Abstract
Dopamine (DA) modifies the motor pattern generated by the pyloric network in the stomatogastric ganglion (STG) of the spiny lobster, Panulirus interruptus, by directly acting on each of the circuit neurons. The 14 pyloric neurons fall into six cell types, and DA actions are cell type specific. The transient potassium current mediated by shal channels (IA) is a common target of DA modulation in most cell types. DA shifts the voltage dependence of IA in opposing directions in pyloric dilator (PD) versus lateral pyloric (LP) neurons. The mechanism(s) underpinning cell-type specific DA modulation of IA is unknown. DA receptors (DARs) can be classified as type 1 (D1R) or type 2 (D2R). D1Rs and D2Rs are known to increase and decrease intracellular cAMP concentrations, respectively. We hypothesized that the opposing DA effects on PD and LP IA were due to differences in DAR expression patterns. In the present study, we found that LP expressed somatodendritic D1Rs that were concentrated near synapses but did not express D2Rs. Consistently, DA modulation of LP IA was mediated by a Gs-adenylyl cyclase-cAMP-protein kinase A pathway. Additionally, we defined antagonists for lobster D1Rs (flupenthixol) and D2Rs (metoclopramide) in a heterologous expression system and showed that DA modulation of LP IA was blocked by flupenthixol but not by metoclopramide. We previously showed that PD neurons express D2Rs, but not D1Rs, thus supporting the idea that cell specific effects of DA on IA are due to differences in receptor expression.
INTRODUCTION
The crustacean pyloric network is a powerful model for studying neuromodulation of rhythmic behaviors. All the major cells and their circuit connections are known. Many projection and sensory neurons that modulate the network have been defined (Blitz et al. 2008; Daur et al. 2009; DeLong et al. 2009; Hedrich et al. 2009; Stein et al. 2007), and the modulatory effects of monoamines and peptides on this circuit have been extensively studied (Marder and Bucher 2007; Nusbaum and Beenhakker 2002; Stein 2009).
Pyloric neurons receive both neuromodulatory and -hormonal dopamine (DA) transmissions. A few DA-containing neurons in the two commissural ganglia (COGs) project to the stomatogastric ganglion (STG) via the superior esophageal nerve (Goldstone and Cooke 1971; Kushner and Barker 1983; Kushner and Maynard 1977; Pulver et al. 2003; Sullivan et al. 1977; Tierney et al. 2003) and release DA to pyloric neurons largely in a paracrine fashion (Oginsky et al. 2010). Additionally, the STG resides in a major blood vessel, and the L-cells located in the COGs project to the cardiac ganglion and release neurohormonal DA into the hemolymph (Pulver and Marder 2002; Tierney et al. 2003).
Bath applied DA alters circuit output by differentially modulating pyloric neuron synaptic and intrinsic firing properties (Harris-Warrick et al. 1998). This is partially mediated by DA modulation of several ion channels (Cleland and Selverston 1997; Harris-Warrick et al. 1995a,b, 1998; Johnson et al. 2003a; Kloppenburg et al. 1999, 2000; Peck et al. 2001, 2006). Here we focus on DA modulation of IA, which helps determine the rate of postinhibitory rebound and spike frequency in pyloric neurons and influences pyloric cycle frequency and phase constancy (Ayali and Harris-Warrick 1998, 1999; Golowasch et al. 1992; Harris-Warrick et al. 1995a,b; Hooper 1997; Johnson et al. 2005;Tierney and Harris-Warrick 1992).
DA targets IA to differentially alter pyloric neuron activity. For example, in pyloric dilator (PD) neurons bath-applied DA increases IA maximal conductance and shifts the voltage dependence for activation to more hyperpolarized potentials without altering the voltage dependence for inactivation (Kloppenburg et al. 1999). As a result, the transient (peak) current is increased at all depolarizing membrane potentials where IA is activated. Further, the window current between the activation and inactivation curves is enlarged, and this increase in the tonic (sustained or steady-state) IA causes the PD neuron to hyperpolarize. On the other hand, bath applied DA modulates the lateral pyloric (LP) IA by reducing its maximal conductance and shifting the voltage dependence of both activation and inactivation in the depolarizing direction (Harris-Warrick et al. 1995b). This reduces the transient IA at all depolarizing potentials and shifts the window current so that the maximal tonic IA occurs at more depolarized potentials. Ultimately this differential modulation of IA in PD versus LP neurons contributes to a decrease versus increase in action potential firing and a phase delay versus advancement of neuronal activity, respectively.
The mechanism(s) underpinning this opposing DA modulation of PD and LP IA is unknown. There are no obvious differences in the ion channels mediating PD and LP IA. Shal channels mediate IA and appear to have the same subcellular distribution in both identified cell types such that they are found throughout the somatodendritic compartment and in axon terminals (Baro et al. 1996, 1997, 2000). We therefore hypothesized that differences in DA transduction cascades may underpin the opposing effects of DA on each cell type.
DA acts on several highly conserved DARs that belong to the G-protein-coupled receptor (GPCR) superfamily. In the traditional view, a given type of GPCR signals through one of four classes of G proteins: Gs, Gi/o, Gq/11, and G12/13 (Cabrera-Vera et al. 2003). All DARs can be classified into two types based on their G protein coupling: D1Rs and D2Rs (Neve et al. 2004). In the canonical pathways, D1Rs couple with Gαs to increase adenylyl cyclase (AC) activity and D2Rs couple with Gαi/o to decrease AC activity, thereby increasing and decreasing cAMP levels, respectively. The change in cAMP concentration will then alter protein kinase A (PKA) activity, which in turn will alter the phosphorylation state of a number of substrates. The two spiny lobster D1Rs, D1αPan and D1βPan, and the single D2R, D2αPan, signal through canonical G-protein-coupled pathways when overexpressed in human embryonic kidney cells (HEK) cells (Clark and Baro 2006, 2007) and in native stomatogastric membrane preparations (Clark et al. 2008). In addition to canonical G-protein-coupled signaling, GPCRs have recently been found to signal through noncanonical pathways that may or may not involve G proteins. We have shown that both types of lobster DARs can signal through evolutionarily conserved, noncanonical pathways. In STG membrane preparations, D1αPan can couple with Gq to activate phospholipase Cβ ( PLCβ) as well as Gs (Clark et al. 2008). It is not clear whether both types of coupling occur in a single neuron and/or whether the distinct cascades alter the same targets. D2αPan can couple with PLCβ via Gβγi/o when expressed in HEK cells (Clark and Baro 2007). We have recently shown that PD neurons express D2Rs but not D1Rs (Oginsky et al. 2010). PD DARs are concentrated in perisynaptic regions in the somatodendritic but not axonal compartments. In the present study, we test the hypothesis that DA has opposing effects on PD and LP IA because LP neurons express D1Rs while PD neurons express D2Rs.
METHODS
Drugs
Drugs were all purchased from Sigma (St. Louis, MO) except Rp-cAMP, tetrodotoxin (TTX), and H-89 (Tocris Bioscience, Bristol, UK). The strategy we used to choose drugs that would block the actions of AC and PKA is as follows: the sites of action for H-89 and Rp cAMP on PKA, and foskolin on AC, were determined from the literature. Using Megalign (DNASTAR), we then aligned these sites in homologues from mammals and invertebrates, including crustaceans. In addition we performed Blast searches with these regions. Together the data suggested that these sites were well conserved across species, a finding also noted in several previous publications. Moreover we only used drugs that were previously shown to work in invertebrates in general and/or specifically in crustaceans.
Animals
California spiny lobsters, Panulirus interruptus, were purchased from Don Tomlinson Commercial Fishing (San Diego, CA) and kept in aerated and filtered artificial saltwater.
Dissection and cell identification
Lobsters were cold-anesthetized for ≥30 min and the stomatogastric nervous system (STNS) was dissected as previously described (Bierman and Tobin 2009; Tobin and Bierman 2009). The STNS was pinned in a silicone elastomer (Sylgard)-lined dish and continuously superfused with Panulirus (P.) saline, which contained (in mM) 479 NaCl, 12.8 KCl, 13.7 CaCl2, 39 Na2SO4, 10 MgSO4, 2 glucose, 4.99 HEPES, and 5 TES; pH 7.4.
Cells were identified using standard intra- and extracellular recording techniques as previously described (Harris-Warrick et al. 1995a,b). Neuronal activity was monitored with intracellular somatic recordings using 20–40 MΩ glass microelectrodes filled with 3 M KCl and Axoclamp 2B or 900A amplifiers (Axon Instruments, Foster City, CA). Extracellular recordings of identified motoneurons were obtained using a differential AC amplifier (A-M Systems, Everett, WA) and stainless steel pin electrodes. Neurons were identified by their distinct waveforms, the timing of their voltage oscillations, and correlation of spikes on the extracellular and intracellular recordings.
Immunohistochemistry (IHC)
The identified LP neuron was filled with a lysine fixable, dextran coupled Texas Red fluorophore that was impermeable to gap junctions (MW 10,000; Molecular Probes), as previously described (Clark et al. 2008). A 1% solution of the fluorophore in 0.2 mol/l KCl was pressure injected (8–20 psi, 200 ms pulse, 0.05 Hz) using an 8–15 MΩ glass microelectrode and a PicoSpritzerIII (General Valve/Parker Hannifin). The fluorophore was injected until the cell became dark purple (typically 15–25 min, depending on the microelectrodes resistance), and the preparation was incubated for 4–24 h at room temperature to allow the fluorophore to diffuse. The preparation was then fixed and DAR protein distributions were determined using whole mount STG preparations in IHC experiments, followed by confocal microscopy. The IHC protocol was as previously described (Baro et al. 2000; Clark et al. 2004). The primary, affinity purified antibodies against the three lobster DARs (D1αPan, D1βPan, and D2αPan) and their respective specificities were previously described (Clark et al. 2008; Oginsky et al. 2010). Data were acquired with a LSM510 confocal laser scanning microscope from Carl Zeiss Microimaging (Oberkochen, Germany).
cAMP assays in a heterologous expression system
Receptors were transiently expressed in HEK as previously described (Spitzer et al. 2008a). Cells that overexpressed a given receptor were exposed to DA or DA plus varying concentrations of a given antagonist. The change in cAMP levels induced by DA (10−5M) or DA plus antagonist were measured using an ELISA assay kit (Assay Designs) as previously described (Clark and Baro 2006, 2007; Spitzer et al. 2008a,b). Data were analyzed with Prism (GraphPad) and Excel (Microsoft) software.
TEVC
For two electrode voltage clamp (TEVC), the desheathed stomatogastric nerve (stn) and STG were isolated in separate petroleum jelly (Vaseline) wells. The STG was superfused continuously at room temperature with Panulirus saline using a Rainin Dynamax peristaltic pump (Rainin). For all experiments, temperature was continuously monitored with a miniature probe in the bath. After cell identification, descending inputs were removed with a sucrose block applied into the well surrounding the stn for 1 h. Glutamatergic synaptic inputs were blocked with picrotoxin (10−6M). The known voltage-dependent ion channels except IA were blocked with bath-applied TTX (10−7 M, INa), TEA (2 × 10−2 M, IK(V) and IK(Ca)), CsCl (5 × 10−3 M, Ih) and CdCl2 (2 × 10−4 M, ICa).
IA was analyzed as previously described (Baro et al. 1997) using Axoclamp 2B and 900A amplifiers and Clampex 8.2 and 10.2 software (Axon Instruments). The LP neuron was impaled with low resistance (5–9 MΩ) microelectrodes filled with 3 M KCl. Cells were held at −50 mV holding potential between experimental protocols.
The voltage dependence of activation was measured by a series of sweeps in which a hyperpolarizing prepulse to −90 mV for 200 ms was followed by a 500 ms depolarizing test pulse that ranged from −50 to +60 mV with 10 mV increments. Steady-state inactivation was measured with a series of sweeps in which the membrane potential was stepped to a prepulse between −110 and −20 mV with 10 mV increments (200 ms) followed by a constant test pulse to +20 mV (500 ms). Besides the pharmacological isolation described in the preceding text, IA was further isolated by digital subtraction of the leak conductance. For activation, IA was evoked by test pulses between −50 and +60 mV from −50 mV holding potential without a negative prepulse. This leaves a relatively linear leak current, which, however, contains a transient component of IA that is not inactivated at −50 mV. Typically this does not exceed 10% of the peak conductance and was therefore tolerated. These current traces were subtracted from those evoked with negative prepulse. For inactivation, the subtraction protocol contained a depolarizing prepulse to −20 mV before the test pulse to +20 mV.
Data were analyzed with Clampfit v.8.2 and 10.2 (Axon Instruments), Prism v.4 and 5 (Graphpad) and Excel (Microsoft). After digital subtraction, peak currents measured at each voltage step were converted into conductance using the formula G = Ipeak/(V − EK), assuming EK = −86 mV (Eisen and Marder 1982). The calculated conductance and the corresponding voltage were then used to construct conductance-voltage plots. Plots were fit with a first-order Boltzmann equation to obtain the maximal conductance (Gmax), the apparent voltage of half activation (V1/2 activation), and voltage of half inactivation (V1/2 inactivation).
Many biophysical channel properties are temperature dependent. In preliminary studies, we performed experiments either at room temperature (which did not vary during an experiment and never varied by >3°C across experiments, from 19 to 22°C) or at 16°C (maintained by placing the perfusion tubing in an ice water bath). The results of these studies showed that the DA induced shifts in IA voltage dependencies were not temperature sensitive. Average shifts were almost exactly the same and not significantly different when measured at room temperature or at 16°C. Therefore for convenience, experiments were performed at room temperature, and all data included in this manuscript were obtained at room temperature.
Drug application
In most experiments, drugs were continuously superfused into the STG well. However, to save on costs, during Rp-cAMP application, perfusion pumps were stopped after the drug was applied to the bath and the bath volume had been replaced at least five times. Pumps were started again for washout. No changes in holding currents or temperature were observed during this process. In all experiments, the concentration of DA was −10−5M, except the Rp-cAMP experiments. To save on costs, DA was reduced to 5 × 10−6 M so that less blocker was required. In some experiments (n ≥ 1), the order of application was reversed to show that the drug had the same effect regardless of whether or not it was preceded by DA application (e.g., antagonist alone and antagonist +DA applications were before the DA application). We also confirmed that serial applications of DA separated by a 30 min wash had the same effects on LP IA (i.e., no significant differences in the DA induced shifts produced by DA application 1 vs. 2).
Statistical analyses
Unless otherwise indicated, data are shown as means ± SE. Student's t-test were performed with Excel software. One-way (repeated measures) ANOVA was performed with GraphPad Prism software. Tukey's post hoc tests were performed where appropriate. Statistical significance was determined as P < 0.05.
RESULTS
D1Rs, but not D2Rs, are expressed in LP neurons
To understand how DA modulates LP IA, we first defined LP DAR expression. We performed IHC experiments on STG whole mount preparations, each containing a dye-filled LP neuron. Three custom made, affinity purified antibodies, each specific for one of the three lobster DARs, were used in conjunction with confocal microscopy as previously described (Clark et al. 2008; Oginsky et al. 2010). Overlapping 1 μm confocal optical sections throughout the somatodendritic compartment of a given LP neuron were examined for the presence of a given receptor. The data suggested that receptor expression in the somatodendritic compartment was consistent across preparations: D1αPan and D1βPan receptors were always observed in LP neurons, but D2αPan receptors were never detected (n = 5 for each DAR; Fig. 1).
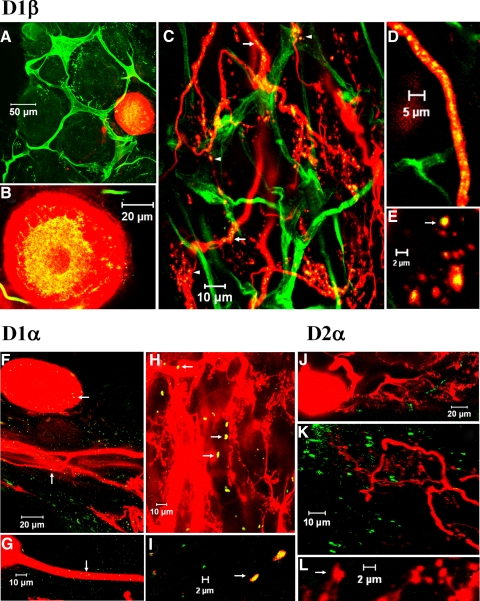
Fig. 1.
D1αPan and D1βPan, but not D2αPan receptors were distributed in the lateral pyloric (LP) somatodendritic compartment. Wholemount stomatogastric ganglion (STG) preparations, each containing a single Texas red filled LP, were stained with anti-D1βPan (A–E), anti-D1αPan (F–I), or anti-D2αPan (J–L). n ≥ 5 wholemount preparations for each receptor type. Yellow staining indicates dopamine receptor (DAR) expression in the LP. Green staining represents DAR expression in unidentified cells. Merged confocal projections were made from serial 1 μm confocal optical slices. A: merged, 3 μm confocal projection from a wholemount preparation showing D1βPan receptor expression in LP perinuclear vesicles (yellow puncta) and unidentified cells (green puncta). Green tubular structures surrounding somata suggests D1βPan receptor expression in glial and /or other support cells. B: a 3 μm merged confocal projection from the center of the LP soma showing D1βPan receptor expression in the perinuclear vesicles. C: a 4 μm merged confocal projection from deep within the synaptic neuropil showing D1βPan receptors in cytoplasmic transport vesicles in higher order neurites (→). ▴, putative synaptic varicosities containing D1βPan receptors. D: high magnification 1 μm optical slice showing cytoplasmic transport vesicles in higher order neurite. E: high magnification 3–4 μm projection showing a cluster of LP synaptic terminals, some of which contain D1βPan receptors.→, the terminal lacking the red cytoplasmic ring structure. F–H: 4 μm merged confocal projections showing D1αPan receptor expression in the LP soma, primary neurite, higher order neurites and synaptic terminals (→). I: high magnification 1 μm optical slice showing the presence of D1αPan receptors on LP synaptic terminals. →, the terminals lacking a red cytoplasmic ring. J: a 37 μm merged confocal projection showing the absence of detectable D2αPan in the LP soma and primary neurites. Green staining represents D2αPan receptors in unidentified neurons. K: a 4 μm confocal projection from deep within the synaptic neuropil showing the absence of D2αPan in LP higher order neurites and terminals. L: high magnification 1 μm optical slice showing a cluster of LP synaptic terminals lacking D2αPanreceptors.
Similar to our findings for PD D2Rs (Clark et al. 2008; Oginsky et al. 2010), LP D1Rs appeared to be located in somatodendritic endomembrane structures (Fig. 1, A, B, and F). D1Rs were also detected in primary and higher order neurites (Fig. 1, C, D, and G). Careful examination of the optical sections showed that receptors were not associated with the plasma membrane in these structures but in endomembrane compartments. Receptors were most highly concentrated in varicosities along, or at the terminals of fine neurites (Fig. 1, C, E, H, and I), which are known to represent synaptic structures (King 1976a,b). D1αPan and D1βPan receptors often appeared to be in the plasma membrane of synaptic varicosities, as there was no rim of red cytoplasm surrounding receptor immunoreactivity (Fig. 1, E and I).
Our previous studies suggested that D1Rs may be expressed in glial cells (Oginsky et al. 2010). Figure 1, A and C, illustrates that D1βPan receptors were highly expressed in the processes of glial and/or other support cells in the STG. It was not clear from our IHC experiments whether or not D1αPan and D2αPan receptors were also expressed in glial cells. They are not obviously in the membrane of glial somata as are shal channels (Baro et al. 2000). If these DARs are expressed in glia, they have a punctate distribution and cannot be differentiated from neuronal staining.
LP D1Rs couple with IA through a Gs-AC-PKA, but not Gq, cascade
We previously showed that D1αPan and D1βPan couple with Gs, and D1αPan can also couple with Gq in STNS membrane preparations (Clark et al. 2008). If DA acts exclusively through D1Rs, then DA effects on LP IA may be mediated by Gs and/or Gq transduction cascades. A pharmacological dissection of the DA induced transduction cascades modulating LP IA was therefore performed using TEVC.
Consistent with previous reports (Harris-Warrick et al. 1995b), a 10 min bath application of 10−5 M DA decreased the LP IA evoked by a depolarizing test pulse following a hyperpolarizing prepulse to remove all channel inactivation (Fig. 2A). First order Boltzmann fits of the conductance voltage relations for activation and inactivation suggested that this decrease was largely the result of reversible DA induced shifts in IA voltage dependencies to more positive potentials (Fig. 2B). Regardless of their initial voltage dependencies, most cells showed similar depolarizing shifts in their apparent voltages of half activation (Fig. 2C) and inactivation (D). The average shifts were statistically significant for both activation (3.4 ± 0.3 mV, n = 41, paired t-test, P < 0.0001) and inactivation (3.9 ± 0.3 mV, n = 33, paired t-test, P < 0.0001). It is noteworthy that <10% of the cells examined did not respond to DA or responded with a smaller negative shift; however, these data were not excluded from the analyses. As previously reported (Harris-Warrick et al. 1995b), we also observed that DA produced a significant decrease the IA maximal conductance (Fig. 2A), but this effect was not reversible and therefore not considered in this study.
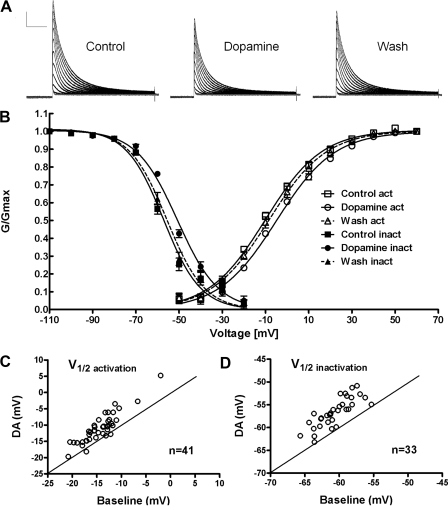
Fig. 2.
DA induced positive shifts in the voltage dependence of LP IA. A: TEVC recordings under control conditions, with 10 min bath application of 10−5 M DA and after 30 min washout of DA. Current traces were obtained in response to a series of depolarizing test pulses (from −50 to +60 mV in 10 mV increments) following a hyperpolarizing prepulse to −90 mV. All voltage dependent ion channels except IA were pharmacologically blocked. Scale bars represent 100 ms and 50 nA. B: normalized conductance–voltage plots for activation (○, □, ▵) and inactivation (●, ■, ▴) fit with a 1st-order Boltzmann equation. Plots were obtained under control condition (□, ■), with 10 min 10−5 M DA application (○, ●), and after 30 min washout of DA (▵, ▴) n ≥ 5 for each data point. C and D: preparation-to-preparation variability. ○, the IA V1/2 activation (C) and inactivation (D) for a single individual in the presence (y axis) versus absence (x axis) of 10−5 M DA. The line indicates unity and points above and below the line indicate that DA shifted the V1/2 to more depolarized or hyperpolarized potentials, respectively.
We first tested whether DA shifted the voltage dependence of LP IA via a Gs cascade. AC converts ATP to cAMP, and Gs can stimulate AC activity. Forskolin directly activates AC in all species by binding to the conserved catalytic core (Yan et al. 1997, 1998; Zhang et al. 1997) (see also methods), and this drug has been successfully used to activate AC in several invertebrates including Drosophila and crustaceans (Kim and Wu 1996; Klein 1993; Nakatsuji et al. 2009). We asked whether or not forskolin could mimic, and at saturating concentrations occlude, the effects of DA on LP IA (Fig. 3). A 10 min 10−5 M DA application was followed by a 30 min washout, and forskolin (5 × 10−5 M) was applied for 10 min followed by a 10 min application of DA (10−5 M) plus forskolin (5 × 10−5 M). LP IA was recorded at the end of each drug application and wash. Forskolin alone produced depolarizing shifts in the LP IA V1/2 activation (Fig. 3A, 6.2 ± 0.8 mV, n = 5) and V1/2 inactivation (Fig. 3B, 3.8 ± 0.7 mV, n = 5). These shifts were significantly different from control (ANOVA, P < 0.01), but not from the shift induced by DA alone. Thus forskolin mimicked the effects of DA on LP IA. Moreover, addition of 10−5 M DA to preparations that previously received 5 × 10−5 M forskolin did not produce further significant shifts in the voltage dependence (ANOVA, P > 0.05). Thus saturating levels of forskolin can occlude the effects of DA on LP IA. To exclude the possibility that forskolin acted directly on shal channels, rather than on AC, we examined the effect of 1,9-dideoxyforskolin (dd-forskolin), a structural analogue of forskolin that does not activate AC. We found that dd-forskolin had no significant effects on LP IA (n = 3). Taken together, these data suggest that DA shifts the voltage dependence of IA at least partially through AC.
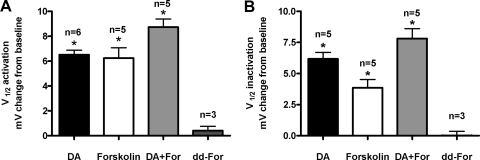
Fig. 3.
An adenylyl cyclase (AC) activator mimicked and, at saturating concentrations, largely occluded the effects of DA on LP IA. IA was recorded before (baseline) and 10 min after application of 10−5 M DA, 5 × 10−5 M forskolin, DA+forskolin, or 5 × 10−5 M dideoxy-forskolin (dd-forskolin). Every preparation received only 1 of the 4 drug treatments. Each drug except dd-forskolin induced a significant and reversible shift in LP IA V1/2 activation (A) and inactivation (B) relative to baseline. *, significant difference from baseline using 1 way ANOVAs with a Tukey post hoc test.
DA produced dose-dependent shifts in the LP IA V1/2 activation (Fig. 4A). If DA signals largely through the Gs cascade, then increasing doses of DA should result in increasing concentrations of cAMP. We therefore asked whether or not cAMP could modulate LP IA in a dose dependent fashion by bath applying varying concentrations of the membrane permeable cAMP analogue, 8-bromo-cAMP, and measuring the V1/2 activation. Note that cAMP is a small second messenger molecule that is not encoded by the genome and that does not vary across species; thus 8-bromo-cAMP will be effective in crustaceans. We found that, like DA, cAMP produced dose-dependent positive shifts in the V1/2 activation (Fig. 4B). Moreover, similar to the effect of bath applied DA, an increase in the cAMP concentration could also shift the V1/2 inactivation to more depolarized membrane potentials (Fig. 4, ●).
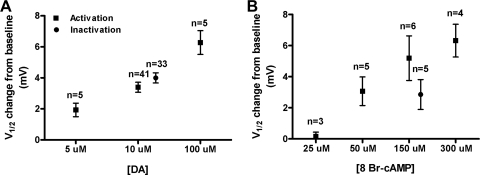
Fig. 4.
The cAMP analogue, 8-Br-cAMP, mimicked DA effects on LP IA. IA was measured before (baseline) and 10 min after application of DA (A) or 8-Br-cAMP (B). Every preparation received only 1 dose of either drug. The change from baseline is plotted for IA V1/2 activation (■) inactivation (●).
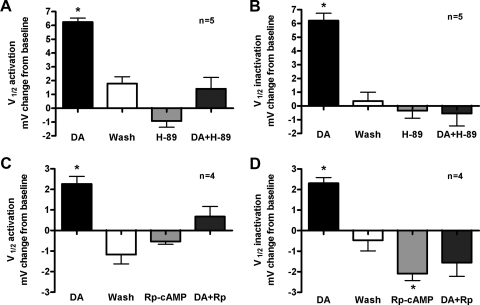
PKA is one of the major effectors of cAMP. To test whether PKA was involved in the signaling pathway mediating DA's affect on LP IA, we examined whether or not the PKA inhibitor, H-89, could block DA's actions. H-89, which has been successfully used in crustaceans (Philipp et al. 2006), inhibits PKA by targeting the ATP binding site (Chijiwa et al. 1990; Engh et al. 1996), which is conserved across species (Gross et al. 1990) (see also methods). In these experiments, the preparation was exposed to 10−5 M DA for 10 min, followed by a 30 min wash. H-89 (2 × 10−5M), was then applied for 10 min followed by an application of H-89 plus DA. IA was recorded prior to DA application, at the end of each drug treatment, and after the 30 min wash. The voltages of half activation and inactivation were determined for each condition, and the change from baseline was plotted in Fig. 5, where baseline is the V1/2 prior to drug treatment (DA) or after the wash (H-89, H-89+DA). The data demonstrated that H-89 reversibly inhibited the DA induced shift in V1/2 activation (ANOVA, P < 0.001, n = 5, Fig. 5A) and inactivation (ANOVA, P < 0.001, n = 5, B). Because H-89 can also inhibit other kinases, such as PKG, we repeated the experiment with a specific and expensive PKA inhibitor, Rp-cAMP. This drug occupies the cAMP binding site on the regulatory subunit of PKA, thus preventing the holoenzyme from dissociating (Rothermel and Parker Botelho 1988). The cAMP binding site on the regulatory subunit is well conserved across species (Canaves and Taylor 2002) (see also methods), and this drug has been used successfully in several arthropods including Drosophila and crustaceans (Erxleben et al. 1995; Kuromi and Kidokoro 2000). To save on the cost of the inhibitor in this experiment, the concentration of DA was reduced to 5 μM. Whereas 5 μM DA induced a significant and reversible shift in IA V1/2 activation (ANOVA, P < 0.001, n = 4, Fig. 5C) and inactivation (ANOVA, P < 0.01, n = 4, Fig. 5D), Rp-cAMP blocked this effect. Interestingly, Rp-cAMP itself significantly altered IA V1/2 inactivation (ANOVA, P < 0.05, n = 4, Fig. 5D). This might suggest that PKA constitutively modulates the IA V1/2 inactivation. Indeed, both PKA blockers, H-89 and Rp-cAMP, shifted IA voltage dependencies in the opposite direction to DA, but in most cases the changes were not statistically significant. Taken together, our data suggest that the Gαs-AC-cAMP-PKA signaling pathway mediates DA modulation of IA in the LP neuron, consistent with the fact that LP expresses somatodendritic D1Rs but not D2Rs.
Fig. 5.
The protein kinase A (PKA) blockers, H-89 and Rp-cAMP, prevented DA induced changes in LP IA. IA was measured before and after a 10 min 10−5 M DA application and after a 30 min wash from DA. Measurements from the 1st recording served as baseline for the DA and wash comparisons. After the wash, a 10 min application of 2 × 10−5 M H89 was followed by a 10 min application of DA+H89. IA was measured at the end of each 10 min application. Measurements from the previous wash served as baseline for H89 comparisons. The change from baseline was plotted for V1/2 activation (A) and V1/2 inactivation (B). C and D: the same experiment was performed except that the concentration of DA was 5 × 10−6 μM and 10−3 M Rp-cAMP was substituted for H89. *, significant difference from baseline as determined with 1 way ANOVAs followed by a Tukey pos thoc test.
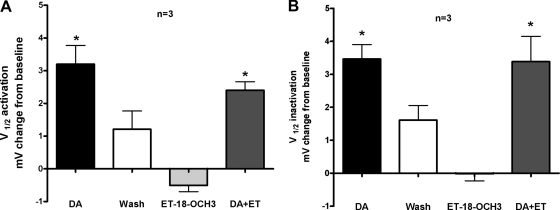
As mentioned in the preceding text, D1αPan receptors also couple with Gq in STNS membrane preparations. PLCβ is the major downstream effector of activated Gαq subunits. The ether lipid analogue ET-18-OCH3 is a known PLCβ inhibitor (Powis et al. 1992). ET-18-OCH3 exerts its effects by being incorporated into the plasma membranes of cells (Aroca et al. 2001; Heczkova and Slotte 2006; Powis et al. 1992), but how ET-18-OCH3 inhibits PLCβ is not clear to date. Nevertheless, ET-18-OCH3 has been successfully used on invertebrate neurons to inhibit PLCβ (Wong et al. 2007). Using the aforementioned experimental paradigm, we asked whether DA modulation of LP IA could be blocked by addition of ET-18-OCH3. Figure 6 illustrates that ET-18-OCH3 did not prevent the DA induced positive shift in LP IA voltage dependencies, suggesting that DA does not modulates LP IA through a Gq cascade.
Fig. 6.
The PLCβ inhibitor, ET-18-OCH3, did not block DA's effects on LP IA. IA was measured before and during a 10 min, 10−5 M DA application, after a 30 min washout, and after sequential 10 min applications of 10−5 M ET-18-OCH3 and ET-18-OCH3+DA. Measurements from the 1st recording served as baseline for DA and washout comparisons. The washout measurements served as baseline for all subsequent drug applications. The change from baseline was plotted for V1/2 activation (A) and V1/2 inactivation (B). *, significant differences from baseline as determined with 1 way ANOVAs followed by Tukey post hoc tests.
DAR specific antagonists confirm that DA acts on LP IA through D1Rs
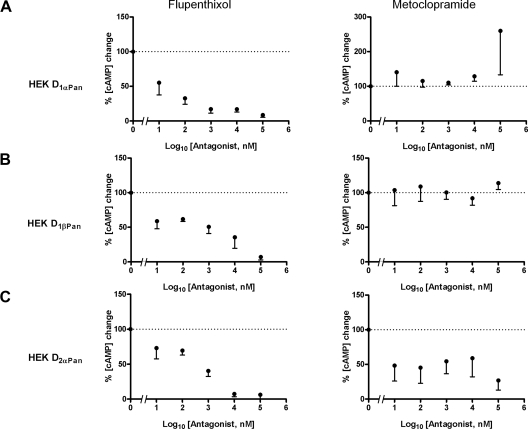
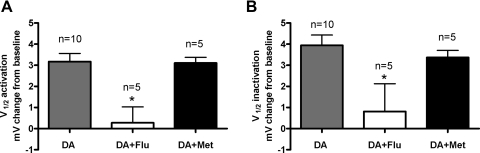
To further confirm that DA modulates LP IA exclusively through D1Rs, we sought to obtain antagonists specific for lobster D1Rs and D2Rs. It is well established that monoamine receptor pharmacology is not well conserved between vertebrate and invertebrate receptors (Blenau and Baumann 2001; Spitzer et al. 2008a,b; Tierney 2001) because many agonists and antagonists do not bind to evolutionarily conserved amino acids. This is in contrast to the blockers of the transduction cascades that we used (i.e., forskolin, H-89, Rp-cAMP, ET-18-OCH3), which act at functional domains that are evolutionarily conserved. We therefore performed a preliminary screen using a cadre of drugs (Table 1) and found candidate antagonists for lobster D1Rs (flupenthixol) and D2Rs (metoclopramide). The effects of these drugs were characterized in detail using a transient, heterologous expression system and assays for DA induced changes in cAMP concentration in the presence and absence of flupenthixol and metoclopramide. Figure 7 illustrates that, consistent with previous studies (Clark and Baro 2006, 2007), DA altered cAMP levels in both D1R and D2R expressing HEK cells. Flupenthixol, but not metoclopramide, blocked the DA induced increase in cAMP in HEK cells expressing D1αPan (Fig. 7A, n ≥ 3) and D1βPan receptors (B, n ≥ 3). On the other hand, Fig. 7C shows that the DA induced changes in cAMP were prevented by metoclopramide in D2αPan expressing HEK cells (n = 3). If DA acts on LP neurons exclusively through D1Rs, then flupenthixol should block DA modulation of LP IA, whereas metoclopramide should not. Figure 8 illustrates that this is indeed the case. Using TEVC, we measured the DA induced change in the V1/2 activation and V1/2 inactivation in LP in the presence and absence of each antagonist. In these experiments, DA (10−5 M) was bath applied for 10 min and washed for 30 min; then, 10−5 M antagonist was bath applied for 5 min, followed by a 10 min application of antagonist plus DA followed by a 30 min wash. IA V1/2 was measured at the end of each drug application and the wash period. In some cases (n ≥ 1 for each antagonist), the application order was altered as described in methods. We found that 10−5 M flupenthixol reversibly blocked the DA induced shift in LP IA activation from 3.18 ± 0.38 mV (n = 10) to 0.27 ± 0.75 mV (n = 5) and inactivation from 3.95 ± 0.49 mV (n = 10) to 0.81 ± 1.3 mV (n = 5). The shifts induced by DA were significantly different from those induced by DA+ flupenthixol (ANOVA, P < 0.01). On the other hand, metoclopramide had no significant effect on the DA induced changes in LP IA. Taken together, the data indicate that DA modulates LP IA exclusively through D1Rs.
Table 1.
List of drugs tested to screen DAR antagonists
| Drugs |
|---|
| (−)-Butaclamol |
| Clozapine |
| Sulpiride |
| Haloperidol |
| Spiperone HCl |
| Methiothepin mesylate |
| Metoclopramide |
| Cyproheptadine HCl |
| N-R(+)-SCH 23390 |
| Chlorpromazine |
| (+)-Butaclamol |
| Domperidone |
| Eticlopride |
| Fluphenazine 2HCI |
| Flupenthixol |
Three criteria were used to select an antagonist: ability to block interval pyloric and pyloric dilator IA; effects on dopamine receptors (DARs) expressed in a heterologous expression system; effects on parental cell lines. Based on these criteria, we chose the best two drugs to examine in detail (flupenthixol and metoclopramide). We have not excluded the possibility that other drugs listed here are effective antagonists for lobster DARs.
Fig. 7.
Dopamine receptor antagonists. cAMP was measured (pmol/mg protein) in cells expressing D1αPan,D1βPan, and D2αPan receptors in the presence of 10−5 M DA and increasing concentration of flupenthixol or metoclopramide. Data for a given experiment were normalized to the change in cAMP evoked by DA in the absence of antagonist. The change induced by DA + antagonist are expressed as a percent of the change induced by DA alone (indicated as 100%, - - -). Plots represent the average of ≥3 separate experiments, and a given point was the average of duplicates within each experiment.
Fig. 8.
Flupenthixol but not metoclopramide blocked DA effects on LP IA. The STG was sequentially exposed to the following treatments: a 10 min, 10−5 M DA application, a 30 min wash, a 5 min, 10−5 M antagonist application, and a 10 min antagonist + DA application. A given preparation received one antagonist, either flupenthixol (□, n = 5) or metoclopramide (■, n = 5). LP IA was recorded at the end of each treatment and immediately before DA application. The change from baseline was plotted for V1/2 activation (A) and V1/2 inactivation (B). Measurements from recordings prior to DA application served as the baseline for DA and wash treatments. Measurements from the wash served as baseline for subsequent treatments. *, significant difference from DA alone (n = 5) as determined with 1 way ANOVAs followed by Tukey post hoc tests.
DISCUSSION
DA is known to modulate intrinsic neuronal firing properties and synaptic strengths in a number of systems by acting on a plethora of targets in a single cell (Harris-Warrick et al. 1998; Nicola et al. 2000; Surmeier et al. 2007). Whereas the effects of DA on pyloric neurons and network output are well studied, little is known about how the signal is transduced. Here, for the first time, we defined the DA signal transduction cascade operating in an identified pyloric neuron. We found that LP exclusively expressed D1Rs perisynaptically but did not express D2Rs. DA modulation of LP IA could be pharmacologically blocked by D1R but not D2R antagonists. Further, we demonstrated that LP D1Rs modulated IA by coupling with the canonical signaling pathway that has been described for D1Rs in all species examined to date: Gs-AC-cAMP-PKA.
Differential effects of DA on LP and PD neurons are due to differences in receptor expression
It was previously shown that DA has opposing effects on two identified cell types of the pyloric motor circuit, LP and PD (Flamm and Harris-Warrick 1986a,b; Harris-Warrick et al. 1995a,b; Johnson et al. 2003a; Kloppenburg et al. 1999, 2000; Peck et al. 2006). DA increases excitability and phase advances LP by increasing calcium currents (ICa) and a hyperpolarization activated inward current (Ih), while decreasing IA. DA inhibits PD firing and phase delays neuronal activity by decreasing ICa and increasing IA. DA has no effect on PD Ih. Here we demonstrated that the cell specific effects of DA were due to differences in the DA transduction cascade in each cell type. In particular, LP expresses D1Rs and PD expresses D2Rs.
DAR expression was consistent at the protein level for both cell types. Every LP examined expressed somatodendritic D1αPan and D1βPan but not D2αPan receptors. Every PD examined expressed D2Rs, but not D1Rs (Oginsky et al. 2010). However, protein levels were not quantified, and receptor number for a given cell type may have varied across preparations. Surface receptors in both cell types appeared to be concentrated in synaptic varicosities within the somatodendritic compartment. These varicosities can represent pre- and/or postsynaptic structures (Oginsky et al. 2010). It is not clear if LP D1Rs are restricted to a subset of synapses as is the case for PD D2Rs (Oginsky et al. 2010).
D1 transduction cascades operate in LP neurons
We showed that DA modulates LP IA by acting on D1αPan and/or D1βPan receptors that couple with a Gs-AC-cAMP-PKA pathway. Interestingly, there is no consensus cAMP-dependent phosphorylation site on shal channels (Baro et al. 1996). Shal channels may contain atypical PKA phosphorylation sites (Shi et al. 2007). Alternatively PKA may not act directly on the pore-forming subunit of the A-channel (i.e., shal subunits) but rather target an auxiliary subunit such as KChip (An et al. 2000) or a downstream enzyme (e.g., phosphatase) that then modifies shal subunits.
The fact that DA shifted LP IA voltage dependencies to more positive potentials through D1R induced increases in cAMP may suggest that DA shifts PD IA voltage dependencies to more negative potentials via a D2-Gi/o coupled cascade that decreases cAMP and PKA activity. Indeed this is the case in D2R expressing striatal medium spiny neurons (Perez et al. 2006). However, DA may not act simply by reciprocally altering cAMP levels in LP and PD as DARs can act through noncanonical cascades: D2Rs can signal through PLCβ (Clark and Baro 2007; Hernandez-Lopez et al. 2000) and D1Rs can couple with Gq (Clark et al. 2008).
LP D1Rs increase cAMP levels. Interestingly, the literature suggests that cAMP signals can have different spatial attributes. In some cases, the cAMP signal is highly restricted to within one micron of the receptor (Zaccolo and Pozzan 2002), whereas in other cases, the signal can show varying degrees of global spread (Nikolaev et al. 2006; Rich et al. 2001). The type of signal generated does not depend on cell type but on the receptor signaling network (Nikolaev et al. 2006). Global cAMP signals are significantly different from local signals: Global signals are small and sustained, whereas local signals are large and transient (Rich et al. 2001). These distinct types of signals will produce different effects. For example, a global signal may only raise PKA activity slightly causing a small shift in the phosphorylation state of constitutively regulated proteins. On the other hand, a local signal may cause a large change in PKA activity that initiates new cascades by phosphorylating low affinity substrates. Indeed phosphorylation of the low affinity substrate, phosphodiesterase, which degrades cAMP to AMP, may underpin the transient versus sustained nature of the local versus global signal.
In pyloric neurons, surface DARs appeared to be concentrated on synaptic varicosities, which are, themselves, concentrated on the terminals of fine neurites. On the other hand, surface shal channels are localized throughout the somatodendritic compartment including the plasmalemma surrounding the soma and large diameter neurites (Baro et al. 2000). Given the spatial vagary of cAMP signals, and the significantly different character of local and global cAMP signals, this difference in protein distributions raises a very important question: are all shal channels modulated equally? Our current understanding of signal transduction suggests that most likely, they are not. However, the extent to which channels are differentially modulated in each compartment is an important matter for future studies.
Divergent and convergent modulatory systems
Both divergent and convergent modulatory systems exist in the STG. Monoaminergic systems appear to be divergent in three respects. First, a given monoamine can target multiple currents within a cell. For example, DA can alter IA, ICa, and Ih in the LP neuron (Harris-Warrick et al. 1995b; Johnson et al. 2003a). Second, a given monoamine can have diverse effects across cell types. For example, DA can increase IA in the PD neuron, decrease it in the LP, anterior burster (AB), inferior cardiac (IC), and pyloric constrictor (PY) neurons, and have no effect on the ventricular dilator (VD) IA although the synaptically isolated VD neuron responds to DA and so presumably has DARs (Flamm and Harris-Warrick 1986a,b; Harris-Warrick et al. 1995a,b; Kloppenburg et al. 1999; Peck et al. 2001). Third, each monoamine can alter a given current in different subsets of cells. For example, the effects of DA, serotonin (5-HT) and octopamine (OCT) on IA were studied in VD, AB, and IC neurons (Peck et al. 2001). All three neurons are known to respond to each modulator in the intact ganglion and in synaptic isolation, i.e., each cell has receptors for each amine (Flamm and Harris-Warrick 1986a,b). Unlike DA, 5-HT alters IA only in the IC neuron and OCT has no effect on IA in AB, IC, or VD neurons. These findings for monoaminergic systems contrast with previously reported convergence in different neuromodulatory systems. Swensen and Marder (2000) demonstrated that five peptides [proctolin, crustacean cardioactive peptide (CCAP), Cancer borealis tachykinin related peptide Ia, red pigment-concentrating hormone, and TNRNFLRFamide] and acetylcholine acting at muscarinic receptors consistently altered the same voltage-gated inward current, IMI (Zhao et al. 2010) in all cell types expressing receptors for the aforementioned modulators. However, not all STG neurons expressed all receptors, and the subset of cells that a given convergent modulator acted on varied (Swensen and Marder 2000, 2001). Thus whereas each convergent and divergent modulator can produce a distinct circuit output, they do so by different mechanisms. Convergent modulators will consistently target the same current but in different subsets of cells. On the other hand, each monoamine can differentially target multiple currents within the same cells.
In cases where a given cell expresses receptors for multiple convergent modulators, application of one modulator can occlude the other (Swensen and Marder 2000). This suggests that convergent modulators either signal globally or that the receptors are colocalized if signals are spatially restricted; otherwise, receptor induced changes in the current should be additive. The extent to which divergent monoaminergic modulators can occlude or potentiate one another is not clear. Future experiments using co-application of monoamines should yield valuable information on monoaminergic receptor localization and signaling.
The difference in divergent and convergent systems may be due, in part, to their molecular organization. Monoaminergic systems have multiple GPCRs in invertebrates: DA signals through at least three receptors, 5-HT acts on at least four receptors, and OCT/tyramine bind to eight receptors (Hauser et al. 2006). On the other hand, many of the aforementioned convergent modulators are known to act through a single GPCR in arthropods. In Drosophila, multiple studies have revealed that there is a single proctolin receptor (Egerod et al. 2003; Hauser et al. 2006; Johnson et al. 2003b). Similarly, there is one muscarinic receptor (Hauser et al. 2006) and one CCAP receptor (Hauser et al. 2006; Park et al. 2002) although there appear to be two CCAP receptors in the flour beetle (Arakane et al. 2008).
Conclusion
Our present work illuminates the molecular mechanisms underlying DA neuromodulation in an identified pyloric neuron, which is a prerequisite for understanding normal pyloric network function. We have shown that DA modulates LP IA by acting on D1αPan and/or D1βPan receptors that couple with a Gs-AC-cAMP-PKA pathway. When considered in light of previous studies, these data not only help to explain the distinct physiological effects of DA on different pyloric cells but also indicate that DAR signal transduction is well conserved across species: DA acts on highly conserved D1Rs and D2Rs in both the crustacean STG and the mammalian striatum, which in turn activate well conserved canonical and noncanonical transduction cascades. The signaling networks activated by homologous DARs in mammals and crustaceans produce similar changes in their target ion channels. Because both the DARs and their ion channel targets can be accurately mapped onto three dimensional reconstructions of identified pyloric neurons, this system can be used for future, broadly applicable studies on the spatial aspects of signal transduction in geometrically complex cells.
GRANTS
H. Zhang was supported by the Georgia State University Molecular Basis of Disease Fellowship. This work was funded by National Institute of Drug Abuse Grant DA024039 to D. J. Baro.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank K. J. Kent and T. Dever for technical assistance.
REFERENCES
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature 403: 553–556, 2000 [DOI] [PubMed] [Google Scholar]
- Arakane Y, Li B, Muthukrishnan S, Beeman RW, Kramer KJ, Park Y. Functional analysis of four neuropeptides, EH, ETH, CCAP and bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum. Mech Dev 125: 984–995, 2008 [DOI] [PubMed] [Google Scholar]
- Aroca JD, Sanchez-Pinera P, Corbalan-Garcia S, Conesa-Zamora P, de Godos A, Gomez-Fernandez JC. Correlation between the effect of the anti-neoplastic ether lipid 1-O-octadecyl-2-O-methyl-glycero-3-phosphocholine on the membrane and the activity of protein kinase Calpha. Eur J Biochem 268: 6369–6378, 2001 [DOI] [PubMed] [Google Scholar]
- Ayali A, Harris-Warrick RM. Interaction of dopamine and cardiac sac modulatory inputs on the pyloric network in the lobster stomatogastric ganglion. Brain Res 794: 155–161, 1998 [DOI] [PubMed] [Google Scholar]
- Ayali A, Harris-Warrick RM. Monoamine control of the pacemaker kernel and cycle frequency in the lobster pyloric network. J Neurosci 19: 6712–6722, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro DJ, Ayali A, French L, Scholz NL, Labenia J, Lanning CC, Graubard K, Harris-Warrick RM. Molecular underpinnings of motor pattern generation: differential targeting of shal and shaker in the pyloric motor system. J Neurosci 20: 6619–6630, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro DJ, Coniglio LM, Cole CL, Rodriguez HE, Lubell JK, Kim MT, Harris-Warrick RM. Lobster shal: comparison with Drosophila shal and native potassium currents in identified neurons. J Neurosci 16: 1689–1701, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro DJ, Levini RM, Kim MT, Willms AR, Lanning CC, Rodriguez HE, Harris-Warrick RM. Quantitative single-cell-reverse transcription-PCR demonstrates that A-current magnitude varies as a linear function of shal gene expression in identified stomatogastric neurons. J Neurosci 17: 6597–6610, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman HS, Tobin AE. Gross dissection of the stomach of the lobster, Homarus americanus. J Vis Exp May22 (27) pii, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol 48: 13–38, 2001 [DOI] [PubMed] [Google Scholar]
- Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol 211: 1000–1011, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev 24: 765–781, 2003 [DOI] [PubMed] [Google Scholar]
- Canaves JM, Taylor SS. Classification and phylogenetic analysis of the cAMP-dependent protein kinase regulatory subunit family. J Mol Evol 54: 17–29, 2002 [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem 265: 5267–5272, 1990 [PubMed] [Google Scholar]
- Clark MC, Baro DJ. Molecular cloning and characterization of crustacean type-one dopamine receptors: D1αPan and D1βPan. Comp Biochem Physiol B Biochem Mol Biol 143: 294–301, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MC, Baro DJ. Arthropod D2 receptors positively couple with cAMP through the Gi/o protein family. Comp Biochem Physiol B Biochem Mol Biol 146: 9–19, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MC, Dever TE, Dever JJ, Xu P, Rehder V, Sosa MA, Baro DJ. Arthropod 5-HT2 receptors: a neurohormonal receptor in decapod crustaceans that displays agonist independent activity resulting from an evolutionary alteration to the DRY motif. J Neurosci 24: 3421–3435, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MC, Khan R, Baro DJ. Crustacean dopamine receptors: localization and G protein coupling in the stomatogastric ganglion. J Neurochem 104: 1006–1019, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Selverston AI. Dopaminergic modulation of inhibitory glutamate receptors in the lobster stomatogastric ganglion. J Neurophysiol 78: 3450–3452, 1997 [DOI] [PubMed] [Google Scholar]
- Daur N, Nadim F, Stein W. Regulation of motor patterns by the central spike-initiation zone of a sensory neuron. Eur J Neurosci 30: 808–822, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong ND, Beenhakker MP, Nusbaum MP. Presynaptic inhibition selectively weakens peptidergic cotransmission in a small motor system. J Neurophysiol 102: 3492–3504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerod K, Reynisson E, Hauser F, Williamson M, Cazzamali G, Grimmelikhuijzen CJ. Molecular identification of the first insect proctolin receptor. Biochem Biophys Res Commun 306: 437–442, 2003 [DOI] [PubMed] [Google Scholar]
- Eisen JS, Marder E. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. III. Synaptic connections of electrically coupled pyloric neurons. J Neurophysiol 48: 1392–1415, 1982 [DOI] [PubMed] [Google Scholar]
- Engh RA, Girod A, Kinzel V, Huber R, Bossemeyer D. Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J Biol Chem 271: 26157–26164, 1996 [DOI] [PubMed] [Google Scholar]
- Erxleben CF, deSantis A, Rathmayer W. Effects of proctolin on contractions, membrane resistance, and non-voltage-dependent sarcolemmal ion channels in crustacean muscle fibers. J Neurosci 15: 4356–4369, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM. Aminergic modulation in lobster stomatogastric ganglion. I. Effects on motor pattern and activity of neurons within the pyloric circuit. J Neurophysiol 55: 847–865, 1986a [DOI] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM. Aminergic modulation in lobster stomatogastric ganglion. II. Target neurons of dopamine, octopamine, and serotonin within the pyloric circuit. J Neurophysiol 55: 866–881, 1986b [DOI] [PubMed] [Google Scholar]
- Goldstone MW, Cooke IM. Histochemical localization of monoamines in the crab central nervous system. Z Zellforsch Mikrosk Anat 116: 7–19, 1971 [DOI] [PubMed] [Google Scholar]
- Golowasch J, Buchholtz F, Epstein IR, Marder E. Contribution of individual ionic currents to activity of a model stomatogastric ganglion neuron. J Neurophysiol 67: 341–349, 1992 [DOI] [PubMed] [Google Scholar]
- Gross RE, Bagchi S, Lu X, Rubin CS. Cloning, characterization, and expression of the gene for the catalytic subunit of cAMP-dependent protein kinase in Caenorhabditis elegans. Identification of highly conserved and unique isoforms generated by alternative splicing. J Biol Chem 265: 6896–6907, 1990 [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Barazangi N, Guckenheimer J, Gueron S. Dopamine modulation of transient potassium current evokes phase shifts in a central pattern generator network. J Neurosci 15: 342–358, 1995a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Levini RM, Gueron S, Guckenheimer J. Dopamine modulation of two subthreshold currents produces phase shifts in activity of an identified motoneuron. J Neurophysiol 74: 1404–1420, 1995b [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Johnson BR, Peck JH, Kloppenburg P, Ayali A, Skarbinski J. Distributed effects of dopamine modulation in the crustacean pyloric network. Ann NY Acad Sci 860: 155–167, 1998 [DOI] [PubMed] [Google Scholar]
- Hauser F, Cazzamali G, Williamson M, Blenau W, Grimmelikhuijzen CJ. A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog Neurobiol 80: 1–19, 2006 [DOI] [PubMed] [Google Scholar]
- Heczkova B, Slotte JP. Effect of anti-tumor ether lipids on ordered domains in model membranes. FEBS Lett 580: 2471–2476, 2006 [DOI] [PubMed] [Google Scholar]
- Hedrich UB, Smarandache CR, Stein W. Differential activation of projection neurons by two sensory pathways contributes to motor pattern selection. J Neurophysiol 102: 2866–2879, 2009 [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLCβ1-IP3-calcineurin-signaling cascade. J Neurosci 20: 8987–8995, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SL. Phase maintenance in the pyloric pattern of the lobster (Panulirus interruptus) stomatogastric ganglion. J Comput Neurosci 4: 191–205, 1997 [DOI] [PubMed] [Google Scholar]
- Johnson BR, Kloppenburg P, Harris-Warrick RM. Dopamine modulation of calcium currents in pyloric neurons of the lobster stomatogastric ganglion. J Neurophysiol 90: 631–643, 2003a [DOI] [PubMed] [Google Scholar]
- Johnson BR, Schneider LR, Nadim F, Harris-Warrick RM. Dopamine modulation of phasing of activity in a rhythmic motor network: contribution of synaptic and intrinsic modulatory actions. J Neurophysiol 94: 3101–3111, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Garczynski SF, Park D, Crim JW, Nassel DR, Taghert PH. Identification and characterization of a G protein-coupled receptor for the neuropeptide proctolin in Drosophila melanogaster. Proc Natl Acad Sci USA 100: 6198–6203, 2003b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YT, Wu CF. Reduced growth cone motility in cultured neurons from Drosophila memory mutants with a defective cAMP cascade. J Neurosci 16: 5593–5602, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DG. Organization of crustacean neuropil. I. Patterns of synaptic connections in lobster stomatogastric ganglion. J Neurocytol 5: 207–237, 1976a [DOI] [PubMed] [Google Scholar]
- King DG. Organization of crustacean neuropil. II. Distribution of synaptic contacts on identified motor neurons in lobster stomatogastric ganglion. J Neurocytol 5: 239–266, 1976b [DOI] [PubMed] [Google Scholar]
- Klein M. Differential cyclic AMP dependence of facilitation at Aplysia sensorimotor synapses as a function of prior stimulation: augmentation versus restoration of transmitter release. J Neurosci 13: 3793–3801, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg P, Levini RM, Harris-Warrick RM. Dopamine modulates two potassium currents and inhibits the intrinsic firing properties of an identified motor neuron in a central pattern generator network. J Neurophysiol 81: 29–38, 1999 [DOI] [PubMed] [Google Scholar]
- Kloppenburg P, Zipfel WR, Webb WW, Harris-Warrick RM. Highly localized Ca2+ accumulation revealed by multiphoton microscopy in an identified motoneuron and its modulation by dopamine. J Neurosci 20: 2523–2533, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Tetanic stimulation recruits vesicles from reserve pool via a cAMP-mediated process in Drosophila synapses. Neuron 27: 133–143, 2000 [DOI] [PubMed] [Google Scholar]
- Kushner PD, Barker DL. A neurochemical description of the dopaminergic innervation of the stomatogastric ganglion of the spiny lobster. J Neurobiol 14: 17–28, 1983 [DOI] [PubMed] [Google Scholar]
- Kushner PD, Maynard EA. Localization of monoamine fluorescence in the stomatogastric nervous system of lobsters. Brain Res 129: 13–28, 1977 [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69: 291–316, 2007 [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Lee CY, Watson RD. Crustacean molt-inhibiting hormone: structure, function, and cellular mode of action. Comp Biochem Physiol A Mol Integr Physiol 152: 139–148, 2009 [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res 24: 165–205, 2004 [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23: 185–215, 2000 [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res 99: 1084–1091, 2006 [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature 417: 343–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginsky MF, Rodgers EW, Clark MC, Simmons R, Krenz WD, Baro DJ. D2 receptors receive paracrine neurotransmission and are consistently targeted to a subset of synaptic structures in an identified neuron of the crustacean stomatogastric nervous system. J Comp Neurol 518: 255–276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Kim YJ, Adams ME. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci USA 99: 11423–11428, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JH, Gaier E, Stevens E, Repicky S, Harris-Warrick RM. Amine modulation of Ih in a small neural network. J Neurophysiol 96: 2931–2940, 2006 [DOI] [PubMed] [Google Scholar]
- Peck JH, Nakanishi ST, Yaple R, Harris-Warrick RM. Amine modulation of the transient potassium current in identified cells of the lobster stomatogastric ganglion. J Neurophysiol 86: 2957–2965, 2001 [DOI] [PubMed] [Google Scholar]
- Perez MF, White FJ, Hu XT. Dopamine D2 receptor modulation of K+channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. J Neurophysiol 96: 2217–2228, 2006 [DOI] [PubMed] [Google Scholar]
- Philipp B, Rogalla N, Kreissl S. The neuropeptide proctolin potentiates contractions and reduces cGMP concentration via a PKC-dependent pathway. J Exp Biol 209: 531–540, 2006 [DOI] [PubMed] [Google Scholar]
- Powis G, Seewald MJ, Gratas C, Melder D, Riebow J, Modest EJ. Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues. Cancer Res 52: 2835–2840, 1992 [PubMed] [Google Scholar]
- Pulver SR, Marder E. Neuromodulatory complement of the pericardial organs in the embryonic lobster, Homarus americanus. J Comp Neurol 451: 79–90, 2002 [DOI] [PubMed] [Google Scholar]
- Pulver SR, Thirumalai V, Richards KS, Marder E. Dopamine and histamine in the developing stomatogastric system of the lobster Homarus americanus. J Comp Neurol 462: 400–414, 2003 [DOI] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci USA 98: 13049–13054, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel JD, Parker Botelho LH. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3′,5′-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem J 251: 757–762, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol 293: R1205–1214, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N, Cymbalyuk G, Zhang H, Edwards DH, Baro DJ. Serotonin transduction cascades mediate variable changes in pyloric network cycle frequency in response to the same modulatory challenge. J Neurophysiol 99: 2844–2863, 2008a [DOI] [PubMed] [Google Scholar]
- Spitzer N, Edwards DH, Baro DJ. Conservation of structure, signaling and pharmacology between two serotonin receptor subtypes from decapod crustaceans, Panulirus interruptus and Procambarus clarkii. J Exp Biol 211: 92–105, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein W. Modulation of stomatogastric rhythms. J Comp Physiol [A] 195: 989–1009, 2009 [DOI] [PubMed] [Google Scholar]
- Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci 26: 1148–1165, 2007 [DOI] [PubMed] [Google Scholar]
- Sullivan RE, Friend BJ, Barker DL. Structure and function of spiny lobster ligamental nerve plexuses: evidence for synthesis, storage, and secretion of biogenic amines. J Neurobiol 8: 581–605, 1977 [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30: 228–235, 2007 [DOI] [PubMed] [Google Scholar]
- Swensen AM, Marder E. Modulators with convergent cellular actions elicit distinct circuit outputs. J Neurosci 21: 4050–4058, 2001a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci 20: 6752–6759, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AJ. Structure and function of invertebrate 5-HT receptors: a review. Comp Biochem Physiol A Mol Integr Physiol 128: 791–804, 2001 [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Harris-Warrick RM. Physiological role of the transient potassium current in the pyloric circuit of the lobster stomatogastric ganglion. J Neurophysiol 67: 599–609, 1992 [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Kim T, Abrams R. Dopamine in crayfish and other crustaceans: distribution in the central nervous system and physiological functions. Microsc Res Tech 60: 325–335, 2003 [DOI] [PubMed] [Google Scholar]
- Tobin AE, Bierman HS. Homarus americanus stomatogastric nervous system dissection J Vis Exp May28(27) pii.1171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Fabian L, Forer A, Brill JA. Phospholipase C and myosin light chain kinase inhibition define a common step in actin regulation during cytokinesis. BMC Cell Biol 8: 15, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SZ, Huang ZH, Andrews RK, Tang WJ. Conversion of forskolin-insensitive to forskolin-sensitive (mouse-type IX) adenylyl cyclase. Mol Pharmacol 53: 182–187, 1998 [DOI] [PubMed] [Google Scholar]
- Yan SZ, Huang ZH, Rao VD, Hurley JH, Tang WJ. Three discrete regions of mammalian adenylyl cyclase form a site for Gsalpha activation. J Biol Chem 272: 18849–18854, 1997 [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295: 1711–1715, 2002 [DOI] [PubMed] [Google Scholar]
- Zhang G, Liu Y, Ruoho AE, Hurley JH. Structure of the adenylyl cyclase catalytic core. Nature 386: 247–253, 1997 [DOI] [PubMed] [Google Scholar]
- Zhao S, Golowasch J, Nadim F. Pacemaker neuron and network oscillations depend on a neuromodulator-regulated linear current. Front Behav Neurosci 4: 1–9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]