Abstract
A pH-sensitive cAMP-gated cation current (INa,cAMP) is widely distributed in neurons of the feeding motor networks of gastropods. In the sea slug Pleurobranchaea this current is potentiated by nitric oxide (NO), which itself is produced by many feeding neurons. The action of NO is not dependent on either cGMP or cAMP signaling pathways. However, we found that NO potentiation of INa,cAMP in the serotonergic metacerebral cells could be blocked by intracellular injection of MOPS buffer (pH 7.2). In neurons injected with the pH indicator BCECF, NO induced rapid intracellular acidification to several tenths of a pH unit. Intracellular pH has not previously been identified as a specific target of NO, but in this system NO modulation of INa,cAMP via pHi may be an important regulator of the excitability of the feeding motor network.
INTRODUCTION
Many gastropod neurons express a cyclic adenosine 3′,5′-monophosphate (cAMP)–induced sodium current (INa,cAMP; Connor and Hockberger 1984a; Green and Gillette 1983) activated by direct cAMP gating (Sudlow et al. 1993). The current takes two forms, types 1 and 2, differentiable on the basis of their voltage dependence (Gillette and Green 1987; Huang and Gillette 1993). Observations that cell signaling pathways could target intracellular pH (pHi) in molluscan neurons (Connor and Hockberger 1984b) was followed by finding that the type 1 INa,cAMP, most often found in neurons of the feeding motor network of Pleurobranchaea, was highly sensitive to small changes in pHi, where current amplitude was increased with acidification (Green and Gillette 1988; also see Aldenhoff et al. 1983). The type 2 INa,cAMP form, found in pedal ganglion neurons, was entirely insensitive to pHi (Huang and Gillette 1991, 1993).
The functional benefit of pH sensitivity of INa,cAMP has been a question without an obvious answer since its discovery. However, recently the pH-sensitive INa,cAMP was shown to be highly sensitive to potentiation by nitric oxide (NO) activity, whereas the pH-insensitive form was not (Hatcher 2003; Hatcher et al. 2006). Thus NO excited many neurons of the feeding motor network expressing both INa,cAMP and apparent resting levels of cAMP, but lacked detectable actions on the pedal locomotor neurons.
NO potentiation of INa,cAMP was not dependent on cyclic guanosine 3′,5′-monophosphate (cGMP) because it was not mimicked or blocked by cGMP analog or by inhibitors of cGMP synthesis or protein kinase G (Hatcher et al. 2006). Effects were specific to NO because they were induced by several different donors and abolished by depletion of donor through exposure to air at room temperature. NO synthase activity is considerable in the feeding network of Pleurobranchaea (Cruz et al. 1997; Floyd et al. 1998; Moroz et al. 1996).
We asked whether NO might act through altering intracellular pH. To study potential effects of NO on pHi, we used the same well-studied, identified neuron previously used to characterize NO-induced potentiation of INa,cAMP (Hatcher et al. 2006), the serotonergic metacerebral giant neuron (MCG; Gillette and Davis 1977; McCrohan and Gillette 1988). This neuron is conserved in cerebral ganglion lobes of many gastropods and provides neuromodulatory excitation to the feeding motor network (Gillette and Davis 1977).
Clamping pHi blocked the potentiating effects of NO on INa,cAMP. Using indicator dyes, we observed that NO induced rapid intracellular acidification. These results not only clarify a mechanism of the excitatory effects of NO on the feeding motor network of Pleurobranchaea, but also document a novel mechanism for NO action in biological systems.
METHODS
Specimens of Pleurobranchaea californica were obtained from Sea Life Supply (Sand City, CA) and maintained in Instant Ocean at 12–13°C. The cell bodies of the identified metacerebral giant neurons (MCGs) were visually identified by their large size and anterior-most position in cerebropleural ganglia, isolated by hand dissection, and stabilized in a perfusion dish against carbon fiber pins. For most experiments, the cell was perfused with 3-(N-morpholino)propanesulfonic acid (MOPS)–buffered saline (composition in mM: NaCl 420, KCl 10, MgSO4 25, MgCl2 25, CaCl2 10, MOPS 5, at pH 7.5) at a rate of 1 ml · min−1 at 13 ± 1°C. Bicarbonate saline was made 30 mM in NaHCO3, substituting equimolar for NaCl, and adjusted to pH 7.5.
INa,cAMP responses to iontophoretic injection of cAMP were measured under two-electrode voltage clamp as described previously (Hatcher et al. 2006; Sudlow and Gillette 1997). Briefly, sharp electrodes were pulled from borosilicate glass (WPI). A single-barreled capillary filled with 3 M KCl served as a voltage-sensing electrode. A second, double-barreled, electrode acted as the current-passing electrode (filled with 3 M KCl) in one half and a cAMP iontophoresis capillary in the other (filled with 200 mM cAMP and 20 mM Tris, adjusted to pH 7.5 with KOH). Iontophoretic injection of cAMP (5 s duration; 5–200 nA) was accomplished with negative current from a constant-current source (Model 260, WPI) that allowed precise, repeatable quantities of cAMP to be injected intracellularly.
Injection amplitudes were kept relatively low to avoid current saturation while yielding a robust INa,cAMP response. Under our conditions, cAMP injection occurs with a constant transport number of 0.1 over the wide range of injection currents used here (Sudlow and Gillette 1997). The kinetics of INa,cAMP is largely determined by intracellular cAMP diffusion and phosphodiesterase activity (Huang and Gillette 1991). INa,cAMP measures in the presence of diethylamine/nitric oxide (DEA/NO) were begun around 10 min after addition of NO donor, at which time responses were nearly maximal and relatively constant in the absence of blocking treatment.
Earlier work in neurons of the mollusk Archidoris showed that pressure-injected cAMP to values of 0.1–1 mM could acidify pHi in the range of 0.1 pH unit, an effect likely mediated by cAMP-dependent kinase (Connor and Hockberger; 1984b). Such an effect could add an interesting dimension to INa,cAMP functioning, but was not visible in these present studies. A previous study by Hatcher et al. (2006) did not find effects of NO on pH-sensitive INa,cAMP to be dependent on cAMP beyond a simple gating role for the current, perhaps because iontophoretic injections were smaller (Sudlow and Gillette 1997) and were not previously found to cause significant changes in pHi themselves (Green and Gillette 1988).
Intracellular recording and voltage clamping were performed with an Axoclamp 2B amplifier (Axon Instruments, Union City, CA) and data were recorded digitally with Biopac hardware and the accompanying Acknowledge software package (Biopac Systems, Goleta, CA). All recordings were performed at a holding potential of −50 mV unless otherwise indicated.
For fluorescence measurements, glass microelectrodes were pulled to resistances in the range 5–15 MΩ when filled with 200 mM KCl, 10 mM MOPS, and the fluorescent pH indicator BCECF [2′,7′-bis(2-carboxyethyl)-5,6-carboxyfluorescein; Sigma], pressure injected from an electrode containing 1 mM dye. Following injection, the electrode was withdrawn and the cell was penetrated with an electrode filled with 3 M KCl for electrical recording via an Axoclamp 2A amplifier and Clampex 8.1 software (Axon Instruments).
Fluorescence measurements were made using an InCyt dual-wavelength fluorescence imaging and photometry system (sampling rate 10 per min; Intracellular Imaging) with a Sutter Lambda 10C optical filter changer (Sutter Instrument) and InCyt Im2 acquisition software. For BCECF measurements, a calibration curve for the 488:436 nm fluorescence ratio was generated using a series of MOPS-saline standards, pH 6.5 to 8. These standards proved reliably stable over time. A slow negative (i.e., acidic) drift occurred in 10 pH recordings, averaging 0.007 ± 0.002 pH units · min−1 (mean ± SD), suggesting that slow dye bleaching or leakage was leading to a greater signal contribution by autofluorescence. This artifact did not interfere with observation of the more rapid dynamic changes caused by NO. Resting membrane potentials in these experiments were −45 to −55 mV.
MOPS was pressure injected as a 1 M solution, adjusted to pH 7.2 with KOH. Injection volumes were estimated at ≥10% of cell volume; thus injections are likely to have raised cell buffering power to fourfold or more that of the resting values (cf. Table 2 in Ahmed and Connor 1980). Injection sequences were composed of multiple small injections where pressures were maintained until a small polarizing membrane response indicated injection success. Subsequent injections were made approximately 1 min after membrane potential recovered. Dye injections were performed similarly. The NO donor diethylamine/nitric oxide (DEA/NO, 1–2 mM; Axxora LLC) was applied in the perfused saline. When measured using a calibrated NO electrode (WPI), freshly prepared 1 mM DEA/NO in Pleurobranchaea saline at this temperature yielded a value of 430 nM of NO, with first-order exponential decay of t1/2 = 418 s (Hatcher et al. 2006). Usually 1–2 half-lives were passed by the time perfusion occurred, so actual NO concentrations may have been in the range of 100–200 nM.
RESULTS
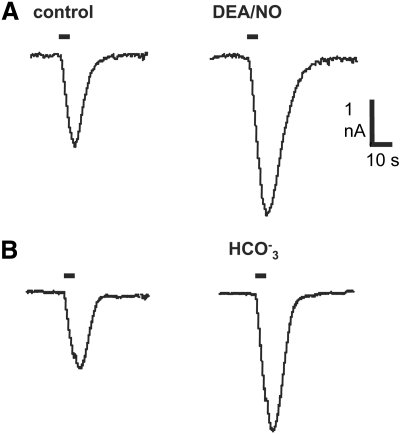
NO donors potentiate the INa,cAMP response to iontophoretically injected cAMP in neurons of the feeding motor network (Hatcher et al. 2006), including the MCG neurons (Fig. 1A). In a total of 16 MCG neurons the NO donor DEA/NO enhanced the INa,cAMP response on average by 85% (24% SE) over control, predonor measures (P < 0.001; two-tailed t-test). Intracellular acidification of a few tenths of pH unit was previously shown to have a similar effect (Green and Gillette 1988), which is recapitulated in the records of Fig. 1B. In the present study, bicarbonate-containing salines increased INa,cAMP amplitude in four neurons by 71.7% (6.3% SE). Thus intracellular acidification mimics the enhancing effect of NO donors on INa,cAMP.
Fig. 1.
Potentiation of the cyclic adenosine 3′,5′-monophosphate (cAMP)–induced sodium current (INa,cAMP) response amplitude to injected cAMP by nitric oxide (NO) donor and intracellular acidification, and NO-induced depolarization. A: diethylamine/NO (DEA/NO, 1 mM) augmented INa,cAMP response amplitude by 78% relative to control measures in saline alone taken just prior to addition of the NO donor. B: saline containing 30 mM bicarbonate mimicked the effect of NO donor, increasing INa,cAMP amplitude by 41%. Such bicarbonate salines acidify Pleurobranchaea neurons by several tenths of a pH unit (Green and Gillette 1988). Holding potentials were −50 mV. Horizontal bars in records indicate 5 s, 100 nA iontophoretic injections of cAMP.
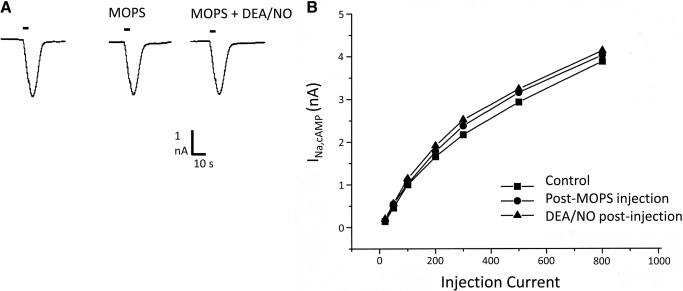
A likely causal linkage of NO and pHi was supported by finding in each of four cells tested that intracellular injection of 1 M MOPS buffer at pH 7.2, in volumes estimated near 0.1× soma volume, eliminated the potentiating effect of NO on INa,cAMP amplitude [Fig. 2; average change 10% (5% SE); P = 0.30, two-tailed t-test]. Similar intracellular injections of BCECF (see following text) did not block NO-induced depolarization or NO effects on pHi. Thus a nonspecific effect of simple pressure injection was unlikely to be a significant factor in the pHi response. The blockade of NO effects by intracellular pH buffering was significant relative to the positive effects of NO donor on the 16 uninjected MCGs (P = 0.0003; Fisher's exact test, two-sided) and led to the following assessment of the effects of NO on pHi.
Fig. 2.
Intracellular injection of the pH buffer MOPS suppresses NO potentiation of INa,cAMP. A: INa,cAMP responses to injected cAMP in normal saline (left), after MOPS injection and in presence of NO donor. Holding potentials were −50 mV. B: a typical dose–response relation for cAMP injection and INa,cAMP response, showing blockade of NO effects over a broad range of injection amplitude.
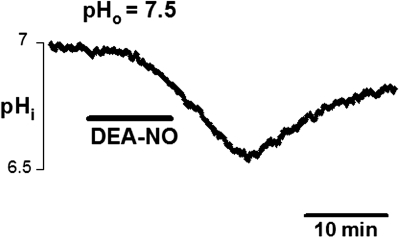
In six of eight MCG somata injected with the pH-indicator dye BCECF, initial pHi lay close to an averaged 7.0 (0.08 SE; values for the remaining two cells were not attained). Bath application of DEA/NO caused strong and rapid intracellular acidification in all eight cells (Fig. 3). Peak response amplitudes were attained within 13–16 min of donor application and ranged from 0.16 to 0.46 pH units (mean 0.22; SE 0.04; n = 8 cells). Decay of the pH response was monitored for seven cells following washout of DEA/NO and lasted ≤26 min with an average half-time to estimated full recovery of 6.5 min (SE 0.72). The minutes-long time course of acidification and recovery of pHi was comparable to observations of effects of NO and its slow washout on INa,cAMP amplitudes (cf. Fig. 4 in Hatcher et al. 2006). The recovery of pHi was consistent with the slow recovery times previously observed following acidification in mollucan neurons (Ahmed and Connor 1980; Thomas and Meech 1982).
Fig. 3.
NO stimulated rapid intracellular acidification in isolated metacerebral giant neuron (MCG) somata, as measured with BCECF fluorescence. Bath application of the NO donor, DEA/NO (1 mM, 10 min, solid bar) to a soma in saline at pH 7.5 caused an acidification of intracellular pH (pHi) >0.3 pH unit, peaking around 15 min after donor application and decaying slowly over minutes following washout.
DISCUSSION
The results show that NO promotes intracellular acidification in neurons whose excitatory state is markedly sensitive to pHi. This demonstration is significant in providing a functional answer to a long-standing question concerning the likely import of the marked pH sensitivity of INa,cAMP in gastropod neurons (Aldenhoff et al. 1983; Green and Gillette 1988).
NO/pHi regulation of type 1 INa,cAMP
Until now, no clearly relevant functional context for the pH sensitivity of INa,cAMP has been available. We previously documented two forms of INa,cAMP. One is the pH-sensitive type 1 form found in many neurons of the feeding motor network in the opisthobranch gastropods Pleurobranchaea (Green and Gillette 1983, 1988), Navanax and Aplysia (unpublished results), and in the pulmonate snails Helix (Aldenhoff et al. 1983) and Lymnaea (McCrohan and Gillette 1988). This current is a highly sensitive indicator of pHi, responding with measurable amplitude change to ΔpHi of 0.03 unit and closely following induced changes in pHi (Green and Gillette 1988). Two unpublished observations in excised patches indicate a population of pH-sensitive INa,cAMP channels (Sudlow and Gillette). In contrast, the type 2 form of INa,cAMP, so far found mainly in neurons of the locomotor network (Huang and Gillette 1993), is relatively insensitive both to changing pHi and to the potentiating effects of NO (Hatcher et al. 2006).
Demonstration of NO-induced acidification in the MCG provides a mechanism for potentiation of type 1 INa,cAMP by NO in the same neuron (Hatcher et al. 2006). It also provides a likely pathway for the ability of exogenous NO to activate the feeding motor network (Moroz et al. 1995). The MCG itself is a neuromodulatory excitor of the feeding network (Gillette and Davis 1977). It lacks chemical markers (unpublished) characteristic of nitrergic neurons (Cruz et al. 1997; Floyd et al. 1998) and is thus a more likely NO target than source. NO has roles in activation of the feeding motor network in diverse gastropod species (Elphick et al. 1995; Moroz et al. 1993; Park et al. 1998) but its potentiation of INa,cAMP has as yet been documented only in Pleurobranchaea.
Significance
The activity of NOS in Pleurobranchaea's CNS is high, comparable to that of mammalian brain when assayed in vitro (Moroz et al. 1996), and localized to numerous neurons in the feeding and locomotion motor networks (Cruz et al. 1997; Floyd et al. 1998b). The presence of NOS in the feeding motor network and the ability of NO to activate the feeding motor rhythm suggest a normal role in regulating the excitability of the feeding neurons, acting on INa,cAMP through pHi. Mechanisms of NO action in altering pHi remain to be explored.
These results indicate a novel action for NO in modulating neuronal excitability through intracellular acidification. A similar, although slower, acidification response to NO donors was observed in cultured hippocampal neurons, leading to apoptosis (Vincent et al. 1999). However, effects of NO in the cultured neurons were protein kinase G dependent (Maiese et al. 1993), unlike for the MCG neurons of Pleurobranchaea (Hatcher et al. 2006). An extensive literature search has otherwise failed to turn up similar results. Thus NO effects on pHi in this system are likely to reflect a new signaling pathway.
Intracellular H+ may interact in complex ways with other signaling pathways and metabolic pathways in the cell to add further dimension to cell signaling and neuronal function. Injections of cAMP in neurons of the mollusk Archidoris were found to acidify pHi ≤0.1 pH unit, an effect likely mediated by cAMP-dependent kinase (Connor and Hockberger 1984b). Ca2+ influx during spike activity can acidify neurons through activation of Ca2+/H+ exchange mechanisms (Ahmed and Connor 1980; Thomas 2009), which in fine neuritic processes can cause swings in pHi up to several tenths of a unit (Schwiening and Willoughby 2002). Such pH shifts can modify many aspects of pH-sensitive ion channels and metabolic processes.
In this system, at levels of cell and circuit NO-induced acidification is liable to amplify effects of neuromodulator-induced cAMP. Thus our emerging picture of the functional role of NO in the feeding motor network of Pleurobranchaea is one in which it acts as a gain-enhancing mechanism, boosting the cellular responses to neuromodulator-induced fluctuations of cAMP. Such gain control will affect the activity state of the feeding network in large part through broad modulation of pH-sensitive INa,cAMP in its neuronal elements.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-26838 and National Science Foundation Grant IOS 08-43621.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present addresses: K. Potgieter: University of Illinois, School of Veterinary Medicine, Urbana, IL 61801; N. G. Hatcher: University of Illinois, Department of Chemistry, 505 S. Matthews Ave., Urbana, IL 61801.
REFERENCES
- Ahmed Z, Connor JA. Intracellular pH changes induced by calcium influx during electrical activity in molluscan neurons. J Gen Physiol 75: 403–426, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldenhoff JB, Hofmeier G, Lux HD, Swandulla D. Stimulation of a sodium influx by cAMP in Helix neurons. Brain Res 276: 289–296, 1983 [DOI] [PubMed] [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003 [DOI] [PubMed] [Google Scholar]
- Connor JA, Hockberger P. A novel membrane sodium current induced by injection of cyclic nucleotides into gastropod neurones. J Physiol 354: 139–162, 1984a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Hockberger P. Intracellular pH changes induced by injection of cyclic nucleotides into gastropod neurons. J Physiol 354: 163–172, 1984b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L, Moroz LL, Gillette R, Sweedler JV. Nitrite and nitrate levels in individual molluscan neurons: single-cell capillary electrophoresis analysis. J Neurochem 69: 110–115, 1997 [DOI] [PubMed] [Google Scholar]
- DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev 83: 475–579, 2003 [DOI] [PubMed] [Google Scholar]
- Elphick MR, Rayne RC, Riveros-Moreno V, Moncada S, O'Shea M. Nitric oxide synthesis in locust olfactory interneurones. J Exp Biol 198: 821–829, 1995 [DOI] [PubMed] [Google Scholar]
- Floyd PD, Moroz LL, Gillette R, Sweedler JV. Capillary electrophoresis analysis of nitric oxide synthase related metabolites in single identified neurons. Anal Chem 70: 2243–2247, 1998 [DOI] [PubMed] [Google Scholar]
- Gillette R, Davis WJ. The role of the metacerebral giant neuron in the feeding behavior of Pleurobranchaea. J Comp Physiol 116: 129–159, 1977 [Google Scholar]
- Green DJ, Gillette R. Patch and voltage clamp analysis of cAMP-stimulated inward current underlying neurone bursting. Nature 306: 784–785, 1983 [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Regulation of cyclic AMP-dependent ion current by intracellular pH, Ca2+, and calmodulin blockers. J Neurophysiol 59: 248–258, 1988 [DOI] [PubMed] [Google Scholar]
- Hatcher N. Neural Mechanisms of Decision-Making: Modulation of the Feeding Neural Network by Nitric Oxide and Serotonin in the Predatory Marine Snail Pleurobranchaea californica (PhD thesis). Urbana, IL: Univ. of Illinois, 2003 [Google Scholar]
- Hatcher N, Moroz LL, Sudlow LC, Gillette R. Nitric oxide potentiates cAMP-gated cation current in feeding neurons of Pleurobranchaea californica independent of cAMP and cGMP signaling pathways. J Neurophysiol 95: 3219–3227, 2006 [DOI] [PubMed] [Google Scholar]
- Huang R-C, Gillette R. Coregulation of cAMP-activated Na+ current by Ca2+. J Physiol 462: 307–320, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrohan CR, Gillette R. Cyclic AMP-stimulated sodium current in identified feeding neurons of Lymnaea stagnalis. Brain Res 438: 115–123, 1988 [DOI] [PubMed] [Google Scholar]
- Meech RW, Thomas RC. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J Physiol 298: 111–129, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz LL, Chen D, Gillette MU, Gillette R. Synthesis, distribution and functional effects of nitric oxide synthase (NOS) in the predatory opisthobranch mollusc Pleurobranchaea californica. Soc Neurosci Abstr 21: 256.3, 1995 [Google Scholar]
- Moroz LL, Chen D, Gillette MU, Gillette R. Nitric oxide synthase activity in the molluscan CNS. J Neurochem 66: 873–876, 1996 [DOI] [PubMed] [Google Scholar]
- Park JH, Straub VA, O'Shea M. Anterograde signaling by nitric oxide: characterization and in vitro reconstitution of an identified nitrergic synapse. J Neurosci 18: 5463–5476, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiening CJ, Willoughby D. Depolarization-induced pH microdomains and their relationship to calcium transients in isolated snail neurons. J Physiol 538: 371–382, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow LC, Gillette R. cAMP levels, adenylyl cyclase activity, and their stimulation by serotonin quantified in intact neurons. J Gen Physiol 110: 243–255, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow LC, Huang RC, Green DJ, Gillette R. cAMP-activated Na+ current of molluscan neurons is resistant to kinase inhibitors and is gated by cAMP in the isolated patch. J Neurosci 13: 5188–5193, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature 299: 826–828, 1982 [DOI] [PubMed] [Google Scholar]