 1 s trains of pulses (Takehara-Nishiuchi and McNaughton 2008). In each animal, one electrode was more medial than the other, and the electrode used for the tone and mPFC-like inputs was counterbalanced across rabbits. The US was presented at the end of the mPFC-like stimulus train. This pattern of mossy fiber input may be analogous to a compound stimulus (e.g., a 500 ms light and 1,000 ms tone). However, standard stimuli like tones and lights provide only modest control over the activation of mossy fibers. Tones produce both phasic and tonic activation of mossy fibers (Aitkin and Boyd 1978), it is not known how light stimuli activate mossy fibers, and worse yet, such stimuli could enable forebrain-activated mossy fiber inputs. For these reasons, we opted to stimulate mossy fibers instead of presenting a compound peripheral stimulus. In one rabbit, the stimulation intensity of the tone-like stimulation was increased to 150 μA to ensure sufficient activation of mossy fibers, because in this animal, changing the length of this stimulation (at 100 μA) had no effect on response timing (see Figs. 3 and 5 for an illustration of this effect). To ensure that the electrode used to deliver tone-like input was functional, two rabbits were trained with stimulation through this electrode as the CS during delay conditioning. In this case, the US was delivered 500 ms into a 550 ms train of mossy fiber stimulation. The six remaining rabbits were given delay conditioning using mossy fiber stimulation as the CS and the same 1,000 ms interstimulus interval as the dual stimulation animals.
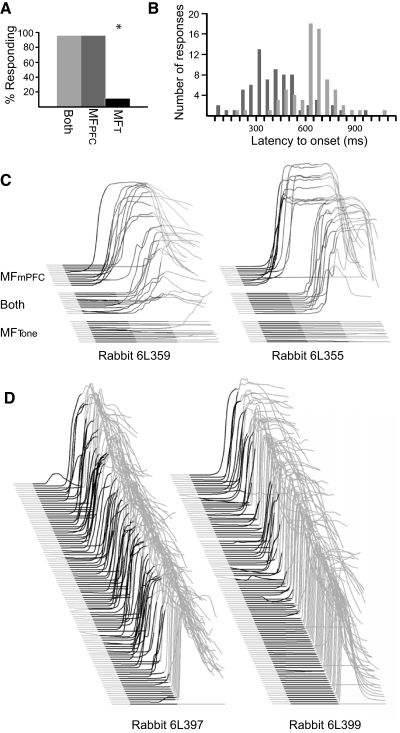
Fig. 3.
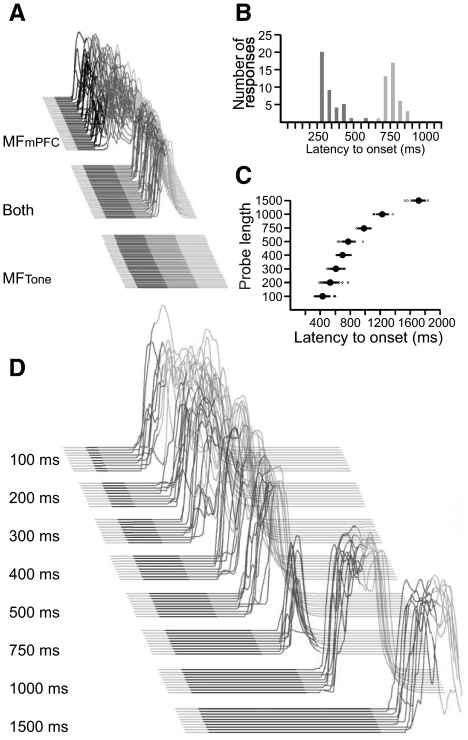
The cerebellum learns to respond to mPFC- but not tone-like mossy fiber input. A: rabbits responded to the mPFC-like input alone and to both inputs together but not tone-like input alone. B: a frequency histogram of latency to onset shows that responses to mPFC-like input alone (dark gray) had shorter latencies to onset than responses to both inputs (light gray). Data are grouped into 50 ms bins. C: behavioral responses to presentations of the mPFC-like input, the tone-like input, and both inputs from 2 representative rabbits. Responses to each trial type are sorted by latency to onset. Note that the latency to onset of responses to the mPFC-like input alone is shorter than that of responses to both inputs. D: stimulation using the electrode that previously delivered the tone-like input supports delay conditioning. Raw data from 2 rabbits are shown.
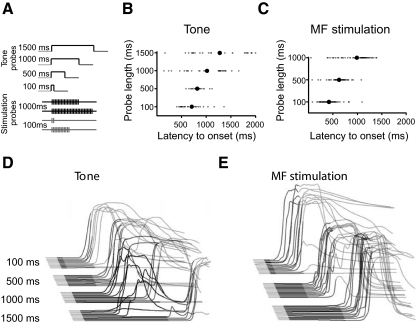
Fig. 5.
The offset of the tone modulates response timing in trace eyelid conditioning. A: diagram of probe trials. B: the timing of tone trained responses depended on the length of the tone. Large filled circles represent the mean latency to onset of responses across rabbits for a given trial type. Small circles are individual data points from individual trials. C: the timing of stimulation-trained responses depends on the length of the tone-like input. D: sample behavioral sweeps from a representative rabbit in response to each probe trial. Responses for a given trial are sorted by latency to onset. E: sample behavioral sweeps from a representative stimulation-trained rabbit in response to each probe trial. Responses are sorted as in D.
Test sessions
After undergoing 10 days of trace conditioning with a tone CS, four rabbits were given three test sessions in which we varied the length of the tone during CS alone trials. During these sessions, which were otherwise identical to training sessions, we gave one probe trial per block. The CS length during probe trials was 100, 500, 1,000, or 1,500 ms.
All rabbits trained with dual mossy fiber stimulation as the CS were given two test sessions in which we manipulated an aspect of the CS during nonreinforced probe trials. In the first test session, we gave probe trials in which stimulation through one electrode was omitted. In the second test session, we varied the length of the stimulation during probe trials. In one type of probe trial, the tone-like and mPFC-like mossy fiber inputs were shortened to 100 and 600 ms, respectively. In the other type of probe trial, the tone-like and mPFC-like mossy fiber inputs were lengthened to 1,000 and 1,500 ms, respectively. Two probe trials per block were given in each session. The sessions were otherwise identical to training sessions.
Infusions
In the rabbits trained with dual mossy fiber stimulation as the CS and in five rabbits trained with a tone during trace conditioning, we infused 20 μM of the GABAA antagonist, SR-95531 (gabazine, Tocris), or artificial cerebrospinal fluid (ACSF) into the interpositus nucleus during a test session. Gabazine was dissolved in ACSF consisting of (in mM) 119 NaCl, 2.5 KCl, 1.2 NaH2PO4, 2 MgCl2, 2 CaCl2, 26 NaHCO3, 10 d-glucose, and 20 HEPES, pH 7.30∼7.4. Infusions were performed through a 33-gauge internal cannula that extended 1.2 mm beyond the guide cannula. The internal cannula was coupled to a 50 μl Hamilton syringe that was mounted on an automated injector system (model MD-1001, Bioanalytical Systems, West Lafayette, IN) and driven by an electronic pump (model MD-1020). Infusions during trace conditioning to a tone began after the fourth block and continued at a rate of 0.1 μl/min until training resumed 20 min later. Infusions during mossy fiber stimulation training began after the third block and continued at a rate of 0.1 μl/min until training resumed 20 min later. Each animal received both types of infusion sessions, with session order counterbalanced across rabbits. The mossy fiber stimulation electrodes lost their effectiveness in one rabbit before a gabazine infusion could be made. Gabazine infusions in another stimulation-trained rabbit failed to affect the timing of responses. Histological examination showed that the cannula in this rabbit was outside of the interpositus nucleus. Thus there were four stimulation-trained rabbits included in the analysis of infusions.
Data analysis
For each trial, eyelid responses were digitized (1 kHz, 12 bit resolution) and stored on disk for subsequent off-line analyses using custom software. The sweeps capture the 200 ms before CS onset and the next 2300 ms. We analyzed digitized sweeps of eyelid movements made 200 ms before and 2,300 ms after CS onset with custom software. Trials in which eyelid movements >0.3 mm were made in the 200 ms before CS onset were automatically excluded from analysis by the analysis software. A conditioned response was defined as an eyelid movement of at least 0.3 mm within the interval between CS onset and US onset [interstimulus interval (ISI)].
The timing of delay, trace, and mossy fiber stimulation trained responses were quantified and compared using several measures including: latency to criterion (latency to a response of 0.3 mm), latency to onset (latency to first inflection in eyelid sweep of a conditioned response), latency to peak amplitude, peak velocity, latency to peak velocity, latency to ¾ amplitude, and rise time (latency to peak – latency to ¾ amplitude; Fig. 2). For each of these measures, the mean and interquartile range (as a measure of trial-to-trial variability) was computed for each rabbit. Comparisons were made on responses that were >4 mm on the last day before test sessions for mossy fiber training and on days 9 and 10 for delay and trace training. This amplitude criterion was applied to limit analysis to responses at asymptotic performance (e.g., full eyelid closure). There were no differences in the proportion of these large responses or conditioned response rate between groups (P > 0.05). ANOVA followed by Tukey's post hoc comparisons were used to test for within- and between-rabbit differences.
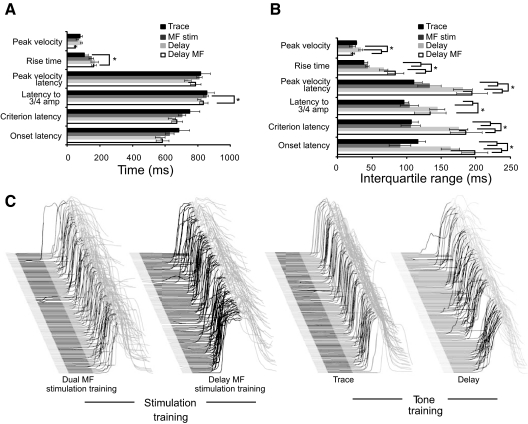
Fig. 2.
A comparison of the timing of trace to tone, delay to tone, delay to mossy fiber stimulation, and dual mossy fiber stimulation conditioned responses shows that the dual stimulation responses quantitatively match normal trace conditioning. A: mean values of 6 timing parameters. The units for peak velocity are mm/s. B: trial-to-trial variability in 6 parameters of response timing. In most instances where trace conditioning and delay conditioning (tone and mossy fiber stimulation) using the same interstimulus interval (ISI) differ, the dual stimulation responses are significantly different from the delay responses (tone and mossy fiber stimulation) and not different from trace responses. C: sample eyelid sweeps from representative rabbits during their last day of training. *P < 0.05.
Histology
Infusion and electrode sites were marked by passing 200 μA anodal current for 15 s and switching polarity for 15 s through a wire (cut to the length of the internal cannula and threaded through the guide cannula) or electrode. Animals were killed with an overdose of pentobarbital sodium and were perfused transcardially with 10% formalin. Brains were embedded in gelatin and sectioned at 80 μm using a microtome. Sections were mounted and stained with cresyl violet.
Computer simulations
Because details of the simulation have been described elsewhere, only a brief description is provided here (Mauk and Ohyama 2004; Medina and Mauk 1999, 2000; Medina et al. 2001, 2002).
The construction of the simulation was intended to emulate the known synaptic organization and physiology of the cerebellum and its mossy fiber and climbing fiber inputs. To approximate the ratio of cell types within the cerebellum, the simulation contained leaky integrate-and-fire representations of 600 mossy fibers, 12,000 granule cells, 900 Golgi cells, 200 basket/stellate cells, 24 Purkinje cells, 8 deep cerebellar nuclei (DCN) cells, and 4 climbing fibers. Synaptic connectivity between cell types was determined by their known synaptic convergence/divergence ratios. The membrane potential of each cell was calculated based on synaptic and leak conductances. Synaptic conductances were simulated by using step increases on a spike in the presynaptic cell followed by an exponential decay with time constants derived from in vitro recording studies. Spikes were initiated when the membrane potential exceeded threshold. The threshold for spike initiation changed with spiking history to mimic absolute and relative refractory periods. The time step for calculating the membrane potential was 1 ms.
To capture the geometric relationships and divergence and convergence ratios of synaptic connections between neurons, granule cells, Golgi cells, and mossy fiber glomeruli were arranged in a two-dimensional grid (120 × 100 for granule cells, 30 × 30 for Golgi cells, and 30 × 30 for glomeruli). We specified areas over which a simulated neuron could contact a member of the population of target cells. This process can be illustrated by considering the synaptic connections of granule cells onto Gogli cells. For granule cells, the contact area was a long, narrow rectangle to capture the long length of the granule cell axons (the parallel fibers) and the two diameters of Golgi cell apical dendrites. This area constrained the spatial range over which cells could make connections, but the actual connections between a given granule cell and Golgi cells within the rectangle were determined randomly in a way that obeyed divergence ratios and convergence ratios of the granule to Golgi connections. This general process was repeated for all simulated neurons to determine the connectivity of the entire simulations.
Two synapses—the granule cell-to-Purkinje cell synapse and the mossy fiber-to-DCN synapse—were modifiable. Plasticity at the granule cell-to-Purkinje cell synapse was controlled by climbing fiber input (Coesmans et al. 2004; Ito and Kano 1982; Lev-Ram et al. 2002, 2003). A granule cell-to-Purkinje cell synapse was eligible to undergo either long-term depression (LTD) or long-term potentiation (LTP) depending on whether or not its activity fell within a 100 ms time widow preceding a climbing fiber input (Raymond and Lisberger 1998; Safo and Regehr 2008). Active synapses within this window underwent LTD, whereas those active outside underwent LTP. The size of the weight change produced by these events was small, and the ratio of changes was adjusted to produce a self-regulating 1 Hz level of spontaneous climbing fiber activity before training. Mossy fiber-to-DCN synapse strength was under the control of Purkinje cell activity (Medina and Mauk 1999; Pugh and Raman 2006, 2008). Mossy fiber-to-DCN synapses active within a time window of an abrupt pause in Purkinje cell activity underwent LTP. These synapses underwent LTD when active during strong Purkinje cell activity.
Eyelid conditioning was simulated by altering the activity of a subset of mossy fiber and climbing fiber inputs according to empirical data (Aitkin and Boyd 1978; Boyd and Aitkin 1976; Freeman and Muckler 2003; Sears and Steinmetz 1991). Tone-driven mossy fibers are known to be activated either phasically or tonically. Thus to simulate the presentation of a 500 ms tone, the activity of 3% of mossy fibers was increased for the first 20 ms of the tone and the activity of 1% of mossy fibers was increased for 500 ms. To simulate the putative activity of mossy fibers driven by mPFC during trace conditioning (Takehara-Nishiuchi and McNaughton 2008), the activity of a randomly chosen subset of 1% of mossy fibers was increased for 1 s at CS onset. This activity occurred against a background of activity in the remaining mossy fibers. This was accomplished by assigning the remaining mossy fibers a range of average firing rates that were unchanged by the presentation of the CS. US-driven climbing fiber input was simulated by presenting a brief excitatory conductance to the climbing fiber. The output of the simulation was represented by the activity of the eight DCN cells.
Once the simulation acquired responses to its inputs, we presented it with two types of test sessions. During both sessions, synaptic plasticity was turned off to avoid changes in weight that might be caused by the inputs presented and the climbing fiber was no longer activated by the US. During the first session, the simulation was presented with equal numbers of trials in which tone and mPFC-like inputs were presented together, only tone inputs were presented, or only mPFC-like inputs were presented. During the second session, the simulation was presented with trials that were designed to mimic the presentation of various tone lengths. For these trials, the activity of tonically activated tone-driven mossy fibers was increased by the length of the presented tone, and the activity of the mPFC-like inputs increased 500 ms longer than that. For example, to simulate the presentation of a 300 ms tone, the activity of tonically activated tone-driven mossy fibers increased for 300 ms and the activity of mPFC-like inputs increased for 800 ms.
RESULTS
The basic approach was to stimulate one set of mossy fibers with a pattern that mimicked tone-elicited mossy fiber responses (Aitkin and Boyd 1978; Boyd and Aitkin 1976; Freeman and Muckler 2003) and a second set with a pattern that mimicked persistent activity in mPFC (Takehara-Nishiuchi and McNaughton 2008) and in the pontine nuclei (Siegel et al. 2009) during trace eyelid conditioning. For convenience, we will refer to these inputs as “mPFC-like” and “tone-like.” The intent was not to identify or selectively activate the same mossy fibers engaged during trace conditioning with a tone. Rather, the use of stimulation addresses the rules that govern how the cerebellum reacts to the temporal patterns of inputs putatively present during trace conditioning. Thus rabbits were implanted with two closely spaced stimulating electrodes (laterally separated by 1 mm) in the middle cerebellar peduncle (where mossy fibers enter the cerebellum) ipsilateral to the training eye (Fig. 1B). Electrodes were placed here rather than in the pontine nuclei, where mossy fibers originate, to avoid unwanted stimulation of fibers of passage and antidromic activation of forebrain inputs that might confound the experiments.
Stimulation through the electrodes was designed to mimic a forebrain-dependent trace conditioning procedure (Kalmbach et al. 2009; Kim et al. 1995; Kronforst-Collins and Disterhoft 1998; Moyer et al. 1990; Powell et al. 2001; Weible et al. 2000) in which the offset of a 500 ms tone is followed 500 ms later by the reinforcing US. Because the activity of tone-driven mossy fibers does not outlast the presentation of a tone (Aitkin and Boyd 1978; Boyd and Aitkin 1976; Freeman and Muckler 2003), stimulation through one electrode lasted for 500 ms to mimic mossy fiber inputs activated by a 500 ms tone. To mimic the activity of mPFC neurons known to persist beyond the offset of the tone (Takehara-Nishiuchi and McNaughton 2008), stimulation through the second electrode began at the same time as the other electrode, but lasted for 1,000 ms (Fig. 1B). We used periorbital stimulation as the US to activate climbing fiber input to the cerebellum (Hesslow 1994; Sears and Steinmetz 1991). If trace eyelid conditioning is mediated by cerebellar learning in response to a similar pattern of input, learned responses produced by stimulation should quantitatively match the behavioral features of normal trace conditioned responses using a tone CS.
Six rabbits trained in this manner learned robust conditioned responses (an average of 86.6 ± 9.4% responding during the last day of training). Because rabbits received different numbers of training sessions (see methods), we do not report group acquisition data. Instead, to show the acquisition of responses across training blocks, Fig. 1, C and D, shows learning curves from each rabbit and raw data from one representative rabbit.
To address how well these conditioned eyelid responses match normal trace eyelid responses using a tone CS, we made various measures of response timing. We used these measures to compare the timing of responses produced by 1) eyelid conditioning using dual mossy fiber stimulation, 2) trace conditioning using a tone, 3) delay conditioning using a tone and the same 1,000 ms ISI, and 4) delay conditioning using mossy fiber stimulation and the same 1,000 ms ISI. These comparisons allow us to ask 1) how well the dual mossy fiber stimulation responses quantitatively match normal trace responses to tone and 2) when there are differences between tone delay and trace responses, do the dual mossy fiber stimulation responses better resemble delay (both tone and mossy fiber stimulation) or trace responses?
There were no significant differences in various measures of response timing (Fig. 2, A and C) but consistent differences in the trial-to-trial variability of these same measures (Fig. 2, B and C). To quantify trial-to-trial variability, we computed the mean interquartile range for each timing parameter, which showed significantly greater variability for delay (tone and mossy fiber stimulation) than trace conditioned responses in five of six parameters. Importantly, the trial-to-trial variability in the timing of dual mossy fiber stimulation responses differed from delay responses (tone and mossy fiber stimulation) on five of six measures and did not differ from trace responses on any measure (Fig. 2, B and C). These data indicate that, for measures in which there are clear differences between delay and trace conditioning (trial-to-trial variability of timing), stimulation-trained responses quantitatively replicate trace responses and are significantly different from delay responses using mossy fiber stimulation or a tone as the CS and the same 1,000 ms ISI. These data show that both a mPFC-like input and a tone-driven input are necessary to produce responses that match trace eyelid responses to a tone and that are different from delay conditioning (tone or stimulation produced) with the same 1,000 ms ISI. Perhaps during trace eyelid conditioning the mPFC- and tone-driven inputs combine to enable the cerebellum to make more precise and consistent responses than during delay conditioning where learning occurs primarily to just the tone input (Steinmetz et al. 1987). This relates to the general question of the relative contributions of the two inputs, which we addressed next.
Cerebellum responds to mPFC- but not tone-like mossy fiber input
Previous work showed that learning in the cerebellum does not occur when the US-activated climbing fiber input is presented 500 ms or more after the offset of a mossy fiber input (Kalmbach et al. 2009). However, the data in Fig. 2 imply that presenting the cerebellum with two mossy fiber inputs, one that overlaps with climbing fiber input and one that does not, promotes learned responses that differ from those mediated by only a tone input. We used probe trials in which each of the two mossy fiber inputs was stimulated separately to address the relative contributions of these two inputs. Is learning supported by only the input that overlaps with the US (mPFC-like), by the shorter input (tone-like), or by an interaction of both inputs?
We first tested well-trained animals with probe trials in which only the mPFC- or only the tone-like mossy fiber input was presented. Although rabbits failed to respond to the tone-like input (t(5) = 11.17, P < 0.001; t-test comparing response rates to tone-like input versus both inputs together), they responded to the mPFC-like input at a rate comparable to both inputs presented together (P = 0.97; t-test comparing response rates to mPFC-like input vs. both inputs; Fig. 3, A and C). This is consistent with previous findings that suggested indirectly that the cerebellum learns to respond to mPFC- and not tone-driven input during trace eyelid conditioning (Kalmbach et al. 2009). Nonetheless, the data also suggest that the tone-driven input is not entirely irrelevant, because the latency to onset of responses to the mPFC-like input was significantly shorter than that to both stimuli (t(5) = 4.45, P = 0.007; Fig. 3, B and C). We return to this interesting finding later, which may show new insights on cerebellar timing mechanisms.
Plasticity in deep cerebellar nuclei and short latency responses
We addressed the relative contribution of the two inputs further by asking whether plasticity in the DCN that occurs during cerebellar learning (Medina and Mauk 1999; Ohyama et al. 2006; Pugh and Raman 2006, 2008; Raymond et al. 1996) is established to mPFC-like, tone-like, or both mossy fiber inputs. Preventing the cerebellar cortex from influencing DCN activity by making lesions of the cerebellar cortex or temporarily inactivating the Purkinje cell–DCN synapse unmasks learned responses with short and relatively fixed latencies to onset (Bao et al. 2002; Kalmbach et al. 2010; Ohyama et al. 2003, 2006; Perrett et al. 1993). These short-latency responses provide a behavioral readout of learning-induced, stimulus-specific plasticity at the mossy fiber-to-DCN synapse (Ohyama et al. 2003, 2006). Therefore the presence of short-latency responses would identify the mossy fiber input that undergoes learning-related plasticity. For example, if learning occurs to the mPFC-like but not the tone-like input, short-latency responses should occur to the mPFC-like input but not the tone-like input.
We began by determining whether short-latency responses established during dual stimulation training are similar to those established during trace conditioning to a tone (Kalmbach et al. 2010). To test for short latency responses in stimulation-trained and tone-trained trace conditioned rabbits, the GABAA antagonist, SR-95531 (gabazine) was infused into the interpositus nucleus of the cerebellum (Fig. 4A). The response latencies during trace conditioning to a tone decreased after infusions (Fig. 4, B and C) (n = 5; F(11,44) = 6.11, P < 0.001; F test for simple effect of training block on response rate), as did those of responses to both inputs of four stimulation-trained rabbits (Fig. 4, D and E; n = 4; F(11,33) = 5.17, P < 0.001). Infusing ACSF did not affect the latency to onset of either response (P > 0.5 for both F tests). The apparent difference between the latency to onset of tone trained and stimulation trained responses after the infusion was not statistically significant (P > 0.05). Consistent with previous observations (Kalmbach et al. 2010), the short-latency responses unmasked in tone-trained animals began before the offset of the tone suggesting that DCN plasticity during trace conditioning occurs for mossy fiber inputs activated near CS onset.
Fig. 4.
Deep cerebellar nucleus plasticity is established to mPFC-like input. A: coronal section of the cerebellum showing a representative infusion site. The cannula tip for all rabbits was in the interpositus nucleus of the cerebellum. B: infusing gabazine into the deep cerebellar nuclei (DCN) during test sessions (break in the abscissa) decreased the latency to onset of responses (●), whereas infusing artificial cerebrospinal fluid (ACSF) did not (○). C: sample behavioral sweeps from a tone-trained rabbit during a gabazine infusion session. Break in sweeps denotes infusion. D: infusing gabazine into the DCN during test sessions decreased the latency to onset of stimulation trained responses, whereas infusing ACSF did not. E: sample behavioral sweeps from a dual stimulation-trained rabbit during a gabazine infusion session. F: rabbits responded to mPFC-like input alone but not tone-like input alone during gabazine infusion sessions. Sweeps from 2 rabbits are shown.
This input could either be mPFC- or tone-driven mossy fiber input, because both begin shortly after tone onset (Aitkin and Boyd 1978; Boyd and Aitkin 1976; Freeman and Muckler 2003; Takehara-Nishiuchi and McNaughton 2008). As before, the use of mossy fiber stimulation as a CS permitted a test of which input undergoes plasticity in the DCN. We presented trained rabbits with tone- or mPFC-like input alone during infusion sessions and found that rabbits responded to mPFC-like input (73.70% of trials) but rarely to the tone-like input (5% of trials; t(3) = 4.80, P = 0.009; Fig. 4F). The failure to respond to tone-like input was not caused by incorrect electrode placement because both electrodes were located in the middle cerebellar peduncle in all rabbits (Fig. 1B). Nor is it caused by electrode failure because subsequent use of the tone-like input because the CS in two rabbits supported robust delay conditioning with a 500 ms ISI (Fig. 3D).
Together, these data suggest that, during trace eyelid conditioning, plasticity in the DCN is established to the mPFC-driven and not the tone-driven mossy fiber input. This is consistent with previous evidence that cerebellar learning requires temporal overlap in climbing fiber and mossy fiber input (Kalmbach et al. 2009).
Cerebellum uses mPFC- and tone-driven input to time trace responses
The above data seemingly indicate a lack of learning to the tone-driven input during trace eyelid conditioning. The hint to the contrary is the observation that omitting the tone-like input resulted in responses with shorter onset latencies than responses to both inputs (Fig. 3, B and C). This suggests that the cerebellum uses tone-driven input to time responses, perhaps by learning to suppress responding until after tone offset. This would account for the influence of CS offset on the timing of trace conditioning (Kehoe and Napier 1991; Kehoe and Weideman 1999), despite learning being supported by an input time-locked to CS onset as evidenced by short latency responses to CS onset (Kalmbach et al. 2010) (Figs. 3 and 4). To test this hypothesis more thoroughly, we determined the ability of CS offset to influence the timing of eyelid responses in dual stimulation-trained animals and animals trained with a standard tone CS.
We first attempted to replicate previous demonstrations that CS offset influences response timing (Kehoe and Napier 1991; Kehoe and Weideman 1999) in tone-trained animals using our training conditions. Four rabbits were trained with a 500 ms tone and a 500 ms trace interval for 10 days. During postacquisition test sessions, we intermixed CS alone trials of varying lengths with normal paired trials (Fig. 5A, top). Consistent with previous findings, we observed that the timing of responses to these probe trials was strongly influenced by the length of the probe (n = 4; F(3,9) = 7.54, P = 0.008). Presenting the 1,000 ms probe produced longer latency responses than the 500 ms probe (t(3) = 2.89, P = 0.03), which in turn produced longer latency responses than the 100 ms probe (t(3) = 2.60, P = 0.04; Fig. 5, B and D). These data replicate the basic finding that CS offset exerts a strong influence over the timing of trace eyelid responses (Kehoe and Napier 1991; Kehoe and Weideman 1999).
Next we tested whether the timing of dual mossy fiber stimulation trained responses is similarly sensitive to CS offset. To do so we created two probe trials to mimic a 100 and 1,000 ms tone alone trial (Fig. 5A, bottom). During the 100 ms tone-like probe, we decreased the length of the tone-like and mPFC-like input to 100 and 600 ms, respectively. During the 1,000 ms tone-like probe, we increased the length of the tone-like and mPFC-like input to 1,000 and 1,500 ms, respectively. Despite the absence of explicit offset information in the mossy fiber input (i.e., no mossy fiber input driven by the offset of the tone), we found that the length of the probe affected the latency to onset (Fig. 5, C and E; n = 6; F(2, 10) = 49.23, P < 0.001). Presenting the 1,000 ms tone-like probe produced longer latency responses than the 500 ms probe trial (t(5) = 5.03, P = 0.004), which in turn produced longer latency responses than the 100 ms probe (t(5) = 4.41, P = 0.007; Fig. 5, C and E). These data suggest that the cerebellum does not require a mossy fiber input driven by CS offset to time trace responses relative to CS offset. Rather, this timing seems to arise from the cerebellum learning to suppress responding to the tone-driven input while learning to initiate responding to the mPFC-driven input.
Potential cerebellar contributions to the timing of trace conditioned response timing: Simulations
The ability of the cerebellum to suppress responding until after CS offset is reminiscent of what seems to occur during delay conditioning where the cerebellum learns to inhibit responding early in the tone and learns to respond late in the tone (Medina et al. 2000). Previous computational analyses suggest that this relies on temporal coding mechanisms in the cerebellar cortex in which different sets of granule cells are active at different times during a time-invariant mossy fiber input (Medina and Mauk 2000; Medina et al. 2000). Thus the tendency of trace conditioned responses to be delayed until after CS offset may be caused by similar mechanisms, where the cerebellum learns to inhibit responding to granule cells active early during the tone while learning to respond to granule cells whose activity increases after the offset of the tone, late in the trace interval. To formalize this hypothesis more precisely, we presented a computer simulation of the cerebellum with simulated tone-driven and putative mPFC-driven mossy fiber inputs in a manner consistent with trace conditioning with a 500 ms tone and 500 ms trace interval (see methods). We asked whether there is granule cell activity that specifically increases after the offset of the tone-driven input regardless of the length of the tone and whether this activity contributes to the precise timing of learned responses.
First, we determined whether the simulation learns to produce responses that are similar to those seen during trace conditioning and dual mossy fiber stimulation training. The simulated cerebellum learned to increase its output over several trials of conditioning, and once well trained, responded to mPFC- (100% of trials) but not tone-like input presented alone (0% of trials; Fig. 6A). Consistent with our stimulation results (Fig. 3), the onset latency of the simulation responses to the mPFC-like input alone was shorter than that of simulation responses to both inputs (Fig. 6, A and B). Also consistent with the learning in the rabbit (Fig. 5), the timing of the simulation responses was affected by the duration of the tone-like input (Fig. 6, C and D). Thus like the real cerebellum, the simulation only learns to increase its output to mossy fiber inputs that overlap with climbing fiber input while using mossy fiber input that does not overlap with the US to improve the timing of its output. These parallels with the rabbit data suggest that how the simulation processes the two inputs represents an initial but specific working hypothesis for cerebellar information processing in response to mPFC- and tone-driven input during trace eyelid conditioning.
Fig. 6.
The output of computer simulations of the cerebellum in response to tone- and mPFC-like mossy fiber input. All data in this figure are from simulations trained with a 500 ms mossy fiber input to mimic a 500 ms tone and a 1,000 ms input to mimic input driven by mPFC. A: a well-trained simulation responds to mPFC- but not tone-like input presented alone. B: responses to mPFC-like input alone (dark gray) have a shorter latency to onset than responses to both inputs (light gray). C: the offset of the tone-like input affected the timing of responses. D: responses of the simulation during probe trials where the length of the tone-like input was varied.
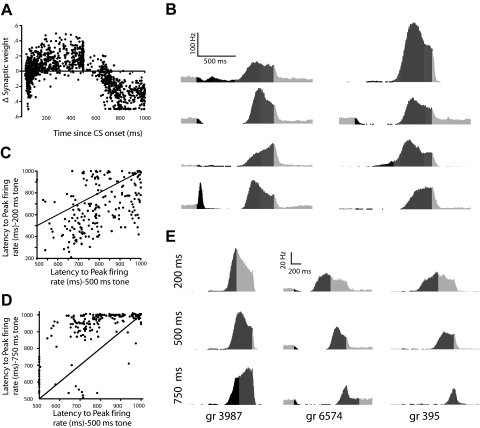
In this light, we analyzed the temporal profile of granule cell activity in the simulation during trace conditioning. We found that the activity of a subset of cells peaked during the tone while the activity of others peaked during the trace interval (the activity of a randomly chosen subset of these granule cells can be found in Fig. 7A; the activity of most granule cells did not change during the trials—data not shown). Sample histograms of granule cells whose activity peaked during the trace interval can be seen in Fig. 7B. The strength of granule cell-to-Purkinje cell synapses active during the trace interval decreased, whereas those active early during the trial increased (Fig. 7A). Because Purkinje cells, as the sole output of cerebellar cortex, inhibit the output of the cerebellum (Ito 1984), the activation of strengthened synapses would tend to inhibit cerebellar output, and the activation of weakened synapses would tend to increase cerebellar output (Fig. 8A). This suggests that granule cells active during the CS and activity early during the trace interval are important for suppressing responding, whereas granule cells active late in the trace interval are especially important for learning the responses.
Fig. 7.
The cerebellum generates granule cell activity driven by the offset of the tone-like input and uses this to time its output. A: the strength of granule cell-to-Purkinje cell synapses active early during the trial increases and those active late during the trial decrease. Plotted is the latency to peak activity vs. change in synaptic weight as the result of training for individual granule cells. B: sample peristimulus histograms of granule cells whose peak activity occurred during the trace interval. The light gray region marks the 200 ms before the onset of the trial and 500 ms after US offset, black marks the duration of the tone, and dark gray marks the trace interval. C: the activity of a subset of granule cells peaked earlier during a 200 ms probe than during a 500 ms probe. Plotted is the latency to peak activity of individual granule cells during the 500 ms trial vs. the 200 ms trial. Data points below the unity line represent granule cells whose peak activity occurred earlier during the 200 ms probe than during the 500 ms probe. D: the activity of a subset of granule cells peaked later during a 750 ms probe than during a 500 ms probe. Plotted is the latency to peak activity of individual granule cells during the 500 ms trial vs. the 750 ms trial. Data points above the unity line represent granule cells whose peak activity occurred later during the 750 ms probe than during the 500 ms probe. E: sample histograms of granule cells whose activity peaked during the trace interval and varied with the length of the tone input.
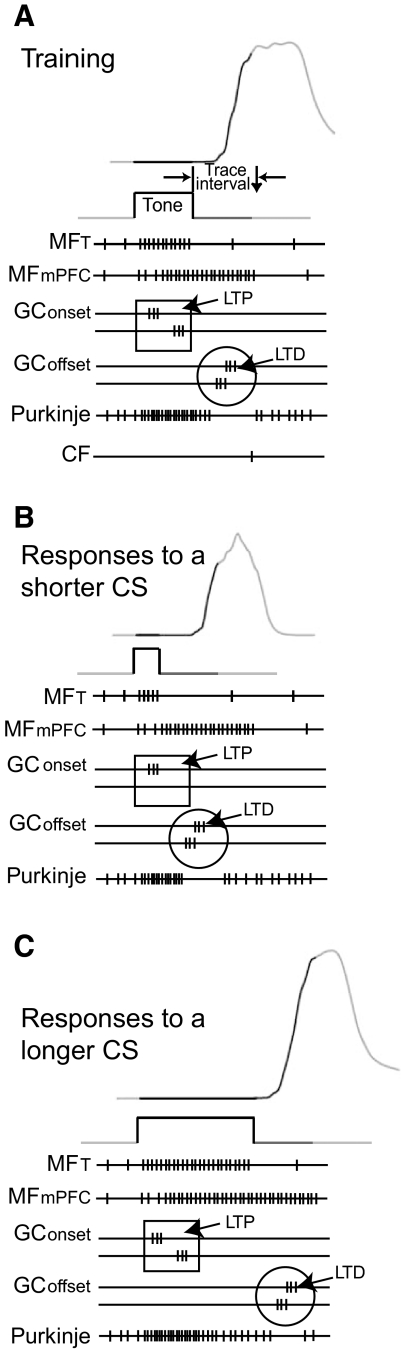
Fig. 8.
Schematic illustration of potential cerebellar mechanisms of response timing during trace eyelid conditioning. A: during trace eyelid conditioning, tone-driven and mPFC-driven mossy fiber input generates 2 general classes of granule cell activity. The peak activity of some granule cells is tied to the onset of the conditions stimulus (CS) (GConset) and the peak activity of others is tied to the offset of the CS (GCoffset). Granule cell-to-Purkinje cell synapses active early during a trial tend to increase in strength (long-term potentiation) and those active late during the trial tend to decrease in strength (long-term depression). When strengthened synapses are active, Purkinje cell activity tends to increase, and when weakened synapses are active, Purkinje cell activity tends to decrease. Because Purkinje cells are the sole output of the cerebellar cortex and inhibit the output of the cerebellum, early during the trial, when strengthened synapses are active, responding is suppressed, whereas late during the trial, when weakened synapses are active, responding is initiated. B: the peak activity of granule cells driven by the onset of the CS does not vary during the presentation of a CS shorter than the one used during training. However, the peak activity of granule cell-to Purkinje cell synapses driven by the offset of the CS occurs earlier. Because these synapses were weakened by training, their activation results in a response that is initiated shortly after CS offset. C: the peak activity of granule cells driven by the onset of the CS does not vary during the presentation of a CS longer than the one used during training. However, the peak activity of granule cells driven by the offset of the CS occurs later, resulting in a response that is initiated shortly after CS offset.
Finally, we examined whether varying the offset of the tone-like input to the simulation changed the timing of activity for granule cells normally active during the trace interval. Because this activity is essential for simulation response expression, changes in timing could contribute to the observed influence of CS offset on response timing. We presented the simulation with two probes that mimicked the presentation of a 200 and 750 ms tone. The activity of many granule cells peaked earlier during the 200 ms tone than during the 500 ms tone (Fig. 7, C and E). Similarly, the activity of many granule cells peaked later during the 750 ms probe than during the 500 ms tone (Fig. 7, D and E). Taken as a whole, these data suggest that the timing of trace conditioned responses arises from the cerebellum learning to suppress responding to granule cells active early during the trial and learning to respond to granule cells whose activity specifically code for the trace interval (Fig. 8).
DISCUSSION
Temporal properties of mossy fiber input necessary and sufficient for trace eyelid conditioning
We found that presenting the cerebellum with mossy fiber inputs whose temporal patterns mimic persistently active neurons in mPFC and mossy fiber inputs driven by the tone is necessary and sufficient for the acquisition and expression behavioral responses having the properties of trace conditioned responses to a tone. Stimulation-trained responses exhibited two properties of the timing of trace conditioned responses to a tone: 1) the trial-to-trial variability in response timing was indistinguishable from that of trace responses to a tone but was significantly lower than that of delay conditioning to a tone (Fig. 2) and 2) the timing of responses was modulated by the offset of the tone (Fig. 5) (Kehoe and Napier 1991; Kehoe and Weideman 1999). Furthermore, the cerebellum learned to increase its output only to the mPFC-like input (Figs. 3 and 4), consistent with evidence that the cerebellum learns to respond to mPFC-driven rather than to tone-driven input (Kalmbach et al. 2009).
At the same time, our data indicate that the tone-driven input is not irrelevant. The duration of the tone strongly influenced the timing of responses (Fig. 5), as did omitting the tone-like mossy fiber input (Fig. 3). Also, quantitative analysis of the timing of responses indicated that the tone-driven input contributes to the cerebellum's ability to make precisely timed responses (Fig. 2). These observations suggest that tone-driven input contributes to timing mechanisms in the cerebellum representing a neural instantiation of Pavlov's inhibition of delay hypothesis (Pavlov 1927). Specifically, we suggest that the cerebellum learns to respond to mossy fiber input that overlaps with climbing fiber input and learns to suppress responding to mossy fiber input that does not. In the case of trace conditioning, the net result of this learning is well-timed responses that often begin after CS offset.
Finally, computer simulations provided insight into the neural mechanisms of this learned timing. Presenting a computer simulation of the cerebellum with tone-like and putative mPFC-like mossy fiber input showed subsets of granule cells whose activity peaked after the offset of the tone-like input, regardless of tone length. These granule cells were important for initiating responding, whereas granule cells active during the tone-like input were important for suppressing responding (Fig. 8). The simulation results represent a working hypothesis of cerebellar processing required to produce well-timed trace conditioned responses and more generally for how the cerebellum processes multiple mossy fiber inputs of different durations.
In summary, these data extend previous work suggesting that trace eyelid conditioning is mediated by cerebellar learning in response to mPFC-driven mossy fiber inputs that persist beyond CS offset to overlap with the US. This collaboration overcomes the cerebellum's inability to learn when mossy fiber and climbing fiber inputs do not overlap in time (Kalmbach et al. 2009)—essentially by converting, from the cerebellum's perspective, trace eyelid conditioning into delay eyelid conditioning. This interpretation explains why trace conditioning is sensitive to lesions of cerebellum and forebrain (Kalmbach et al. 2009; Kim et al. 1995; Kronforst-Collins and Disterhoft 1998; Moyer et al. 1990; Powell et al. 2001; Weible et al. 2000; Woodruff-Pak et al. 1985) and explains how persistent activity in mPFC (Takehara-Nishiuchi and McNaughton 2008) may be used by the cerebellum to produce well-timed trace conditioned responses. This interpretation is not inconsistent with observations that lesions of certain areas of mPFC do not affect trace conditioning or only affect trace conditioning under certain circumstances; these regions may not be involved in trace conditioning or may participate in trace conditioning in other ways than providing the cerebellum with input that overlaps with the US (Oswald et al. 2006, 2008). Nor are these data inconsistent with classic observations that lesions of hippocampus prevent acquisition of trace responses (Moyer et al. 1990). Indeed, hippocampal involvement in the acquisition or regulation of persistent activity in mPFC is an attractive hypothesis worth future attention. Trace conditioning therefore seems to be a rare instance of a well-characterized and experimentally tractable behavior that is mediated by interactions between two brain systems.
The data do not identify the anatomical location, or the identity of, mPFC-driven mossy fibers. If this was the goal, stimulation of mPFC or pontine nuclei rather than mossy fibers in the middle cerebellar peduncle might be useful. However, stimulating the mPFC or pontine nuclei has the disadvantage of potentially activating fibers of passage or antidromically activating forebrain structures involved in trace conditioning. This would open the door to mossy fiber inputs arriving at the cerebellum at unknown times, which would completely obviate the ability to establish the necessity and sufficiency of temporal input patterns. By stimulating mossy fibers in the middle cerebellar peduncle, we sacrificed the ability to identify the precise anatomical source(s) of mPFC-driven mossy fibers in favor of the ability for precise identification of the temporal pattern of input that is necessary and sufficient for trace conditioning.
Trace conditioning and cerebellar cortex
There is no principled reason to expect that the cerebellum operates under different sets of rules for delay and trace conditioning. Indeed, this work suggests that, from the cerebellum's standpoint, the mPFC-driven input essentially converts trace conditioning into delay conditioning. As during delay conditioning (Bao et al. 2002; Garcia and Mauk 1998; Ohyama et al. 2003, 2006; Perrett et al. 1993), blocking Purkinje cell input to the DCN during trace conditioning unmasks short latency responses (Fig. 4). Because short latency responses reflect stimulus-specific plasticity in the DCN (Ohyama et al. 2003, 2006; see Bracha et al. 2009 for an alternate interpretation), our data indicate that cerebellar learning during trace conditioning involves DCN plasticity. Furthermore, together with previous work (Kalmbach et al. 2010), our data indicate that, without the cerebellar cortex, the forebrain–cerebellum circuitry that remains is incapable of timing trace conditioned responses. Because these results parallel those for delay conditioning, we suggest that the role of the cerebellum (including the cerebellar cortex) in trace conditioning parallels that in delay conditioning.
Our hypothesis of forebrain–cerebellum interactions during trace eyelid conditioning therefore differs from hypotheses that predict that trace eyelid conditioning does not require cerebellar cortex (Woodruff-Pak and Disterhoft 2008; Woodruff-Pak et al. 1985, 2006). The acquisition of delay, but not trace eyelid conditioning, in mice is affected by various genetic manipulations that compromise the integrity of cerebellar cortex (Kishimoto et al. 2001a,b; Koekkoek et al. 2003, 2005; Woodruff-Pak et al. 2006). This has led to the interpretation that trace eyelid conditioning engages cerebellar learning via forebrain-driven mossy fibers that bypass cerebellar cortex and project only to the interpositus nucleus (Woodruff-Pak and Disterhoft 2008). Our data suggest that forebrain driven mossy fibers project to cerebellar cortex, which is necessary for the timing of trace conditioned responses. Because the induction of plasticity in the DCN seems to depend on plasticity in cerebellar cortex (Garcia et al. 1999; Medina and Mauk 1999), we predict that making permanent lesions of cerebellar cortex will permanently prevent trace eyelid conditioning just as in delay conditioning (Garcia et al. 1999).
Implications for PFC–cerebellum interactions
The brain is organized into multiple systems that interact with one another. To understand the brain, it is therefore necessary to determine how different brain systems interact. One facet of this complex problem is how the output of a given brain region contributes to computations in downstream regions. Here we provided evidence that persistent activity in mPFC, together with tone-driven mossy fiber input, is necessary and sufficient for the cerebellum to learn properly timed trace conditioned responses. Although ongoing experiments will test whether persistent activity is conveyed to mossy fibers in the LPN necessary for trace conditioning, these data may have implications for analyses of persistent activity in PFC and its use by downstream structures such as the cerebellum. First, persistent activity in PFC has long been hypothesized to represent the short-term storage of information that is used by downstream structures to guide behavior or make associations between temporally separate events (Goldman-Rakic 1996; Pasternak and Greenlee 2005). Consistent with this hypothesis, our evidence suggests that the cerebellum, a downstream target of PFC (Buchanan et al. 1994; Middleton and Strick 2000; Schmahmann and Pandya 1995; Weible et al. 2007), can use persistent input to learn associations between the temporally separate CS and US during trace eyelid conditioning. Previous evidence indicates that the cerebellum cannot learn to respond to mossy fiber input driven directly by the CS because it does not overlap in time with the US-driven climbing fiber input (Aitkin and Boyd 1978; Boyd and Aitkin 1976; Freeman and Muckler 2003; Kalmbach et al. 2009). This suggests that mPFC-driven input engages cerebellar learning when the inputs driven relatively directly by the outside world fail to do so. Likewise, PFC-driven input may serve a similar function in other brain regions. For example, given that lesions of the mPFC impair amygdala-dependent trace fear conditioning (Blum et al. 2006; Quinn et al. 2008), the amygdala may learn in response to persistent PFC-driven input during trace fear conditioning.
Second, cortical persistent activity, particularly in frontal regions, is generally interpreted in the context of the mechanisms for working memory (Goldman-Rakic 1995, 1996). Trace eyelid conditioning may be useful in analyses of the mechanisms of working memory in general and of persistent activity in PFC in particular. It is more tractable than many other behaviors used to study persistent activity in PFC. For instance, compared with the complex stimuli involved in delayed-matching to sample tasks, the stimuli used in trace eyelid conditioning are simple. Thus understanding how the nervous system encodes trace conditioning stimuli is likely to be easier than understanding how it encodes stimuli used during delayed-matching to sample tasks. This in turn may facilitate research on how sensory-driven input to the mPFC is converted into persistent activity. Finally, the acquisition of trace conditioned responses, unlike the acquisition of responses during delayed matching to sample tasks in primates, is relatively fast, allowing the development of delay cell activity to be observed across initial learning. These factors make trace eyelid conditioning an attractive tool for analyses of how persistent activity in PFC activity is established, maintained and used by other brain systems.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
GRANTS
This work was supported by National Institute of Mental Health Grants MH-74006 and MH-46904.
ACKNOWLEDGMENTS
We thank F. Riusech for assistance with histology and surgery.
REFERENCES
- Aitkin LM, Boyd J. Acoustic input to the lateral pontine nuclei. Hear Res 1: 67–77, 1978 [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proc Natl Acad Sci USA 99: 1592–1597, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport 17: 341–344, 2006 [DOI] [PubMed] [Google Scholar]
- Boyd J, Aitkin LM. Responses of single units in the pontine nuclei of the cat to acoustic stimulation. Neurosci Lett 3: 259–263, 1976 [DOI] [PubMed] [Google Scholar]
- Bracha V, Zbarska S, Parker K, Carrel A, Zenitsky G, Bloedel JR. The cerebellum and eye-blink conditioning: learning versus network performance hypotheses. Neuroscience 162: 787–796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan SL, Thompson RH, Maxwell BL, Powell DA. Efferent connections of the medial prefrontal cortex in the rabbit. Exp Brain Res 100: 469–483, 1994 [DOI] [PubMed] [Google Scholar]
- Clark RE, Manns JR, Squire LR. Classical conditioning, awareness, and brain systems. Trends Cogn Sci 6: 524–531, 2002 [DOI] [PubMed] [Google Scholar]
- Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44: 691–700, 2004 [DOI] [PubMed] [Google Scholar]
- Flores LC, Disterhoft JF. Caudate nucleus is critically involved in trace eyeblink conditioning. J Neurosci 29: 14511–14520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Muckler AS. Developmental changes in eyeblink conditioning and neuronal activity in the pontine nuclei. Learn Mem 10: 337–345, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Weible AP, Disterhoft JF. Cortical barrel lesions impair whisker-CS trace eyeblink conditioning. Learn Mem 14: 94–100, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology 37: 471–480, 1998 [DOI] [PubMed] [Google Scholar]
- Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. J Neurosci 19: 10940–10947, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron 14: 477–485, 1995 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA 93: 13473–13480, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. J Physiol 476: 229–244, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. New York: Raven Press, 1984, p. 300–302 [Google Scholar]
- Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett 33: 253–258, 1982 [DOI] [PubMed] [Google Scholar]
- Kalmbach BE, Davis T, Ohyama T, Riusech F, Nores WL, Mauk MD. Cerebellar cortex contributions to the expression and timing of conditioned eyelid responses. J Neurophysiol 103: 2039–2049, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD. Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learn Mem 16: 86–95, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe EJ, Napier RM. In the blink of an eye: real-time stimulus factors in delay and trace conditioning of the rabbit's nictitating membrane response. Q J Exp Psychol B 43: 257–277, 1991 [PubMed] [Google Scholar]
- Kehoe EJ, Weideman G. Within-stimulus competition in trace conditioning of the rabbit's nictitating membrane response. Psychobiology 27: 72–84, 1999 [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci 109: 195–203, 1995 [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Fujimichi R, Mori H, Mishina M, Kirino Y. Impairment of eyeblink conditioning in GluRdelta2-mutant mice depends on the temporal overlap between conditioned and unconditioned stimuli. Eur J Neurosci 14: 1515–1521, 2001a [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Suzuki M, Mori H, Mishina M, Kirino Y. Classical eyeblink conditioning in glutamate receptor subunit delta 2 mutant mice is impaired in the delay paradigm but not in the trace paradigm. Eur J Neurosci 13: 1249–1253, 2001b [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Hulscher HC, Dortland BR, Hensbroek RA, Elgersma Y, Ruigrok TJ, De Zeeuw CI. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science 301: 1736–1739, 2003 [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, De Schutter E, Oostra BA, Ito M, De Zeeuw CI. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron 47: 339–352, 2005 [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol Learn Mem 69: 147–162, 1998 [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Mehta SB, Kleinfeld D, Tsien RY. Reversing cerebellar long-term depression. Proc Natl Acad Sci USA 100: 15989–15993, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc Natl Acad Sci USA 99: 8389–8393, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Ohyama T. Extinction as new learning versus unlearning: considerations from a computer simulation of the cerebellum. Learn Mem 11: 566–571, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Thompson RF. Retention of classically conditioned eyelid responses following acute decerebration. Brain Res 403: 89–95, 1987 [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and pavlovian conditioning: trace versus delay conditioning. Behav Neurosci 116: 37–47, 2002 [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. J Neurosci 21: 4081–4089, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: testing predictions of a large-scale computer simulation. J Neurosci 20: 5516–5525, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Mauk MD. Simulations of cerebellar motor learning: computational analysis of plasticity at the mossy fiber to deep nucleus synapse. J Neurosci 19: 7140–7151, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Mauk MD. Computer simulation of cerebellar information processing. Nat Neurosci 3: 1205–1211, 2000 [DOI] [PubMed] [Google Scholar]
- Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature 416: 330–333, 2002 [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 31: 236–250, 2000 [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci 104: 243–252, 1990 [DOI] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Mauk MD. Stimulus generalization of conditioned eyelid responses produced without cerebellar cortex: implications for plasticity in the cerebellar nuclei. Learn Mem 10: 346–354, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD. Learning-induced plasticity in deep cerebellar nucleus. J Neurosci 26: 12656–12663, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald B, Knuckley B, Mahan K, Sanders C, Powell DA. Prefrontal control of trace versus delay eyeblink conditioning: role of the unconditioned stimulus in rabbits (Oryctolagus cuniculus). Behav Neurosci 120: 1033–1042, 2006 [DOI] [PubMed] [Google Scholar]
- Oswald BB, Maddox SA, Powell DA. Prefrontal control of trace eyeblink conditioning in rabbits: role in retrieval of the CR? Behav Neurosci 122: 841–848, 2008 [DOI] [PubMed] [Google Scholar]
- Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nat Rev Neurosci 6: 97–107, 2005 [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes; An Investigation of the Physiological Activity of the Cerebral Cortex. London: Oxford UP, 1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci 13: 1708–1718, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Skaggs H, Churchwell J, McLaughlin J. Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus). Behav Neurosci 115: 1029–1038, 2001 [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron 51: 113–123, 2006 [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. J Neurosci 28: 10549–10560, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn Mem 15: 368–372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. J Neurosci 18: 9112–9129, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science 272: 1126–1131, 1996 [DOI] [PubMed] [Google Scholar]
- Safo P, Regehr WG. Timing dependence of the induction of cerebellar LTD. Neuropharmacology 54: 213–218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neurosci Lett 199: 175–178, 1995 [DOI] [PubMed] [Google Scholar]
- Sears LL, Steinmetz JE. Dorsal accessory inferior olive activity diminishes during acquisition of the rabbit classically conditioned eyelid response. Brain Res 545: 114–122, 1991 [DOI] [PubMed] [Google Scholar]
- Siegel JJ, Kalmbach BE, Chitwood RA, Dembrow N, Johnston D, Mauk MD. Prefrontal cortex and lateral pontine neurons display tone-evoked persistent activity during trace eyelid conditioning. Soc Neurosci Abstr 384.9, 2009 [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci 100: 729–744, 1986 [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proc Natl Acad Sci USA 84: 3531–3535, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci 23: 9897–9905, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science 322: 960–963, 2008 [DOI] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience 162: 732–755, 2009 [DOI] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav Neurosci 114: 1058–1067, 2000 [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Connections of the caudal anterior cingulate cortex in rabbit: neural circuitry participating in the acquisition of trace eyeblink conditioning. Neuroscience 145: 288–302, 2007 [DOI] [PubMed] [Google Scholar]
- Weiss C, Disterhoft JF. Eyeblink conditioning, motor control, and the analysis of limbic-cerebellar interactions. Behav Brain Sci 19: 479–480, 1996 [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends Neurosci 31: 105–112, 2008 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Green JT, Levin SI, Meisler MH. Inactivation of sodium channel Scn8A (Na-sub(v)1.6) in Purkinje neurons impairs learning in Morris water maze and delay but not trace eyeblink classical conditioning. Behav Neurosci 120: 229–240, 2006 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res 348: 249–260, 1985 [DOI] [PubMed] [Google Scholar]