Abstract
Photodynamic inactivation is a rapidly developing antimicrobial treatment that employs a nontoxic photoactivatable dye or photosensitizer in combination with harmless visible light to generate reactive oxygen species that are toxic to cells. Tetrapyrroles (e.g., porphyrins, chlorins, bacteriochlorins) are a class of photosensitizers that exhibit promising characteristics to serve as broad-spectrum antimicrobials. In order to bind to and efficiently penetrate into all classes of microbial cells, tetrapyrroles should have structures that contain (i) one or more cationic charge(s) or (ii) a basic group. In this report, we investigate the use of new stable synthetic bacteriochlorins that have a strong absorption band in the range 720 to 740 nm, which is in the near-infrared spectral region. Four bacteriochlorins with 2, 4, or 6 quaternized ammonium groups or 2 basic amine groups were compared for light-mediated killing against a Gram-positive bacterium (Staphylococcus aureus), a Gram-negative bacterium (Escherichia coli), and a dimorphic fungal yeast (Candida albicans). Selectivity was assessed by determining phototoxicity against human HeLa cancer cells under the same conditions. All four compounds were highly active (6 logs of killing at 1 μM or less) against S. aureus and showed selectivity for bacteria over human cells. Increasing the cationic charge increased activity against E. coli. Only the compound with basic groups was highly active against C. albicans. Supporting photochemical and theoretical characterization studies indicate that (i) the four bacteriochlorins have comparable photophysical features in homogeneous solution and (ii) the anticipated redox characteristics do not correlate with cell-killing ability. These results support the interpretation that the disparate biological activities observed stem from cellular binding and localization effects rather than intrinsic electronic properties. These findings further establish cationic bacteriochlorins as extremely active and selective near-infrared activated antimicrobial photosensitizers, and the results provide fundamental information on structure-activity relationships for antimicrobial photosensitizers.
Photodynamic therapy (PDT) employs a nontoxic dye termed a photosensitizer and low-intensity visible light, which in the presence of molecular oxygen produces reactive oxygen species, such as singlet oxygen, superoxide, and hydroxyl radicals (15). PDT has the advantage of dual selectivity in that the photosensitizer can be targeted to a destination cell or tissue, and in addition the illumination can be spatially directed to the lesion (7, 48). PDT has its origins over a hundred years ago in the discovery of light-mediated killing of microorganisms (35) but since then has been principally developed as a treatment for cancer (8) and age-related macular degeneration (58). Photodynamic inactivation (PDI) is the term used to describe the use of PDT to inactivate an unwanted entity such as a microbial cell.
There has been a relentless rise in antibiotic resistance over many years in most regions of the world and in many different classes of microbial cells (41). In recent times the phenomenon has become even more worrying, with concerns that hitherto fairly trivial infections could again become untreatable as in the days before antibiotics were discovered (3). In fact, the present time has been termed the “end of the antibiotic era” (1). The rise in multidrug resistance among microbial pathogens has motivated an international search for alternative antimicrobial strategies, particularly those which could be applied to infections in wounds and burns (32).
PDI has attracted attention as a possible alternative treatment for localized infections (14, 19, 27). In this treatment, the photosensitizer is topically or locally applied to the infected tissue and, after a relatively short time interval, light is delivered to the area. Depending on the effectiveness of the antimicrobial photosensitizer, up to three logs of bacterial or fungal cells can be killed without causing unacceptable damage to the host tissue (11). PDI is thought to be equally effective against multidrug-resistant and naïve species (49), and in addition the PDI treatment itself is unlikely to cause resistance to arise (26). It should be noted that the lack of development of resistance after PDT is generally difficult to prove experimentally but can be shown in particular instances.
Gram-negative bacteria are resistant to PDI with many commonly used photosensitizers that readily lead to phototoxicity for Gram-positive species (29). On the other hand, photosensitizers bearing a cationic charge (31, 33, 37) or the use of agents that increase the permeability of the outer membrane (38) are known to increase the efficacy of killing of Gram-negative organisms. The ideal photosensitizer for killing bacteria should possess an overall cationic charge and preferably multiple cationic charges (16, 52).
Photosensitizers based on the bacteriochlorin backbone have been studied as potential PDT agents for cancer and nononcological applications (10, 43, 46, 55). The large absorption feature in the near-infrared spectral region, which is characteristic of bacteriochlorins, is considered to be ideal for maximizing light penetration through tissue. This is so because both absorption and scattering of light in the 700- to 800-nm region are minimal (40, 42). However, in addition to good optical properties, it is necessary for a photosensitizer molecule to possess the appropriate structural characteristics that will optimize the binding to and penetration into microbial cells. For antimicrobial applications, the effective molecular features are likely to include the presence of positively charged substituents such as quaternized ammonium groups.
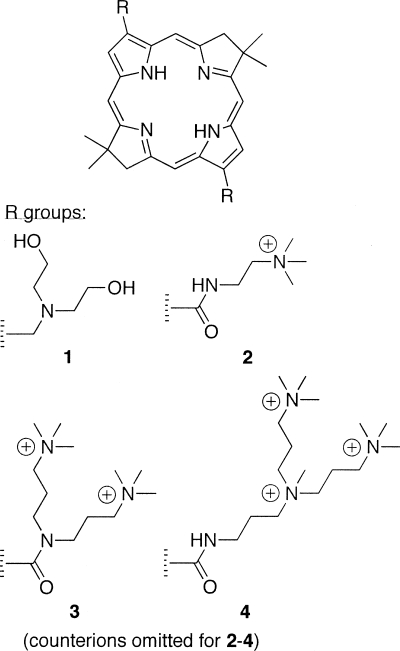
A de novo synthetic pathway to bacteriochlorins that contain a geminal dimethyl group in each pyrroline ring has been developed recently (22). This structural attribute blocks adventitious dehydrogenation (to form the chlorin) and thereby affords a stable macrocycle. This synthetic route has provided a number of bacteriochlorin building blocks, which provided modular access to bacteriochlorins 1 to 4 (Fig. 1). The four molecules were designed to allow investigation of the structure-activity relationship among differently charged bacteriochlorins. Bacteriochlorin 1 is a neutral species with two basic amino groups; bacteriochlorins 2 to 4 contain 2, 4, or 6 cationic charges, respectively. The synthesis of bacteriochlorins 1 to 3 has been reported (44), and the synthesis of bacteriochlorin 4 is described herein (see the supplemental material) making use of known routes to bacteriochlorin (44) and tetraaminoalkane (34) building blocks. The photophysical and molecular orbital characteristics of all four bacteriochlorins have been investigated as part of this study. The goals of the present study were to (i) test bacteriochlorins 1 to 4 as antimicrobial photosensitizers against a panel of human pathogens of different taxonomic classifications and (ii) determine selectivity of the four bacteriochlorins for killing microbial cells versus mammalian (human cancer) cells using the same incubation time and other experimental conditions.
FIG. 1.
Bacteriochlorin photosensitizers.
MATERIALS AND METHODS
Determination of logP values.
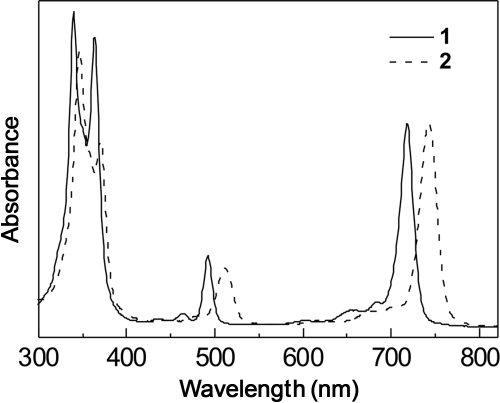
A mixture of 2 ml of octanol and 2 ml of water in a 20-ml scintillation vial was stirred at room temperature for 3 h. Then, less than 0.5 mg of bacteriochlorin was introduced. Stirring was continued at room temperature at 100 to 200 rpm for 24 h. The mixture was allowed to stand for 30 min to allow separation of the phases. A 30-μl aliquot of each phase was placed in 3.0 ml of dimethyl sulfoxide (DMSO), and the absorption spectrum of each phase was measured. The ratio of the peak intensity of the near-infrared feature, the Qy(0,0) band (Fig. 2), for the two phases (octanol/water) was calculated. When there was no detectable amount of bacteriochlorin in a given phase, the noise level (A = 0.001) was used as the limiting reading, and the logP value was bounded accordingly; these measured quantities are here denoted mLogP values. The values were also calculated based simply on the bacteriochlorin structure using ACD Labs (Toronto, Canada) V11.01 software; these calculated quantities are here denoted cLogP values. The cLogP and mLogP values are presented in Table 1. A positive versus negative logP value reflects preferential solubilization in the octanol versus water phases, respectively.
FIG. 2.
Absorption spectra of bacteriochlorins 1 (solid line) and 2 (dashed line) in methanol at room temperature. The long-wavelength, near-infrared feature is the Qy(0,0) band.
Photophysical measurements.
Photophysical measurements were preformed as described previously (20). Measurement of the fluorescence (Φf) and triplet excited state quantum yields (Φisc) and singlet (τS) and triplet (τT) lifetimes utilized Ar-purged solutions (methanol or 2-methyltetrahydrofuran) except that the τT values for bacteriochlorin 2 in methanol and bacteriochlorin 3 in ethanol utilized rigorously degassed (by freeze-pump-thaw) solutions. The Φf values were determined with respect to 8,8,18,18-tetramethylbacteriochlorin (50) in Ar-purged toluene, for which Φf = 0.125 was established with respect to chlorophyll a in benzene (Φf = 0.325 [56]) and free base tetraphenylporphyrin in toluene (Φf = 0.09 [12]) using Soret and Qx excitation. Triplet yields were determined using a reference technique to facilitate comparisons (20). First, a value of Φisc = 0.57 was measured for bacteriochlorin 3. This value, along with the Φf and τS values for this compound (Table 1 ), gives a value of kic = (11.4 ns)−1 for the rate constant for internal conversion of the lowest singlet excited state to the ground state via the expression kic = (τS)−1[1 − Φf − Φisc]. This value is in good agreement with the average value of kic = (10 ns)−1 obtained for a number of analogous 3,13-substituted synthetic bacteriochlorins (unpublished work). This kic value was used to obtain the triplet yield for each of the bacteriochlorins using the expression 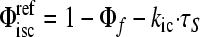 (Table 1).
(Table 1).
TABLE 1.
Chemical and photophysical properties of bacteriochlorinsa
| Compound | Partition coefficient |
Qy absorptionb |
Qy fluorescenceb |
Φfc | τSd (ns) | Φiscrefe | τTf (μs) | Orbital energy |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mLogPg | cLogPh | λ (nm) | fwhm (nm) | λ (nm) | fwhm (nm) | HOMO (eV) | LUMO (eV) | |||||
| 1 | +2.3 | +4.8 ± 1.5 | 718 | 18 | 724 | 23 | 0.095 | 3.8 | 0.53 | 190i | −4.42 | −2.19 |
| 2 | −0.5 | −1.1 ± 1.7 | 742 | 23 | 750 | 25 | 0.13 | 4.0 | 0.48 | 77j | −4.72 | −3.34 |
| 3 | −1.4 | −5.3 ± 1.7 | 729 | 19 | 735 | 24 | 0.12 | 3.5 | 0.53 | 54k | −5.05 | −3.89 |
| 4 | −1.7 | −5.8 ± 1.7 | 740 | 24 | 750 | 25 | −4.56 | −3.92 | ||||
All data measured for compounds at room temperature.
Peak wavelength (λ) and full width at half maximum (fwhm) of spectral feature for compound in aerated methanol.
Fluorescence quantum yield for compound in Ar-purged methanol.
Lifetime of the lowest singlet excited state for compound in Ar-purged methanol determined using fluorescence detection.
Yield of the lowest triplet excited state determined using the expresssion 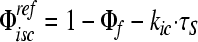 , with kIC = (10 ns)−1 as described in the text.
, with kIC = (10 ns)−1 as described in the text.
Lifetime of the lowest triplet excited state.
Measured logP.
Calculated logP.
In Ar-purged 2-methyltetrahydrofuran.
In freeze-pump-thaw-degassed methanol.
In freeze-pump-thaw-degassed ethanol.
Molecular orbital calculations.
Density-functional-theory (DFT) calculations were performed with Spartan '06 for Windows (Wavefunction, Inc.) using the hybrid B3LYP functional, 6-31G* basis set, and equilibrium geometries were fully optimized using the default program parameters (24).
Microorganisms and culture conditions.
Staphylococcus aureus 8325-4 and Escherichia coli K-12 (both wild type) as well as the DAY286 reference strain of Candida albicans (39) were employed. Planktonic bacterial cells were cultured in brain heart infusion (BHI) broth with aeration at 37°C in mid-log-growth phase (unless otherwise stated) (39). Yeasts were cultured in yeast-peptone-dextrose (YPD) broth with aeration at 30°C. The cell number was assessed with a hemacytometer (47).
In vitro PDI and viability assessment/determination.
A cell suspension consisting of 108 cells/ml for bacteria (107 cells/ml for Candida albicans [6]) was incubated with various concentrations of the bacteriochlorins for 30 min at room temperature in the dark. Aliquots (1 ml) were transferred to a 24-well plate and illuminated at room temperature with a 732-nm laser source (732/6 Diode Laser, Pharmacyclics, Sunnyvale, CA) and a lens adjusted to give a uniform spot of 2.5 cm in diameter with an irradiance of 130 mW/cm2 as measured with a power meter (model DMM 199 with 201 standard head; Coherent, Santa Clara, CA). A fluence of 10 J/cm2 was delivered over a period of 77 s. Cells treated with bacteriochlorin in the dark were incubated covered with aluminum foil for the same time as the PDT groups (30 min).
At the completion of illumination (or dark incubation), the contents of the wells were mixed before sampling. Aliquots (100 μl) were taken from each well to determine CFU. The aliquots were serially diluted 10-fold in phosphate-buffered saline (PBS) to give dilutions of 10−1 to 10−5 times in addition to the original concentration; then, 10-μl aliquots of each of the dilutions were streaked horizontally on square BHI or YPD (for Candida) plates by the method of Jett and colleagues (18). Plates were streaked in triplicate and incubated for 12 to 36 h at 30°C or 37°C in the dark to allow colony formation. A control group of cells treated with light alone (no bacteriochlorin added) showed the same number of CFU as the absolute control (data not shown). Survival fractions were routinely expressed as ratios of CFU of microbial cells treated with light and bacteriochlorin (or bacteriochlorin in the absence of light) to CFU of microbes treated with neither.
Confocal microscopy of Candida.
C. albicans cells (107 cells/ml) were incubated with bacteriochlorin 1 or 2 at a concentration of 100 μM for 30 min in PBS (pH 7.4) at room temperature. Cells were washed in PBS, pelleted, and resuspended in 200 μl PBS, and 10 μl was placed on a microscope slide and covered with a coverslip. An Olympus Fluoview 1000-MPE multiphoton confocal microscope (Olympus Corporation, Tokyo, Japan) was used to image the cells at a resolution of 1,024 × 1,024 pixels with a 100 × 1.4-numerical aperture (NA) oil immersion lens. The microscope used excitation with a 488-nm argon laser and emission bandpass filter (525 ± 10 nm) for green autofluorescence and excitation with a 405-nm violet diode laser and a 655- to 755-nm bandpass filter for near-infrared bacteriochlorin fluorescence (false-colored red). Images were acquired using Fluoview 10-ASW software (version 2.0; Olympus Corporation, Tokyo, Japan).
PDT killing of mammalian cells.
A human cervical cancer cell line, HeLa, was obtained from ATCC (Manassas, VA). The cells were cultured in RPMI-1640 medium with l-glutamine and NaHCO3 (Gibco-Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum and penicillin (100 U/ml) (Sigma, St. Louis, MO) at 37°C in 5% CO2-humidified atmosphere in 75-cm2 flasks (Falcon-Invitrogen, Carlsbad, CA). When the cells reached 80% confluence, they were washed with PBS and harvested with 2 ml of 0.25% trypsin-EDTA solution (Sigma). Cells were then centrifuged and counted in trypan blue to ensure viability and plated at a density of 5,000/well in flat-bottom 96-well plates (Fisher Scientific, Pittsburgh, PA). On the following day, dilutions of bacteriochlorins 1 to 4 were prepared in two different kinds of medium: (i) complete growth medium with 10% serum and (ii) serum-free medium. These dilutions (0.01 to 10 μM concentrations of bacteriochlorins) were added to the cells for 30 min incubation. The dimethyl sulfoxide concentration in the medium did not exceed 0.2%. The medium was replaced, and 10 J/cm2 of illumination was delivered. The light spot covered four wells, which were considered one experimental group illuminated at the same time. Control groups were as follows: no treatment, light alone, and medium with the same bacteriochlorin dilutions described above. Following PDT treatment the cells were returned to the incubator overnight. Then a 4-h MTT assay (4) was carried out the next day and read at 562 nm using a microplate spectrophotometer (Spectra Max 340 PC; Molecular Devices, Sunnyvale, CA). Each experiment was repeated three times.
RESULTS
PDI studies against Gram-positive S. aureus.
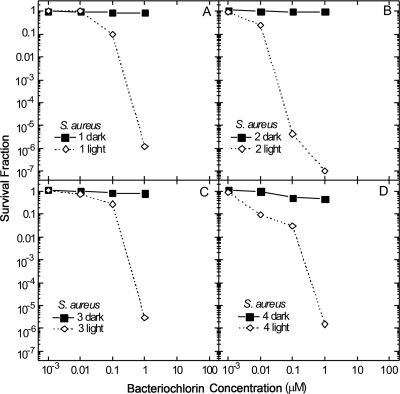
The best way to compare the phototoxicity of a group of photosensitizers with very different potencies is to vary the concentration over several orders of magnitude and determine the survival fraction with and without (dark toxicity) a single light dose. Figure 3 displays the survival fraction curves obtained against the Gram-positive bacterium S. aureus incubated for 30 min using bacteriochlorins 1 to 4 with and without illumination (10 J/cm2 732-nm laser light). The noncationic bacteriochlorin 1 produces 1 log of killing at 100 nM and almost 6 logs at 1 μM, and it eliminates the cells at higher concentrations (Fig. 3A). The most effective compound is the bis-cationic bacteriochlorin 2, which kills a remarkable 5 logs at 100 nM and eliminates the population at 1 μM (Fig. 3B). No dark toxicity is observed. Less effective than bacteriochlorin 2 are the tetrakis-cationic bacteriochlorin 3 (Fig. 3C) and the hexakis-cationic bacteriochlorin 4 (Fig. 3D), both of which have a small degree of phototoxicity at 100 nM and kill 5 logs at 1 μM. Again no dark toxicity is seen.
FIG. 3.
Survival fraction against photosensitizer concentration for the photodynamic killing of S. aureus cells. Cell suspensions of 108/ml were incubated for 30 min with different concentrations of bacteriochlorins 1 (A), 2 (B), 3 (C), and 4 (D) followed by illumination with 10 J/cm2 of 732-nm laser light.
PDI studies against Gram-negative E. coli.
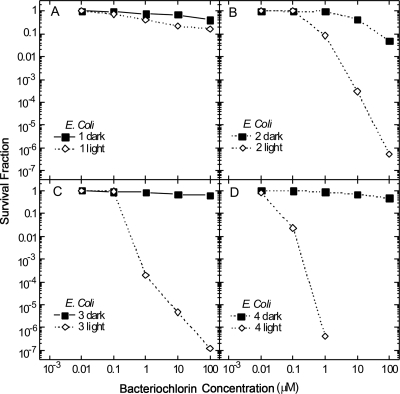
The noncationic bacteriochlorin 1 shows no effects (no phototoxicity and no dark toxicity) against the Gram-negative E. coli (Fig. 4A). The bis-cationic bacteriochlorin 2, however, is effective against E. coli, killing 1 log at 1 μM and 3 logs at 10 μM and eliminating the population at 100 μM (Fig. 4B). Modest dark toxicity (up to 1 log) is observed at the highest concentrations. The tetrakis-cationic bacteriochlorin 3 is significantly more effective, killing 4 logs at 1 μM and almost 6 logs at 10 μM and eliminating the cells at 100 μM (Fig. 4C). The hexakis-cationic bacteriochlorin 4 is even more powerful, killing 1.5 logs at 100 nM and eliminating the cells at 1 μM (Fig. 4D). Interestingly, neither bacteriochlorin 3 nor bacteriochlorin 4 displays dark toxicity.
FIG. 4.
Survival fraction against photosensitizer concentration for the photodynamic killing of E. coli cells. Cell suspensions of 108/ml were incubated for 30 min with different concentrations of bacteriochlorins 1 (A), 2 (B), 3 (C), and 4 (D) followed by illumination with 10 J/cm2 of 732-nm laser light.
PDI studies against fungal yeast C. albicans.
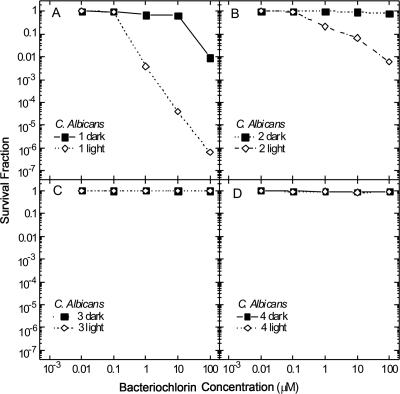
The only bacteriochlorin that exhibits a high degree of phototoxicity against the eukaryotic fungal yeast cell C. albicans is the noncationic bacteriochlorin 1 (Fig. 5A). This compound displays 4 logs of killing at 10 μM and gives total elimination (>6 logs killing) at 100 μM. A much lower degree of phototoxicity is observed with the bis-cationic bacteriochlorin 2, with 1 to 2 logs of killing at 10 to 100 μM (Fig. 5B). Neither the tetrakis-cationic bacteriochlorin 3 (Fig. 5C) nor the hexakis-cationic bacteriochlorin 4 (Fig. 5D) show any PDI effect whatsoever. The only dark toxicity toward C. albicans is 2 logs in the case of bacteriochlorin 1 at 100 μM (Fig. 5A).
FIG. 5.
Survival fraction against photosensitizer concentration for the photodynamic killing of C. albicans cells. Cell suspensions of 107/ml were incubated for 30 min with different concentrations of bacteriochlorins 1 (A), 2 (B), 3 (C), and 4 (D) followed by illumination with 10 J/cm2 of 732-nm laser light.
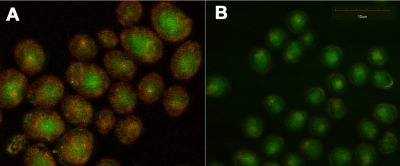
Confocal microscopy was carried out to confirm that the noncationic bacteriochlorin 1 is effective at killing Candida cells because it is able to penetrate inside the cell while the quaternized bacteriochlorin 2 cannot. Figure 6A shows C. albicans incubated with 100 μM bacteriochlorin 1 for 30 min. The bacteriochlorin fluorescence is false-colored red, and the autofluorescence from the Candida cells is false-colored green. It can be seen that the basic bacteriochlorin 1 penetrates into the interior of the yeast cells (Fig. 6A), while the bis-cationic bacteriochlorin 2 under the same conditions gives much less fluorescence, and none was visible inside the yeast cells (Fig. 6B).
FIG. 6.
Two color confocal fluorescence micrographs. C albicans cells were incubated for 30 min with 100 μM bacteriochlorin 1 (A) or 2 (B). Autofluorescence is colored green and near-infrared bacteriochlorin fluorescence is colored red. The scale bar is 10 μm.
PDI studies against mammalian cells.
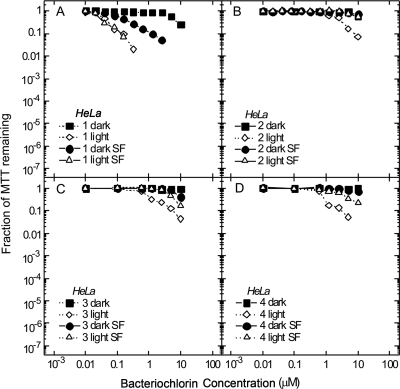
In order to answer the question whether these bacteriochlorins might exhibit selective killing of microbial (bacterial or fungal) cells versus the host mammalian cells, experiments were performed using a human cancer cell line (HeLa) and the same short incubation time (30 min). Tetrapyrroles are thought to be taken up rapidly into microbial cells but much more slowly into mammalian cells. In order to carry out a fair comparison (because the microbial cells were incubated in serum-free medium) two different incubation media were used. These were complete medium with 10% fetal bovine serum and also RPMI medium without serum. All three cationic bacteriochlorins (bacteriochlorin 2 [Fig. 7B], bacteriochlorin 3 [Fig. 7C], and bacteriochlorin 4 [Fig. 7D]) exhibit minimal PDT killing of HeLa cells, exhibiting significant phototoxicity only above 5 μM regardless of the presence of serum. In contrast, the nonquaternized bacteriochlorin 1 (Fig. 7A) shows much greater (at least 50 times) phototoxicity, with significant killing observed at only 100 nM. Although the MTT assay is capable of measuring only 2.5 logs of cell killing, the same y axis was used in Fig. 3 to 5 and 7 to emphasize the observed selectivity of the bacteriochlorins for microbial over mammalian cells. In contrast to expectations, the PDT killing of HeLa cells by compounds 2 to 4 after incubation in serum-free medium was actually lower than that found with conventional serum-containing medium (Fig. 7B to D). It was expected that the uptake of bacteriochlorins into the cells might be higher when there was no competition for binding from serum proteins, but apparently this was not the case. The only case where the killing was higher after incubation in serum-free medium was that of the dark toxicity (not PDT) for compound 1 (Fig. 7A).
FIG. 7.
Survival fraction against photosensitizer concentration for the photodynamic killing of HeLa cells. Cells (5,000/well) were incubated in complete medium or in serum-free medium (SF) for 30 min with different concentrations of compounds 1 (A), 2 (B), 3 (C), and 4 (D) followed by illumination (light) or not (dark) with 10 J/cm2 of 732-nm laser light. Viability was determined 24 h later by MTT assay.
Photophysical properties.
Each compound exhibits a characteristic bacteriochlorin absorption spectrum (23) with a broad near-UV Soret (B) feature and a long-wavelength Qy feature of comparable intensity in the near-infrared spectral region; absorption spectra for bacteriochlorins 1 and 2 are shown in Fig. 2. The wavelength tunability of synthetic bacteriochlorins via functionalization of the 3- and 13-positions has been reported previously (50). The Qy absorption maxima of bacteriochlorins 1 to 4 in methanol are in the range 718 to 742 (Table 1). Lifetimes of the singlet state are in the range 3.5 to 4.0 ns, and the lifetimes of the lowest triplet excited state are in the range 54 to 90 μs (all in the absence of oxygen). The triplet lifetimes are reduced to <1 μs in the presence of atmospheric oxygen, indicating facile excited-state quenching. The yields of the excited triplet state are in the range 0.48 to 0.53. These values are comparable to that of 0.54 for bacteriopheophytin a (17).
Molecular orbital characteristics.
The energies of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of each bacteriochlorin were obtained from DFT calculations. Table 1 shows that the HOMO energy becomes more negative along the following series: bacteriochlorin 1 (−4.42 eV) > bacteriochlorin 3 (−4.56 eV) > bacteriochlorin 2 (−4.72 eV) > bacteriochlorin 4 (−5.05 eV). Along this series, the bacteriochlorins will be progressively harder to oxidize. Table 1 also shows that the LUMO energy becomes more negative along the following series: bacteriochlorin 1 (−2.19 eV) > bacteriochlorin 2 (−3.34 eV) > bacteriochlorin 3 (−3.89 eV) > bacteriochlorin 4 (−3.92 eV). Along this series, the bacteriochlorins will be progressively easier to reduce.
A prior study of a series of zinc chlorins showed excellent linear correlations between the calculated orbital energies and measured redox potentials, with a shift in the HOMO or LUMO energy of 100 meV giving a shift in the oxidation or reduction potential of ∼100 mV (21). These findings suggest that differences in ground-state (S0) oxidation and/or reduction potentials among the bacteriochlorins studied here may be substantial. The one caveat is that the redox properties of a bacteriochlorin may differ considerably depending on the subcellular localization site. A second caveat is that the triplet excited state (T1) redox potentials will differ from those for the ground state (S0) by the T1-to-S0 energy gap, which typically will vary similarly to the S1-to-S0 energy gaps derived from Qy(0,0) spectral positions (Table 1). Potential connections between redox and PDI activity are given below.
DISCUSSION
The present report has demonstrated that stable, synthetic cationic bacteriochlorins are highly promising candidate photosensitizers for antimicrobial PDT. The new method for bacteriochlorin synthesis (22, 44) provides compounds with gem-dimethyl groups in the reduced pyrrole rings at the 8 and 18 positions. This substitution pattern locks in the bacteriochlorin macrocycle by preventing the oxidation reactions that typically occur with derivatives of naturally occurring bacteriochlorins. These reactions lead to instabilities encountered with many other bacteriochlorins previously tested for PDT activity. The versatility of the 3,13-disubstituted bacteriochlorin building blocks enables macrocycles with a variety of substituent patterns to be prepared, including the set of quaternized compounds that were studied herein.
A large number of publications have pointed out the necessity of using cationic charged photosensitizers to efficiently mediate photodynamic inhibition (PDI) of Gram-negative bacteria; however, reports that have compared structure-function relationships of photosensitizers against three different classes of microbial cell (Gram-positive bacteria, Gram-negative bacteria, and fungal yeast) are less common (30). One striking result from the present investigation is that the photosensitizer structure that gives the maximum PDI effect is different for each class of microbial cell.
All four compounds tested were highly active against the Gram-positive S. aureus (Fig. 3). The bis-quaternized bacteriochlorin 2 was most effective, producing a remarkable 5 logs of killing at 100 nM. The other three compounds (basic, tetrakis-quaternized and hexakis-quaternized) exhibited comparable levels of cell killing that were lower than that of bacteriochlorin 2 but still quite substantial (>5 logs at 1 μM). One explanation of this finding is that there exists an optimum level of cationic charge necessary both to bind to bacterial anionic phosphate groups and to allow penetration into the bacterial cell wall, where the reactive oxygen species produced upon illumination can do most damage. Levels of cationic charge less than this optimum value (for instance, the properties of bacteriochlorin 1) will not lead to sufficient binding, and cationic charges greater than this optimum value (for instance, bacteriochlorins 3 and 4) will lead to the binding being too strong to allow greater photosensitizer penetration into the cell wall. A similar finding has been presented in two other reports by one of our groups involving comparisons of conjugates between chlorin(e6) and different sizes of polylysine chains (16) or different sizes of polyethylenimine chains (51). In both cases the smallest conjugate with the least cationic charges had the greatest PDI effect against S. aureus, while the largest conjugate with the most cationic charges was the most effective against E. coli. Maisch et al. (28) also found that a porphyrin with two cationic groups was a better photosensitizer against S. aureus than a molecule with four such groups.
A more straightforward structure-function relationship is found here for the effect of the bacteriochlorins against the Gram-negative E. coli (Fig. 4). In particular, the greater the number of cationic quaternized groups the greater the PDI effect. Bacteriochlorin 4, with six cationic groups, kills measurable numbers of cells at 100 nM and eliminates the population at 1 μM. Bacteriochlorin 3 (four cationic groups) kills 4 logs at 1 μM, while bacteriochlorin 2 (two cationic groups) kills only 1 log at 1 μM, and bacteriochlorin 1 (no cationic groups) has no killing effect at all.
The fungal yeast C. albicans displays yet another structure-function relationship (Fig. 5). Only the noncationic bacteriochlorin 1 has a high PDI killing effect, namely, elimination of the population (>6 logs) at 100 μM. The bis-cationic bacteriochlorin 2 shows a measurable 1 to 2 logs of killing at ≥1 μM, while bacteriochlorins with four (bacteriochlorin 3) or six (bacteriochlorin 4) cationic groups give no killing effect at all. The microscopy studies suggest that the noncationic bacteriochlorin 1 is able to penetrate to the interior of the fungal cells, while the cationic bacteriochlorin 2 cannot; this difference in localization and uptake explains the much greater fungicidal effect of bacteriochlorin 1. The similarity of the structure-function relationships between Candida and HeLa cells is presumably due to the fact that fungal cells are eukaryotic and to some extent resemble mammalian cells in their overall cellular structure. Because both types of cells are classified as eukaryotes, they have many component features in common, including plasma membrane, nucleus and nuclear membrane, mitochondria, endoplasmic reticulum, Golgi apparatus, and cytoskeleton.
While many authors have reported that Candida cells are susceptible to PDI with cationic photosensitizers (9, 25, 36), there are other reports that photosensitizers commonly used to kill cancer cells, such as Photofrin (5), are also effective against yeast cells. Further study is necessary to understand the precise structural features of photosensitizer molecules for optimal PDI of fungal cells while preserving selectivity over the host mammalian cells. The overall goal of antimicrobial PDT is to be able to kill microbes that are infecting tissue after local application of the photosensitizer solution to the infected area and subsequent illumination. Thus, it is necessary to also study the PDT killing of mammalian cells that would comprise the host tissue. To this end, a human cancer cell line (HeLa cells) was investigated using the same incubation time (30 min) employed for the microbial cells. The structure-function relationship was to some extent similar to that found for C. albicans, with only the basic bacteriochlorin (bacteriochlorin 1) giving any significant level of killing at concentrations lower than 1 μM. Therefore, selective PDT killing of bacteria (and to a lesser extent fungal cells) compared to that of mammalian cells is accomplished with quaternized bacteriochlorins, with the bis-cationic compound giving the highest selectivity for S. aureus and the hexakis-cationic compound giving the highest selectivity for E. coli.
To our knowledge there has been only one prior investigation of bacteriochlorins as antimicrobial photosensitizers. Schastak et al. (45) compared the photodynamic killing of S. aureus, methicillin-resistant S. aureus (MRSA), E. coli, and Pseudomonas aeruginosa using a meso-substituted tetramethylpyridinium bacteriochlorin with that with a chorin(e6) derivative called Photolon. The cationic bacteriochlorin was able to kill both Gram-positive and Gram-negative bacteria, while the anionic Photolon was only able to kill Gram-positive species. Several groups have studied bacteriochlorins to kill cancer cells and to treat tumors in vivo. The long-wavelength light between 700 and 800 nm that is absorbed by bacteriochlorins is believed to be ideally suited to penetrate living tissue due to reduced absorption by tissue chromophores and reduced Mie scattering (53). The large extinction coefficient (>100,000 M−1cm−1) typical of the bacteriochlorin Qy band is also advantageous for strong absorption of near-infrared light by the photosensitizer. The Pd-containing bacteriochlorins known as TOOKAD (13, 57) and Stakel (2) have been extensively investigated in laboratory studies, and, in addition, TOOKAD has been studied in clinical trials of PDT for prostate cancer (54).
The photophysical studies and DFT calculations indicate that the activity differences observed among bacteriochlorins 1 to 4 must stem from cellular binding and localization effects rather than photochemical properties. Indeed, the yield of the triplet excited state (from which the reactive oxygen species is produced) is essentially identical (0.48 to 0.53) for the four bacteriochlorins, and in each case the lifetime is reduced to <1 μs in the presence of atmospheric oxygen, indicating facile excited-state quenching. Moreover, there is no specific correlation between the anticipated differences in redox properties (based on the molecular orbital energies) for the four bacteriochlorins and their PDI activities against any of the organisms studied. The only broad trend is that bacteriochlorins 1 and 2 are typically more active than bacteriochlorins 3 and 4, which, all other things being equal, would favor a mechanism of activity that involves reduction rather than oxidation of the photoexcited bacteriochlorin to the extent that electron transfer is involved.
In conclusion, bacteriochlorins with constitutive cationic charges provided by quaternized ammonium groups are highly active antibacterial photosensitizers. The hexakis-cationic bacteriochlorin 4 is capable of eliminating (>6 logs killing) both Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria at the remarkably low concentration of 1 μM. Good selectivity (4 to 5 logs) for bacteria over mammalian cells is observed. Only the nonquaternized bacteriochlorin 1 shows good PDT killing of the yeast (C. albicans), and selectivity over mammalian cells is lower in this case because both cell types are eukaryotic organisms.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (R01AI050875 to M.R.H. and R01GM36238 to J.S.L.), a Burroughs-Wellcome fellowship (to M.K.), and the JimmyV NCSU Cancer Therapeutics Training Program. L.H., Y.-Y.H. and T.B. were supported by a grant (R41AI072854) from the National Institute of Allergy and Infectious Diseases to NIRvana Pharmaceuticals, Inc. P.M. was partly supported by a Genzyme-Partners Translational Research Grant. G.P.T was partly supported by a Massachusetts Technology Transfer Center Award. Characterization of the photophysical and redox properties of the bacteriochlorins described herein was initially motivated by solar-energy studies and supported by grants from the Division of Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences of the U.S. Department of Energy, to D.F.B. (DE-FG02-05ER15660) and D.H. (DE-FG02-05ER15661).
We thank Aaron Mitchell, Department of Microbiology, Columbia University, New York, NY, for the gift of DAY286 reference strain of C. albicans. We thank Jie (Jenny) Zhao and Margaret E. Sherwood, Wellman Center for Photomedicine, Massachusetts General Hospital for help with confocal microscopy.
Footnotes
Published ahead of print on 12 July 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Bell, S. G. 2003. Antibiotic resistance: is the end of an era near? Neonatal Netw. 22:47-54. [DOI] [PubMed] [Google Scholar]
- 2.Berdugo, M., R. A. Bejjani, F. Valamanesh, M. Savoldelli, J. C. Jeanny, D. Blanc, H. Ficheux, A. Scherz, Y. Salomon, D. BenEzra, and F. Behar-Cohen. 2008. Evaluation of the new photosensitizer Stakel (WST-11) for photodynamic choroidal vessel occlusion in rabbit and rat eyes. Invest. Ophthalmol. Vis. Sci. 49:1633-1644. [DOI] [PubMed] [Google Scholar]
- 3.Carmeli, Y. 2008. Strategies for managing today's infections. Clin. Microbiol. Infect. 14(Suppl. 3):22-31. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael, J., W. G. DeGraff, A. F. Gazdar, J. D. Minna, and J. B. Mitchell. 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 47:936-942. [PubMed] [Google Scholar]
- 5.Chabrier-Rosello, Y., T. H. Foster, N. Perez-Nazario, S. Mitra, and C. G. Haidaris. 2005. Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob. Agents Chemother. 49:4288-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demidova, T. N., and M. R. Hamblin. 2005. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Chemother. 49:2329-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demidova, T. N., and M. R. Hamblin. 2004. Photodynamic therapy targeted to pathogens. Int. J. Immunopathol. Pharmacol. 17:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolmans, D. E., D. Fukumura, and R. K. Jain. 2003. Photodynamic therapy for cancer. Nat. Rev. Cancer 3:380-387. [DOI] [PubMed] [Google Scholar]
- 9.Foley, J. W., X. Song, T. N. Demidova, F. Jilal, and M. R. Hamblin. 2006. Synthesis and properties of benzo[a]phenoxazinium chalcogen analogues as novel broad-spectrum antimicrobial photosensitizers. J. Med. Chem. 49:5291-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuzumi, S., K. Ohkubo, X. Zheng, Y. Chen, R. K. Pandey, R. Zhan, and K. M. Kadish. 2008. Metal bacteriochlorins which act as dual singlet oxygen and superoxide generators. J. Phys. Chem. B. 112:2738-2746. [DOI] [PubMed] [Google Scholar]
- 11.Gad, F., T. Zahra, K. P. Francis, T. Hasan, and M. R. Hamblin. 2004. Targeted photodynamic therapy of established soft-tissue infections in mice. Photochem. Photobiol. Sci. 3:451-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gradyushko, A. T., A. N. Sevchenko, K. N. Solovyov, and M. P. Tsvirko. 1970. Energetics of photophysical processes in chlorophyll-like molecules. Photochem. Photobiol. 11:387-400. [DOI] [PubMed] [Google Scholar]
- 13.Gross, S., A. Gilead, A. Scherz, M. Neeman, and Y. Salomon. 2003. Monitoring photodynamic therapy of solid tumors online by BOLD-contrast MRI. Nat. Med. 9:1327-1331. [DOI] [PubMed] [Google Scholar]
- 14.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamblin, M. R., and P. Mroz. 2008. Advances in photodynamic therapy: basic, translational and clinical. Artech House, Boston, MA.
- 16.Hamblin, M. R., D. A. O'Donnell, N. Murthy, K. Rajagopalan, N. Michaud, M. E. Sherwood, and T. Hasan. 2002. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J. Antimicrob. Chemother. 49:941-951. [DOI] [PubMed] [Google Scholar]
- 17.Holten, D., M. Gouterman, W. W. Parson, M. W. Windsor, and M. G. Rockley. 1976. Electron transfer from photoexcited singlet and triplet bacteriopheophytin. Photochem. Photobiol. 23:415-420. [DOI] [PubMed] [Google Scholar]
- 18.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. Biotechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 19.Jori, G., C. Fabris, M. Soncin, S. Ferro, O. Coppellotti, D. Dei, L. Fantetti, G. Chiti, and G. Roncucci. 2006. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg. Med. 38:468-481. [DOI] [PubMed] [Google Scholar]
- 20.Kee, H. L., J. Bhaumik, J. R. Diers, P. Mroz, M. R. Hamblin, D. F. Bocian, J. S. Lindsey, and D. Holten. 2008. Photophysical characterization of imidazolium-substituted Pd(II), In(III), and Zn(II) porphyrins as photosensitizers for photodynamic therapy. J. Photochem. Photobiol. A 200:346-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kee, H. L., C. Kirmaier, Q. Tang, J. R. Diers, C. Muthiah, M. Taniguchi, J. K. Laha, M. Ptaszek, J. S. Lindsey, D. F. Bocian, and D. Holten. 2007. Effects of substituents on synthetic analogs of chlorophylls. Part 2: Redox properties, optical spectra and electronic structure. Photochem. Photobiol. 83:1125-1143. [DOI] [PubMed] [Google Scholar]
- 22.Kim, H. J., and J. S. Lindsey. 2005. De novo synthesis of stable tetrahydroporphyrinic macrocycles: bacteriochlorins and a tetradehydrocorrin. J. Org. Chem. 70:5475-5486. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, M., M. Akiyama, H. Kano, and H. Kise. 2006. Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications, p. 79-94. In B. Grimm, R. J. Porra, W. Rüdiger, and H. Scheer (ed.), Advances in photosynthesis and respiration, vol. 25. Springer, Dordrecht, Netherlands. [Google Scholar]
- 24.Kong, J., C. A. White, A. I. Krylov, D. Sherrill, R. D. Adamson, T. R. Furlani, M. S. Lee, A. M. Lee, S. R. Gwaltney, T. R. Adams, C. Ochsenfeld, A. T. B. Gilbert, G. S. Kedziora, V. A. Rassolov, D. R. Maurice, N. Nair, Y. Shao, N. A. Besley, P. E. Maslen, J. P. Dombroski, H. Daschel, W. Zhang, P. P. Korambath, J. Baker, E. F. C. Byrd, T. Van Voorhis, M. Oumi, S. Hirata, C.-P. Hsu, N. Ishikawa, J. Florian, A. Warshel, B. G. Johnson, P. M. W. Gill, M. Head-Gordon, and J. A. Pople. 2000. Q-Chem. 2.0: a high-performance ab initio electronic structure program package. J. Comput. Chem. 21:1532-1548. [Google Scholar]
- 25.Lambrechts, S. A., M. C. Aalders, and J. Van Marle. 2005. Mechanistic study of the photodynamic inactivation of Candida albicans by a cationic porphyrin. Antimicrob. Agents Chemother. 49:2026-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauro, F. M., P. Pretto, L. Covolo, G. Jori, and G. Bertoloni. 2002. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem. Photobiol. Sci. 1:468-470. [DOI] [PubMed] [Google Scholar]
- 27.Maisch, T. 2007. Anti-microbial photodynamic therapy: useful in the future? Lasers Med. Sci. 22:83-91. [DOI] [PubMed] [Google Scholar]
- 28.Maisch, T., C. Bosl, R. M. Szeimies, N. Lehn, and C. Abels. 2005. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob. Agents Chemother. 49:1542-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malik, Z., H. Ladan, and Y. Nitzan. 1992. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J. Photochem. Photobiol. B. 14:262-266. [DOI] [PubMed] [Google Scholar]
- 30.Mantareva, V., V. Kussovski, I. Angelov, E. Borisova, L. Avramov, G. Schnurpfeil, and D. Wohrle. 2007. Photodynamic activity of water-soluble phthalocyanine zinc(II) complexes against pathogenic microorganisms. Bioorg. Med. Chem. 15:4829-4835. [DOI] [PubMed] [Google Scholar]
- 31.Merchat, M., G. Bertolini, P. Giacomini, A. Villanueva, and G. Jori. 1996. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B. 32:153-157. [DOI] [PubMed] [Google Scholar]
- 32.Michel, M., and L. Gutmann. 1997. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: therapeutic realities and possibilities. Lancet 349:1901-1906. [DOI] [PubMed] [Google Scholar]
- 33.Minnock, A., D. I. Vernon, J. Schofield, J. Griffiths, J. H. Parish, and S. B. Brown. 2000. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob. Agents Chemother. 44:522-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizzoni, R. H., M. A. Hennessey, and C. R. Scholz. 1954. Polyamine salts with autonomic blocking properties. J. Am. Chem. Soc. 76:2414-2417. [Google Scholar]
- 35.Moan, J., and Q. Peng. 2003. An outline of the hundred-year history of PDT. Anticancer Res. 23:3591-3600. [PubMed] [Google Scholar]
- 36.Munin, E., L. M. Giroldo, L. P. Alves, and M. S. Costa. 2007. Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT). J. Photochem. Photobiol. B. 88:16-20. [DOI] [PubMed] [Google Scholar]
- 37.Nitzan, Y., R. Dror, H. Ladan, Z. Malik, S. Kimel, and V. Gottfried. 1995. Structure-activity relationship of porphines for photoinactivation of bacteria. Photochem. Photobiol. 62:342-347. [DOI] [PubMed] [Google Scholar]
- 38.Nitzan, Y., M. Gutterman, Z. Malik, and B. Ehrenberg. 1992. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem. Photobiol. 55:89-96. [DOI] [PubMed] [Google Scholar]
- 39.Nobile, C. J., and A. P. Mitchell. 2005. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr. Biol. 15:1150-1155. [DOI] [PubMed] [Google Scholar]
- 40.Oertel, M., S. I. Schastak, A. Tannapfel, R. Hermann, U. Sack, J. Mossner, and F. Berr. 2003. Novel bacteriochlorine for high tissue-penetration: photodynamic properties in human biliary tract cancer cells in vitro and in a mouse tumour model. J. Photochem. Photobiol. B. 71:1-10. [DOI] [PubMed] [Google Scholar]
- 41.Owens, R. C., Jr. 2008. Antimicrobial stewardship: concepts and strategies in the 21st century. Diagn. Microbiol. Infect. Dis. 61:110-128. [DOI] [PubMed] [Google Scholar]
- 42.Rovers, J. P., M. L. de Jode, and M. F. Grahn. 2000. Significantly increased lesion size by using the near-infrared photosensitizer 5,10,15,20-tetrakis (m-hydroxyphenyl)bacteriochlorin in interstitial photodynamic therapy of normal rat liver tissue. Lasers Surg. Med. 27:235-240. [DOI] [PubMed] [Google Scholar]
- 43.Rovers, J. P., M. L. de Jode, H. Rezzoug, and M. F. Grahn. 2000. In vivo photodynamic characteristics of the near-infrared photosensitizer 5,10,15,20-tetrakis(M-hydroxyphenyl) bacteriochlorin. Photochem. Photobiol. 72:358-364. [DOI] [PubMed] [Google Scholar]
- 44.Ruzie, C., M. Krayer, T. Balasubramanian, and J. S. Lindsey. 2008. Tailoring a bacteriochlorin building block with cationic, amphipathic, or lipophilic substituents. J. Org. Chem. 73:5806-5820. [DOI] [PubMed] [Google Scholar]
- 45.Schastak, S., B. Gitter, R. Handzel, R. Hermann, and P. Wiedemann. 2008. Improved photoinactivation of gram-negative and gram-positive methicillin-resistant bacterial strains using a new near-infrared absorbing meso-tetrahydroporphyrin: a comparative study with a chlorine e6 photosensitizer photolon. Methods Find. Exp. Clin. Pharmacol. 30:129-133. [DOI] [PubMed] [Google Scholar]
- 46.Schuitmaker, J. J., J. A. van Best, J. L. van Delft, T. M. Dubbelman, J. A. Oosterhuis, and D. de Wolff-Rouendaal. 1990. Bacteriochlorin a, a new photosensitizer in photodynamic therapy. In vivo results. Invest. Ophthalmol. Vis. Sci. 31:1444-1450. [PubMed] [Google Scholar]
- 47.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 48.Solban, N., I. Rizvi, and T. Hasan. 2006. Targeted photodynamic therapy. Lasers Surg. Med. 38:522-531. [DOI] [PubMed] [Google Scholar]
- 49.Tang, H. M., M. R. Hamblin, and C. M. Yow. 2007. A comparative in vitro photoinactivation study of clinical isolates of multidrug-resistant pathogens. J. Infect. Chemother. 13:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taniguchi, M., D. L. Cramer, A. D. Bhise, H. L. Kee, D. F. Bocian, D. Holten, and J. S. Lindsey. 2008. Accessing the near-infrared spectral region with stable, synthetic, wavelength-tunable bacteriochlorins. New J. Chem. 32:947-958. [Google Scholar]
- 51.Tegos, G. P., M. Anbe, C. Yang, T. N. Demidova, M. Satti, P. Mroz, S. Janjua, F. Gad, and M. R. Hamblin. 2006. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob. Agents Chemother. 50:1402-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tegos, G. P., T. N. Demidova, D. Arcila-Lopez, H. Lee, T. Wharton, H. Gali, and M. R. Hamblin. 2005. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 12:1127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torricelli, A., A. Pifferi, P. Taroni, E. Giambattistelli, and R. Cubeddu. 2001. In vivo optical characterization of human tissues from 610 to 1010 nm by time-resolved reflectance spectroscopy. Phys. Med. Biol. 46:2227-2237. [DOI] [PubMed] [Google Scholar]
- 54.Trachtenberg, J., R. A. Weersink, S. R. Davidson, M. A. Haider, A. Bogaards, M. R. Gertner, A. Evans, A. Scherz, J. Savard, J. L. Chin, B. C. Wilson, and M. Elhilali. 2008. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: a study of escalating light doses. BJU Int. 102:556-562. [DOI] [PubMed] [Google Scholar]
- 55.van Duijnhoven, F. H., J. P. Rovers, K. Engelmann, Z. Krajina, S. F. Purkiss, F. A. Zoetmulder, T. J. Vogl, and O. T. Terpstra. 2005. Photodynamic therapy with 5,10,15,20-tetrakis(m-hydroxyphenyl) bacteriochlorin for colorectal liver metastases is safe and feasible: results from a phase I study. Ann. Surg. Oncol. 12:808-816. [DOI] [PubMed] [Google Scholar]
- 56.Weber, G., and F. W. J. Teale. 1957. Determination of the absolute quantum yield of fluorescent solutions. Trans. Faraday Soc. 53:646-655. [Google Scholar]
- 57.Woodhams, J. H., A. J. MacRobert, M. Novelli, and S. G. Bown. 2006. Photodynamic therapy with WST09 (Tookad): quantitative studies in normal colon and transplanted tumours. Int. J. Cancer 118:477-482. [DOI] [PubMed] [Google Scholar]
- 58.Wormald, R., J. Evans, L. Smeeth, and K. Henshaw. 2005. Photodynamic therapy for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 4:CD002030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.