Abstract
The objective of the present prospective pharmacokinetic study was to describe the variability of plasma gentamicin concentrations in critically ill patients with acute kidney injury (AKI) necessitating extended daily diafiltration (EDD-f) using a population pharmacokinetic model and to subsequently perform Monte Carlo dosing simulations to determine which dose regimen achieves the pharmacodynamic targets the most consistently. We collected data from 28 gentamicin doses in 14 critically ill adult patients with AKI requiring EDD-f and therapeutic gentamicin. Serial plasma samples were collected. A population pharmacokinetic model was used to describe the pharmacokinetics of gentamicin and perform Monte Carlo simulations with doses of between 3 mg/kg of body weight and 7 mg/kg and at various time points before commencement of EDD-f to evaluate the optimal dosing regimen for achieving pharmacodynamic targets. A two-compartment pharmacokinetic model adequately described the gentamicin clearance while patients were on and off EDD-f. The plasma half-life of gentamicin during EDD-f was 13.8 h, whereas it was 153.4 h without EDD-f. Monte Carlo simulations suggest that dosing with 6 mg/kg every 48 h either 30 min or 1 h before the commencement of EDD-f results in 100% attainment of the target maximum concentration drug in plasma (<10 mg/liter) and sufficient attainment of the target area under the concentration-time curve from 0 to 24 h (AUC0-24; 70 to 120 mg·h/liter). None of the simulated dosing regimens satisfactorily achieved the targets of the minimum concentrations of drug in plasma (<1.0 mg/liter) at 24 h. In conclusion, dosing of gentamicin 30 min to 1 h before the commencement of an EDD-f treatment enables attainment of target peak concentrations for maximal therapeutic effect while enhancing drug clearance to minimize toxicity. Redosing in many patients should occur after 48 h, and we recommend the use of therapeutic drug monitoring to guide dosing to optimize achievement of the AUC0-24 targets.
Acute kidney injury (AKI) is an independent risk factor for mortality in critically ill patients (5) and has a mortality rate as high as 60% (20). Of all patients admitted to a critical care unit, approximately 5% will develop AKI and at least 70% of these patients will require renal replacement therapy (RRT) (11). Extended daily diafiltration (EDD-f), also known as sustained low-efficiency dialysis, is an emerging form of RRT increasingly being used worldwide. EDD-f combines the convenience and efficiency of intermittent hemodialysis with the stability of traditional continuous renal replacement therapy into a 10- to 12-h treatment (16). This hybrid type of dialysis is reported to have advantages over intermittent hemodialysis and continuous RRT, including efficient solute removal with minimum solute disequilibrium, improved hemodynamic stability, low anticoagulant needs, diminished cost, and improved patient mobility (16). This has been confirmed with critically ill patients (1). However, drug dosing remains a challenge to critical care physicians, and to date few pharmacokinetic studies of this specific dialysis modality have been undertaken (7, 10).
In critically ill patients, a wide array of pathophysiological changes which complicate antibiotic dosing can occur (17). Changes in clearances and volumes of distribution for different dialysis treatments have been widely described, although the number and type of dialysis modalities and the consequent altered capacity for drug clearance necessitate that pharmacokinetic studies be undertaken to determine the precise dosing requirements for a drug in the chosen dialysis modality (6). Such information is vital in the context of antibiotic dosing, particularly in patients with AKI, who have higher mortality rates than other critically ill patients (5, 20).
Gentamicin is a widely used aminoglycoside antibiotic that has an important role in treatment of infections in critical care units. Gentamicin exhibits concentration-dependent bacterial killing (13), requiring a maximum concentration in plasma (Cmax) during a dosing period of at least 10 mg/liter (4). Further to this, an area under the concentration-time curve from 0 to 24 h (AUC0-24) of 70 to 120 mg·h/liter has been reported to be desirable for efficacy (4). Minimizing aminoglycoside toxicity in patients receiving gentamicin is best achieved by minimizing the minimum concentration (Cmin) during a dosing period. Emerging data support maintenance of an AUC0-24 of <120 mg·h/liter/day to minimize toxicity as well (4).
The aims of the present study were to describe the variability of plasma gentamicin concentrations in critically ill patients with AKI necessitating EDD-f using a population pharmacokinetic model and to subsequently perform Monte Carlo dosing simulations to determine a dose regimen that most consistently achieves the pharmacodynamic targets.
MATERIALS AND METHODS
Patients.
The present study was performed in a 13-bed intensive care unit of a 530-bed hospital. Ethical approval to conduct the study was obtained from the local institutional ethics committee (protocol 200620). Consent to participate was obtained from the patient's legally authorized representative.
Procedures.
Critically ill adult patients with AKI requiring EDD-f and therapeutic gentamicin treatment were studied. In accordance with usual practice, all patients had an indwelling arterial cannula. Patients were administered gentamicin at the discretion of the treating physician.
Dialysis prescription.
EDD-f was performed in all patients with a 4008S hemodialysis machine (Fresenius Medical Care, Bad Homburg, Germany) using Fresenius AV600S filters (surface area, 0.6 m2; Fresenius Medical Care). For each patient, a central vein was cannulated with a standard dialysis vascular catheter. A standardized prescription consisted of hemodiafiltration with a target duration of 10 h (with 300 ml/min of blood and dialysate flow and 50 ml/min of predilution). The target duration was rarely achieved, with EDD-f typically occurring for a duration of 6 h. The biochemical composition of the dialysate and bicarbonate-based replacement fluid was set according to the patient's biochemistry. Data on the precise times for EDD-f commencement and cessation, due to blood clotting on the filter or the end of treatment, were recorded.
Sample collection.
Gentamicin was administered by a central venous line as a 30-min infusion. Samples of arterial blood were collected from an indwelling arterial cannula before the drug administration (T0) and at 15 min (T15), T30, T60, T120, T180, T300, T480, and T600 after the drug administration. Further arterial blood samples were collected from each patient to establish the plasma concentrations between EDD-f sessions. Sampling occurred during the first dosing period and at subsequent dosing intervals where possible thereafter. EDD-f was commenced at the discretion of the clinician and did not uniformly correspond with the timing of gentamicin dosing.
Drug assay.
Plasma gentamicin concentrations were determined using a Synchron LX system assay (Beckman-Coulter Inc., Fullerton, CA). The within-run coefficients of variation were estimated to be 7.1% at 2.2 mg/liter and 2.1% at 9.7 mg/liter. The lowest level of detection was 0.3 mg/liter.
Pharmacokinetic and statistical analysis.
The concentration-versus-time data for gentamicin in plasma were analyzed by a nonlinear mixed-effects modeling approach (2) using NONMEM software (version 6.1; GloboMax LLC, Hanover, MD) with double precision with a Compaq Visual Fortran compiler. Cmax and Cmin were the observed values from the intensive sampling schedule. The NONMEM runs were executed using Wings for NONMEM (WFN; version 6.1.3). Data were analyzed using the first-order conditional estimation method with the Interaction program.
For the population pharmacokinetic analysis, the plasma gentamicin concentrations were fitted to one-, two-, or three-compartment linear models using subroutines from the NONMEM library (2). The concentration-time profile can be described as (equation 1):
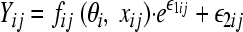 |
(1) |
where yij is the jth observed concentration at time points xij for the ith subject. θi represents the fixed-effects parameter of the structural model to be estimated. fij is the function for the prediction of the jth response for the ith subject. Finally, ɛij denotes the jth measurement error for the ith subject. In other words, ɛij is the difference of the observed concentration from the predicted concentration. It is assumed to be independent and identically distributed with a normal distribution around the mean zero and variance σ2.
Between-subject variability and between-occasion variability.
Between-subject variability was modeled using an exponential variability model (equation 2):
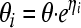 |
(2) |
where θi is the value of the parameter for the ith subject; θ is the typical value of the parameter in the population; and finally, ηi is a random vector with normal distribution, zero mean, and a variance-covariance matrix of between-subject variability (Ω) to be estimated.
Model diagnostics.
To assess model validity, statistical comparison of nested models was undertaken in the NONMEM program on the basis of a χ2 test of the difference in the objective function. A decrease in the objective function of 3.84 units (P < 0.05) was considered significant. The goodness of fit was evaluated by visual inspection of diagnostic scatter plots, including the observed and predicted concentrations versus time, weighted residual versus time, and residual versus predicted concentrations.
Bootstrap.
A nonparametric bootstrap method (15) (n = 1,000) was used to study the uncertainty of all pharmacokinetic parameter estimates in the final base model. From the bootstrap empirical posterior distribution, we have been able to obtain the 95% confidence interval (2.5 to 97.5%) for the parameters, as described previously (14).
Covariate screening.
EDD-f was considered an essential covariate, and therefore, a base EDD-f model was established before other covariates were considered. The covariates analyzed were age, total body weight (normalized to 70 kg), lean body weight (LBW; equation from Janmahasatian et al. [9]) normalized to 55 kg, the final plasma creatinine concentration prior to commencement of EDD-f, and creatinine clearance estimated via the Cockroft-Gault equation (using total body weight) normalized to 6 liters h−1 and an adjusted aminoglycoside dosing weight (3). Possible covariates were added into the model in a stepwise fashion. Covariates were considered for inclusion in the model if they were biologically plausible and there was improvement in the overall fit of the base model, i.e., a decrease in the objective function (at least 3.84 units), a decrease in the unexplained between-subject variability of the parameter, and a decrease in residual unexplained variability.
Dosing simulations.
Monte Carlo dose simulations of different weight-based dosing regimens with differential delay between gentamicin dosing and commencement of a 10-h treatment of EDD-f were undertaken using NONMEM. The simulations used a 50-year-old male (total body weight, 70 kg; body mass index, 23.6 kg/m2) with daily doses of 3 mg/kg of body weight, 4 mg/kg, 5 mg/kg, or 7 mg/kg administered either at the beginning of EDD-f (no delay) or at 30 min, 1 h, 2 h, or 4 h before EDD-f commencement. Simulations of dosing every 48 h were also undertaken. Post-EDD-f dosing was not simulated, in line with findings from a previous pharmacokinetic simulations study by Teigen et al., which demonstrated unacceptable gentamicin exposure when gentamicin was administered posthemodialysis (18). Each Monte Carlo simulation generated concentration-time profiles for 1,000 subjects per dosing regimen using the parameters from the final covariate model. The abilities of the dosing regimens to achieve predefined pharmacodynamic targets, a Cmax/MIC ratio of ≥10 or an AUC0-24 of 70 to 120 mg·h/liter (4), were then compared.
RESULTS
Fourteen patients were enrolled, and 265 plasma samples for gentamicin concentration determination were collected throughout 28 dosing intervals. All patients were sampled during the first dosing interval, with one patient being sampled from one additional EDD-f treatment, three patients being sampled during an additional two EDD-f treatments, and two patients being sampled during an additional three EDD-f treatments. Patient demographic and clinical details are described in Table 1.
TABLE 1.
Demographic and clinical details of enrolled patientsa
| Patient no. | Age (yr) | Sex | Ht (cm) | Wt (kg) | Admission diagnosis | Ventilator duration (days) | ICU LOS (days) | APACHE III score | SOFA score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 73 | M | 166 | 78 | Postlaparotomy | 28 | 38 | 98 | 12 |
| 2 | 57 | M | 180 | 127 | Mulitorgan failure | 11 | 19 | 94 | 14 |
| 3 | 49 | M | 187 | 182 | Sepsis | 8 | 9 | 124 | 14 |
| 4 | 52 | M | 166 | 111 | Cardiac arrest | 4 | 5 | 98 | 9 |
| 5 | 72 | M | 175 | 80 | Sepsis | 7 | 7 | 104 | 12 |
| 6 | 55 | M | 175 | 110 | Respiratory failure | 9 | 10 | 93 | 15 |
| 7 | 81 | M | 174 | 70 | Repair of ruptured AAA | 12 | 13 | 44 | 12 |
| 8 | 79 | M | 180 | 90 | Subclavian-bifemoral graft | 10 | 12 | 103 | 13 |
| 9 | 64 | M | 168 | 95 | Ventriculoperitoneal shunt revision | 8 | 13 | 135 | 11 |
| 10 | 87 | M | 166 | 79 | Laparotomy | 2 | 2 | 112 | 12 |
| 11 | 68 | M | 173 | 95 | Sepsis | 22 | 24 | 95 | 12 |
| 12 | 57 | F | 157 | 85 | Respiratory failure | 13 | 16 | 80 | 11 |
| 13 | 75 | M | 170 | 115 | Laparotomy | 9 | 20 | 96 | 7 |
| 14 | 62 | M | 185 | 80 | Neutropenic sepsis | 11 | 14 | 142 | 9 |
| Median (interquartile range) | 66.0 (57.0-74.5) | 12 M, 1 F | 173.5 (166.5-178.8) | 92.5 (80.0-111.1) | 9.5 (8.0-11.8) | 13.0 (9.3-18.3) | 98 (94-110) | 12.0 (11.0-12.8) |
Group data are presented as medians (interquartile ranges). ICU LOS, intensive care unit length of stay; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; AAA, abdominal aortic aneurysm; F, female; M, male.
Population pharmacokinetic modeling was performed using the concentration data from the plasma samples. The best base model, obtained using the model-building criteria, consisted of a two-compartment linear model with zero-order input and exponential residual unknown variability. Other models could not be supported, as they did not result in an improvement in the objective function value or between-subject variability. Between-subject variability was included for all parameters except non-EDD-f clearance (CLNEDD-f), which was considered to be not appropriate for inclusion because standard dialysis filters were used. A more mechanistic model contrasting dialysis clearance with different dialysis membrane characteristics was not explored, as all patients received a standard dialysis dose with the same dialyzers and membranes. A variance-covariance matrix between clearance, central volume of distribution, and peripheral volume of distribution was supported. The model did not support between-occasion variability between any of the parameters. The final objective function for the base model was 131.853.
The covariates that described gentamicin clearance were patient lean body weight (normalized to 55 kg) and the last plasma creatinine concentration before commencement of EDD-f. The addition of these parameters reduced the objective function by 10.032 and 45.023, respectively (a statistically significant change is 3.84 units). The covariate that described the central volume of distribution was total body weight normalized to 70 kg. The addition of this parameter reduced the objective function by 4.925. The objective function for the final covariate model was 76.875. The final model for gentamicin clearance was represented by equation 4:
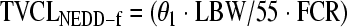 |
(3) |
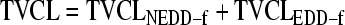 |
(4) |
where TVCLNEDD-f is the typical value of non-EDD-f clearance, LBW is lean body weight described by the equation from Janmahasatian et al. (9) normalized to 55 kg, FCR is the inverse of the final plasma creatinine concentration recorded in micromoles/liter before commencement of EDD-f, and TVCLNEDD-f is the typical value of clearance during EDD-f. None of the other covariates statistically significantly improved the model and therefore could not be included.
The values of the parameters for the final model are given in Table 2. Table 2 presents the 95% confidence interval for the parameters computed from all bootstrap runs. The population value for the clearance of gentamicin during EDD-f (CLEDD-f) was 2.54 liters/h (half-life [t1/2], 13.8 h), whereas it was 0.23 liter/h (t1/2 153.4 h) when EDD-f was not being used (CLNEDD-f). The population value for the volume of distribution was 0.55 liter/kg.
TABLE 2.
Bootstrap parameter final estimates of the final covariate model
| Parametera | Mean | 95% confidence interval |
|---|---|---|
| Fixed effects | ||
| CLEDD-f (liters h−1) | 2.59 | 1.91-4.16 |
| CLNEDD-f (liters h−1) | 0.24 | 0.17-0.32 |
| Central vol of distribution (liters) | 14.1 | 10.8-20.5 |
| Peripheral vol of distribution (liters) | 32.8 | 27.25-47.4 |
| Intercompartmental clearance (liters h−1) | 2.76 | 1.48-6.7 |
| Random effects, between-subject variability ΩBSV (% coefficient of variation) | ||
| Clearance | 39.9 | 7.8-52 |
| Central vol of distribution | 16.4 | 3.7-42.9 |
| Intercompartmental clearance | 20.5 | 57.7-137 |
| Peripheral vol of distribution | 110.5 | 9.1-56.4 |
| Random error, residual unexplained variability (% coefficient of variation) | 20.8 | 15.5-23.8 |
CLEDD-f, clearance of gentamicin during EDD-f; CLNEDD-f, clearance of gentamicin not due to EDD-f.
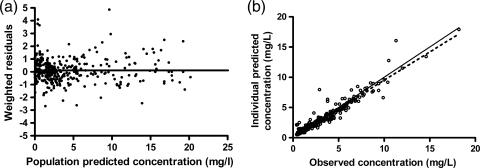
Figure 1 displays the goodness-of-fit plots for the final model. The weighted residual graph shows no apparent visual or statistical bias for the prediction. Of the 265 samples included in the analysis, 10 samples had a concentration greater than 2 standard deviations outside that predicted by the model, which we considered acceptable, given the level of sickness severity and the likely pharmacokinetic heterogeneity of the patient cohort. It was noted that the model underpredicted the higher concentrations to a small extent. All subsequent dosing simulations were then based on this model. All other visual predictive checks were acceptable and confirmed the goodness of fit of the model. The plots show that the final pharmacokinetic model adequately describes the measured gentamicin concentrations when EDD-f is being used and when it is not being used.
FIG. 1.
Goodness-of-fit plots for the final pharmacokinetic model. (a) Population predicted concentrations (mg/liter) versus weighted residuals; (b) individual predicted concentrations versus observed concentrations. The black dotted line is the line of linear regression with an R2 value of 0.91, and the gray unbroken line is the line of x equal to y.
Dosing simulations.
Multiple dosing schedules were evaluated on the basis of the final covariate model and by assuming an MIC of 1 mg/liter. The abilities of the different dosing schedules to achieve predefined pharmacodynamic end points (4) were evaluated on the basis of the final covariate model and assuming an MIC of 1 mg/liter. None of the simulated doses adequately achieved a target Cmin of <1.0 mg/liter, with the best-performing dosage being a 3-mg/kg infusion finishing at the time of EDD-f commencement, which achieved attainment in 29% of the simulated patients. Achievement of the maximal AUC0-24 (70 to 120 mg·h/liter) and Cmax (>10 mg/liter) pharmacodynamic targets for the simulated patients was by the 4-mg/kg or 5-mg/kg dose given either 30 min or 1 h before commencement of EDD-f.
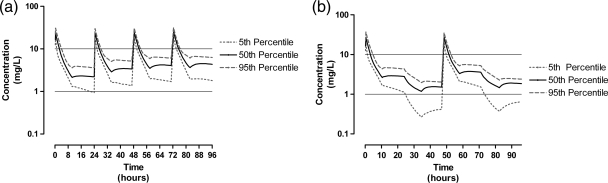
Simulations performed with dosing every 48 h achieved targets more successfully than dosing every 24 h. We compared a 96-h duration of therapy of 5 mg/kg every 24 h with 6 mg/kg every 48 h. The median daily AUC0-24 for 5 mg/kg was 135.7 mg·h/liter (range, 85.8 to 188.3 mg·h/liter), which exceeded the recommended range (70 to 120 mg·h/liter), whereas the median AUC0-24 for dosing with 6 mg/kg every 48 h was more favorable at 88.7 mg·h/liter (range, 48.4 to 122.0 mg·h/liter). Both doses achieved a Cmax of >10 mg/liter in 99.9% of the simulated patients. Figure 2 describes the comparative simulated concentrations for the regimen of 5 mg/kg every 24 h and the regimen of 6 mg/kg every 48 h. Figure 2 shows that the schedule of 6 mg/kg every 48 h minimized the Cmin at 1.5 mg/liter, whereas dosing with 5 mg/kg every 24 h minimized the Cmin at 2.2 mg/liter.
FIG. 2.
Simulation data for gentamicin administered at 5 mg/kg every 24 h (with EDD-f commencing daily at 30 min postdosing) (a) and 6 mg/kg every 48 h (with daily EDD-f) (b). Simulations are presented as the expected concentrations for the 5th, 50th, and 95th percentiles of simulated patients (n = 1,000).
DISCUSSION
Knowledge of the significant clearance of gentamicin caused by EDD-f can be used to procure dosing regimens that will facilitate achievement of pharmacodynamic targets that are associated with optimal antibiotic activity and a reduced potential for toxicity. Administration of gentamicin before commencement of EDD-f allows a high Cmax to be reached and then utilizes EDD-f for rapid drug clearance to minimize the Cmin and optimize the AUC0-24. Specifically, in the present study we have shown that administering 6 mg/kg every 48 h (for obese patients, we would recommend that this dose be based on lean body weight) will ensure the maximal achievement of AUC0-24 and Cmax targets (4) in patients with EDD-f for infections caused by organisms with an MIC of ≤1 mg/liter. It is likely that under this scenario an organism with an MIC of ≥2 mg/liter cannot receive treatment that will achieve the Cmax/MIC or AUC0-24/MIC pharmacodynamic targets or a low Cmin.
Using a 24-h gentamicin dosing regimen with EDD-f does not appear to enable drug exposures that would be desired to minimize both nephro- and ototoxicity for critically ill patients with AKI. The simulations in this paper show that none of the simulated dosing regimens achieve a satisfactory Cmin (<1.0 mg/liter). Although the 3-mg/kg regimens enable the highest proportion of simulated patients to achieve a Cmin of <1.0 mg/liter, it is still achieved in only 29% of patients. This suggests that the clearance of gentamicin by EDD-f is not sufficient to reduce the likelihood of drug toxicity from once-daily dosing. Extended-interval dosing, i.e., dosing every 48 h, may be the solution to this problem, and the simulations performed in the present study support use of dosing with 6 mg/kg every 48 h to maximize achievement of Cmax targets (assuming an MIC of ≤1 mg/liter).
The correct prescription of gentamicin during EDD-f is likely to be guided by the clinical scenario. While daily 7-mg/kg dosing achieves the pharmacodynamic targets for maximal bacterial kill, it risks toxicity, whereas dosing gentamicin at 6 mg/kg every 48 h achieves appropriate Cmax and AUC0-24 targets with an improvement in Cmin targets as well. Smaller doses do not achieve Cmax or AUC0-24 targets as proficiently as higher doses and confer only a small decrease in the potential for gentamicin toxicity (target Cmin). In the absence of achieving a target Cmin, minimizing the cumulative gentamicin exposure is the most appropriate method to ensure prevention of toxicity. Pursuant to this, we would advocate that short courses (≤4 days) of gentamicin at 6 mg/kg every 48 h be used where possible. The inherent pharmacokinetic variability observed in critically ill patients mandates that therapeutic drug monitoring still be undertaken to guide the timing of redosing after the administration of the first dose. Achievement of AUC0-24 targets can then be facilitated by inputting two concentration-time points (prelevel and postlevel) into freely available Bayesian dosing software, such as TCI Works (available at www.tciworks.info). Such an approach may enable more accurate dosing as well as the use of longer courses of therapy with a decreased likelihood of toxicity.
The volume of distribution of gentamicin in this cohort of patients with AKI requiring EDD-f is largely similar to that observed in previous studies of critically ill patients without RRT requirements. The mean volume of distribution in our study was 0.55 liter/kg, which compares favorably with aminoglycoside data from Marik (0.41 liter/kg) (12), Dasta and Armstrong (0.36 liters/kg) (8), and Triginer et al. (0.43 liters/kg) (19). The incrementally higher volume of distribution for this cohort compared with the result from previous studies is likely to be due to the high level of sickness severity of the patients enrolled in the present study.
There are some limitations to the present study that we would like to declare. First, due to the high volumes of dialysate generated by EDD-f, it was not possible to determine the concentration of gentamicin in the dialysate, and therefore, it was assumed that clearance during EDD-f is due solely to EDD-f. Second, our model was based on the homogeneous EDD-f settings used in our intensive care unit, and therefore, we are unable to extrapolate from these data the effect of different blood flow or ultrafiltrate flow rates. Third, the simulations in the present study assume that EDD-f continues for the planned 10-h duration of RRT treatment. In patients, blood clotting on the filter frequently prevents this, and therefore, therapeutic drug monitoring remains an essential part of redosing in such situations. Finally, the convenient sampling used in the present study meant that this cohort had a high proportion of obese patients (5 patients weighed greater than 100 kg) and 13 of the 14 patients were male. However, these anomalies were explained pharmacokinetically in our model by the inclusion of total body weight as a covariate of the central volume of distribution. It is unlikely that clearance would be significantly affected by gender or obesity because the predominant clearance is caused by EDD-f.
Conclusions.
Gentamicin remains an important antibiotic for use in critical care units, but it is often withheld or inadequately dosed for fears of accumulation and toxicity in renal failure. When it is used in patients with AKI, EDD-f results in significant gentamicin clearance. In this paper, we have been able to show that when it is dosed for 48 h, a dose of 6 mg/kg gentamicin 30 min before the commencement of EDD-f results in sufficient drug clearance to achieve the pharmacodynamic targets associated with maximal bacterial killing. To achieve the optimal therapeutic effect of gentamicin while minimizing toxicity, inputting predose and postdose therapeutic drug monitoring data into pharmacokinetic dosing software can be used to determine personalized dosing regimens for critically ill patients receiving EDD-f.
Acknowledgments
We thank Fresenius Medical Care for funding the cost of the blood assays and the medical and nursing staff of the Gold Coast Hospital intensive care unit for supporting the project.
J.A.R. is funded by an Australian National Health and Medical Research Council Health Professional Research Fellowship (fellowship 569917), and we acknowledge the Australian National Health and Medical Research Council for project grant 519702.
Footnotes
Published ahead of print on 14 June 2010.
REFERENCES
- 1.Baldwin, I., T. Naka, B. Koch, N. Fealy, and R. Bellomo. 2007. A pilot randomised controlled comparison of continuous veno-venous haemofiltration and extended daily dialysis with filtration: effect on small solutes and acid-base balance. Intensive Care Med. 33:830-835. [DOI] [PubMed] [Google Scholar]
- 2.Beal, S. L., and L. B. Sheiner. 1998. NONMEM user guides (I to VIII). University of California at San Francisco, San Francisco, CA.
- 3.Bearden, D. T., and K. A. Rodvold. 2000. Dosage adjustments for antibacterials in obese patients: applying clinical pharmacokinetics. Clin. Pharmacokinet. 38:415-426. [DOI] [PubMed] [Google Scholar]
- 4.Begg, E. J., M. L. Barclay, and S. B. Duffull. 1995. A suggested approach to once-daily aminoglycoside dosing. Br. J. Clin. Pharmacol. 39:605-609. [PMC free article] [PubMed] [Google Scholar]
- 5.Chertow, G. M., E. M. Levy, K. E. Hammermeister, F. Grover, and J. Daley. 1998. Independent association between acute renal failure and mortality following cardiac surgery. Am. J. Med. 104:343-348. [DOI] [PubMed] [Google Scholar]
- 6.Choi, G., C. D. Gomersall, Q. Tian, G. M. Joynt, R. C. Freebairn, and J. Lipman. 2009. Principles of antibacterial dosing in continuous renal replacement therapy. Crit. Care Med. 37:2268-2282. [DOI] [PubMed] [Google Scholar]
- 7.Czock, D., C. Husig-Linde, A. Langhoff, T. Schopke, C. Hafer, K. de Groot, S. Swoboda, E. Kuse, H. Haller, D. Fliser, F. Keller, and J. T. Kielstein. 2006. Pharmacokinetics of moxifloxacin and levofloxacin in intensive care unit patients who have acute renal failure and undergo extended daily dialysis. Clin. J. Am. Soc. Nephrol. 1:1263-1268. [DOI] [PubMed] [Google Scholar]
- 8.Dasta, J. F., and D. K. Armstrong. 1988. Variability in aminoglycoside pharmacokinetics in critically ill surgical patients. Crit. Care Med. 16:327-330. [DOI] [PubMed] [Google Scholar]
- 9.Janmahasatian, S., S. B. Duffull, S. Ash, L. C. Ward, N. M. Byrne, and B. Green. 2005. Quantification of lean bodyweight. Clin. Pharmacokinet. 44:1051-1065. [DOI] [PubMed] [Google Scholar]
- 10.Kielstein, J. T., D. Czock, T. Schopke, C. Hafer, S. M. Bode-Boger, E. Kuse, F. Keller, and D. Fliser. 2006. Pharmacokinetics and total elimination of meropenem and vancomycin in intensive care unit patients undergoing extended daily dialysis. Crit. Care Med. 34:51-56. [DOI] [PubMed] [Google Scholar]
- 11.Levy, E. M., C. M. Viscoli, and R. I. Horwitz. 1996. The effect of acute renal failure on mortality. A cohort analysis. JAMA 275:1489-1494. [PubMed] [Google Scholar]
- 12.Marik, P. E. 1993. Aminoglycoside volume of distribution and illness severity in critically ill septic patients. Anaesth. Intensive Care 21:172-173. [DOI] [PubMed] [Google Scholar]
- 13.Marik, P. E., J. Lipman, S. Kobilski, and J. Scribante. 1991. A prospective randomized study comparing once- versus twice-daily amikacin dosing in critically ill adult and paediatric patients. J. Antimicrob. Chemother. 28:753-764. [DOI] [PubMed] [Google Scholar]
- 14.Matthews, I., C. Kirkpatrick, and N. Holford. 2004. Quantitative justification for target concentration intervention—parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br. J. Clin. Pharmacol. 58:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parke, J., N. H. Holford, and B. G. Charles. 1999. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput. Methods Programs Biomed. 59:19-29. [DOI] [PubMed] [Google Scholar]
- 16.Ricci, Z., and C. Ronco. 2008. Dose and efficiency of renal replacement therapy: continuous renal replacement therapy versus intermittent hemodialysis versus slow extended daily dialysis. Crit. Care Med. 36:S229-S237. [DOI] [PubMed] [Google Scholar]
- 17.Roberts, J. A., and J. Lipman. 2006. Antibacterial dosing in intensive care: pharmacokinetics, degree of disease and pharmacodynamics of sepsis. Clin. Pharmacokinet. 45:755-773. [DOI] [PubMed] [Google Scholar]
- 18.Teigen, M. M., S. Duffull, L. Dang, and D. W. Johnson. 2006. Dosing of gentamicin in patients with end-stage renal disease receiving hemodialysis. J. Clin. Pharmacol. 46:1259-1267. [DOI] [PubMed] [Google Scholar]
- 19.Triginer, C., I. Izquierdo, R. Fernandez, J. Rello, J. Torrent, S. Benito, and A. Net. 1990. Gentamicin volume of distribution in critically ill septic patients. Intensive Care Med. 16:303-306. [DOI] [PubMed] [Google Scholar]
- 20.Uchino, S., J. A. Kellum, R. Bellomo, G. S. Doig, H. Morimatsu, S. Morgera, M. Schetz, I. Tan, C. Bouman, E. Macedo, N. Gibney, A. Tolwani, and C. Ronco. 2005. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813-818. [DOI] [PubMed] [Google Scholar]