Abstract
Rapid eye movement (REM) sleep is generally associated with a withdrawal of parasympathetic activity and heart rate increases; however, episodic vagally mediated heart rate decelerations also occur during REM sleep. This alternating pattern of autonomic activation provides a physiological basis for REM sleep-induced cardiac arrhythmias. Medullary neurons within the lateral paragigantocellular nucleus (LPGi) are thought to be active after REM sleep recovery and play a role in REM sleep control. In proximity to the LPGi are parasympathetic cardiac vagal neurons (CVNs) within the nucleus ambiguus (NA), which are critical for controlling heart rate. This study examined brain stem pathways that may mediate REM sleep-related reductions in parasympathetic cardiac activity. Electrical stimulation of the LPGi evoked inhibitory GABAergic postsynaptic currents in CVNs in an in vitro brain stem slice preparation in rats. Because brain stem cholinergic mechanisms are involved in REM sleep regulation, we also studied the role of nicotinic neurotransmission in modulation of GABAergic pathway from the LGPi to CVNs. Application of nicotine diminished the GABAergic responses evoked by electrical stimulation. This inhibitory effect of nicotine was prevented by the α7 nicotinic receptor antagonist α-bungarotoxin. Moreover, hypoxia/hypercapnia (H/H) diminished LPGi-evoked GABAergic current in CVNs, and this inhibitory effect was also prevented by α-bungarotoxin. In conclusion, stimulation of the LPGi evokes an inhibitory pathway to CVNs, which may constitute a mechanism for the reduced parasympathetic cardiac activity and increase in heart rate during REM sleep. Inhibition of this pathway by nicotinic receptor activation and H/H may play a role in REM sleep-related and apnea-associated bradyarrhythmias.
INTRODUCTION
Non–rapid eye movement (NREM) sleep in both humans and animals is associated with a relatively stable pattern of reduced heart rate and the highest level of parasympathetic activity compared with wakefulness and rapid eye movement (REM) sleep (Toscani et al. 1996; Verrier et al. 1998). In contrast, REM sleep is characterized by an increased sympathetic and attenuated vagal tone in adults, children, and infants (Berlad et al. 1993; Valladares et al. 2008; Villa et al. 2000). The cardiovascular alterations that occur during rat REM sleep are similar to those in human REM sleep (DeMesquita and Hale 1992). Blood pressure and heart rate are higher in REM sleep than in NREM sleep, with levels in both sleep states being lower than those during wakefulness (DeMesquita and Hale 1992; Sei et al. 2002; Snyder et al. 1964). The results from other studies also suggest that ambient temperature affects heart rate during REM sleep in rats. At 28°C, heart rate increases during transition from NREM to REM sleep, whereas at 16°C, it decreases (Sei and Morita 1996). REM sleep also results in a highly variable heart rate with episodic abrupt both increases and decreases in heart rates in both humans and animals (del Bo et al. 1982; George and Kryger 1985; Koehler et al. 1998; Sato and Seto 1993; Verrier et al. 1998). In cats, abrupt episodic decreases in heart rate are thought to occur because of centrally mediated episodic bursting in cardiac vagal efferent fiber activity (Verrier et al. 1998). Similarly, in rats, bradyarrhythmias related to vagal excitation have been observed during REM sleep (Kuo and Yang 2004; Sato and Seto 1993). Human infants spend almost 50% of their sleep time in REM sleep (Gaultier 1995; Sandyk 1992), and episodes of apnea and bradyarrhythmia are common in infants who succumb to sudden cardiac death during sleep (Meny et al. 1994). Relative risk for sudden death during REM sleep may be as high as 1.2 times the risk during wakefulness (Verrier et al. 1996). It is possible that mechanisms shown in animal studies including both vagally mediated episodic heart rate decelerations (Dickerson et al. 1993a; Verrier et al. 1998) and sympathetic nervous system-mediated episodic accelerations in heart rates during REM sleep (Dickerson et al. 1993b) may be the mechanisms responsible for the increased risk of arrhythmias and sudden cardiac death during REM sleep in humans.
The neurons responsible for the onset and maintenance of REM sleep are restricted to the brain stem (Jouvet 1962; Verret et al. 2005). Medullary neurons located in the lateral paragigantocellular nucleus (LPGi) increase their activity after REM sleep deprivation in rats (Verret et al. 2005, 2006). In addition, neurons with an activity specific to REM sleep were identified in the cat LPGi (Sakai 1988). Within the medulla, neurons active after REM sleep recovery from REM sleep deprivation and projecting to the mesopontine locus coeruleus (LC) have been localized in the dorsal paragigantocellular nucleus and to a lesser extent in the LPGi. Some of these LPGi neurons were postulated to be GABAergic and (in addition to the GABAergic neurons of the dorsal paragigantocellular nucleus) responsible for the inhibition of LC noradrenergic neurons during REM sleep (Verret et al. 2005, 2006). Supporting this framework GABAergic neurons have been identified in the LPGi (Dehkordi et al. 2007).
In proximity to the LPGi in the brain stem are preganglionic parasympathetic cardiac vagal neurons (CVNs) within the nucleus ambiguus (NA), which are critical for controlling heart rate. CVNs in the NA are the major determinant of parasympathetic cardiac activity to the heart (Cheng et al. 2004). Electrophysiological studies have shown CVNs within the NA do not possess pacemaker activity, and their activity is controlled by activation and modulation of synaptic inputs, including glutamatergic, GABAergic, and glycinergic neurotransmission (Wang et al. 2001).
Neurons in the rostral ventral medulla, including the LPGi, are important for autonomic regulation of cardiovascular function (Varner et al. 1992). Microinjection of orexin into the LGPi evokes tachycardia mediated in part by inhibition of parasympathetic activity to the heart (Ciriello et al. 2003). Anterograde labeling results (Babic and Ciriello 2004) show neurons from the LPGi project to the external formation of the NA, where preganglionic CVNs are located (Ciriello and Calaresu 1980). Injections of the retrograde transneuronal virus into the heart result in transsynaptically labeling neurons in the rostral ventral medulla, including the LPGi (Standish et al. 1995; Ter Horst et al. 1993).
This study identified and characterized the functional connections between neurons located in the LPGi and fluorescently identified preganglionic CVNs within the NA that are critical for controlling heart rate. In addition, because hypoxia/hypercapnia (H/H) occurs during apnea-associated events during REM sleep; H/H and/or hypoxia alone impacts cardiac parasympathetic (Dergacheva et al. 2009; Kamendi et al. 2008; Neff et al. 2004) as well as REM sleep control mechanisms (Huertas and McMillin 1968; Laszy and Sarkadi 1990); and H/H evokes release of acetylcholine within the medulla oblongata (Metz 1966), we studied the role of H/H and nicotinic receptor activation in modulation of GABAergic pathways to CVNs activated by electrical stimulation of the LPGi.
METHODS
All animal procedures were performed in compliance with the institutional guidelines at George Washington University and are in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and the National Institutes of Health publication Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and any possible discomfort.
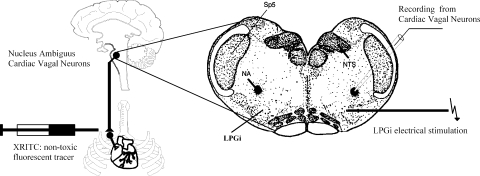
To identify cardiac vagal neurons in vitro, a two-stage procedure was used. In an initial surgery, Sprague-Dawley rats (postnatal days 2–6, Hilltop, Scottdale, PA) were anesthetized with hypothermia and received a right thoracotomy. The heart was exposed, and 0.05 ml of 1–5% rhodamine (XRITC, Molecular Probes, Eugene, OR) was injected into the pericardial sac to retrogradely label CVNs (Fig. 1). On the day of experiment (2–4 days later), the animals were anesthetized with isoflurane and killed by rapid cervical dislocation. The brain was submerged in cold (4°C) buffer composed of 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 5 mM glucose, and 10 mM HEPES and continually gassed with 100% O2. Using a dissection microscope, the cerebellum was removed, and the hindbrain was isolated. A single slice of the medulla (400-μm thickness) that included the NA and the LPGi was obtained and submerged in a recording chamber, which allowed perfusion (5–10 ml/min) of artificial cerebrospinal fluid (ACSF) at room temperature containing 125 mM NaCl, 3 mM KCl, 2 mM CaCl2, 26 mM NaHCO3, 5 mM glucose, and 5 mM HEPES equilibrated with carbogen (95% O2-5% CO2, pH 7.4).
Fig. 1.
Schematic drawing of the experimental preparation. Cardiac vagal neurons (CVNs) were identified by retrograde fluorescent labeling. Slices of 400-μm thickness were obtained and individual identified. CVNs in the nucleus ambiguus (NA) were studied using whole cell patch-clamp technique. The location of the lateral paragigantocellular nucleus (LPGi) was identified using stereotaxic coordinates in addition to the location relative to fluorescently identified CVNs in the NA. Square wave current injections of 0.1–0.5 mA and 1-ms duration were applied to evoke GABAergic pathways from the LPGi to CVNs.
Individual CVNs in the NA were identified by the presence of the fluorescent tracer. These identified CVNs were imaged with differential interference contrast optics, infrared illumination, and infrared-sensitive video detection cameras to gain better spatial resolution. Patch pipettes (2.5–3.5 MΩ) were filled with a solution consisting of 150 mM KCl, 2 mM MgCl2, 2 mM EGTA, 10 mM HEPES, and 2 mM Mg-ATP, pH 7.35. With this pipette solution, the chloride current induced by activation of GABA receptors was recorded as an inward current. Voltage-clamp whole cell recordings were made at a holding potential of −80 mV with an Axopatch 200B and pClamp 8 software (Axon Instruments, Union City, CA).
GABAergic inhibitory postsynaptic currents (IPSCs) were isolated by continuous focal application of strychnine (1 μM), d-2-amino-5-phosphonovalerate (AP-5, 50 μM), and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 50 μM) to block glycine, N-methyl-d-asparate (NMDA), and non-NMDA receptors, respectively. Drugs were focally released using a picrospritzer and pressure ejected from a patch pipette positioned within 30 μm of the patched CVN. The maximum range of drug application was determined previously to be 100–120 μm downstream from the drug pipette and was considerably less behind the drug pipette (Wang et al. 2002). Continual focal drug applications were performed using a pneumatic picopump pressure system (WPI, Sarasota, FL). Other drugs used in this study included the acetylcholine receptor agonist nicotine (1, 10, and 100 μM), the α7 nicotinic receptor antagonist α-bungarotoxin (α-BgTX, 0.1 μM), and the non-α7 nicotinic receptor antagonist dihydro-β-erythrodine (DHβE, 100 μM). Nicotine, α-BgTX, and DHβE were applied by inclusion in the perfusate. All drugs used in this study were purchased from Sigma-Aldrich Chemical (St. Louis, MO).
In experiments that examined the role of H/H in modulation of GABAergic response in CVNs, slices were exposed for 10 min to H/H by changing control-ACSF to ACSF equilibrated with 85% N2-6% O2-9% CO2, and slices were reoxygenated for 10 min by returning the perfusate to initial control ACSF.
The location of the LPGi was identified using stereotaxic coordinates (Paxinos 1997) in addition to the location relative to fluorescently identified CVNs in the NA (Fig. 1). The LPGi was electrically stimulated using a stimulus isolator (A.M.P.I., Jerusalem, Israel). Electrical stimuli of 1-ms duration were used for both single stimulation and burst stimulation (1-s trains of stimulus, 100-Hz frequency). Stimulus intensity was 1.5 times of the minimum intensity that evoked a response in CVNs. Synaptic events were measured using pClamp 8 software (Molecular Devices, Sunnyvale, CA). Synaptic latency of the evoked responses was measured as the time between the onset of the stimulus artifact and the onset of synaptic current. The responses to a series of 10 consecutive stimulations in each neuron were averaged in all series of experiments. The mean value from each neuron in the population was averaged for the population of neurons to create a summary of results for each condition. During H/H, a series of 10 consecutive stimulations were applied on minutes 1, 4, and 9 after onset of H/H.
Focal uncaging of caged glutamate was performed, as described previously (Frank et al. 2009), using a DPSS 355 nm UV laser launch system (Prairie Technologies, Madison, WI) to focally photoexcite caged glutamate (Sigma, St. Louis, MO). The laser was focused through an acousto-optic tunable filter on the slice surface with location of the LGPi (Paxinos 1997). Caged glutamate was bath-applied at a concentration of 20 μM and uncaged for a duration of 5 ms. Analysis of spontaneous synaptic currents in CVNs was performed using MiniAnalysis (version 5.6.12, Synaptosoft) with a threshold determined by the amplitude at which all synaptic events were blocked in the presence of gabazine (25 μM). Data were analyzed for the 5 s before and 20 s after glutamate uncaging and plotted in 5-s bins. The responses to a series of four consecutive photoexcitations in each neuron were averaged. The mean value from each neuron in the population was averaged to create a summary of results for each condition. In these experiments, AP-5 and CNQX were omitted from the perfusate.
Results are presented as mean ± SE and statistically compared using ANOVA with repeated measurement and Dunnett's posttest, unless otherwise stated. Only one experiment was performed per preparation.
RESULTS
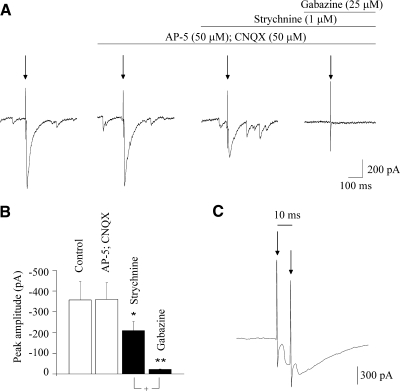
Stimulation of the LGPi evoked a postsynaptic current (average peak current of – 357 ± 89 pA, n = 10) in CVNs (Fig. 2, A and B). Focal application of the NMDA and non-NMDA receptor blockers AP-5 (50 μM) and CNQX (50 μM), respectively, did not elicit any significant changes in the evoked IPSC (–357 ± 89 vs. –360 ± 80 pA, n = 10, P > 0.05; Fig. 2, A and B). However, focal application of strychnine (1 μM), a glycinergic receptor antagonist, produced a significant decrease in the evoked postsynaptic current (from –360 ± 80 to –209 ± 43 pA, P < 0.05, n = 10; Fig. 2, A and B), suggesting glycinergic inhibitory neurotransmission contributes to this evoked postsynaptic response. Application of gabazine (25 μM), a GABAergic receptor blocker, nearly abolished the activation of this synaptic pathway, decreasing the inhibitory evoked events from –209 ± 43 to –22 ± 2 pA (n = 10, P < 0.01; Fig. 2, A and B). In further experiments, we focused on the inhibitory GABAergic current isolated by focal application of strychnine (1 μM), AP-5 (50 μM), and CNQX (50 μM), which were evoked by stimulation of the LPGi in each CVN tested (n = 65). The average latency of the evoked GABAergic IPSCs was 4.6 ± 0.2 ms (n = 22; range, 3.4–7.7 ms). The mean standard variation of the latencies (jitter) of individual traces was 0.85 ± 0.08 ms. Failures were observed only in 4 of 22 CVNs, with an average failure rate of 2.4%. In addition, GABAergic responses in CVNs were consistently evoked by paired pulse stimulations with an interval of 10 ms (n = 10; Fig. 2C).
Fig. 2.
A: stimulation of the LPGi evoked a postsynaptic current in CVNs. Application of the N-methyl-d-aspartate (NMDA) and non-NMDA receptor blockers AP-5 and CNQX, respectively, did not alter the evoked inhibitory postsynaptic current (IPSC) as shown in a typical example (A) and in the summary data (B). However, application of strychnine, a glycinergic receptor antagonist, significantly decreased the evoked postsynaptic current (A, typical experiment; B, summary data). Finally, gabazine, a GABAergic receptor antagonist, nearly abolished the activation of this synaptic pathway (A, typical experiment; B, summary data). As shown in C, paired pulse stimulations with a delay of 10 ms evoked postsynaptic GABAergic responses (isolated by focal application of strychnine, AP-5, and CNQX) in CVNs with a constant latency. +P < 0.05. In this and all subsequent figures, arrow indicates electrical stimulation (unless other stated), ■ with asterisks indicates statistically significant differences, *P < 0.05, **P < 0.01, and ***P < 0.001.
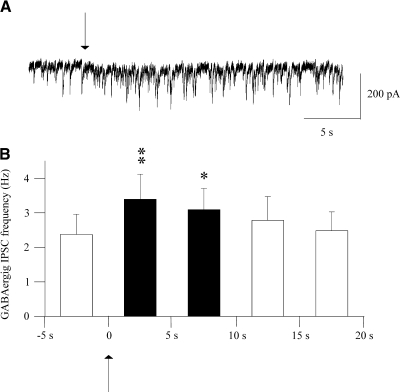
To test whether activation of somata or passing axonal fibers en route the LPGi recruits a GABAergic pathway to CVNs, photoexcitation of caged glutamate was used instead of electrical stimulation to only activate LPGi somata. Stimulation of the LPGi for 5 ms with 20 μM uncaged glutamate elicited a significant increase in the frequency of GABAergic IPSCs in the CVNs (n = 10; Fig. 3), and this pathway was subsequently blocked by application of gabazine (25 μM). Although both photo-uncaging of caged glutamate and electrical stimulation elicited the GABAergic pathway from the LPGi to CVNs, chemical stimulation is less temporally precise; therefore electrical stimulation was used in subsequent experiments.
Fig. 3.
Photoexcitation of LPGi neurons with uncaged glutamate elicited a significant increase in the frequency of GABAergic IPSCs in the CVNs. A typical experiment is shown in A, whereas the summary data are shown in B. Arrow indicates 5-ms stimulation of the LPGi with uncaged glutamate.
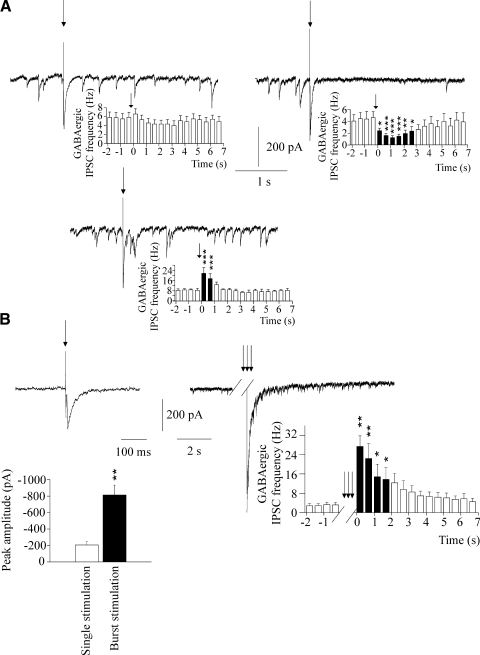
In addition to the initial relatively fast (<8-ms latency) IPSCs described above, electrical stimulation of the LGPi often evoked changes in spontaneous IPSC frequency that followed the initial response and lasted 1–3 s. In 11 of 26 CVNs (42%), only an initial IPSC was evoked (Fig. 4A). However, in other CVNs tested, the initial evoked sympathetic current was followed by either a reduction in the GABAergic IPSC frequency (42%, n = 26; Fig. 4A) or, in a minority of CVNs, an enhancement of GABAergic IPSCs (16%, n = 26; Fig. 4A).
Fig. 4.
As shown in A (left), in a typical experiment and in the histogram from 11 cells, the frequency of IPSCs followed by the initial short-latency GABAergic current was not altered by the LPGi stimulation (42% of CVNs tested). However, in other CVNs tested, there was either decrease (42%, A, right, typical experiment and histogram from 11 cells) or increase (16%, A, bottom, typical experiment and histogram from 4 cells) in the frequency of IPSCs followed the initial GABAergic response. Burst stimulation evoked GABAergic current (B, right) significantly larger than GABAergic currents evoked by single stimulation (B, left, and B, bottom, the summary data from 10 cells). This initial GABAergic current evoked by burst electrical stimulation was followed by an increase in the IPSC frequency with long latencies, as shown in the histogram obtained from 8 cells (B, right).
A pattern of bursting, rather than a single stimulation evoked a GABAergic current with average peak amplitude of – 814 ± 124 pA, which was significantly larger (P < 0.01, Student's t-test, n = 8), as shown in Fig. 4B, than GABAergic postsynaptic response evoked by a single stimulation (–207 ± 39 pA). Moreover, the initial response evoked by burst stimulation was consistently followed by a persistent increase in the frequency of GABAergic IPSCs in each neuron tested (n = 8; Fig. 4B).
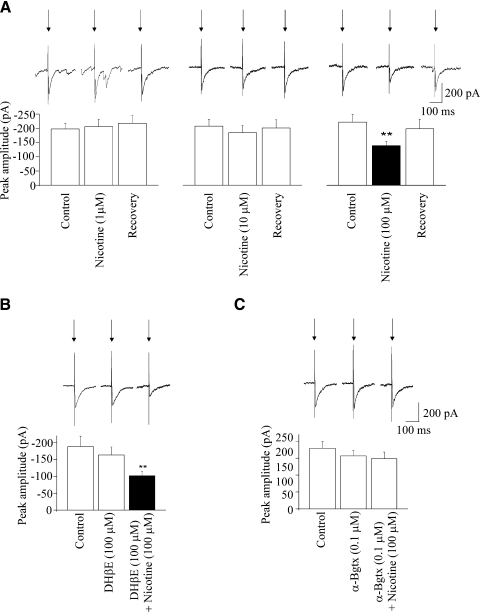
Application of nicotine, at concentrations of both 1 and 10 μM, did not elicit any significant change in the peak amplitude of the GABAergic response in CVNs evoked by stimulation of the LPGi (−198 ± 19 vs. −206 ± 26 pA, n = 7, P > 0.5, and −207 ± 24 vs. −185 ± 25 pA, n = 10, P > 0.05, respectively; Fig. 5A). However, application of nicotine at a concentration of 100 μM significantly and reversibly decreased the peak amplitude of the GABAergic postsynaptic response (from −222 ± 28 to −139 ± 15 pA, n = 10, P < 0.01; Fig. 5A). To identify the specific nicotinic receptors involved and test whether α7 and non-α7 nicotinic receptors modulate this inhibitory GABAergic pathway, the effects of different nicotinic receptor antagonists were tested. Application of the non-α7 nicotinic receptor antagonist DHβE at a concentration of 100 μM did not elicit any significant change in the evoked GABAergic response (−188 ± 31 vs. −163 ± 24 pA, n = 9, P > 0.05; Fig. 5B). DHβE also did not prevent the inhibition elicited by nicotine; in the presence of DHβE (100 μM), nicotine (100 μM) continued to elicit a decrease in the evoked GABAergic response (from −188 ± 31 to −102 ± 13 pA, n = 9, P < 0.01; Fig. 5B). The α7 nicotinic receptor antagonist α-BgTX at a concentration of 0.1 μM did not change the peak amplitude of the GABAergic response (−179 ± 20 vs. −156 ± 16 pA, n = 10, P > 0.05; Fig. 5B). However, application of α-BgTX (0.1 μM) prevented the inhibition of evoked GABAergic response elicited by nicotine at concentration of 100 μM (−179 ± 20 vs. −148 ± 20 pA, n = 10, P > 0.05; Fig. 5B).
Fig. 5.
As shown in a single experiment (A, top) and in the summary data (A, bottom), application of nicotine at concentrations of either 1 or 10 μM did not significantly alter the peak amplitude of the GABAergic current evoked by LGPi electrical stimulation. However, nicotine, at a concentration of 100 μM, significantly inhibited the evoked GABAergic current (A, top, single experiment; A, bottom, summary data). As shown in a single experiment (B, top) and in the summary data (B, bottom), application of the non α7 receptor antagonist DHβE did not alter the evoked GABAergic response and DHβE also did not alter the inhibitory effect of nicotine on the evoked GABAergic current. However, the inhibitory effect of nicotine was antagonized with α-Bgtx, an α7 receptor blocker, whereas α-Bgtx alone did not evoke any effect on the inhibitory GABAergic current evoked by LGPi stimulation, as shown (C, top, single experiment; C, bottom, summary data).
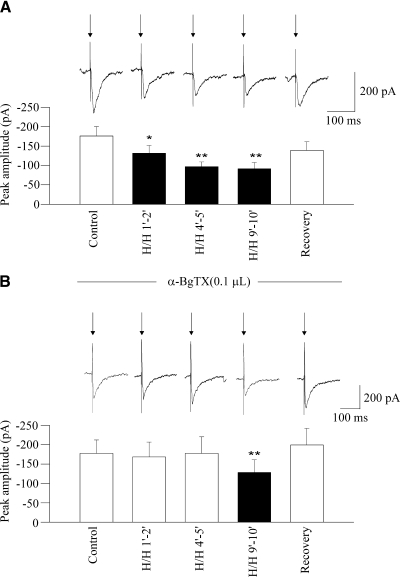
Changing the perfusate from ACSF equilibrated with 95% O2-5% CO2 to ACSF equilibrated with 9% CO2-6% O2-85% N2 (H/H) induced a significant decrease in the peak amplitude of the GABAergic response evoked by LPGi electrical stimulation throughout H/H (tested periods of 1–2, 4–5, and 9–10 min, from –177 ± 24 to –131 ± 19 pA, P < 0.05; then to –97 ± 12 pA, P < 0.01, then to –91 ± 16 pA, P < 0.01, respectively, n = 9; Fig. 6A). This inhibitory effect of H/H was prevented by the α7 nicotinic receptor antagonist α-BgTX (0.1 μM) in periods of 1–2 and 4–5 min (–177 ± 35 vs. –168 ± 38 pA and –177 ± 35 vs. –177 ± 43 pA, respectively, n = 9, P > 0.05; Fig. 6B). However, in the last period of H/H (9–10 min), H/H decreased the evoked GABAergic response in the presence of α-BgTX (from –177 ± 35 to –128 ± 33 pA, n = 9, P < 0.01; Fig. 6B). Because electrical stimulation of the LGPi under normal conditions often evoked changes in spontaneous IPSC frequency that followed the initial response and lasted 1–3 s, the frequency of GABAergic IPSCs that followed the initial response was also analyzed throughout H/H. Changing the perfusate to H/H did not evoke any significant changes in the frequency of the GABAergic IPSCs in a period of 1–2 min after onset of H/H (2.9 ± 0.9 vs. 2.4 ± 0.5 Hz, P > 0.05, n = 6) However, H/H induced a significant decrease in the frequency of the GABAergic IPSCs in H/H periods of 4–5 min and 9–10 min (from 2.9 ± 0.9 to 1.5 ± 0.5 Hz, P < 0.01; then to 1.0 ± 0.3 Hz, P < 0.001, respectively, n = 6). This inhibitory effect of H/H was prevented by the α7 nicotinic receptor antagonist α-BgTX (0.1 μM) in the 4- to 5-min period after H/H onset (2.6 ± 0.7 vs. 1.8 ± 0.5 Hz, n = 7, P > 0.05). However, in the period 9–10 min after H/H, the frequency of GABAergic IPSCs continued to decrease in the presence of α-BgTX (from 2.6 ± 0.7 to 0.8 ± 0.2 Hz, n = 7, P < 0.05).
Fig. 6.
As shown in a typical experiment (A, top) and in the summary data (A, bottom), hypoxia/hypercapnia (H/H) elicited a significant and reversible decrease in the peak amplitude of GABAergic current evoked by electrical stimulation of the LPGi. This inhibition of evoked GABAergic response was prevented by application of the α7 receptor antagonist α-Bgtx in periods of both 1–2 and 4–5 min, whereas in a period of 9–10 min, α-Bgtx did not alter the H/H-elicited inhibition in evoked GABAergic current, as shown in a typical experiment (B, top) and in the summary data (B, bottom).
DISCUSSION
The results from this study indicate the LPGi contains neurons that project to CVNs within the NA. Synaptic input to CVNs evoked by electrical stimulation of the LPGi is predominantly inhibitory because synaptic responses in CVNs evoked by LPGi stimulation are significantly decreased and subsequently nearly abolished by the glycinergic receptor blocker strychnine and the GABAergic receptor blocker gabazine, respectively. Although electrical stimulation may activate not only neuronal somata but also axonal fibers passing through the stimulated area of the brain, the results from this study indicate both electrical stimulation of the LPGi and stimulation of the LPGi by photo-uncaging of caged glutamate recruit a GABAergic neurotransmission to CVNs. Taken in conjunction with the results from other studies (Christian and Dudek 1988; Wuarin and Dudek 2001) indicating glutamate only activates the somatodendritic area, but does not generate action potential firing when applied to axons, the result from this study indicate activation of neuronal somata within the LPGi, rather than fibers within the stimulated site, is responsible for the evoked GABAergic pathway to CVNs.
The most common approach to distinguishing monosynaptic from polysynaptic pathways relies on shortness of the latency, the failure rate, and whether evoked synaptic events faithfully follow pairs of closely timed stimuli (Doyle and Andresen 2001; Karayannis et al. 2007; Zhang and Mifflin 2000). The latency of the initial evoked GABAergic IPSCs in this study can be characterized as a relatively short latency. These data in conjunction with other results from this study such as the low failure rate of evoked events and the ability of the evoked events to faithfully follow paired pulse stimulations with a delay of 10 ms suggest the pathway from LPGi to CVNs is likely monosynaptic. In support of this hypothesis, GABAergic neurons have been found in the LPGi (Dehkordi et al. 2007; Song et al. 2000). Also consistent with this hypothesis, injections of the retrograde transneuronal pseudorabies virus into the heart results in transsynaptically labeling neurons in the rostral ventral medulla oblongata, including the LPGi (Standish et al. 1995; Ter Horst et al. 1993). In addition, anterograde labeling results (Babic and Ciriello 2004) indicates neurons from the rostral ventromedial medulla, including the LPGi, project to the external formation of the NA, where preganglionic CVNs are located (Bieger and Hopkins 1987; Ciriello and Calaresu 1980; Stuesse 1982). However, we cannot rule out the possibility that the evoked pathway from the LPGi to CVNs is polysynaptic. Jitter is often used as a the most reliable discriminator of mono- versus polysynaptic pathways (Doyle and Andresen 2001). The data from this study indicate the jitter of evoked IPSCs in CVNs is high, and this is consistent with transmission via polysynaptic pathways (Doyle and Andresen 2001). Because the latency of monosynaptic response could be as short as 2.3–2.8 ms (Doyle and Andresen 2001; Yoshimura and Nishi 1995), and the average latency of evoked GABAergic responses in CVNs in this study is 4.6 ms, it is possible that some GABAergic responses observed in this study are disynaptic. It is also possible that the population of GABAergic responses evoked by stimulation of the LPGi represent a mixture of mono- and disynaptic response pathways.
During REM sleep, neurons from different brain areas, including the brain stem, switch from firing single spikes to a prominent bursting pattern (Dahan et al. 2007; Eguchi and Satoh 1980; Nelson et al. 1983), which is known to enhance neurotransmitter release (Dahan et al. 2007). In this study, we found stimulation of the LGPi with bursts evokes significantly larger GABAergic postsynaptic currents in CVNs than a single stimulation. In addition, with burst stimulation, the initial GABAergic response is followed by an increase in the frequency of the IPSCs in all CVNs tested. In contrast, a single stimulation elicits, in addition to the initial evoked IPSCs, facilitation of IPSC events only in a minority of CVNs, whereas in a majority of CVNs, there is either only an initial response or a reduction of IPSCs after the initial response. Burst stimulation therefore evokes a more robust and a longer-lasting activation of inhibitory postsynaptic pathway to CVNs than a single stimulation.
Cholinergic neurotransmission in the brain stem is an important component of the neuronal network generating REM sleep (Vazquez and Baghdoyan 2004; Verret et al. 2005). There is an REM sleep-associated increase in acetylcholine release in discrete brain region including the pons, the thalamus, and the medulla oblongata (Benedito and Camarini 2001; Kodama et al. 1992). Moreover, recent work has found a subpopulation of cholinergic LPGi neurons that increase their activity-dependent c-Fos levels specifically after REM sleep recovery from REM sleep deprivation (Verret et al. 2005). In this study, we found application of nicotine significantly diminishes the inhibitory GABAergic response evoked by electrical stimulation of the LPGi. This inhibitory effect of nicotine is prevented by the α7 nicotinic receptor antagonist α-BgTX (0.1 μM), suggesting an important role of cholinergic α7 receptor in modulation of inhibitory GABAergic pathway to CVNs. Although we do not know the site of action of nicotine in this study, it is not likely nicotine acts on GABAergic presynaptic terminals, because in close proximity to CVNs nicotine has been shown to facilitate spontaneous GABAergic IPSCs in CVNs acting on α4β2 nicotinic receptors at presynaptic terminals surrounding CVNs, whereas α7 receptors did not contribute to this nicotinic effect (Wang et al. 2003). It is possible that, in this study, nicotine application activates α7 receptors directly on the LPGi neuron at their soma because the LPGi contains GABAergic neurons that express α7 receptors (Dehkordi et al. 2007).
Both hypercapnia alone and H/H produce acetylcholine release in the medulla oblongata (Metz 1966). The results from this study indicate H/H elicits a significant decrease in the GABAergic response evoked by LPGi stimulation. Application of the α7 nicotinic receptor antagonist α-BgTX (0.1 μM) abolishes this inhibitory effect for ≤5 min of H/H. However, after 9–10 min of H/H, α-BgTX does not block the H/H-evoked inhibition. It is possible that during the initial H/H period (1–5 min) acetylcholine release evoked by H/H activates α7 nicotinic receptors to diminish the GABAergic pathway to CVNs evoked by LGPi electrical stimulation. However, during the final period of H/H exposure (9–10 min), neurochemical mechanisms, other than nicotinic receptor activation, are likely recruited and inhibit GABAergic pathway to CVNs from the LPGi.
Implications for heart rate control during REM sleep
Considerable evidence indicates that LPGi neurons play an important role in REM sleep control. Following lesions of the medullary reticular formation encompassing the LGPi, there is a significant reduction in the amount of REM sleep (Holmes and Jones 1994). The rat LGPi contains a significant number neurons with activity-dependent increases in c-Fos levels specifically after REM sleep recovery from REM sleep deprivation (Verret et al. 2005, 2006). Because Fos-labeled neurons after REM sleep rebound correspond to neurons active during REM sleep, it has been postulated that the LGPi contains numerous neurons active during REM sleep (Verret et al. 2005, 2006). Supporting this postulate, neurons with an activity specific to REM sleep have been identified in the cat LPGi (Sakai 1988). A large number of LPGi neurons project to the sublaterodorsal nucleus known to be critical for REM sleep onset and maintenance (Boissard et al. 2002). The LPGi sends GABA and glycine inputs to the LC and the dorsal raphe, a major wakefulness-promoting nuclei containing noradrenergic and serotonergic neurons, respectively, that are active during wakefulness and stop firing during REM sleep (Rampon et al. 1999; Verret et al. 2006). Electrical stimulation of the LPGi evoked purely inhibitory responses in 16% of the LC neurons (Rampon et al. 1999). A substantial number of LPGi neurons that project to the LC are active following REM sleep deprivation (Verret et al. 2006), and the GABAergic neurons in the medulla oblongata projecting to the LC have been proposed to provide the tonic inhibition of LC noradrenergic neurons during REM sleep (Verret et al. 2005, 2006). Furthermore, inhibitory LPGi neurons have been proposed to regulate the excitability of the noradrenergic neurons of the LC and serotonergic neurons of the dorsal raphe across the sleep-waking cycle (Rampon et al. 1999). The results from this study suggest another important GABAergic pathway is from the LPGi to CVNs in the NA. During REM sleep, activation of GABAergic neurons in the LPGi may exert an inhibitory action at both monoaminergic neurons promoting wakefulness and CVNs in the NA, maintaining parasympathetic activity to the heart. In support of this framework, microinjection of orexin into the LGPi evokes tachycardia mediated in part by inhibition of parasympathetic activity to the heart (Ciriello et al. 2003). It is worth noting that considerable evidence suggests hypothalamic orexin plays a role in regulation and/or maintenance of REM sleep in addition to its crucial role in the control of wakefulness (Burlet et al. 2002; Kiyashchenko et al. 2002; Xi and Chase 2006; Xi et al. 2002, 2003). Switching LGPi neurons to a bursting pattern of firing during REM sleep would enhance this inhibitory pathway to CVNs and likely contributes to withdrawal of parasympathetic activity that occurs during REM sleep (Berlad et al. 1993; Valladares et al. 2007).
Implications for REM sleep-related bradyarrhythmias
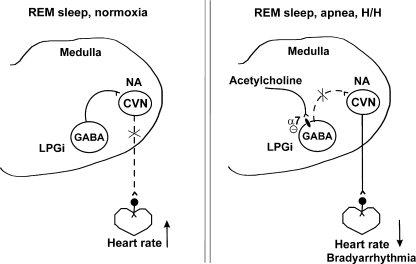
Cardiac arrhythmias are a major cause of sudden death during sleep (Cobb et al. 1980; Meny et al. 1994; Steinschneider 1972). Respiratory and cardiac disturbance are known to occur selectively during REM sleep (Gaultier 1995). Victims of sudden infant death syndrome typically have an increased frequency of apnea events during REM sleep (Franco et al. 1999). Apnea-associated bradyarrhythmias are considered to be caused by heightened parasympathetic tone (Koehler et al. 1998) and have a clear predominance during REM sleep (Gabriel and Albani 1976; Koehler et al. 1998). Although REM sleep is associated with a withdrawal of parasympathetic activity (Berlad et al. 1993; Valladares et al. 2008), episodic abrupt vagally mediated decreases in heart rates also occur during REM sleep (George and Kryger 1985; Koehler et al. 1998; Verrier et al. 1998). Supranormal activation of parasympathetic pathways during REM sleep may result in extreme bradyarrhythmia and sudden death. The results from this study suggest that neurochemical mechanisms for bradyarrhythmias evoked by H/H that typically occurs during apnea-associated events likely include an α7 nicotinic receptor mediated attenuation of the inhibitory pathway to parasympathetic CVNs evoked by activation of LPGi neurons. This framework is shown in Fig. 7.
Fig. 7.
Hypothetical framework for rapid eye movement (REM) sleep-dependent changes in heart rate. REM sleep-related activation of the LPGi neurons evokes GABAergic pathway to CVNs in the NA, which results in withdrawal of parasympathetic activity to the heart and increases in heart rate (right). H/H that typically occurs during apnea-associated events during REM sleep induces an α7 nicotinic receptor–mediated attenuation of the inhibitory pathway to parasympathetic CVNs that results in CVN disinhibition and activation of vagal tone, which may cause life-threatening bradyarrhythmias (left).
Although fullterm newborn humans spend almost 50% of their sleep time in REM sleep, this percentage is even greater in preterm newborns (Gaultier 1995; Sandyk 1992). It is important to emphasize that this study was performed on newborn rats, which, similar to newborn humans, spend very little time in wakefulness but rather spend most of sleep time in an activated state (which is comparable to REM sleep in adults) (Mirmiran 1986). Such predominance of REM sleep is a risk factor for abnormal sleep-related events for neonates (Gaultier 1995). Accordingly, the inhibitory pathway from the LPGi to CVNs and its attenuation with H/H may have particular significance for neonates.
In conclusion, this study established likely neuroanatomical substrates and cellular mechanisms underlying REM sleep-related general reduction in parasympathetic cardiac activity and increase in heart rate and apnea-associated bradyarrhythmias. Activation of LPGi neurons elicits an inhibitory GABAergic pathway to CVNs. Diminishing this pathway to the NA by both nicotinic receptor activation and H/H may play a critical role in REM sleep-related and apnea-associated bradyarrhythmias.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-59895, HL-49965, and HL-72006 to D. Mendelowitz.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Babic T, Ciriello J. Medullary and spinal cord projections from cardiovascular responsive sites in the rostral ventromedial medulla.J Comp Neurol 469: 391–412, 2004 [DOI] [PubMed] [Google Scholar]
- Benedito MA, Camarini R. Rapid eye movement sleep deprivation induces an increase in acetylcholinesterase activity in discrete rat brain regions.Braz J Med Biol Res 34: 103–109, 2001 [DOI] [PubMed] [Google Scholar]
- Berlad II, Shlitner A, Ben-Haim S, Lavie P. Power spectrum analysis and heart rate variability in Stage 4 and REM sleep: evidence for state-specific changes in autonomic dominance.J Sleep Res 2: 88–90, 1993 [DOI] [PubMed] [Google Scholar]
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus.J Comp Neurol 262: 546–562, 1987 [DOI] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study.Eur J Neurosci 16: 1959–1973, 2002 [DOI] [PubMed] [Google Scholar]
- Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications for wakefulness and narcolepsy.J Neurosci 22: 2862–2872, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Zhang H, Yu J, Wurster RD, Gozal D. Attenuation of baroreflex sensitivity after domoic acid lesion of the nucleus ambiguus of rats.J Appl Physiol 96: 1137–1145, 2004 [DOI] [PubMed] [Google Scholar]
- Christian EP, Dudek FE. Characteristics of local excitatory circuits studied with glutamate microapplication in the CA3 area of rat hippocampal slices.J Neurophysiol 59: 90–109, 1988 [DOI] [PubMed] [Google Scholar]
- Ciriello J, Calaresu FR. Distribution of vagal cardioinhibitory neurons in the medulla of the cat.Am J Physiol 238: R57–R64, 1980 [DOI] [PubMed] [Google Scholar]
- Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla.Brain Res 991: 84–95, 2003 [DOI] [PubMed] [Google Scholar]
- Cobb LA, Werner JA, Trobaugh GB. Sudden cardiac death. I. A decade's experience with out-of-hospital resuscitation.Mod Concepts Cardiovasc Dis 49: 31–36, 1980 [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep.Neuropsychopharmacology 32: 1232–1241, 2007 [DOI] [PubMed] [Google Scholar]
- Dehkordi O, Millis RM, Dennis GC, Jazini E, Williams C, Hussain D, Jayam-Trouth A. Expression of alpha-7 and alpha-4 nicotinic acetylcholine receptors by GABAergic neurons of rostral ventral medulla and caudal pons.Brain Res 1185: 95–102, 2007 [DOI] [PubMed] [Google Scholar]
- del Bo A, Ledoux JE, Tucker LW, Harshkfield GA, Reis DJ. Arterial pressure and heart rate changes during natural sleep in rat.Physiol Behav 28: 425–429, 1982 [DOI] [PubMed] [Google Scholar]
- DeMesquita S, Hale GA. Cardiopulmonary regulation after rapid-eye-movement sleep deprivation.J Appl Physiol 72: 970–976, 1992 [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Kamendi H, Wang X, Pinol RM, Frank J, Jameson H, Gorini C, Mendelowitz D. The role of 5-HT3 and other excitatory receptors in central cardiorespiratory responses to hypoxia: implications for sudden infant death syndrome.Pediatr Res 65: 625–630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson LW, Huang AH, Nearing BD, Verrier RL. Primary coronary vasodilation associated with pauses in heart rhythm during sleep.Am J Physiol 264: R186–R196, 1993a [DOI] [PubMed] [Google Scholar]
- Dickerson LW, Huang AH, Thurnher MM, Nearing BD, Verrier RL. Relationship between coronary hemodynamic changes and the phasic events of rapid eye movement sleep.Sleep 16: 550–557, 1993b [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro.J Neurophysiol 85: 2213–2223, 2001 [DOI] [PubMed] [Google Scholar]
- Eguchi K, Satoh T. Convergence of sleep-wakefulness subsystems onto single neurons in the region of cat's solitary tract nucleus.Arch Ital Biol 118: 331–345, 1980 [PubMed] [Google Scholar]
- Franco P, Szliwowski H, Dramaix M, Kahn A. Decreased autonomic responses to obstructive sleep events in future victims of sudden infant death syndrome.Pediatr Res 46: 33–39, 1999 [DOI] [PubMed] [Google Scholar]
- Frank JG, Jameson HS, Gorini C, Mendelowitz D. Mapping and identification of GABAergic neurons in transgenic mice projecting to cardiac vagal neurons in the nucleus ambiguus using photo-uncaging.J Neurophysiol 101: 1755–1760, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M, Albani M. Cardiac slowing and respiratory arrest in preterm infants.Eur J Pediatr 122: 257–261, 1976 [DOI] [PubMed] [Google Scholar]
- Gaultier C. Cardiorespiratory adaptation during sleep in infants and children.Pediatr Pulmonol 19: 105–117, 1995 [DOI] [PubMed] [Google Scholar]
- George CF, Kryger MH. Sleep and control of heart rate.Clin Chest Med 6: 595–601, 1985 [PubMed] [Google Scholar]
- Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience 62: 1179–1200, 1994 [DOI] [PubMed] [Google Scholar]
- Huertas J, McMillin JK. Paradoxical sleep: effect of low partial pressures of atmospheric oxygen.Science 159: 745–746, 1968 [DOI] [PubMed] [Google Scholar]
- Jouvet M. Research on the neural structures and responsible mechanisms in different phases of physiological sleep.Arch Ital Biol 100: 125–206, 1962 [PubMed] [Google Scholar]
- Kamendi HW, Cheng Q, Dergacheva O, Frank JG, Gorini C, Jameson HS, Pinol RA, Wang X, Mendelowitz D. Recruitment of excitatory serotonergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus post hypoxia and hypercapnia.J Neurophysiol 99: 1163–1168, 2008 [DOI] [PubMed] [Google Scholar]
- Karayannis T, Huerta-Ocampo I, Capogna M. GABAergic and pyramidal neurons of deep cortical layers directly receive and differently integrate callosal input.Cereb Cortex 17: 1213–1226, 2007 [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states.J Neurosci 22: 5282–5286, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Enhancement of acetylcholine release during REM sleep in the caudomedial medulla as measured by in vivo microdialysis.Brain Res 580: 348–350, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler U, Fus E, Grimm W, Pankow W, Schafer H, Stammnitz A, Peter JH. Heart block in patients with obstructive sleep apnoea: pathogenetic factors and effects of treatment.Eur Respir J 11: 434–439, 1998 [DOI] [PubMed] [Google Scholar]
- Kuo TB, Yang CC. Scatterplot analysis of EEG slow-wave magnitude and heart rate variability: an integrative exploration of cerebral cortical and autonomic functions.Sleep 27: 648–656, 2004 [DOI] [PubMed] [Google Scholar]
- Laszy J, Sarkadi A. Hypoxia-induced sleep disturbance in rats.Sleep 13: 205–217, 1990 [PubMed] [Google Scholar]
- Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home.Pediatrics 93: 44–49, 1994 [PubMed] [Google Scholar]
- Metz B. Hypercapnia and acetylcholine release from the cerebral cortex and medulla.J Physiol 186: 321–332, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmiran M. The importance of fetal/neonatal REM sleep.Eur J Obstet Gynecol Reprod Biol 21: 283–291, 1986 [DOI] [PubMed] [Google Scholar]
- Neff RA, Simmens SJ, Evans C, Mendelowitz D. Prenatal nicotine exposure alters central cardiorespiratory responses to hypoxia in rats: implications for sudden infant death syndrome.J Neurosci 24: 9261–9268, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JP, McCarley RW, Hobson JA. REM sleep burst neurons, PGO waves, and eye movement information.J Neurophysiol 50: 784–797, 1983 [DOI] [PubMed] [Google Scholar]
- Paxinos G. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 1997 [Google Scholar]
- Rampon C, Peyron C, Gervasoni D, Pow DV, Luppi PH, Fort P. Origins of the glycinergic inputs to the rat locus coeruleus and dorsal raphe nuclei: a study combining retrograde tracing with glycine immunohistochemistry.Eur J Neurosci 11: 1058–1066, 1999 [DOI] [PubMed] [Google Scholar]
- Sakai K. Executive mechanisms of paradoxical sleep.Arch Ital Biol 126: 239–257, 1988 [PubMed] [Google Scholar]
- Sandyk R. Melatonin and maturation of REM sleep.Int J Neurosci 63: 105–114, 1992 [DOI] [PubMed] [Google Scholar]
- Sato T, Seto K. Centrally administered ouabain aggravates rapid-eye-movement-sleep-related bradyarrhythmias in freely moving rats.Br J Pharmacol 110: 253–256, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sei H, Morita Y. Effect of ambient temperature on arterial pressure variability during sleep in the rat.J Sleep Res 5: 37–41, 1996 [DOI] [PubMed] [Google Scholar]
- Sei H, Sano A, Ohno H, Yamabe K, Nishioka Y, Sone S, Morita Y. Age-related changes in control of blood pressure and heart rate during sleep in the rat.Sleep 25: 279–285, 2002 [DOI] [PubMed] [Google Scholar]
- Snyder F, Hobson JA, Morrison DF, Goldfrank F. Changes in respiration, heart rate, and systolic blood pressure in human sleep.J Appl Physiol 19: 417–422, 1964 [DOI] [PubMed] [Google Scholar]
- Song G, Li Q, Shao FZ. GABAergic neurons in Kolliker-Fuse nucleus and Botzinger complex with axons projecting to phrenic nucleus.Sheng Li Xue Bao 52: 167–169, 2000 [PubMed] [Google Scholar]
- Standish A, Enquist LW, Escardo JA, Schwaber JS. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus.J Neurosci 15: 1998–2012, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinschneider A. Prolonged apnea and the sudden infant death syndrome: clinical and laboratory observations.Pediatrics 50: 646–654, 1972 [PubMed] [Google Scholar]
- Stuesse SL. Origins of cardiac vagal preganglionic fibers: a retrograde transport study.Brain Res 236: 15–25, 1982 [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Van den Brink A, Homminga SA, Hautvast RW, Rakhorst G, Mettenleiter TC, De Jongste MJ, Lie KI, Korf J. Transneuronal viral labelling of rat heart left ventricle controlling pathways.Neuroreport 4: 1307–1310, 1993 [DOI] [PubMed] [Google Scholar]
- Toscani L, Gangemi PF, Parigi A, Silipo R, Ragghianti P, Sirabella E, Morelli M, Bagnoli L, Vergassola R, Zaccara G. Human heart rate variability and sleep stages.Ital J Neurol Sci 17: 437–439, 1996 [DOI] [PubMed] [Google Scholar]
- Valladares EM, Eljammal SM, Motivala S, Ehlers CL, Irwin MR. Sex differences in cardiac sympathovagal balance and vagal tone during nocturnal sleep.Sleep Med 9: 310–316, 2008 [DOI] [PubMed] [Google Scholar]
- Varner KJ, Rutherford DS, Vasquez EC, Brody MJ. Identification of cardiovascular neurons in the rostral ventromedial medulla in anesthetized rats.Hypertension 19: II193–II197, 1992 [DOI] [PubMed] [Google Scholar]
- Vazquez J, Baghdoyan HA. GABAA receptors inhibit acetylcholine release in cat pontine reticular formation: implications for REM sleep regulation.J Neurophysiol 92: 2198–2206, 2004 [DOI] [PubMed] [Google Scholar]
- Verret L, Fort P, Gervasoni D, Leger L, Luppi PH. Localization of the neurons active during paradoxical (REM) sleep and projecting to the locus coeruleus noradrenergic neurons in the rat.J Comp Neurol 495: 573–586, 2006 [DOI] [PubMed] [Google Scholar]
- Verret L, Leger L, Fort P, Luppi PH. Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery.Eur J Neurosci 21: 2488–2504, 2005 [DOI] [PubMed] [Google Scholar]
- Verrier RL, Lau TR, Wallooppillai U, Quattrochi J, Nearing BD, Moreno R, Hobson JA. Primary vagally mediated decelerations in heart rate during tonic rapid eye movement sleep in cats.Am J Physiol 274: R1136–R1141, 1998 [DOI] [PubMed] [Google Scholar]
- Verrier RL, Muller JE, Hobson JA. Sleep, dreams, and sudden death: the case for sleep as an autonomic stress test for the heart.Cardiovasc Res 31: 181–211, 1996 [PubMed] [Google Scholar]
- Villa MP, Calcagnini G, Pagani J, Paggi B, Massa F, Ronchetti R. Effects of sleep stage and age on short-term heart rate variability during sleep in healthy infants and children.Chest 117: 460–466, 2000 [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus.Ann NY Acad Sci 940: 237–246, 2001 [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Synaptic activation of hypoglossal respiratory motorneurons during inspiration in rats.Neurosci Lett 332: 195–199, 2002 [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Irnaten M, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Endogenous acetylcholine and nicotine activation enhances GABAergic and glycinergic inputs to cardiac vagal neurons.J Neurophysiol 89: 2473–2481, 2003 [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment.J Neurophysiol 85: 1067–1077, 2001 [DOI] [PubMed] [Google Scholar]
- Xi MC, Chase MH. Neuronal mechanisms of active (rapid eye movement) sleep induced by microinjections of hypocretin into the nucleus pontis oralis of the cat.Neuroscience 140: 335–342, 2006 [DOI] [PubMed] [Google Scholar]
- Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat.J Neurophysiol 87: 2880–2888, 2002 [DOI] [PubMed] [Google Scholar]
- Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Hypocretinergic facilitation of synaptic activity of neurons in the nucleus pontis oralis of the cat.Brain Res 976: 253–258, 2003 [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro.J Physiol 482: 29–38, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Integration of aortic nerve inputs in hypertensive rats.Hypertension 35: 430–436, 2000 [DOI] [PubMed] [Google Scholar]