Abstract
Bidirectional interactions between neurons and glial cells are crucial to the genesis of pathological pain. The mechanisms regulating these interactions and the role of this process in relaying synaptic input in the spinal dorsal horn remain to be established. We studied the role of glutamate transporters in the regulation of such interactions. On pharmacological blockade of glutamate transporters, slow inward currents (SICs) appeared spontaneously and/or were evoked by peripheral synaptic input in the spinal superficial dorsal horn neurons, including the spinothalamic tract neurons. We showed that the SICs were induced by the release of glutamate from glial cells. On inhibition of glutamate uptake, the stimulation-induced, synaptically released glutamate activated glial cells and caused glial cells to release glutamate. Glial-derived glutamate acted on extrasynaptic N-methyl-d-aspartate (NMDA) receptors mainly composed of NR2B receptors and generated SICs, which led to depolarization and action potential generation in superficial spinal dorsal horn neurons. Thus glutamate transporters regulate glutamatergic neuron–glia interactions at spinal sensory synapses. When glutamate uptake is impaired, glial cells function like excitatory interneurons—they are activated by peripheral synaptic input and release glutamate to activate postsynaptic neurons in spinal pain pathways.
INTRODUCTION
Bidirectional interactions between neurons and glial cells are crucial to the functional regulation of the tripartite synapse (composed of pre- and postsynaptic neuronal elements and surrounding astrocytes) and to the genesis of pathological pain. The mode and impact of these interactions on synaptic transmission are region specific and vary depending on the functional status of the neurons and astrocytes (Nie and Weng 2009; Oliet et al. 2001; Santello and Volterra 2009; Weng et al. 2007). Many studies have shown that activation of glial cells and the subsequent release of many active substances like proinflammatory cytokines, nitric oxide, and brain-derived neurotrophic factor (BDNF) (Coull et al. 2005; Milligan and Watkins 2009) are related to neuronal hyperexcitability (or central sensitization) in the spinal dorsal horn. However, the mechanisms underlying the interactions between neurons and glial cells are not fully established.
Glutamate is a principal transmitter mediating bidirectional interactions between neurons and astrocytes. Homeostasis of extracellular glutamate depends on glutamate uptake by glutamate transporters, because glutamate cannot be metabolized extracellularly (Danbolt 2001). The amount of glutamate acting on neuronal glutamate receptors depends not only on the glutamate released from presynaptic neurons but also on the function of astrocytes. Astrocytes regulate extracellular glutamate in two opposite manners: either by importing glutamate through glutamate transporters (Huang and Bergles 2004) or by releasing glutamate (Agulhon et al. 2008). We recently reported that deficiency of glial glutamate uptake results in increased extracellular glutamate concentrations and enhanced responses of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors in the spinal dorsal horn neurons to peripheral synaptic input (Nie and Weng 2009, 2010; Weng et al. 2006, 2007). Depending on the different synaptic structures in the CNS, astrocyte-derived glutamate can either target presynaptic terminals and modulate glutamate release from the terminals (Jourdain et al. 2007) or directly activate postsynaptic glutamate receptors (Angulo et al. 2004; Bardoni et al. 2010; Fellin et al. 2004; Xu et al. 2007). Despite the pivotal role of astrocyte-derived glutamate in regulating the synaptic transmission, the mechanisms that trigger glutamatergic neuron–glia communications and cause glial cells to release glutamate, and the functional implications of such communications for the relay of signals from peripheral synaptic input to postsynaptic neurons in the spinal dorsal horn, remain to be established.
In this study, we found that pharmacological blockade of glutamate transporters caused glial cells in the spinal dorsal horn to release glutamate spontaneously and in response to peripheral synaptic input elicited by a single stimulus. Glial-derived glutamate activates extrasynaptic NMDA receptors and generates slow inward currents (SICs) and action potentials in superficial spinal dorsal horn neurons, including spinothalamic tract (STT) neurons projecting to the thalamic sensory nuclei. Our study shows that glutamate transporters are key endogenous regulators of bidirectional glutamatergic interactions between neurons and glial cells and that glial cells can enhance and relay signals from presynaptic neurons to postsynaptic neurons by releasing glutamate to activate postsynaptic neurons in spinal pain pathways.
METHODS
Animals
All experiments were approved by the Institutional Animal Care and Use Committee at The University of Texas M. D. Anderson Cancer Center and were fully compliant with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Electrophysiology
Young adult male Sprague-Dawley rats (150–200 g) were used. Rats were deeply anesthetized via isoflurane inhalation and underwent laminectomy for removal of the lumbar spinal cord. The lumbar spinal cord section was placed in ice-cold sucrose artificial cerebrospinal fluid (ACSF) presaturated with 95% O2-5% CO2. The sucrose ACSF contained (in mM) 234 sucrose, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 12.0 glucose, and 25.0 NaHCO3. The pia-arachnoid membrane was removed from the section. The L4–L5 spinal segment, identified by the lumbar enlargement and large dorsal roots, was attached with cyanoacrylate glue to a cutting support, which was glued onto the stage of a vibratome (Series 1000, Technical Products International, St. Louis, MO). Transverse spinal cord slices (400 μm) were cut in the ice-cold sucrose ACSF and preincubated in Krebs solution oxygenated with 95% O2-5% CO2 at 35°C for ≥2 h before they were transferred to the recording chamber. The Krebs solution contained (in mM) 117.0 NaCl, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 11.0 glucose, and 25.0 NaHCO3.
Recordings were made from spinal slices placed in a recording chamber perfused with Kreb's solution (bubbled with 95% O2-5% CO2). All experimental solutions contained 1.2 mM MgCl2, except those shown in Fig. 4A, where no Mg2+ was added. Whole cell patch-clamp recordings were obtained from the spinal dorsal horn laminae I and II neurons visualized with an infrared Nomarski microscope system (Leads Instruments). When lamina I STT neurons labeled with a retrograde fluorescent marker were recorded, a fluorescence filter was first used to identify neurons filled with the fluorescent marker. In most experiments, borosilicate glass recording electrodes (resistance, 3–5 MΩ) were filled with an internal solution containing (in mM) 135 K-gluconate, 5 KCl, 2.0 MgCl2, 0.5 CaCl2, 5.0 HEPES, 5.0 EGTA, 5.0 ATP-Mg, 0.5 Na-GTP, 1 guanosine 5′-O-(2-thiodiphosphate) (GDP)-βS, and 10 lidocaine N-ethyl bromide (QX314) (pH 7.3, 290–300 mOsm). GDP-βS was used to suppress the signaling pathways activated by G protein–coupled receptors. QX314 was used to suppress the generation of action potentials from the recorded cell. Excitatory postsynaptic currents (EPSCs) and SICs were recorded from neurons held at −70 mV in a voltage clamp. In a subset of experiments, where neurons were recorded at a holding potential of +40 mV, potassium-gluconate and KCl in the pipette solution were replaced by Cs2SO4 (110 mM). The access resistance ranged from 20 to 35 MΩ and was constantly monitored throughout the experiments. Peripheral sensory input was activated by electrical stimuli (0.2 ms duration) through a concentric bipolar electrode placed at the apex of dorsal root entry zone (Nie and Weng 2009; Ramer et al. 2000), which was ≥80–160 μm away from the spinal gray matter. Recordings were made with 10 μM bicuculline and 5 μM strychnine in the external solution to block the GABAA receptors and glycine receptors at a holding membrane potential of −70 mV and temperature of 35°C, unless otherwise indicated.
Fig. 4.
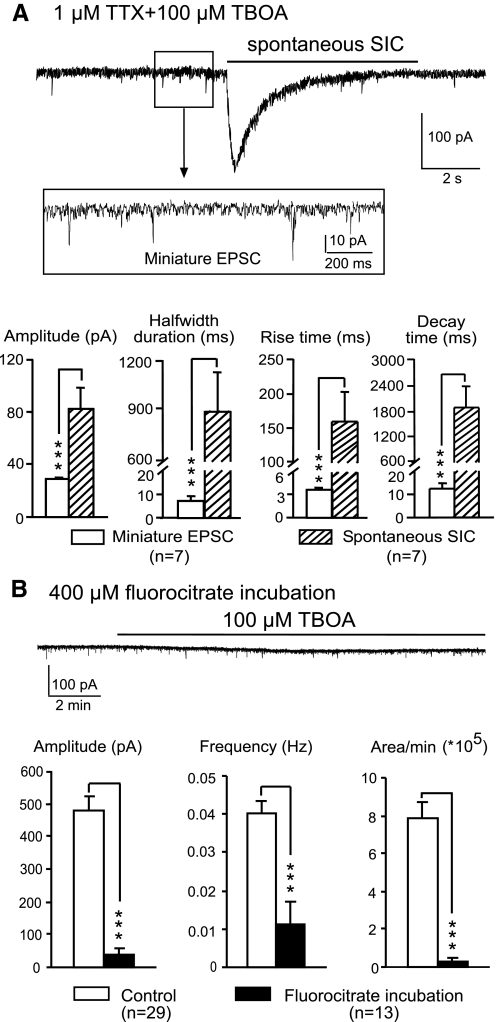
SICs are caused by glutamate released from glial cells. A: an original recording showing a sample of spontaneous SICs induced by 100 μM TBOA in the presence of 1 μM TTX and 0 Mg2+ at 20°C. Miniature EPSCs are shown in the enlarged inset. Comparisons of the mean (±SE) amplitude, half-width duration, and rise and decay times of both the SICs and miniature EPSCs collected at the same time are shown in the bar graphs. ***P < 0.001. B: an original recording showing that 100 μM TBOA did not induce spontaneous SICs in a neuron from a slice treated with 400 μM FC. Bar graphs show that the mean (±SE) amplitude, frequency, and areas of spontaneous SICs per minute recorded in neurons from FC-treated slices were significantly lower than those from untreated slices. ***P < 0.001.
Retrograde labeling of STT neurons
In some experiments, lamina I neurons projecting to the thalamus (STT neurons) were labeled with the retrograde fluorescent marker DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Invitrogen). This was accomplished by injecting 5 μl of 2% DiI [in dimethylsulfoxide (DMSO)] into bilateral ventroposterolateral thalamic nuclei in rats deeply anesthetized with isofluorane inhalation 1 wk before the electrophysiological recording.
Reagents
6,7-Dinitroquinoxaline-2,3-dione (DNQX), bicuculline, strychnine, (+)-MK-801 maleate (MK-801), GDP-βS, TTX, and sodium fluorocitrate were obtained from Sigma-Aldrich. d-Threo-β-benzyloxyaspartate (TBOA), Ro 25–6981, and d-aminophosphonovaleric acid (d-AP5) were obtained from Tocris Bioscience. When the chemicals had to be dissolved in DMSO, the final concentration of DMSO was <0.1% in the Krebs solution.
Data analysis
Data were recorded with Axopatch 700B amplifiers, digitized at 10 kHz, and analyzed off-line. Mini-Analysis software (Synaptosoft) and Clampfit 10.2 software (Molecular Devices) were used to analyze the EPSCs and SICs, including frequency, onset latency (measured from the onset of stimulation to the onset of EPSCs or SICs), peak amplitude, half-width duration (measured at 50% of peak amplitude), rise time (measured from 10 to 90% of peak amplitude), and decay time (measured from 90 to 10% of peak amplitude). Data are presented as means ± SE. Student's t-test was used to determine statistical differences within the same group (paired t-test) or between groups (unpaired t-test). Fisher's test was used to compare incidence rates of SICs between groups. P < 0.05 was considered statistically significant.
RESULTS
We used spinal superficial dorsal horn neurons as biosensors to detect glutamate release from neurons or glial cells and to study the interactions between neurons and glial cells. To ensure sensory synapses were studied, all neurons recorded had synaptic inputs from peripheral afferents, as verified by the EPSCs that were evoked by electrical stimulation of the dorsal root entry zone (Nie and Weng 2009; Weng et al. 2007). We inhibited glutamate uptake in the spinal slice by bath application of TBOA, a nontransportable competitive glutamate transporter blocker that nonselectively blocks both neuronal and glial glutamate transporters (Shimamoto et al. 1998) without any effects on postsynaptic glutamate receptors or presynaptic glutamate release (Demarque et al. 2004; Jabaudon et al. 1999). It has been established that blockade of glutamate uptake by TBOA results in increased ambient glutamate concentrations (Jabaudon et al. 1999; Liaw et al. 2005) and extrasynaptic glutamate spillover (Arnth-Jensen et al. 2002; Nie and Weng 2009; Szapiro and Barbour 2007) in the forebrain and spinal dorsal horn.
Blockade of glutamate transporters induces novel spontaneous SICs in spinal doral horn neurons
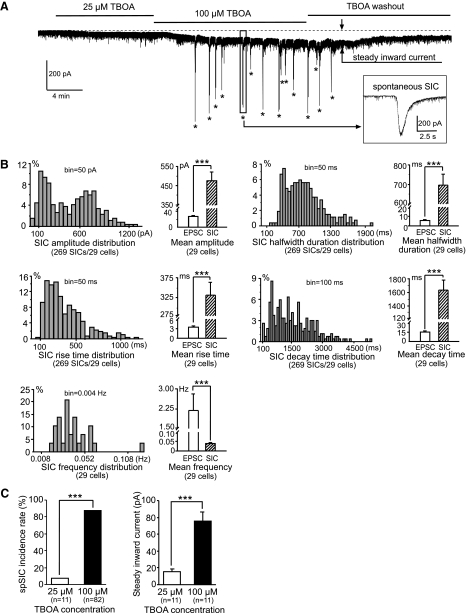
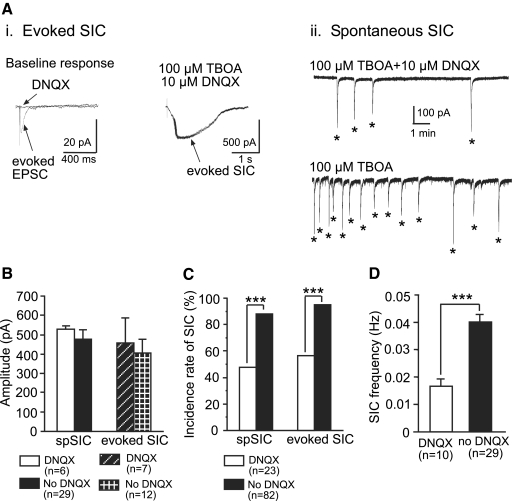
Among 82 neurons recorded, perfusion of TBOA (bath concentration, 100 μM) induced novel spontaneous SICs in 72 (87.80%) neurons, including 5 STT neurons (Fig. 1A). We analyzed spontaneous SICs in 29 cells with ≥2 min of continuous recordings. Compared with the spontaneous EPSCs recorded under the same conditions, the SICs showed significantly different properties (Fig. 1B), including larger amplitudes (477.00 ± 43.42 vs. 28.45 ± 2.30 pA), longer durations (half-width duration, 696.78 ± 56.45 vs. 5.51 ± 0.61 ms), slower rise (332.46 ± 31.46 vs. 3.42 ± 0.40 ms) and decay (1,630.61 ± 153.87 vs. 14.41 ± 1.50 ms) times, and lower frequencies (0.04 ± 0.003 vs. 2.18 ± 0.62 Hz). The rise time and half-width duration measured from all the conventional spontaneous EPSCs were completely separated from those measured at the same time from the SICs. All the spontaneous EPSCs had a rise time <20 ms and half-width duration <30 ms in the presence of TBOA. By contrast, all the SICs recorded at the same time had a rise time >51 ms and half-width duration >118 ms. Thus events with rise time >50 ms were classified as SICs. The appearance of spontaneous SICs induced by TBOA was dose dependent. One (9.10%) of 11 neurons in slices exposed to 25 μM TBOA developed spontaneous SICs, which was significantly less (P < 0.001) than the 87.80% SIC incidence rate in neurons exposed to 100 μM TBOA (Fig. 1C, left). No SICs were observed in the 82 neurons before perfusion of TBOA or after TBOA washout (tested in 7 neurons).
Fig. 1.
Blockade of glutamate transporters with d-threo-β-benzyloxyaspartate (TBOA) elicits novel spontaneous slow inward currents (SICs) and steady inward current in spinal superficial dorsal horn neurons. A: the original recording trace shows that TBOA at 100 μM, but not at 25 μM, induced spontaneous SICs (marked by *). TBOA at 100 μM produced a larger steady inward current (marked by arrows) than did TBOA at 25 μM. The enlarged inset shows typical features of SICs, including high amplitude, long duration, and slow kinetics during the rising and decaying phases. B: histograms show the percentage distributions of the SIC amplitudes, durations (half-width duration), rise and decay times, and frequencies collected from 269 SICs from 29 cells. Bar graphs show means ± SE of amplitudes, half-width durations, rise and decay times, and frequencies of both the SICs and spontaneous excitatory postsynaptic currents (EPSCs) collected under the same conditions. C: TBOA at 100 μM induced a higher incidence rate of spontaneous SICs (left) and a higher amplitude of steady inward currents (right) than did 25 μM TBOA. ***P < 0.001.
TBOA also induced a steady inward current (Fig. 1, A and C, right). The steady inward currents induced by TBOA at two different concentrations (25 and 100 μM) in the same cell were analyzed. The steady inward currents induced by 100 μM TBOA (−75.50 ± 11.25 pA, n = 11) were significantly greater than those induced by 25 μM TBOA (-15.60 ± 2.99 pA, n = 11, P < 0.001) in the same cells. These findings indicate that higher ambient glutamate concentrations were induced by 100 μM TBOA, because the shift of steady inward currents is proportional to the ambient glutamate concentrations (Herman and Jahr 2007; Jabaudon et al. 1999; Le Meur et al. 2007). Thus a significant elevation of ambient glutamate concentrations induced by impairment of glutamate uptake is required for the generation of SICs in spinal dorsal horn neurons in our experimental conditions.
Peripheral synaptic input elicits SICs in the presence of TBOA
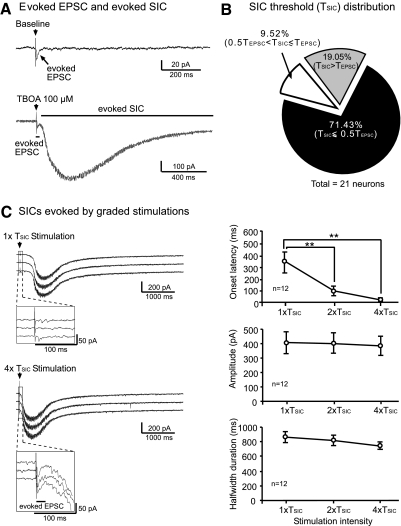
To determine the relationship between the SICs and peripheral sensory input, we recorded the neuronal response to electrical stimulation (given every 60 s) of the spinal dorsal root entry zone at 2 times the EPSC activation threshold (2× TEPSC) before and after perfusion of 100 μM TBOA. In addition to the EPSC, a novel SIC was elicited by the same stimulation in the presence of TBOA (Fig. 2A) in 78 (95.12%) of 82 neurons, including 6 STT neurons. In the presence of 25 μM TBOA, the SICs were evoked in only 1 of 11 neurons recorded. No SICs were evoked before (n = 82) or after (n = 7) washout of TBOA.
Fig. 2.
Peripheral synaptic input elicits SICs in the presence of TBOA. A: original recordings show the average of 3 responses to electrical stimulation (marked by arrows) applied to the spinal dorsal root entry zone at 2× the EPSC activation threshold (2× TEPSC) at baseline and after perfusion of 100 μM TBOA. An EPSC was evoked at baseline (top). In the presence of TBOA, a novel SIC was evoked by the same stimulation (bottom). B: percentage distribution of stimulating intensities needed to activate SICs (TSIC) relative to those needed to activate EPSCs (TEPSC) in the same neurons (n = 21). C: original recordings show SICs evoked by stimulation of spinal dorsal root entrance at graded stimulation intensities (1× and 4× TSIC). Three sweeps of recordings show 3 SICs evoked by the same stimulation intensity given every 60 s. The enlarged insets show that EPSCs were evoked by 4× TSIC but not by 1× TSIC. Lines and scatterplots show the effect of the stimulation intensities (1× to 4× TSIC) on the mean (±SE) onset latency, peak amplitude, and half-width duration of the stimulation-evoked SICs. The average of 3 responses to consecutive stimulation was calculated. Significant differences between the SIC latency evoked by stimulation at 1× TSIC and at 2× or 4× TSIC,are indicated by *. **P < 0.01.
In the same neuron, the stimulation intensity required to evoke the SICs (i.e., the activation threshold for evoked SICs, or TSIC) was different from the threshold to activate EPSCs (TEPSC). Of the 21 neurons examined, 15 (71.43%) had a TSIC that was ≤50% of the TEPSC (Fig. 2B). The mean onset latency for the SICs (i.e., the time from the stimulation to the onset of the SICs evoked at 1× TSIC was 336.44 ± 86.03 ms (range, 66.00–814.29 ms, n = 12), which was two orders of magnitude longer than the mean onset latency of the stimulation-evoked EPSCs (2.29 ± 0.14 ms) measured in the same neurons. The onset latencies of the SICs evoked by 2× TSIC (81.29 ± 26.29 ms) and by 4× TSIC (26.03 ± 8.88 ms) were significantly shorter (P < 0.01) than those evoked by 1× TSIC. In contrast, the peak amplitude and half-width duration of the stimulation-evoked SICs were not significantly altered as the stimulation intensity was increased (Fig. 2C).
Because both blockade of glutamate transporters (Arnth-Jensen et al. 2002; Diamond 2002; Isaacson 1999; Nie and Weng 2009; Weng et al. 2007) and train stimulation (Hires et al. 2008) produce glutamate spillover, we examined whether train stimulation of dorsal root entrance zone could evoke SICs in the absence of TBOA. Repetitive stimulation (10–20 pulses at 10–200 Hz and maximum intensity that evoked maximum EPSCs in the neuron recorded) did not evoke any SICs (n = 18).
Overlap in the cellular circuits generating spontaneous and evoked SICs
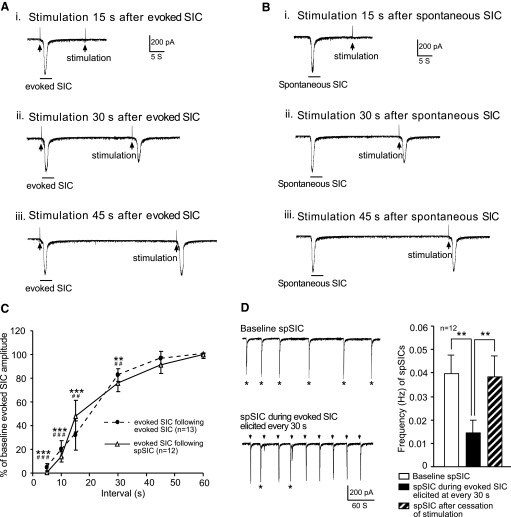
The SICs evoked by repeated stimulation at 45- to 60-s intervals were rather stable. However, the amplitudes of the SICs were gradually reduced as the stimulation intervals shortened. The amplitudes of the SICs evoked 30, 15, and 5 s after the previously evoked SIC were reduced by 17.50 ± 12.13, 68.20 ± 5.41, and 95.00 ± 3.11% (P < 0.01, n = 13), respectively. The SICs could not be elicited in 28.57 and 66.67% of neurons when the stimulation was applied 15 and 5 s, respectively, after the previously evoked SIC (Fig. 3, A and C). These data indicate that appearance of SICs produces an SIC-refractory period of ≤30 s. We reasoned that if the cellular circuits generating spontaneous and evoked SICs overlap, appearance of spontaneous SICs should inhibit the evoked SICs in a similar fashion. As expected, SICs evoked between 30 and 5 s after the onset of spontaneous SICs were inhibited as tested in 12 neurons (Fig. 3, B and C). Furthermore, spontaneous SICs were suppressed by evoked SICs elicited every 30 s, as shown by the significantly (n = 12, P < 0.01) reduced frequency of spontaneous SICs during the period when evoked SICs were repeatedly elicited by stimulation at 30-s intervals (Fig. 3D). These data indicate that the cellular circuits responsible for spontaneous and evoked SICs overlap.
Fig. 3.
Cellular circuits generating spontaneous and evoked SICs overlap. A: original recordings show that the stimulation-evoked SICs produced a refractory period of ≤30 s on evoked SICs. B: the spontaneous SICs produced a similar refractory period (≤30 s) on the stimulation-evoked SICs. C: lines and scatterplots show the percent inhibition produced by the stimulation-evoked (dashed line) and spontaneous SICs (spSIC; solid line) on the amplitudes of the SICs evoked at various time points after the onset of the stimulation-evoked and spontaneous SICs. Symbols mark the differences between each time point and baseline: #stimulation-evoked SICs; *spontaneous SICs. Two symbols, P < 0.01; 3 symbols, P < 0.001. D: original recordings show spontaneous SICs (marked by asterisks) before (top) and during (bottom) the stimulation-evoked SICs. The stimulation-evoked SICs are marked by arrows. Bar graphs show the mean (±SE) frequency of the spontaneous SICs before and during the stimulation-evoked SICs elicited every 30 s and after cessation of the stimulation-evoked SICs. **P < 0.01.
Glutamate released from glial cells causes SICs
Glutamate is released from either neurons or glial cells in the CNS. The high amplitude and slow kinetics of spontaneous SICs found in this study are very similar to the SICs found in the forebrain (Angulo et al. 2004; Fellin et al. 2004; Xu et al. 2007) and in the spinal dorsal horn (Bardoni et al. 2010), in which spontaneous release of glutamate from glial cells has been shown to account for the generation of SICs. To study whether this is the case for the SICs in our study, after inducing spontaneous SICs with 100 μM TBOA, we applied 1 μM TTX to the bath to block the vesicular release of glutamate induced by the opening of voltage-gated sodium channels in neurons. Unexpectedly, spontaneous SICs were completely abolished in all six neurons tested. We reasoned that the abolishment of spontaneous SICs by TTX resulted from the inhibition of the sodium channel–dependent glutamate release from neurons and the consequent decrease in the ambient glutamate concentrations exposed to glial cells.
To overcome the reduced ambient glutamate concentrations induced by TTX, we took advantage of the fact that the activity of the glutamate transporters significantly decreases at low temperatures (Asztely et al. 1997; Danbolt 2001) and tested whether 100 μM TBOA induces spontaneous SICs at 20°C. Under such conditions, spontaneous SICs were induced by 100 μM of TBOA in two of seven neurons tested. We also performed the recordings in Mg2+-free medium at 20°C, a condition used by others to show SICs in the forebrain (Fellin et al. 2004) and spinal dorsal horn (Bardoni et al. 2010). After 100 μM TBOA was added to the recording bath in the presence of TTX, one to seven spontaneous SICs were observed during the 10-min recording period in all seven neurons tested. SICs recorded under this condition also displayed typical features of SICs—large amplitudes, long durations, and slow kinetics—which were completely different from the features of the miniature EPSCs recorded at the same time (Fig. 4A). These results indicate that the size of the glutamatergic vesicles (the amount of glutamate) inducing the SICs is significantly larger than the size of the glutamatergic vesicles inducing the miniature EPSCs, and/or the receptors responsible for the SICs were different from those responsible for the miniature EPSCs. Importantly, these data show that the glutamate that induced SICs in postsynaptic neurons was independent of synaptic activity and suggest that SICs are induced by glutamate released from glial cells but not from neurons.
To further support this conclusion, we selectively depressed the function of astrocytes by incubating spinal slices with a specific gliotoxin, fluorocitrate (FC, 400 μM), for a minimum of 1.5 h (Andersson et al. 2007; Fonnum et al. 1997; Gordon et al. 2005; Largo et al. 1996; Martin et al. 2007). In the presence of 100 μM TBOA, the spontaneous SIC amplitudes, frequencies, and areas per minute recorded in neurons from FC-treated slices were significantly lower (P < 0.001) than those recorded in untreated slices (Fig. 4B). Only 5 (38.46%) of 13 neurons from FC-treated slices developed spontaneous SICs, which was significantly lower (P < 0.001) than the 87.80% SIC incidence rate in untreated slices. Although prolonged incubation with FC might potentially compromise neuronal function, in agreement with others (Gordon et al. 2005; Martin et al. 2007), we do not think this is the case on the basis of the following facts. First, the mean baseline spontaneous EPSC frequency (0.93 ± 0.17 Hz) and amplitude (25.76 ± 1.77 pA) recorded in 13 neurons from FC-treated slices were not significantly different from those recorded in 15 neurons from untreated slices (frequency, 1.06 ± 0.23 Hz; amplitude, 32.47 ± 2.83 pA), indicating that the activities in the presynaptic neurons and the responses of the postsynaptic neurons remained intact. Second, the mean neuronal resting membrane potential (−53.44 ± 1.45 mV), membrane resistance (643.84 ± 146.50 MΩ), and membrane capacitance (47.77 ± 4.27 pF) in FC-treated slices (n = 13) were not significantly different from those collected from untreated slices (n = 15; resting membrane potential, −57.80 ± 1.62 mV; membrane resistance, 618.07 ± 145.64 MΩ; membrane capacitance, 55.79 ± 4.70 pF). Such passive membrane properties were similar to those measured in neurons from the same area reported by others (Balasubramanyan et al. 2006; Ruscheweyh and Sandkuhler 2002). Thus the reduction of SICs in FC-treated spinal slices in the presence of TBOA was caused by the selective impairment of astrocytes rather than of neurons. These findings confirm the conclusion made from the TTX experiments that SICs are caused by glutamate released from glial cells.
Blockade of AMPA receptors does not alter the SIC amplitude but reduces the spontaneous SIC frequency
We studied the role of AMPA receptors in the generation of SICs. The amplitudes of the stimulation-evoked and spontaneous SICs induced by 100 μM TBOA in the presence or absence of a non-NMDA receptor antagonist DNQX (10 μM) were similar (Fig. 5, A and B). However, the incidence rates of the spontaneous (10/23; 43.48%) and evoked (13/23; 56.65%) SICs, as well as the frequencies of the spontaneous SICs (0.016 ± 0.002 Hz, n = 10), in the presence of DNQX were significantly lower (P < 0.001) than those in the absence of DNQX (Fig. 5, C and D). This indicates that the SICs were not caused by the activation of AMPA receptors on the recorded postsynaptic neurons, and the amount of glutamate released from glial cells was not controlled by the activation of non-NMDA receptors in glial cells. It is likely that the blockade of the AMPA receptors reduced neuronal activities and glutamate release from neurons, which resulted in lower ambient glutamate concentrations acting on glial cells.
Fig. 5.
Blockade of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors does not alter the SIC amplitude but reduces the frequency of spontaneous SICs and the incidence rates of TBOA-induced spontaneous and stimulation-evoked SICs. Ai: original recordings collected from a neuron show an EPSC evoked by stimulation of the spinal dorsal root entry zone at 2× TEPSC at baseline, which was blocked by 10 μM DNQX (left); the SICs evoked by the same stimulation in the presence of 6,7-dinitroquinoxaline-2,3-dione (DNQX) plus 100 μM TBOA (right). Aii: original recordings taken from the same neuron in the presence (upper trace) and absence (lower trace) of 10 μM DNQX during blockade of glutamate transporters by 100 μM TBOA. B: amplitudes of spontaneous and evoked SICs induced by 100 μM TBOA in the presence of DNQX were not significantly different from that in the absence of DNQX. C: incidence rates of spontaneous and evoked SICs induced by 100 μM TBOA in the presence of DNQX were significantly lower than those in the absence of DNQX. D: mean frequencies of spontaneous SICs induced by 100 μM TBOA in the presence of DNQX were significantly lower than that in the absence of DNQX. ***P < 0.001.
SICs are generated by activation of extrasynaptic NMDA receptors mainly composed of NR2B subunits on postsynaptic neurons
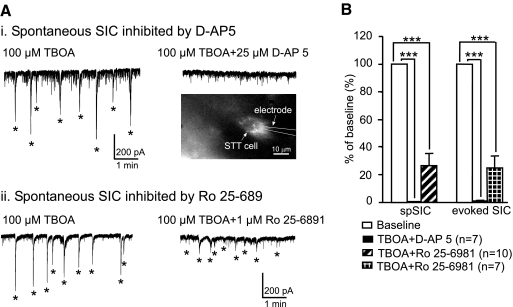
Next, we studied the role of NMDA receptors in the generation of SICs. After the appearance of the SICs induced by 100 μM TBOA, application of the selective NMDA receptor antagonist d-AP5 (25 μM) into the recording chamber completely abolished both the stimulation-evoked and spontaneous SICs (tested in 7 neurons, including 4 STT neurons; Fig. 6). Furthermore, bath application of a selective NR2B receptor antagonist, Ro 25–6981 (1 μM), reduced the amplitude of the evoked SICs by 76.13 ± 7.93% (n = 7, P < 0.001) and the spontaneous SICs by 73.27 ± 8.36% (n = 10, P < 0.001; Fig. 6). The spontaneous SICs in 4 of 10 neurons and evoked SICs in 2 of 7 neurons were completely abolished by Ro 25–6981. The abolition of SICs by NMDA antagonists is consistent with the finding that DNQX did not alter the SIC amplitude, indicating that postsynaptic AMPA receptors do not contribute to the generation of the SICs. These data suggest that the SICs are generated by glutamate released outside the synaptic cleft, which activates extrasynaptic NMDA receptors mainly composed of NR2B receptors on postsynaptic neurons, because the AMPA receptors inside the synaptic cleft would be activated together with the NMDA receptors by the glutamate released inside the synaptic cleft.
Fig. 6.
SICs are inhibited by blocking and N-methyl-d-aspartate (NMDA) or NR2B receptors. Ai: spontaneous SICs induced by 100 μM TBOA (left) were completely inhibited by bath perfusion (right) of 25 μM d-AP5 in an spinothalamic tract (STT) neuron. The inset shows the STT neuron labeled by a retrograde fluorescent marker. Aii: spontaneous SICs induced by 100 μM TBOA (left) were strongly inhibited by bath perfusion (right) of 1 μM Ro 25–6981 in an unidentified neuron. B: mean (±SE) percent inhibition of the amplitudes of the spontaneous and stimulation-evoked SICs by 25 μM d-AP5 and 1 μM Ro 25–6981. ***P < 0.001.
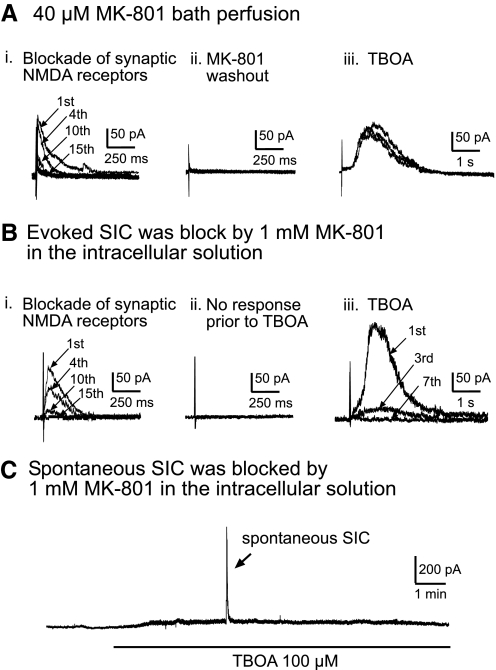
To show the extrasynaptic origin of SICs, we used a use-dependent NMDA channel blocker, MK-801, to functionally remove all intrasynaptic NMDA receptors activated by peripheral synaptic input (Huettner and Bean 1988; Massey et al. 2004; Nie and Weng 2009; Tovar and Westbrook 2002) and observed whether the SICs still could be evoked by the same synaptic input when the glutamate transporters were blocked. Experiments were conducted in the presence of 10 μM DNQX. To completely block all NMDA receptors inside the active synapses, we evoked NMDA EPSCs at a holding membrane potential of +40 mV by repeated stimulations at maximum intensity (0.1 Hz) in the presence of 40 μM MK-801 (Chen and Diamond 2002; Nie and Weng 2009; Tovar and Westbrook 2002). The maximum stimulation intensity was defined as the stimulation intensity that evoked a maximum EPSC for the recorded neuron. Synaptically activated NMDA EPSCs were gradually blocked following each stimulus in the presence of MK-801 (Fig. 7Ai). After MK-801 blocked the synaptically activated NMDA EPSCs, MK-801 was completely washed out for 10 min. Stimulation at the same intensity after washout did not evoke any new response, indicating that the NMDA receptors located inside the active synapse remained effectively blocked by MK-801 (Fig. 7Aii). After bath application of TBOA (100 μM), a novel SIC was evoked by the same stimulation in 6 of 12 neurons recorded (Fig. 7Aiii). The SICs recorded under these conditions (n = 6) displayed the typical features of stimulation-evoked SICs: long onset latencies (233.50 ± 103.22 ms), slow kinetics (rise time, 392.00 ± 130.80 ms; decay time, 2,293.00 ± 794.66 ms), high amplitudes (137.15 ± 21.43 pA), and long durations (half-width duration, 1,045.83 ± 248.93 ms). Because the intrasynaptic NMDA receptors activated by the same stimulation were already blocked by MK801 and the AMPA receptors were blocked by DNQX, the data indicate that the stimulation-evoked SICs were not generated by NMDA receptors located inside the original synapses but rather by extrasynaptic NMDA receptors.
Fig. 7.
SICs are generated by activation of extrasynaptic NMDA receptors in postsynaptic neurons. Recordings were made at a holding potential of +40 mV in the presence of 10 μM DNQX to block non-NMDA receptors. Traces in A–C were taken from different neurons. Ai: synaptic NMDA currents (channels) were gradually blocked by stimulation repeated at 0.1 Hz at the intensity that evoked maximum NMDA EPSCs during perfusion of 40 μM MK-801 (responses to 1st, 4th, 10th, and 15th stimulations are shown). Aii: no synaptic NMDA EPSCs were evoked by the same stimulation 10 min after washout of MK-801. Aiii: 3 sweeps of recordings show 3 SICs evoked by the same stimulation intensity given every 60 s in the presence of 100 μM TBOA. Bi: synaptic NMDA currents channels were gradually blocked by stimulation repeated at 0.1 Hz at the maximum intensity with 1 mM MK-801 included in the intracellular solution (responses to 1st, 4th, 10th, and 15th stimulations are shown). Bii: no synaptic NMDA EPSCs were evoked by the same stimulation before perfusion of TBOA. Biii: in the presence of 100 μM TBOA, a novel SIC was evoked by the same stimulation but was immediately and completely blocked by MK-801 inside the recording electrode after repeated stimulations (responses to 1st, 3rd, and 7th stimulations are shown). C: after synaptic NMDA receptors were blocked by 1 mM MK-801 in the intracellular solution, a spontaneous SIC was induced by 100 μM TBOA but was immediately and completely blocked by MK-801 inside the recording electrode.
To verify that the site of SIC inhibition induced by bath perfusion of NMDA receptor antagonists is on postsynaptic neurons rather than on glial cells, we conducted experiments in which we included 1 mM MK-801 in the intracellular solution inside the recording electrode. Intrasynaptic NMDA receptors responding to peripheral input elicited by electrical stimulation at maximum intensity were first completely blocked by intracellular MK-801 through repetitive stimulation (0.1 Hz) of the dorsal root entry zone. The evoked and spontaneous SICs were induced by bath perfusion of 100 μM TBOA but were abolished rapidly by the MK-801 in the intracellular solution in all six neurons tested (Fig. 7, B and C). These data confirm that the SICs are generated by the activation of NMDA receptors located at the extrasynaptic site on postsynaptic neurons. It is worth mentioning that only the NMDA receptors inside the synapses activated by the peripheral stimulation were blocked by MK-801 before TBOA perfusion. The SICs induced under this condition could originate from NMDA receptors located outside the original active synapse (extrasynaptic NMDA receptors) and/or those inside the synapses not activated by the peripheral stimulation. However, the fact that 100% of the SICs are caused by the activation of NMDA receptors, without any contribution from AMPA receptors, rules out the possibility that NMDA receptors inside the synapses contribute to the SIC generation.
SIC induces membrane depolarization and activation of action potentials under physiological conditions
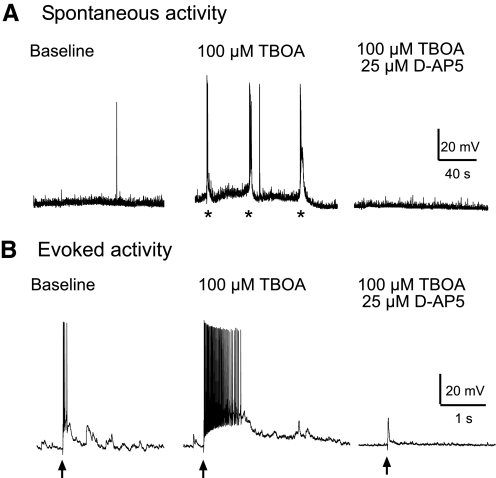
To define the impact of SICs on neuronal activity under physiological conditions, we recorded neurons in bridge mode with potassium-filled electrodes in a normal external solution without bicuculline or strychnine. Similar to the results from experiments performed in voltage-clamp mode with bicuculline and strychnine in the external solution, TBOA (100 μM) induced a d-AP5–sensitive spontaneous slow depolarization, which could also be evoked by synaptic input from the periphery (Fig. 8). The amplitudes of the slow depolarization were so large that they induced a burst of action potentials in all six neurons recorded. These data indicate that blockade of GABAA and glycine receptors is not required for the generation of SICs and that the SICs directly activate postsynaptic neurons with sensory synapses in the spinal dorsal horn under physiological conditions.
Fig. 8.
SICs induced large membrane depolarization and activation of action potentials under physiological conditions. Original recordings made in a bridge mode with potassium-filled electrodes in normal external solution (i.e., in the absence of bicuculline and strychnine) show spontaneous neuronal activities (A) and responses evoked by stimulation of the dorsal root entry zone (B) at baseline, during perfusion of 100 μM TBOA, and after addition of 25 μM d-AP5. In the presence of TBOA, the spontaneous depolarization (marked by *) and depolarization elicited by stimulation (marked by arrows) of the dorsal root entry zone was so large that it caused activation of action potentials. Both spontaneous and evoked depolarizations were abolished by d-AP5 (tested in 4 neurons).
DISCUSSION
Our study identifies, for the first time, the role of glutamate transporters in regulating neuronal–glial interactions and the impact of glial-derived glutamate in spinal sensory transmission. Specifically, we showed that, on inhibition of glutamate uptake, SICs appear spontaneously and are evoked by peripheral synaptic input in the spinal superficial dorsal horn neurons, including the STT neurons. We further showed that the SICs result from the activation of extrasynaptic NMDA receptors in postsynaptic neurons by the glutamate released from glial cells. On deficient glutamate uptake, the stimulation-induced, synaptically released glutamate activates glial cells and causes glial cells to release glutamate and activate postsynaptic neurons. Thus we identified a novel cellular mechanism underlying neuronal–glial–neuronal interactions in the spinal dorsal horn, which may be related to hyperactivation of spinal dorsal horn neurons under certain types of pathological pain conditions.
Glutamate released from glial cells induces SICs
Glutamate is released from either neurons or glial cells in the CNS. Neurons release glutamate through neuronal terminals within the synapse, which activates postsynaptic glutamate receptors located inside the same synapse and/or outside the synapses by extrasynaptic glutamate spillover (Arnth-Jensen et al. 2002; Nie and Weng 2009; Szapiro and Barbour 2007). This study provided compelling evidence that the glutamate that evokes SICs in spinal dorsal horn neurons in the presence of TBOA does not come from presynaptic neurons, but rather from glial cells. First, the features of the SICs (slow kinetics, large amplitude, and long duration), which were completely different from those of the conventional spontaneous EPSCs or miniature EPSCs recorded at the same time, suggest that the mechanism by which the SICs were induced was different from the mechanism by which the EPSCs are induced. This result may reflect differences in the kinetics, amount, and site of glutamate release, as well as differences in the site and properties of the activated glutamate receptors between the conventional EPSCs and SICs (Clements 1996; Savtchenko and Rusakov 2007).
Second, both the spontaneous and stimulation-evoked SICs were generated by glutamate released outside the synapse, but not by glutamate released within the synapse, as shown in the MK-801 experiments. These findings indicate that the glutamate eliciting the SICs does not come from presynaptic neurons, but rather from glial cells, because the glutamate released from the presynaptic neuronal terminals within the synapse must activate the glutamate receptors inside the synapse before it spills over to the extrasynaptic sites. This notion is further supported by the following: 1) activation of the AMPA receptor, a major ionotropic glutamate receptor located inside the postsynaptic density (Dingledine et al. 1999; Nie and Weng 2009), did not contribute to the SIC generation; 2) the SICs were caused, either solely or predominantly, by the activation of the NR2B receptors. These findings also exclude the possibility that the long duration of SICs was caused by the asynchronous release of glutamate from presynaptic neuronal terminals, because the AMPA receptors inside the synapse are activated regardless of the pattern by which glutamate is released from the presynaptic terminals (Iremonger and Bains 2007; Otsu and Murphy 2004; Scheuss et al. 2007).
Third, the onset latency for the SICs evoked at TSIC was two orders of magnitude longer than the onset latency of the stimulation-evoked EPSCs obtained in the same neurons. The long latency of the stimulation-evoked SICs is incompatible with the conducting time required for the neuronal network in spinal slices to transmit input from the stimulating site and generate synaptic currents in postsynaptic neurons by glutamate released directly from apposed presynaptic neuronal terminals or by extrasynaptic glutamate spilled from neighboring synapses (Arnth-Jensen et al. 2002; Nie and Weng 2009; Szapiro and Barbour 2007). These findings suggest that the cellular circuit responsible for the generation of the SICs must include a non-neuronal component, glial cells. Indeed, the long latency of the stimulation-evoked SICs is in agreement with the long latency for elevation of astrocytic Ca2+ activity induced by a train of synaptic inputs (Beierlein and Regehr 2006; Schipke et al. 2008). The dramatic changes in the onset latencies (from hundreds to tens of milliseconds) and the constant amplitudes of the stimulation-evoked SICs on increasing the stimulating intensity suggest that the SICs are not activated by glutamate released from a pure neuronal network. The long refractory period (≤30 s) of the evoked SICs is also inconsistent with signal transmission through the neuronal circuit. This notion is further supported by the differences in the activation thresholds between the EPSCs and SICs, which indicates that the glutamate that elicits the SICs is from a different origin than the glutamate that elicits the EPSCs. Furthermore, these findings exclude the possibility that the SICs result from the activation of NMDA receptors in silent synapses (Kullmann and Asztely 1998; Li and Zhuo 1999), because the long latency of the stimulation-evoked SICs is not compatible with the conducting time required for the neuronal signals to induce synaptic currents in the silent synapses used in our preparation.
Finally, the SICs were still induced by TBOA when neuronal synaptic transmission was arrested with TTX, but they were not induced or their induction was greatly reduced in spinal slices treated with the glial-specific toxin FC. Given all of these findings, we conclude that the glutamate activating SIC is not released within the synaptic cleft from neuronal terminals but, rather, is released outside the synaptic cleft from glial cells. This conclusion is in agreement with a recent study showing that spontaneous SICs recorded from spinal lamina II neurons are caused by glutamate released from astrocytes (Bardoni et al. 2010).
Mechanisms triggering glial cells to release glutamate
Activation of astrocytic purinergic receptors in the spinal dorsal horn causes astrocytes to release glutamate, which evokes spontaneous SICs in the spinal lamina II neurons (Bardoni et al. 2010). Much remains unknown about the role of astrocytic glutamate receptors in triggering glial cells to release glutamate and factors regulating the activation of astrocytic glutamate receptors in the spinal dorsal horn. In this study, we showed, for the first time, that the release of glutamate from glial cells in the spinal dorsal horn depends on the function of glutamate transporters. Deficiency in glutamate uptake results in increased ambient glutamate concentrations (Jabaudon et al. 1999; Liaw et al. 2005; Weng et al. 2006) and extrasynaptic glutamate spillover (Arnth-Jensen et al. 2002; Nie and Weng 2009; Szapiro and Barbour 2007), which in turn triggers glutamatergic communications between neurons and glial cells. Consequently, glial cells release glutamate both spontaneously and in response to presynaptic input in spinal sensory synapses.
A significant elevation of ambient glutamate concentrations and glutamate spillover induced by TBOA are required for the appearance of spontaneous and evoked SICs in spinal dorsal horn neurons of adult rats in our experimental conditions. Although train stimulation produces glutamate spillover, we failed to evoke SICs with train stimulation in our preparation. Furthermore, in the data collected for our previous published study where we examined the effects of a selective glial glutamate transporter inhibitor [dihydrokainate (DHK), 300 μM concentration] on AMPA EPSCs, we did not observe the appearance of SICs (observed in 44 neurons) either (Weng et al. 2007). The failure of train stimulation and DHK to induce SICs most likely is because the levels of glutamate spillover and ambient concentrations produced by these two conditions are less than those by 100 μM TBOA. This notion is supported by the following results collected by us from the same preparation. We previously reported that TBOA at 50 μM increased the NMDA EPSC amplitude by 182.51 ± 65.09% and duration by 298.69 ± 40.51% (Nie and Weng 2009), but 200-Hz train stimulation (10 pulses) only increased the NMDA EPSC amplitude by 72.75 ± 22.39% (n = 5) and duration by 59.21 ± 26.13% (n = 5) in comparison with those evoked by single pulse stimulation (Nie and Weng, unpublished observations). The degree of blockade on glial glutamate uptake by 300 μM DHK (46%) is less than that (83%) by 100 μM TBOA (Zhang et al. 2009). It is noteworthy that spontaneous SICs were observed in normal conditions in neonatal spinal dorsal horn neurons (Bardoni et al. 2010; Thomson et al. 2006). This discrepancy may be due to a lower protein expression of glutamate transporters in the spinal dorsal horn in neonates than adults (Furuta et al. 1997).
Target of glutamate released from glial cells
We found that glial-derived glutamate mainly targets the NR2B receptors located outside the synaptic cleft at the spinal sensory synapses. This is in agreement with findings from the hippocampal CA1 area (Angulo et al. 2004; Fellin et al. 2004; Xu et al. 2007), thalamus (Parri et al. 2001), and nucleus accumbens (D'Ascenzo et al. 2007). However, our results differ from those found at perforant path–granule cell synapses in the hippocampal dentate gyrus, where astrocytic glutamate acts on NR2B receptors at the presynaptic terminals (Jourdain et al. 2007). Our findings strengthen a long-held view that neuronal–glial communications depend on the synaptic microstructure that is region- and synapse-specific (Agulhon et al. 2008) and the functional status of both glial cells and neurons (Huang and Bergles 2004; Nie and Weng 2009; Panatier et al. 2006; Weng et al. 2007). In agreement with other studies of the ventrobasal thalamus (Parri et al. 2001), hippocampus (Angulo et al. 2004; Fellin et al. 2004; Xu et al. 2007), nucleus accumbens (D'Ascenzo et al. 2007), cortex (Ding et al. 2007), and spinal lamina II area (Bardoni et al. 2010), we also found that NMDA receptors were activated by glial-derived glutamate in normal Mg2+-containing medium at resting membrane potentials. Other features of the SICs that we observed in this study, which are consistent with SICs in the forebrain area (Angulo et al. 2004; Fellin et al. 2004; Parri et al. 2001; Xu et al. 2007) and spinal lamina II area, include their slow kinetics, prolonged duration, and large amplitudes. These features may be ascribed to the large size of the glutamatergic vesicles in astrocytes (Xu et al. 2007). Thus the nature of glutamate release from glial cells and the properties of receptors activated by glial-derived glutamate in postsynaptic neurons appear similar in these different CNS areas.
Functional implications
It was reported recently that activation of astrocytic purinergic receptors causes astrocytes to release glutamate, which evokes spontaneous SICs in the spinal lamina II neurons, and that the incidence rate of the SICs increases in hyperalgesic rats induced by intraplantar injection of zymosam (Bardoni et al. 2010). Our results confirm the findings of SICs in the spinal dorsal horn, and identify deficiency of glutamate uptake as another factor causing glial cells to release glutamate. More importantly, we show a novel bidirectional neuron–glia–neuron communication in relaying signals from peripheral synaptic input to postsynaptic neurons including STT neurons in the spinal dorsal horn.
The elevated extracellular glutamate concentrations and deficiencies in glutamate uptake or the expression of glutamate transporters in the spinal dorsal horn are seen in neuropathic animal models induced by chronic constriction of the sciatic nerve (Sung et al. 2003), partial sciatic nerve ligation (Nie and Weng 2010; Xin et al. 2009), or chemotherapy (Weng et al. 2005). Glutamate release from astrocytes has also been proposed to be a key component contributing to neuronal hyperactivity in the spinal dorsal horn (Ren and Dubner 2008). The impact of deficient glutamate uptake on neuron–glial interactions in these pain models is unknown. It remains to be determined whether our findings underlie synaptic mechanisms contributing to neuronal hyperactivity in the spinal dorsal horn in pathological conditions. Nevertheless, we showed deficient glutamate uptake as a condition causing glial cells to release glutamate spontaneously and in response to peripheral synaptic input. We also identified extrasynaptic NMDA receptors, particularly NR2B receptors, on postsynaptic neurons as the target for glial-derived glutamate. Glial-derived glutamate alters synaptic transmission in the spinal dorsal horn by at least two mechanisms. First, the spontaneous release of glutamate from glial cells produces periodic bursts of action potentials in the spinal dorsal horn neurons of nociceptive pathways without peripheral synaptic input, a condition that might be related to the spontaneous and persistent pain seen in pathological conditions. Second, the glutamate released from glial cells that is elicited by peripheral synaptic input results in the additional activation of dorsal horn neurons. More importantly, the activation thresholds for evoked SICs in most of cases were lower than those for evoked EPSCs. In other words, neurons are activated by glutamate released from glial cells that are activated by the primary afferents (or neuronal circuits) not functionally projected to the neurons. Our experimental approaches in this study would not allow us to define the types of afferent fibers needed for evoking EPSCs and SICs. It should be noted that the differences in activation thresholds indicate that different fibers were activated, but do not necessarily imply that different functional classes of fibers (Aβ, Aδ, or C fibers) were activated. Hence, the activation of postsynaptic neurons by glial-derived glutamate magnifies the activation of postsynaptic neurons by peripheral synaptic inputs, leading to impairments in spatial, temporal, and intensity coding and to loss of the modality specificity in relaying peripheral afferent input.
GRANTS
This project was supported by National Institutes of Health RO1 Grant NS-064289 to H.-R. Weng.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron 59: 932–946, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Blomstrand F, Hanse E. Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region.J Physiol 585: 843–852, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus.J Neurosci 24: 6920–6927, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake.Nat Neurosci 5: 325–331, 2002 [DOI] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake.Neuron 18: 281–293, 1997 [DOI] [PubMed] [Google Scholar]
- Balasubramanyan S, Stemkowski PL, Stebbing MJ, Smith PA. Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons.J Neurophysiol 96: 579–590, 2006 [DOI] [PubMed] [Google Scholar]
- Bardoni R, Ghirri A, Zonta M, Betelli C, Vitale G, Ruggieri V, Sandrini M, Carmignoto G. Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn.J Physiol 588: 831–846, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Regehr WG. Brief bursts of parallel fiber activity trigger calcium signals in bergmann glia.J Neurosci 26: 6958–6967, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina.J Neurosci 22: 2165–2173, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function.Trends Neurosci 19: 163–171, 1996 [DOI] [PubMed] [Google Scholar]
- Coull JAM, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, DeKoninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain.Nature 438: 1017–1021, 2005 [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake.Prog Neurobiol 65: 1–105, 2001 [DOI] [PubMed] [Google Scholar]
- D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens.Proc Natl Acad Sci USA 104: 1995–2000, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarque M, Villeneuve N, Manent JB, Becq H, Represa A, Ben Ari Y, Aniksztejn L. Glutamate transporters prevent the generation of seizures in the developing rat neocortex.J Neurosci 24: 3289–3294, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS. A broad view of glutamate spillover.Nat Neurosci 5: 291–292, 2002 [DOI] [PubMed] [Google Scholar]
- Ding S, Fellin T, Zhu Y, Lee SY, Auberson YP, Meaney DF, Coulter DA, Carmignoto G, Haydon PG. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus.J Neurosci 27: 10674–10684, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels.Pharmacol Rev 51: 7–61, 1999 [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors.Neuron 43: 729–743, 2004 [DOI] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism.Glia 21: 106–113, 1997 [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development.J Neurosci 17: 8363–8375, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy.Nat Neurosci 8: 1078–1086, 2005 [DOI] [PubMed] [Google Scholar]
- Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice.J Neurosci 27: 9736–9741, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hires SA, Zhu Y, Tsien RY. Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters.Proc Natl Acad Sci USA 105: 4411–4416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse.Curr Opin Neurobiol 14: 346–352, 2004 [DOI] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels.Proc Natl Acad Sci USA 85: 1307–1311, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. Integration of asynchronously released quanta prolongs the postsynaptic spike window.J Neurosci 27: 6684–6691, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb.Neuron 23: 377–384, 1999 [DOI] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin.Proc Natl Acad Sci USA 96: 8733–8738, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength.Nat Neurosci 10: 331–339, 2007 [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus: evidence and implications.Trends Neurosci 21: 8–14, 1998 [DOI] [PubMed] [Google Scholar]
- Largo C, Cuevas P, Somjen GG, Martin dR, Herreras O. The effect of depressing glial function in rat brain in situ on ion homeostasis, synaptic transmission, and neuron survival.J Neurosci 16: 1219–1229, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus.J Physiol 580: 373–383, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord.Nature 393: 695–698, 1999 [DOI] [PubMed] [Google Scholar]
- Liaw WJ, Stephens RL, Jr, Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord.Pain 115: 60–70, 2005 [DOI] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission.Glia 55: 36–45, 2007 [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression.J Neurosci 24: 7821–7828, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain.Nat Rev Neurosci 10: 23–36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Weng HR. Glutamate transporters prevent excessive activation of NMDA receptors and extrasynaptic glutamate spillover in the spinal dorsal horn.J Neurophysiol 101: 2041–2051, 2009 [DOI] [PubMed] [Google Scholar]
- Nie H, Weng HR. Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats.J Neurophysiol 103: 2570–2580, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons.Science 292: 923–926, 2001 [DOI] [PubMed] [Google Scholar]
- Otsu Y, Murphy TH. Optical postsynaptic measurement of vesicle release rates for hippocampal synapses undergoing asynchronous release during train stimulation.J Neurosci 24: 9076–9086, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory.Cell 125: 775–784, 2006 [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation.Nat Neurosci 4: 803–812, 2001 [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord.Nature 403: 312–316, 2000 [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity.Curr Opin Anaesthesiol 21: 570–579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R, Sandkuhler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro.J Physiol 541: 231–244, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Volterra A. Synaptic modulation by astrocytes via Ca(2+)-dependent glutamate release.Neuroscience 158: 253–259, 2009 [DOI] [PubMed] [Google Scholar]
- Savtchenko LP, Rusakov DA. The optimal height of the synaptic cleft.Proc Natl Acad Sci USA 104: 1823–1828, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuss V, Taschenberger H, Neher E. Kinetics of both synchronous and asynchronous quantal release during trains of action potential-evoked EPSCs at the rat calyx of Held.J Physiol 585: 361–381, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke CG, Haas B, Kettenmann H. Astrocytes discriminate and selectively respond to the activity of a subpopulation of neurons within the barrel cortex.Cereb Cortex 18: 2450–2459, 2008 [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters.Mol Pharmacol 53: 195–201, 1998 [DOI] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats.J Neurosci 23: 2899–2910, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover.Nat Neurosci 10: 735–742, 2007 [DOI] [PubMed] [Google Scholar]
- Thomson LM, Zeng J, Terman GW. An N-methyl-d-aspartate receptor mediated large, low-frequency, spontaneous excitatory postsynaptic current in neonatal rat spinal dorsal horn neurons.Neuroscience 141: 1489–1501, 2006 [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses.Neuron 34: 255–264, 2002 [DOI] [PubMed] [Google Scholar]
- Weng HR, Aravindan N, Cata JP, Chen JH, Shaw AD, Dougherty PM. Spinal glial glutamate transporters downregulate in rats with taxol-induced hyperalgesia.Neurosci Lett 386: 18–22, 2005 [DOI] [PubMed] [Google Scholar]
- Weng HR, Chen JH, Cata JP. Inhibition of glutamate uptake in the spinal cord induces hyperalgesia and increased responses of spinal dorsal horn neurons to peripheral afferent stimulation.Neuroscience 138: 1351–1360, 2006 [DOI] [PubMed] [Google Scholar]
- Weng HR, Chen JH, Pan ZZ, Nie H. Glial glutamate transporter 1 regulates the spatial and temporal coding of glutamatergic synaptic transmission in spinal lamina II neurons.Neuroscience 149: 898–907, 2007 [DOI] [PubMed] [Google Scholar]
- Xin WJ, Weng HR, Dougherty PM. Plasticity in expression of the glutamate transporters GLT-1 and GLAST in spinal dorsal horn glial cells following partial sciatic nerve ligation.Mol Pain 5: 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Peng H, Kang N, Zhao Z, Lin JH, Stanton PK, Kang J. Glutamate-induced exocytosis of glutamate from astrocytes. J Biol Chem 282: 24185–24197, 2007 [DOI] [PubMed] [Google Scholar]
- Zhang H, Xin W, Dougherty PM. Synaptically evoked glutamate transporter currents in spinal dorsal horn astrocytes.Mol Pain 5: 36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]