Abstract
Repeated cocaine exposure and withdrawal leads to long-term changes, including behavioral and dopamine sensitization to an acute cocaine challenge, that are most pronounced after long withdrawal periods. However, the changes in dopamine neurotransmission after short withdrawal periods are less well defined. To study dopamine neurotransmission after 1-day withdrawal, we used fast-scan cyclic voltammetry (FSCV) to determine whether repeated cocaine alters rapid dopamine release and uptake in the nucleus accumbens (NAc) core and shell. FSCV was performed in urethane anesthetized male Sprague-Dawley rats that had previously received one or seven daily injections of saline or cocaine (15 mg/kg, ip). In response to acute cocaine, subjects showed increased dopamine overflow that resulted from both increased dopamine release and slowed dopamine uptake. One-day cocaine pre-exposure, however, did not alter dopaminergic responses to a subsequent cocaine challenge. In contrast, 7-day cocaine-treated subjects showed a potentiated rapid dopamine response in both the core and shell after an acute cocaine challenge. In addition, kinetic analysis during the cocaine challenge showed a greater increase in apparent Km of 7-day cocaine exposed subjects. Together, the data provide the first in vivo demonstration of rapid dopamine sensitization in the NAc core and shell after a short withdrawal period. In addition, the data clearly delineate cocaine's release and uptake effects and suggest that the observed sensitization results from greater uptake inhibition in cocaine pre-exposed subjects.
INTRODUCTION
Cocaine acts in the brain to slow dopamine uptake through inhibition of the dopamine transporter (DAT) (Ritz et al. 1987) and thus increases the amount of time that dopamine is present in the extraneuronal space. Cocaine's ability to increase dopamine overflow, particularly in the nucleus accumbens (NAc), has been shown to play an important role in several models of addiction (Goeders and Smith 1983; Kalivas and Duffy 1993a; Ritz et al. 1988; Rodd-Henricks et al. 2002). Specifically, dopaminergic mechanisms in the NAc shell have been shown to alter motivation for rewards such as cocaine, whereas dopamine in the NA core is important for goal-directed behavior (Ikemoto 2007; Ito et al. 2000, 2004). In addition, repeated cocaine administration also leads to a progressive, or sensitized, increase of dopamine overflow (Heidbreder et al. 1996; Kalivas and Duffy 1993a,b) because of changes in the brain (Vanderschuren and Kalivas 2000; Wolf 1998). Specifically, cocaine has been shown to alter synaptic plasticity (Argilli et al. 2008; Borgland et al. 2004; Saal et al. 2003) and to modulate second messenger signaling proteins implicated in neuronal plasticity (Bibb et al. 2001; Nishi et al. 2000; Scheggi et al. 2004; Valjent et al. 2005).
An extensive amount of microdialysis literature has shown modulation of extracellular dopamine levels in the nucleus accumbens after acute and repeated cocaine exposure (reviewed in Pierce and Kalivas 1997). Normally, dopaminergic neurons fire at a low frequency of ∼5 Hz (Hyland et al. 2002; Wightman and Zimmerman 1990), but these neurons also exhibit burst firing patterns that are characterized by multiple action potentials at frequencies of 20 Hz or more (Chiodo 1988; Grace and Bunney 1983, 1984). This burst firing leads to rapid and transient changes in dopamine concentration at terminals, and this change in dopamine overflow can be readily observed using fast-scan cyclic voltammetry (FSCV) (Hyland et al. 2002; Sombers et al. 2009; Wightman 2006; Wightman and Zimmerman 1990). Burst firing can be mimicked by high-frequency stimulation of dopaminergic neurons (Sombers et al. 2009), and the time course of this evoked overflow has been shown to arise from the balance of uptake and release (Wightman and Zimmerman 1990). Here we examine the effect of repeated cocaine on these rapid dopamine dynamics.
Recent FSCV studies have shown an important role for rapid dopamine signaling in reward-related behavior where dopamine concentration transients have been observed in response to cocaine and to cues that predict cocaine availability (Cheer et al. 2007a; Heien et al. 2005; Owesson-White et al. 2009; Phillips et al. 2003; Stuber et al. 2005). Given these data, the question addressed here is how repeated cocaine exposure may alter in vivo rapid dopamine dynamics. While previous voltammetry studies of sensitization have been performed in brain slices, in vivo voltammetry provides the advantage of addressing sensitization in the brain with much of the neuronal circuitry intact. In addition, the design allows for comparison of the NAc core and shell that receive topographically distinct inputs from different regions of the ventral tegmental area (reviewed in Ikemoto 2007) and may exhibit differential responses to repeated cocaine exposure. Specifically, we used FSCV to monitor electrically stimulated dopamine overflow in the NAc core and shell of anesthetized rats. This experimental design has been used extensively to characterize dopamine uptake and release under different conditions (Jones et al. 1995; Mateo et al. 2004; Ng et al. 1991; Oleson et al. 2008; Walker et al. 2006; Wightman and Zimmerman 1990; Wu et al. 2001a). Furthermore, this in vivo design is critical for kinetic analysis because the modeling of release and uptake used here cannot be performed without electrically stimulated dopamine overflow. These results provide the first in vivo comparison and characterization of repeated cocaine effects on rapid dopamine overflow in the core and shell. Furthermore, the results show that the neurobiological changes associated with repeated cocaine exposure are sufficient to drive a sensitized rapid dopamine response to a subsequent cocaine challenge that originates from a greater inhibition of dopamine uptake in cocaine pre-exposed subjects.
METHODS
Subjects
Male Sprague-Dawley rats were supplied by Charles River Laboratories (Raleigh, NC) and weighed between 200 and 250 g (7–8 wk in age) on arrival. Animals were fed ad libtum throughout the duration of the study and were housed two to three per cage on a light/dark cycle with lights on at 7:00 am and off at 7:00 pm. Animal handling and procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the University of North Carolina Animal Care and Use Committee.
Drug administration
After arrival, rats were allowed to acclimate to the facility for 5–7 days before drugs were administered. An intraperitoneal (ip) dose of 15 mg/kg cocaine was selected based on previous studies showing behavioral and neurochemical changes with this dose in rats (Kalivas and Duffy 1993a). An intravenous (iv) dose of 0.3 mg/kg cocaine is sufficient to produce the feeling of a “high” in humans (Volkow et al. 1997) and is readily self-administered in rats (Stuber et al. 2005). Although a 10-mg/kg ip dose results in cocaine concentrations in the brain similar to that observed with a 1.0-mg/kg iv dose (Orona et al. 1994), self-administration in humans and rodents is characterized by repeated exposure in short time intervals that would lead to a greater cumulative dose. In our first experiment, rats received a single intraperitoneal injection of saline vehicle (0.9% sodium chloride, Hospira, Lake Forest, IL) or 15 mg/kg cocaine (NIDA, Bethesda, MD) in a non-home cage environment followed by a 1-day withdrawal period before electrochemical analysis with FSCV. In the second experiment, saline vehicle or 15 mg/kg cocaine was administered (ip) for 7 consecutive days in a non-home cage environment followed by a 1-day withdrawal and subsequent electrochemical analysis.
Surgery
After the 1-day withdrawal period, all rats were anesthetized with 1.5 g/kg urethane (ip) (Sigma-Aldrich, St. Louis, MO) in preparation for in vivo voltammetric recordings. Surgery was performed using a stereotaxic instrument (David Kopf Instruments, Tujunga, CA) to locate brain regions of interest for electrode placement. For electrical stimulation, a stainless-steel bipolar stimulating electrode (Plastics One, Roanoke, VA) was placed in the ventral tegmental area (VTA) using the following coordinates (A/P −5.2 mm, M/l +0.5 to 1.5 mm, D/V 8.0 mm below dura). To prepare carbon fiber microelectrodes, glass capillaries (Stoelting, Wood Dale, IL) were aspirated with a carbon fiber (T650, Amoco, Greenville, SC) and pulled with an electrode puller (Narishige International) to produce a glass microelectrode with a 5 μm diam at the tip and a protruding carbon fiber. The carbon fiber was cut at a length of 75–100 μm beyond the tip of the glass under a light microscope (Olympus, Center Valley, PA) with a scalpel blade (Cahill et al. 1996). During the experiment, carbon-fiber microelectrodes were placed either into the NAc core (A/P + 1.2 mm, M/l +1.4 mm, D/V 6.5 to 7.5 mm below dura) or shell (A/P + 1.7 mm, M/l +0.8 mm, D/V 6.5 to 7.5 mm below dura). In addition, an Ag/AgCl reference electrode was placed in the contralateral hemisphere of the brain 5–8 mm from the carbon-fiber electrode.
Electrochemistry
Background subtracted FSCV was used to monitor dopamine in vivo. To elicit dopamine overflow in the NAc, electrical stimulation was applied to the VTA and consisted of 24 biphasic pulses (300 μA), 2 ms each phase, applied at frequencies of 10, 20, 40, and 60 Hz with train lengths of 2.4, 1.2, 0.6, and 0.4 s, respectively. During voltammetric recordings, the carbon-fiber microelectrode was held at −0.4 V versus the Ag/AgCl reference electrode. A triangular waveform was applied every 100 ms in which the potential was ramped from −0.4 to 1.3 V and back to −0.4 V at a rate of 400 V/s. The triangular waveform was generated with a multifunction data acquisition board (PCI 6052E, National Instruments, Austin, TX). Waveform acquisition, stimulation delivery, and data collection were synchronized using PCI-6711E (National Instruments). Data processing and signal transduction were performed using custom-built instrumentation (University of North Carolina, Department of Chemistry Electronics Facility) while cyclic voltammograms were visualized and analyzed with Tarheel CV software (ESA, Chelmsford, MA).
For each individual animal, a fresh carbon-fiber microelectrode was initially inserted 4 mm into the brain above the region of interest, and the triangular waveform was applied at a frequency of 60 Hz for 5–10 min. This conditioning phase increases the sensitivity of the electrode as a result of oxidative etching of the carbon-fiber surface that occurs with 60-Hz application of the −0.4 to 1.3 V waveform (Takmakov et al. 2010). However, this process is not likely to alter dopamine release because cell firing is not affected by waveform application (Cheer et al. 2005). After the conditioning phase, waveform application was changed to 10 Hz, and the electrode was lowered into the region of interest (NAc core or shell). Stimulating electrode placement was optimized along the dorsal-ventral axis by the presence of a slight whisker twitch in response to a 60-Hz stimulation of the VTA. Microelectrode depth was subsequently optimized based on depth and the presence of a characteristic cyclic voltammogram for dopamine, with an oxidation peak at 0.6 V and a reduction peak at −0.2 V. In each experimental subject, predrug stimulations were performed using 20-, 40-, and 60-Hz stimulations. Each subject received an acute challenge of 15 mg/kg cocaine (ip) followed by 60-Hz stimulations of the VTA performed every 5 min until the cocaine effect on rapid dopamine overflow reached an asymptote. Once this point was reached, VTA stimulation (postdrug) was applied at 10, 20, 40, and 60 Hz to evaluate the cocaine effect at multiple stimulation frequencies.
Histology
To verify microelectrode placement at the conclusion of the experiment, a 20-μA current was applied to the carbon-fiber microelectrode (Supplementary Fig. S1).1 The lesion disrupts the tip of the carbon fiber; therefore it was necessary to preserve some of the microelectrodes for postcalibration with known dopamine concentrations. In such instances, a tungsten wire was placed at the site of the carbon-fiber microelectrode, and an electrolytic lesion was created as indicated above (Supplementary Fig. S1). Each subject was killed with a lethal dose of urethane (3 mg/kg, ip), and the brain was removed by dissection and stored in 10% formalin (Sigma-Aldrich). Brain tissue was sliced into 50-μm sections on a cryostat (Leica Microsystems, Bannockburn, IL), and lesions were assessed under a light microscope (Olympus), as indicated by representative placements in pictorial coronal sections (Supplementary Fig. S1). Subjects with lesions outside of the target region were excluded from analysis.
Data analysis
The electrochemical data presented in the study were background subtracted and filtered with Tarheel CV software (ESA, Chelmsford, MA). Data for each trial were represented in a two-dimensional graph with the y-axis corresponding to the applied potential of the waveform, the x-axis representing time, and the z-axis (color scale) representing changes in current at the electrode (Fig. 1B). Changes in current were thus visualized in the color plot and those attributable to dopamine were identified by its characteristic cyclic voltammogram. Because dopamine is oxidized at 0.6 V on the positive scan of the voltage, examination of current at this potential showed changes in dopamine current versus time during the trial (I vs. t; Fig. 1C). After the experiment, changes in current were converted to changes in dopamine concentration by postcalibration. The carbon-fiber microelectrode was removed from the brain and placed into a flow-injection analysis system in which known dopamine concentrations, ranging from 100 nM to 3 μM, were presented at the electrode surface. The calibration curve was generated using 20 different electrodes and gave a linear response across dopamine concentrations with an r2 value of 0.9961 (data not shown). The equation generated from the linear response was used to determine electrode response of 12.4 nA/μM. The area of the carbon-fiber cylinder was calculated to be 1,221 μm2 (based on 2.55 μm radius and 75 μm carbon fiber length), and calculated sensitivity of the electrode was 10.2 pA/μM/μm2. This calibration was used to determine the peak concentration of dopamine observed in the brain, [DA]max, after stimulation of the VTA.
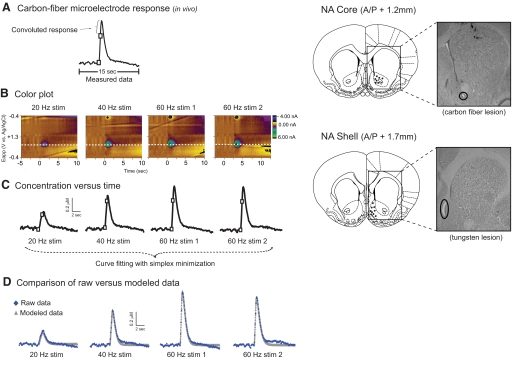
Fig. 1.
In vivo rapid dopamine overflow at the carbon-fiber microelectrode. A: dopamine overflow in the nucleus accumbens (NAc) shell after electrical stimulation of the ventral tegmental area (VTA). At high stimulation frequencies, a continual rise in dopamine concentration even after the stimulation has ceased (2nd white box on trace). This slowed, or convoluted, response is caused by the kinetics of the electrode response time and can be accounted for using a mathematical algorithm. B: predrug rapid dopamine responses at multiple stimulation frequencies represented in a 3-demenisional image in which current is indicated in false color. C: dopamine concentration vs. time plots obtained using the 0.6-V current (dashed line in B) associated with the oxidation of dopamine and calculated using postcalibration values. A simplex minimization algorithm was used to determine values for [DA]p, Vmax, and Km. D: comparison of modeled data (obtained with simplex minimization) to data measured in vivo.
In accord with prior work, dopamine release was considered to be instantaneous with each stimulation pulse that was followed by uptake that is governed by Michaelis-Menton kinetics (Garris and Wightman 1995; Wu et al. 2001b). Specifically, the change in dopamine overflow during the stimulation was defined as follows: d[DA]/dtoverflow = [DA]p × f − d[DA]/dtuptake, where [DA]p was the amount of dopamine released per stimulation pulse, f was the stimulation frequency and d[DA]/dtuptake was the rate of uptake. Thus observed changes in rapid dopamine overflow during stimulation resulted from both dopamine release and uptake mechanisms. After stimulation, extracellular dopamine concentration was governed solely by uptake and was defined as d[DA]/dtoverflow = −Vmax/{(Km/[DA]) + 1}, where Vmax was the maximal rate of uptake and Km was the dopamine concentration at which the rate of uptake is one half of Vmax.
In our experimental design, the dopamine cell body region (VTA) was stimulated at various frequencies ranging from 10 to 60 Hz. During stimulation, a fraction of released dopamine is removed by uptake in the interval between stimulation pulses. For example, a stimulation consisting of 24 pulses applied at a 10-Hz frequency results in a 100-ms delay between each stimulus pulse. In contrast, 24 pulses at a 60-Hz frequency allows for a 16.67-ms pause between each pulse. By comparison, the lower stimulation frequencies provide more time for uptake between each stimulation pulse, whereas higher stimulation frequencies provide less time for uptake between pulses. Thus [DA]max obtained with low frequency stimulation results from a combination of uptake and release components, whereas [DA]max values with high frequency stimulation are governed primarily by dopamine release. As a result, high stimulation frequencies lead to greater [DA]max values compared with lower stimulation frequencies. These characteristics also allow for determination of release and uptake components contributing to the rapid dopamine signals.
As depicted in Fig. 1A, high stimulation frequencies often lead to increases in dopamine concentration that continue after the completion of the stimulation (2nd white box on trace). This time delay is caused by the kinetics associated with the electrode response time (Venton et al. 2002), which results in convoluted, or slowed, response (Garris and Wightman 1995; Venton et al. 2002). Because this time delay can be measured in vitro after removal from brain tissue, the convolution associated with in vivo measurements can be accounted for using a mathematical algorithm (Wu et al. 2001b). Accounting for this convolution also allows for subsequent determination of rate constants from in vivo FSCV recordings.
Analysis of release and uptake kinetics
To determine the values of [DA]p, Vmax, and Km, a simplex minimization algorithm (Press et al. 1989) was used as previously described (Wu et al. 2001b). Briefly, predrug dopamine responses at one location (NAc core or shell) in the anesthetized rat brain were obtained using stimulation frequencies of 20, 40, and 60 Hz (Fig. 1B). The current versus time response was converted to dopamine concentration versus time using values obtained during postcalibration (Fig. 1C). Simulated curves for 20-, 40-, and 60-Hz stimulations were constructed based on initial estimates of values for [DA]p, Vmax, and Km. The simulated curves were convoluted with a measured delay to generate a modeled curve of dopamine concentration versus time. Next, the modeled curves were compared with curves measured in vivo (Fig. 1D), and simplex minimization was repeated until the best fit for all three kinetic parameters ([DA]p, Vmax, and Km) was obtained. After an acute challenge of the competitive inhibitor, cocaine, Vmax was held constant at the predrug value for each subject, and [DA]p and Km were determined with simplex minimization using 60-Hz stimulation frequencies.
Statistics
An independent samples t-test was performed to compare predrug [DA]max responses in subjects with previous saline or cocaine exposure. To measure the effect of acute cocaine exposure on rapid dopamine responses, predrug [DA]max values were normalized to 100% and compared with postdrug responses after an acute cocaine challenge. A one-sample t-test was performed in a within-subjects design to determine significant differences between predrug and postdrug [DA]max values. In addition, an independent samples t-test was used to compare acute cocaine responses in subjects that had received either saline or cocaine pre-exposure before the FSCV experiment. Changes in [DA]p and Km after an acute cocaine challenge were represented as a percent change from predrug values and statistical significance between treatment groups was determined using a 2 × 2 univariate ANOVA with brain region (NAc core or shell) and pre-exposure (saline or cocaine) variables. For all experiments, P < 0.05 was considered significant. All statistical tests were performed using SPSS 14.0 software (SPPS, Chicago, IL).
RESULTS
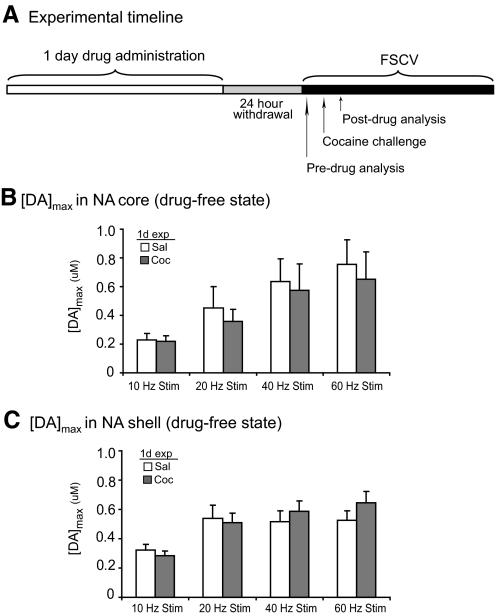
One-day cocaine pre-exposure does not alter dopamine responses in the nucleus accumbens core during a drug-free state
To evaluate the effects of 1-day cocaine exposure on rapid dopamine dynamics, we first examined [DA]max responses in subjects that previously received 1-day saline/cocaine followed by a 24-h withdrawal period. The experimental timeline consisted of a 1-day pre-exposure with a 24-h withdrawal before FSCV recordings (Fig. 2A). Baseline FSCV recordings were first performed before drug administration (Fig. 2A). Dopamine responses in the NAc core showed no significant differences between [DA]max in subjects pretreated with saline compared with cocaine for all of the frequencies tested (P > 0.05, independent samples t-test; Fig. 2B). Similarly, [DA]max in the NAc shell showed no significant difference between saline- and cocaine-treated subjects at all tested frequencies (P > 0.05, independent samples t-test; Fig. 2C). Thus 1-day pre-exposure to cocaine did not alter [DA]max responses in either the NAc core or shell after a subsequent cocaine challenge.
Fig. 2.
Drug-free [DA]max responses after 1-day saline or cocaine followed by 24-h withdrawal. A: experimental timeline for 1-day drug-exposure and fast-scan cyclic voltammetry (FSCV) recordings. B and C: pre-exposure to 1-day cocaine did not alter peak dopamine responses in the NAc core or shell compared with 1-day saline-treated subjects. [DA]max in the NAc core (n = 9) (B) and NAc shell (n = 16) (C) after 10-, 20-, 40-, and 60-Hz stimulations of the VTA. [DA]max data presented as ±SE.
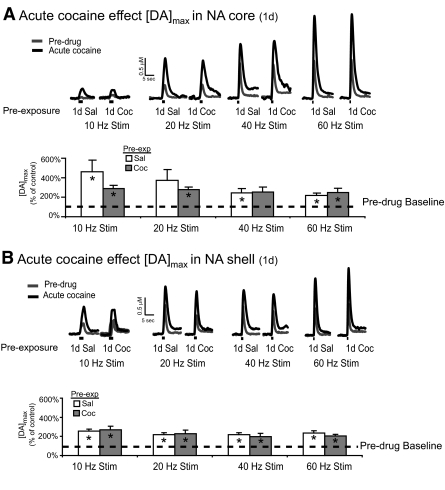
Acute cocaine administration increase [DA]max in the nucleus accumbens before and after 1-day cocaine pre-exposure
A single cocaine exposure has been shown to increase both basal dopamine levels (Kalivas and Duffy 1990) and rapid dopamine dynamics in the NAc (Heien et al. 2005). Here we sought to determine whether a single cocaine exposure could alter rapid dopamine responses to a subsequent cocaine challenge. In the saline control group (1-day saline), [DA]max showed a significant increase after acute cocaine (black traces) in the NAc core compared with the predrug baseline (gray traces, Fig. 3A). This increase was evident at all tested stimulation frequencies (P < 0.05, 1-sample t-test) with the exception of the 20-Hz stimulation frequency (P = 0.07, 1-sample t-test). Likewise, 1-day previous cocaine exposure 24 h before did not alter the [DA]max response to an acute cocaine challenge in the NAc core because 1-day cocaine-treated subjects showed increased [DA]max after acute cocaine (P < 0.05 at 10, 20, and 60 Hz; 1-sample t-test; Fig. 3A), with no significant difference compared with 1-day saline-treated subjects (P > 0.05 at all frequencies, independent samples t-test; Fig. 3A). Similar effects were also observed in the NAc shell where an acute cocaine challenge led to increased [DA]max compared with predrug values in both 1-day saline- and 1-day cocaine-treated subjects (P < 0.05, 1-sample t-test at a frequencies tested; Fig. 3B). Consistent with the results in the NAc core, 1-day pre-exposure to cocaine did not alter responses in the NAc shell to an acute cocaine challenge compared with 1-day saline pre-exposure (P > 0.05, independent samples t-test; Fig. 3B). Together, the data show that a 1-day pre-exposure to cocaine is not sufficient to alter rapid dopamine responses to cocaine as assessed by FSCV. In terms of the rapid dopamine response to cocaine, the increase in [DA]max after acute cocaine occurred at almost all tested frequencies in the core and shell. These results suggest that cocaine was able to increase the [DA]max responses that resulted from a combination of uptake and release components (low frequency stimulations) and those that were governed primarily by release (high frequency stimulations).
Fig. 3.
Cocaine pre-exposure for 1 day does not alter rapid dopamine responses in the NAc core or shell after an acute cocaine challenge. A: rapid dopamine responses in the NAc core after application of various stimulation frequencies to the VTA (n = 9). Dopamine responses are represented as concentration vs. time for both the predrug state (gray trace) and after an acute cocaine challenge (black trace). Both pre-exposure groups showed an increase in [DA]max after acute cocaine at all frequencies tested (*P < 0.05 vs. baseline, 1-sample t-test), with the exception of the 20-Hz response in the saline-treated subjects (+P < 0.07 vs. baseline, 1-sample t-test). No significant differences were observed when comparing acute cocaine responses in 1-day saline vs. 1-day cocaine-treated subjects (P > 0.05, independent samples t-test). B: rapid dopamine responses in the NAc shell after application of various stimulation frequencies to the VTA (n = 16). Both 1-day saline- and 1-day cocaine-treated subjects showed increased [DA]max after an acute cocaine challenge at all frequencies tested (*P < 0.05, 1-sample t-test), with no difference between the two treatment groups (P > 0.05, 1-sample t-test). [DA]max data presented as ±SE.
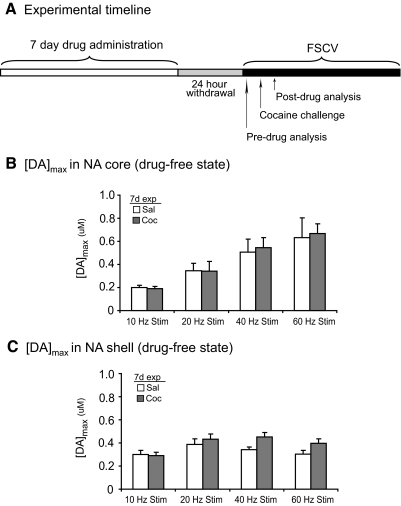
Altered rapid dopamine release and uptake in 7-day saline versus 7-day cocaine-treated subjects
To address whether a longer cocaine exposure could alter rapid dopamine responses in the NAc core and shell, we first examined [DA]max responses in 7-day treated subjects during a drug-free state (Fig. 4A). In the NAc core, analysis of [DA]max showed no significant differences in [DA]max between 7-day saline- and 7-day cocaine-treated subjects (P > 0.05 at all tested frequencies, independent samples t-test; Fig. 4B). In the NAc shell, 7-day cocaine exposure did not alter [DA]max responses during the drug-free state (P > 0.05 at all tested frequencies, independent samples t-test; Fig. 4B). Thus similar to 1-day cocaine pre-exposure (Fig. 2), 7-day pre-exposure to cocaine did not alter [DA]max responses in the NAc core or shell after cocaine challenge.
Fig. 4.
Drug-free [DA]max responses after 7-day saline or cocaine followed by 24-h withdrawal. A: experimental timeline for 1-day drug-exposure and FSCV recordings. B and C: pre-exposure to 7-day cocaine did not alter peak dopamine responses in the NAc core or shell compared with 7-day saline-treated subjects. [DA]max in the NAc core (n = 9) (B) and NAc shell (n = 17) (C) in response to electrical stimulation of the VTA. [DA]max data presented as ±SE.
Seven-day cocaine pre-exposure alters rapid dopamine responses to an acute cocaine challenge
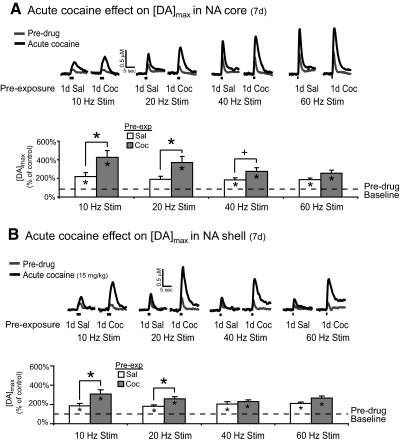
Repeated exposure to cocaine is sufficient to alter basal dopamine levels in response to an acute cocaine challenge as assessed by microdialysis (Kalivas and Duffy 1993a). In this study, we hypothesized that 7-day repeated cocaine pre-exposure would also alter rapid dopamine responses to a subsequent cocaine challenge. In the NAc core of 7-day pretreated subjects, comparison of [DA]max in predrug (gray traces, Fig. 5A) versus acute cocaine (black traces, Fig. 5A) responses showed a significant difference at several of the stimulation frequencies tested (P < 0.05 for 10, 40, and 60 Hz; 1-sample t-test; Fig. 5A). Similarly, 7-day cocaine-treated subjects showed increased [DA]max in the NAc core in response to an acute cocaine challenge (P < 0.05 for all frequencies tested, 1-sample t-test; Fig. 5A). In contrast to the 1-day experiment, however, 7-day cocaine-treated subjects showed a potentiated cocaine response in the NAc core with 10- and 20-Hz VTA stimulation frequencies (P < 0.05, independent samples t-test; Fig. 5A) Thus while rapid dopamine increases after an acute cocaine challenge were observed at both low (10 Hz) and high (60 Hz) stimulation frequencies, the sensitized responses after 7-day cocaine exposure were restricted to low stimulation frequencies.
Fig. 5.
Cocaine pre-exposure for 7 days potentiates the NAc core and shell rapid dopamine response to an acute cocaine challenge. A: concentration vs. time traces of rapid dopamine responses in the NAc core obtained after application of various stimulation frequencies to the VTA (n = 9). Both groups showed increases in [DA]max after an acute cocaine challenge at all frequencies tested (*P < 0.05 vs. baseline, 1-sample t-test) with the exception of the 20-Hz stimulation frequency in 7-day saline-treated subjects (P < 0.06 vs. baseline, 1-sample t-test). Subjects previously exposed to 7-day cocaine showed a potentiated response to the cocaine challenge compared with 7-day saline-treated subjects (*P < 0.05 for 10- and 20-Hz stimulations, independent samples t-test). B: concentration vs. time traces in the NAc shell after application of various stimulation frequencies to the VTA (n = 17). An acute cocaine challenge led to an increased [DA]max response at all frequencies tested in both 7-day saline- and 7-day cocaine-treated subjects (P < 0.05 vs. baseline, 1-sample t-test). Subjects that were pre-exposed to 7-day cocaine also showed a potentiated response to the acute cocaine challenge at 10- and 20-Hz stimulation frequencies compared with 7-day saline-treated subjects (*P < 0.05, independent samples t-test). [DA]max data presented as ±SE.
In separate animals, we also examined rapid dopamine responses in the NAc shell of 7-day saline- or cocaine-treated subjects. Both 7-day saline- and 7-day cocaine-treated subjects showed increased [DA]max after acute cocaine (Fig. 5B, black trace) compared with their predrug responses (Fig. 5B, gray trace) at all frequencies tested (P < 0.05, 1-sample t-test; Fig. 5B). In addition, 7-day cocaine-treated subjects showed a potentiated response to cocaine at the 10- and 20-Hz stimulation frequencies (P < 0.05, independent samples t-test; Fig. 5B). This sensitized response in the shell mimicked those observed in the NAc core of 7-day cocaine-treated subjects. In light of the components that contribute to [DA]max in our experimental design, the sensitization that was observed with low stimulation frequencies suggests that this effect of repeated cocaine likely resulted from uptake mechanisms as opposed to release mechanisms.
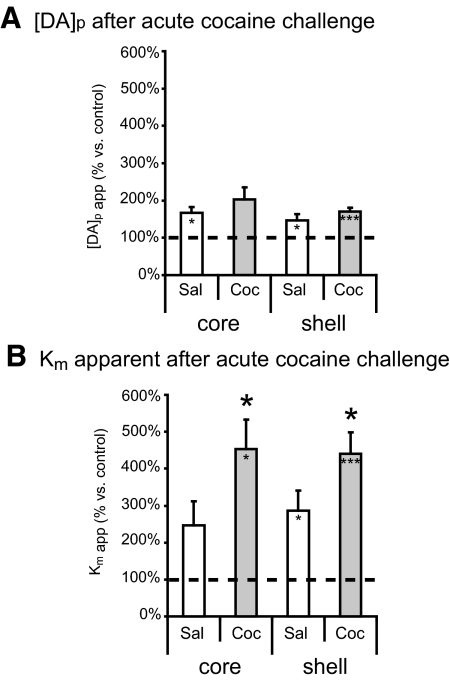
To further examine the possible role of release and uptake components in the sensitized rapid dopamine response, we determined Km and [DA]p values after acute cocaine challenge. Here, we used kinetic analysis to determine whether the potentiated increase in [DA]max after 7-day cocaine pre-exposure was caused by changes in release, uptake, or both. The analysis showed that an acute cocaine challenge increased [DA]p in the NAc core and shell of 7-day saline pre-exposed subjects (P < 0.05, 1-sample t-test; Fig. 6A) and in the NAc shell of 7-day cocaine pre-exposed subjects (P < 0.05, 1-sample t-test; Fig. 6A). The acute cocaine challenge also increased the apparent Km value in the shell of 7-day saline-treated subjects (P < 0.05, 1-sample t-test; Fig. 6B) and in the core and shell of 7-day cocaine-treated subjects (P < 0.05, 1-sample t-test; Fig. 6B). Furthermore, subjects that had previously received cocaine for 7 days showed a potentiated increase in apparent Km (F3,25 = 5.942, P < 0.05 vs. cocaine subjects; Fig. 6B). Together, the data suggest that the changes associated with cocaine's ability to alter the apparent Km value played a greater role in the sensitized response to an acute cocaine challenge. Given that such effects were not observed with 1-day pre-exposure, the ability of a cocaine challenge to augment the [DA]max seems to be dependent on the duration of the intermittent cocaine pre-exposure.
Fig. 6.
Cocaine pre-exposure for 7 days leads to a greater uptake inhibition during an acute cocaine challenge. A: 1-day saline- and cocaine-treated subjects showed increased [DA]p in response to an acute cocaine challenge (*P < 0.05 vs. baseline, **P < 0.005 vs. baseline, ***P < 0.001 vs. baseline, 1-sample t-test). B: all groups also showed a significant increase in apparent Km after the acute cocaine challenge (*P < 0.05 vs. baseline, **P < 0.005 vs. baseline, 1-sample t-test) with the exception of the NAc core response in 1-day cocaine subjects. In addition, 7-day cocaine-treated subjects showed a potentiated change in apparent Km while cocaine was on board, as shown by a main effect of cocaine (F3,25 = 5.942, *P < 0.05, univariate ANOVA). Data presented as ±SE.
DISCUSSION
The work presented here provides the first in vivo characterization and comparison of acute and repeated cocaine effects on rapid dopamine dynamics in the NAc core and shell. Specifically, we showed that 7-day cocaine exposure and 1-day withdrawal is sufficient to potentiate rapid dopamine signals in both subregions of the NAc after an acute cocaine challenge. Given that experiments were performed in anesthetized animals, these data showed that this potentiation can occur independent of contextual cues at the time of the cocaine challenge. Thus we propose that repeated cocaine exposure leads to underlying changes in the mesolimbic dopamine system that modulate rapid dopamine responses to subsequent cocaine exposure. The fact this potentiation was not observed with 1-day cocaine pre-exposure suggests that these underlying neurobiological changes emerge with longer cocaine exposure. Specifically, the 7-day sensitization was restricted to low stimulation frequencies (10 and 20 Hz), where rapid dopamine overflow is governed more strongly by uptake and was not observed at higher stimulation frequencies where overflow is primarily governed by release. Consistent with this observation, kinetic analysis showed a potentiated change in apparent Km for uptake measured in vivo during an acute cocaine challenge, showing an increased potency of cocaine's uptake inhibiting actions in pretreated animals.
This study builds on the microdialysis literature concerning extracellular dopamine responses to cocaine and extends the evidence for neurochemical sensitization to rapid dopamine signaling. Indeed, previous studies have shown increased extracellular dopamine in the NAc after acute cocaine exposure in cocaine-naïve rats, an effect that is accompanied by increased locomotor activity (Heidbreder et al. 1996; Kalivas and Duffy 1990; Parsons and Justice 1993). In our work with rapid dopamine, acute cocaine in naïve animals also led to an increase in [DA]max. Previous microdialysis studies have shown that repeated cocaine exposure for 4–10 days can elicit a sensitized extracellular dopamine response to an acute cocaine challenged presented after 24-h withdrawal (Kalivas and Duffy 1993a,b; Parsons and Justice 1993; Pettit et al. 1990). Sensitized extracellular dopamine levels after 24-h cocaine withdrawal are often correlated with locomotor behavior, and, although we did not examine behavior in this study, we used a dose (15 mg/kg, ip) that has been previously shown to initiate behavioral sensitization (Kalivas and Duffy 1993a). It should be noted, however, that the evidence for extracellular dopamine sensitization is mixed for withdrawal periods of <1 wk, whereas withdrawal periods of 2 wk or greater lead to a more robust extracellular dopamine sensitization (Heidbreder et al. 1996; Hooks et al. 1994; Hurd et al. 1989; Kalivas and Duffy 1993a; Pierce and Kalivas 1997; Segal and Kuczenski 1992). Nevertheless, the observed rapid dopamine sensitization in the absence of contextual cues and behavior during the challenge suggests that repeated cocaine exposure is sufficient to induce changes in the neural mechanisms governing rapid dopamine signaling responses to a cocaine challenge after a 24-h withdrawal.
The primary distinctive aspect of our sensitization study is the use of FSCV and kinetic analysis, which allows for the examination of both release and uptake components of the dopamine signal. To date, there are a limited number of voltammetric analyses that have addressed dopaminergic effects of chronic cocaine exposure. In one previous study, [DA]max was assessed in rats after a 10-day cocaine exposure and 24-h withdrawal. In that work, Ng et al. (1991) used a prolonged length of stimulation (10 s) that led to a steady-state [DA]max response during stimulation with the goal of depleting the dopamine releasable pool. Importantly, they compared the maximal amount of dopamine available for release and observed a 44% [DA]max increase in cocaine-treated versus saline-treated subjects. In fact, the difference in cocaine-treated subjects emerged after 7–8 s of stimulation, and the authors suggested that the potentiated increase in [DA]max arose from a greater pool of available dopamine in cocaine-treated animals (Ng et al. 1991). In contrast, such changes were not detected in our design where shorter stimulation periods (0.4–2.4 s) were used. Thus our work shows that the [DA]max potentiation can emerge in the presence of cocaine, even if it is not apparent during the drug-free state. Although previous voltammetric analysis was limited to the NAc core, we extended the work to show sensitization in both the core and the shell of the NAc. Given that DATs are more densely expressed in the NAc core (Nirenberg et al. 1997), one might suggest that cocaine would have a greater effect on dopamine transmission in the NAc shell because of its ability to inhibit a greater number of transporters in this lower density region. In addition, studies have shown that rats will self-administer cocaine directly to the NAc shell but not to the NAc core. Despite these anatomical and functional differences, we observed similar cocaine-mediated sensitization in both subregions of the NAc, suggesting that repeated cocaine exposure is sufficient to potentiate cocaine effects in both the core and shell. However, differences in repeated cocaine effects may emerge with different withdrawal periods or with the addition of behavioral analysis. Indeed, rapid dopamine sensitization can persist after long withdrawal periods; a sensitized [DA]max response to a cocaine challenge has been observed in NAc slices after a 14-day withdrawal (Williams et al. 1995). Thus our voltammetry data provide the foundation for future combined voltammetry and behavioral examinations of rapid dopamine effects after short and long withdrawal periods.
By using FSCV and kinetic analysis to distinguish release and uptake components of the dopamine signal, our work provides unique insight into cocaine's mechanism of action at a level that cannot be described using standard microdialysis techniques. Although cocaine effects on dopamine dynamics are often described in terms of DAT inhibition, our observed increase in rapid dopamine release, [DA]p, after cocaine is consistent with other voltammetry studies using electrical stimulation (Lee et al. 1998; Venton et al. 2006; Walker et al. 2006). There are several potential mechanisms by which cocaine could increase dopamine release. Indeed, in freely moving animals, intravenous cocaine administration increases the frequency and amplitude of transient dopamine events in the NAc shell (Aragona et al. 2008; Cheer et al. 2007b; Sombers et al. 2009). These effects can be blocked by lidocaine-mediated inhibition of the VTA and likely result from cocaine-mediated increases in burst firing of dopamine neurons (Sombers et al. 2009). In addition to these VTA mechanisms, another factor that could contribute to dopamine release is the synapsin dependent mobilization of vesicles from the reserve pool to the readily releasable pool. Indeed, recent work from our laboratory with synapsin knockout mice showed a reduced dopaminergic response to cocaine that is caused by an attenuation of cocaine's ability to increase [DA]p (Venton et al. 2006). Thus synapsin mechanisms likely contribute to the cocaine-mediated increase in [DA]p that was observed in this study as well.
Importantly, our kinetic data during the acute cocaine challenge provide a unique in vivo demonstration of the enhanced cocaine-mediated uptake inhibition in cocaine pre-exposed animals. Our in vivo results are consistent with previous in vitro voltammetry data from 7-day cocaine-treated subjects that showed greater uptake inhibition after bath application of cocaine in NAc slices, an effect that persisted after a 2-wk withdrawal period. To date, however, the majority of the data has suggested that DAT-cocaine effects are caused by changes in trafficking and expression rather than changes in activity or function (Kahlig and Galli 2003; Zahniser and Sorkin 2004, 2009). Indeed, changes in DAT cell surface expression and dopamine clearance have been shown after cocaine exposure both in vivo and in vitro (Cass et al. 1993; Daws et al. 2002; Little et al. 2002; Sabeti et al. 2002). However, our findings of a potentiated uptake inhibition during a cocaine challenge serve to compliment previous in vitro literature (Izenwasser and Cox 1990; Lee et al. 1998) and suggest that changes in DAT function and activity may also play an important role in dopaminergic responses to repeated cocaine. In addition, such DAT effects are also likely to affect extracellular dopamine responses to cocaine. For instance, stronger DAT inhibition in the NAc would allow for potentiated increases in extracellular dopamine levels. In addition to these effects in the NAc, the potentiated Km shift could also modulate rapid and extracellular dopamine overflow in the VTA to influence downstream dopaminergic responses in the NAc.
Overall, the data presented in this study provide evidence for in vivo rapid dopamine sensitization in both the NAc core and shell after repeated cocaine exposure and clearly distinguish the contribution of release and uptake components to this sensitization. Given that repeated exposure to cocaine is characteristic of self-administration, the effects of repeated cocaine on rapid dopamine overflow may in fact influence drug-seeking behavior in the self-administration task. Indeed, both burst firing of dopamine neurons in the VTA and rapid dopamine overflow in the NAc have been shown to play an important role in responses to reward-predictive cues (Cheer et al. 2007a; Hyland et al. 2002; Phillips et al. 2003; Schultz 1998). Furthermore, changes in the potency of cocaine's effects on DAT during self-administration could modulate the hedonic value of the drug, especially given the fact that cocaine-induced DAT inhibition contributes to euphoria in humans (Volkow et al. 1999). With such implications, the current demonstration of rapid dopamine sensitization highlights the need for additional studies to further examine the mechanisms of such effects as well as their behavioral consequences.
GRANTS
This work was supported by National Institute of Drug Abuse Grants DA-026698 to N. A. Addy, DA-109000 to R. M. Wightman, and DA-024036 to P. A. Garris and the Carolina Postdoctoral Program for Faculty Diversity to. N. A. Addy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank C. McKinney and the University of North Carolina Electronics Facility for instrumentation, T. S. Guillot and Z. A. McElligott for critically reading the manuscript, and J. Park and K. Fontillas for assistance with histology.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events.J Neurosci 28: 8821–8831, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area.J Neurosci 28: 9092–9100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5.Nature 410: 376–380, 2001 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats.J Neurosci 24: 7482–7490, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Microelectrodes for the measurement of catecholamines in biological systems.Analyt Chem 68: 3180–3186, 1996 [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Gillespie K, Curella P, Mayfield RD, Zahniser NR. Reduced clearance of exogenous dopamine in rat nucleus accumbens, but not in dorsal striatum, following cocaine challenge in rats withdrawn from repeated cocaine administration.J Neurochem 61: 273–283, 1993 [DOI] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior.Neuron 54: 237–244, 2007a [DOI] [PubMed] [Google Scholar]
- Cheer JF, Heien ML, Garris PA, Carelli RM, Wightman RM. Simultaneous dopamine and single-unit recordings reveal accumbens GABAergic responses: implications for intracranial self-stimulation.Proc Natl Acad Sci USA 102: 19150–19155, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation.J Neurosci 27: 791–795, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LA. Dopamine-containing neurons in the mammalian central nervous system: electrophysiology and pharmacology.Neurosci Biobehav Rev 12: 49–91, 1988 [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, Galli A. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters.Biochem Biophys Res Commun 290: 1545–1550, 2002 [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Regional Differences in Dopamine Release, Uptake, and Diffusion by Fast-Scan Cyclic Voltammetry. Totowa, NJ: Humana Press, 1995 [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement.Science 221: 773–775, 1983 [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–1. Identification and characterization.Neuroscience 10: 301–315, 1983 [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing.J Neurosci 4: 2877–2890, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine.J Pharmacol Exp Ther 278: 490–502, 1996 [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats.Proc Natl Acad Sci USA 102: 10023–10028, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Duffy P, Striplin C, Kalivas PW. Behavioral and neurochemical sensitization following cocaine self-administration.Psychopharmacology (Berl) 115: 265–272, 1994 [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, And N, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study.Brain Res 498: 199–203, 1989 [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat.Neuroscience 114: 475–492, 2002 [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex.Brain Res Rev 56: 27–78, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats.J Neurosci 20: 7489–7495, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell.Nat Neurosci 7: 389–397, 2004 [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Cox BM. Daily cocaine treatment produces a persistent reduction of [3H]dopamine uptake in vitro in rat nucleus accumbens but not in striatum.Brain Res 531: 338–341, 1990 [DOI] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Kilts CD, Wightman RM. Comparison of dopamine uptake in the basolateral amygdaloid nucleus, caudate-putamen, and nucleus accumbens of the rat.J Neurochem 64: 2581–2589, 1995 [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine.Eur J Pharmacol 479: 153–158, 2003 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens.Synapse 5: 48–58, 1990 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals.J Neurosci 13: 266–275, 1993a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. II. Dopamine perikarya.J Neurosci 13: 276–284, 1993b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Gee KR, Ellinwood EH, Seidler FJ. Altered cocaine potency in the nucleus accumbens following 7-day withdrawal from intermittent but not continuous treatment: voltammetric assessment of dopamine uptake in the rat.Psychopharmacology (Berl) 137: 303–310, 1998 [DOI] [PubMed] [Google Scholar]
- Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Cocaine induction of dopamine transporter trafficking to the plasma membrane.Molec Pharmacol 61: 436–445, 2002 [DOI] [PubMed] [Google Scholar]
- Mateo Y, Budygin EA, Morgan D, Roberts DC, Jones SR. Fast onset of dopamine uptake inhibition by intravenous cocaine.Eur J Neurosci 20: 2838–2842, 2004 [DOI] [PubMed] [Google Scholar]
- Ng JP, Hubert GW, Justice JB., JR Increased stimulated release and uptake of dopamine in nucleus accumbens after repeated cocaine administration as measured by in vivo voltammetry.J. Neurochem 56: 1485–1492, 1991 [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Pohorille A, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter: comparative ultrastructure of dopaminergic axons in limbic and motor compartments of the nucleus accumbens.J Neurosci 17: 6899–6907, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Amplification of dopaminergic signaling by a positive feedback loop.Proc Natl Acad Sci USA 97: 12840–12845, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Talluri S, Childers SR, Smith JE, Roberts DC, Bonin KD, Budygin EA. Dopamine uptake changes associated with cocaine self-administration.Neuropsychopharmacology 34: 1174–1184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orona RA, Mayfield RD, Cline EJ, Zahniser NR. Repeated intravenous cocaine administration to rats produces behavioral sensitization without changing brain cocaine levels.Neurosci Lett 167: 121–124, 1994 [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM, Carelli RM. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell.Eur J Neurosci 30: 1117–1127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration.J Neurochem 61: 1611–1619, 1993 [DOI] [PubMed] [Google Scholar]
- Pettit HO, Pan HT, Parsons LH, Justice JB., Jr Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration.J Neurochem 55: 798–804, 1990 [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking.Nature 422: 614–618, 2003 [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants.Brain Res Brain Res Rev 25: 192–216, 1997 [DOI] [PubMed] [Google Scholar]
- Press WH, Flanery BP, Teukolsy SA, Fetterline WT. Numerical Recipes in Pascal. Cambridge: Cambridge University Press, 1989 [Google Scholar]
- Ritz M, Lamb R, Goldberg S, Kuhar M. Cocaine receptors on dopamine transporters are related to self-administration of cocaine.Science 237: 1219–1223, 1987 [DOI] [PubMed] [Google Scholar]
- Ritz M, Lamb R, Goldberg S, Kuhar M. Cocaine self-administration appears to be mediated by dopamine uptake inhibition.Prog Neuropsychopharmacol Biol Psychiatry 12: 233–239, 1988 [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats.J Pharmacol Exp Ther 303: 1216–1226, 2002 [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons.Neuron 37: 577–582, 2003 [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats.J Pharmacol Exp Ther 302: 1201–1211, 2002 [DOI] [PubMed] [Google Scholar]
- Scheggi S, Rauggi R, Gambarana C, Tagliamonte A, De Montis MG. Dopamine and cyclic AMP-regulated phosphoprotein-32 phosphorylation pattern in cocaine and morphine-sensitized rats.J Neurochem 90: 792–799, 2004 [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons.J Neurophysiol 80: 1–27, 1998 [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Repeated cocaine administration induces behavioral sensitization and corresponding decreased extracellular dopamine response in caudate and accumbens.Brain Res 577: 351–355, 1992 [DOI] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area.J Neurosci 29: 1735–1742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration.Neuropsychopharmacology 30: 853–863, 2005 [DOI] [PubMed] [Google Scholar]
- Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, McCarty GS, Wightman RM. Carbon microelectrodes with a renewable surface.Analytic Chem 82: 2020–2028, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum.Proc Natl Acad Sci USA 102: 491–496, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies.Psychopharmacology (Berl) 151: 99–120, 2000 [DOI] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool.J Neurosci 26: 3206–3209, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Troyer KP, Wightman RM. Response times of carbon fiber microelectrodes to dynamic changes in catecholamine concentration.Analytic Chem 74: 539–546, 2002 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans.J Psychopharmacol 13: 337–345, 1999 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy.Nature 386: 827–830, 1997 [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum.Neuropsychopharmacology 31: 1193–1202, 2006 [DOI] [PubMed] [Google Scholar]
- Wightman RM. Detection technologies. Probing cellular chemistry in biological systems with microelectrodes.Science 311: 1570–1574, 2006 [DOI] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake.Brain Res Brain Res Rev 15: 135–144, 1990 [DOI] [PubMed] [Google Scholar]
- Williams JE, Wieczorek W, Willner P, Kruk ZL. Parametric analysis of the effects of cocaine and cocaine pretreatment on dopamine release in the nucleus accumbens measured by fast cyclic voltammetry.Brain Res 678: 225–232, 1995 [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants.Prog Neurobiol 54: 679–720, 1998 [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission.J Neurosci 21: 6338–6347, 2001a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry.J Neurosci Methods 112: 119–133, 2001b [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology 47Suppl 1: 80–91, 2004 [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions.Semin Cell Dev Biol 20: 411–417, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.