Abstract
Diarrhea is one of the most common infirmities affecting international travelers, occurring in 20 to 50% of persons from industrialized countries visiting developing regions. Enterotoxigenic Escherichia coli (ETEC) is the most common causative agent and is isolated from approximately half of the cases of traveler's diarrhea. Rifaximin, a largely water-insoluble, nonabsorbable (<0.4%) antibiotic that inhibits bacterial RNA synthesis, is approved for use for the treatment of traveler's diarrhea caused by diarrheagenic E. coli. However, the drug has minimal effect on the bacterial flora or the infecting E. coli strain in the aqueous environment of the colon. The purpose of the present study was to evaluate the antimicrobial effect and bioavailability of rifaximin in aqueous solution in the presence and absence of physiologic concentrations of bile acids. The methods used included growth measurement of ETEC (strain H10407), rifaximin solubility measurements, total bacterial protein determination, and assessment of the functional activity of rifaximin by monitoring inhibition of bacterial β-galactosidase expression. Solubility studies showed rifaximin to be 70- to 120-fold more soluble in bile acids (approximately 30% in 4 mM bile acids) than in aqueous solution. Addition of both purified bile acids and human bile to rifaximin at subinhibitory and inhibitory concentrations significantly improved the drug's anti-ETEC effect by 71% and 73%, respectively, after 4 h. This observation was confirmed by showing a decrease in the overall amount of total bacterial protein expressed during incubation of rifaximin plus bile acids. Rifaximin-treated samples containing bile acids inhibited the expression of ETEC β-galactosidase at a higher magnitude than samples that did not contain bile acids. The study provides data showing that bile acids solubilize rifaximin on a dose-response basis, increasing the drug's bioavailability and antimicrobial effect. These observations suggest that rifaximin may be more effective in the treatment of infections in the small intestine, due to the higher concentration of bile in this region of the gastrointestinal tract than in the colon. The water insolubility of rifaximin is the likely explanation for the drug's minimal effects on colonic flora and fecal pathogens, despite in vitro susceptibility.
Diarrhea is one of the most common illnesses of international travelers, occurring in 20 to 50% of persons visiting developing regions from industrialized countries (2, 4). The diarrhea-producing Escherichia coli strains important in traveler's diarrhea are enterotoxigenic Escherichia coli (ETEC) and enteroaggregative E. coli (EAEC) (1, 30); both ETEC and EAEC are known to be pathogens of the small bowel. ETEC is the most common causative agent, being identified in approximately half of the cases of traveler's diarrhea. It is also the most commonly isolated bacterial enteropathogen in children under age 5 years in developing countries (5) and is responsible for approximately 200 million diarrheal episodes and 380,000 deaths annually (43, 49). ETEC colonizes the intestinal lumen by binding to specific receptors on the enterocytic surfaces. It produces two notable enterotoxins that cause pathology: a cholera-like heat-labile toxin and low-molecular-weight heat-stable toxin.
Bile acids are biosynthesized in the liver from cholesterol through a multistep enzymatic process and form a major part of the organic component of bile (3). Bile acids are highly hydrophobic and contain a perhydrocyclopentanophenanthrene steroid nucleus consisting of three six-membered rings fused to a fourth five-membered ring (26, 27). Following secretion, the primary bile acids (chenodeoxycholic and cholic acids) undergo conjugation through a peptide linkage with either taurine (tauroconjugation) or glycine (glycoconjugation). The ratio of glycoconjugates to tauroconjugates in human bile can be as high as 9:1 in rural African women and as low as 0.1:1 in taurine-fed subjects (24, 42). The conjugated bile acids further undergo modification by the indigenous lumenal bacterial flora during their intestinal transit mainly through deconjugation, 7α-dehydrogenation (chenodeoxycholic acid to 7-oxolithocholic acid), and 7α-dehydroxylation (cholic acid to deoxycholic acid and chenodeoxycholic acid to lithocholic acid) (6, 10, 13, 14, 23, 32, 33, 38, 48). Cholic, chenodeoxycholic, deoxycholic, lithocholic, glycocholic, and taurocholic acids are the most abundant bile acids found in humans (15, 35). The total bile acid concentration in the small bowel ranges from 2 mM to 30 mM (15, 36, 39), depending on the diet and other metabolic conditions. Only 2 to 5% of the bile acids secreted in a healthy human enter the colon after reabsorption in the ileum (15, 22).
Rifaximin, a largely water-insoluble, nonabsorbable (<0.4%), bacterial RNA synthesis-inhibitory drug has been shown to be safe and effective for the treatment of traveler's diarrhea caused by diarrhea-producing Escherichia coli (16, 44, 45). Rifaximin is well tolerated and does not appear to induce important levels of resistance in enteric flora during repeated dosing (17, 18). However, rifaximin has minimal effects on colonic flora (17, 18), and this is likely related to the drug's insolubility in water due to its hydrophobic properties and the aqueous environment of the colon. The purpose of the present study was to evaluate the antimicrobial effect and bioavailability of rifaximin in aqueous solutions in the presence and absence of physiologic concentrations of bile acids.
(The study was presented at an oral session of Digestive Disease Week, New Orleans, LA, 2 May 2010.)
MATERIALS AND METHODS
ETEC strain H10407 was used as a model bacterium in all the susceptibility experiments. This strain produces both the heat-labile and heat-stable enterotoxins (LT and ST, respectively) important in the pathogenesis of traveler's diarrhea. The in vitro MIC of rifaximin for the test strain ranged from 32 to 128 μg/ml (21, 40, 29, 41). Rifaximin powder was obtained from Salix Pharmaceuticals, and bile acids (cholic, chenodeoxycholic, deoxycholic, glycocholic, lithocholic, and taurocholic acids) were purchased from Sigma Aldrich (St. Louis, MO). Equimolar concentrations (0.67 mM) of each of these bile acids were pooled (4 mM total bile acids) for the experiments. This is because the concentrations of total and individual bile acids in the gastrointestinal tract vary and dynamically change with the type of diet and metabolic condition of an individual; thus, the choice of a concentration that reflects the true physiological in vivo conditions is elusive.
Total human bile concentration determination.
A sample of human bile taken after cholecystectomy was kindly provided by David Graham (Department of Medicine, Veterans Affairs Medical Center, and Division of Molecular Virology, Baylor College of Medicine, Houston, TX, and Study Design and Clinical Research Core, Texas Medical Center Digestive Disease Center). The total bile acid concentration was determined using a total bile acids assay (Diazyme Laboratories, CA). This was done by following the protocol provided by the manufacturer.
Rifaximin solubility.
The solubility of rifaximin in different concentrations of synthetic bile acids and water as solvents was determined spectrophotometrically. In order to obtain a standard curve, rifaximin within a concentration range of 0.01 mg/ml to 20 mg/ml in 100% acetone was serially diluted with 100% ethanol. The sample was prepared by adding 12 mg of rifaximin powder to deionized water or a 2.5 mM, 5 mM, 7.5 mM, 10 mM, 15 mM, or 20 mM synthetic bile acids mixture (pH 7.4). The tubes were incubated for an hour and centrifuged at 16,000 × g for 30 min to eliminate undissolved particles. Absorbance measurements at 450 nm of both standards and samples were taken simultaneously using a Multiskan EX spectrophotometer (Thermo Scientific, Waltham, MA). A regression equation from the standard curve was used to estimate the concentration of rifaximin sample in deionized water and bile acids. The percent solubility was calculated as (milligrams of rifaximin determined spectrophotometrically/milligrams of rifaximin dissolved) × 100.
Rifaximin dose-response using turbidity assay.
Escherichia coli strain H10407 was grown in Luria-Bertani (LB) medium overnight to an optical density at 600 nm (OD600) of 0.5 to 1.0. The mixture used in the experiment consisted of 30 ml LB medium containing rifaximin, human bile, or synthetic bile acids (cholic, chenodeoxycholic, deoxycholic, glycocholic, lithocholic, and taurocholic acids) and E. coli H10407. Rifaximin powder was added to LB medium containing synthetic bile acids or human bile at pH 7.4, and the mixture was incubated for 30 min at ambient temperature on a magnetic stirrer. Using the Diazyme assay, the total bile acid concentration of human bile was determined to be approximately 39.7 mM. Addition of a 10th dilution (∼4 mM) of the human bile to rifaximin resulted in a significant increase in the antimicrobial effect of the drug. On the basis of this observation, 4 mM total bile acid was used for all the experiments. In each experiment, an overnight culture of E. coli H10407 was added to the LB medium containing rifaximin and bile acids to an OD600 of 0.04 (approximately 4 × 107 cells/ml), and the mixture was incubated for 4 to 6 h at 37°C in a shaker at 200 rpm. Optical density measurements (OD600) were made every 30 min. The experiment was replicated four times, and the average OD was used for the analysis.
β-Galactosidase assay.
β-Galactosidase is an essential enzyme required by E. coli for lactose metabolism under conditions of low glucose and high lactose concentrations. Inhibition of expression of this enzyme in a medium with limiting glucose and other sources of sugar but a large amount of lactose results in bacterial death when the sugar in the medium is exhausted. Rifaximin acts by binding to the beta subunit of bacterial DNA-dependent RNA polymerase, resulting in inhibition of RNA synthesis and, ultimately, protein synthesis. In a culture medium, the degree of this inhibition depends on the amount of rifaximin available. The extent of inhibition of expression of β-galactosidase by E. coli H10407 grown in the presence of rifaximin with and without bile acids was evaluated as an indirect measure of the bioavailability of rifaximin. The β-galactosidase assay was performed on the basis of the methodologies developed by Zhang and Bremer (50) and Miller (34) with some modifications. Briefly, the optical density (OD600) of an overnight culture was adjusted to 0.5. An aliquot (500 μl) of the culture was pipetted into different tubes (in triplicate) containing 8 μg/ml, 16 μg/ml, and 32 μg/ml of rifaximin in 4 mM total synthetic bile acids. The culture was induced to express β-galactosidase with 1 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG) and incubated in a shaker for 90 min at 30°C. The controls consisted of 500 μl culture; 1 mM IPTG; and 8 μg/ml, 16 μg/ml, or 32 μg/ml rifaximin with no bile acids. To 1.5-ml microcentrifuge tubes, 50 μl of the induced culture was added to 200 μl of permeabilization solution (0.8 mg/ml hexadecyltrimethylammonium bromide [CTAB], 0.4 mg/ml sodium deoxycholate, 200 mM dibasic sodium phosphate [Na2HPO4], 20 mM potassium chloride [KCl], 2 mM magnesium sulfate [MgSO4], 5.4 μl/ml β-mercaptoethanol). After a 30-min incubation at 30°C, 600 μl of substrate solution (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 20 μg/ml CTAB, 1 mg/ml o-nitrophenyl-β-d-galactoside [ONPG], 2.7 μl/ml β-mercaptoethanol) was added and the time of addition was recorded. The incubation was allowed to continue at 30°C for 40 min, after which 350 μl of 2 M sodium carbonate (Na2CO3) was added to stop the reaction. The assay tubes were centrifuged at 15,000 × g for 15 min, and absorbance measurements at 420 nm were made in triplicate using the supernatant. The concentration of β-galactosidase (in Miller units) was calculated as {absorbance at 420 nm of assay/[(absorbance at 600 nm of 0.05 ml of culture) (reaction time)]} × 1,000.
Effect of bile acids on rifaximin inhibition of E. coli H10407 measured by determination of total bacterial protein expression.
The effect of rifaximin and bile acids on total protein expression by the bacteria during the first 5 h of incubation was evaluated. An overnight culture (0.5 ml) at an OD600 of 0.5 was added to 30 ml of LB medium (pH 7.4) containing only rifaximin (16 μg/ml), rifaximin and bile acids (16 μg/ml rifaximin in 4 mM total synthetic bile acids), LB medium only, and 4 mM total synthetic bile acids only. Aliquots (1 ml) of the culture were taken every hour for total protein determination. To lyse the cells, 300 μl of permeabilization solution (2.4 mg/ml hexadecyltrimethylammonium bromide, 1.2 mg/ml sodium deoxycholate, 600 mM dibasic sodium phosphate, 60 mM potassium chloride, 6 mM magnesium sulfate, 16 μl/ml β-mercaptoethanol) was added. The tubes were incubated for 1 h at room temperature and centrifuged at 15,000 × g for 20 min. The protein concentration was determined in triplicate using the Bradford assay (7) with bovine serum albumin as the standard. Each experiment was repeated three times, and the averages and standard deviations are reported.
Statistical analysis.
To determine the significance level of the differences observed between the samples, Mann-Whitney two-tailed nonparametric tests of significance were performed using Prism (version 5.02) software for Windows (GraphPad Software, San Diego, CA). In all cases, statistical significance was defined as a P value of <0.05.
RESULTS
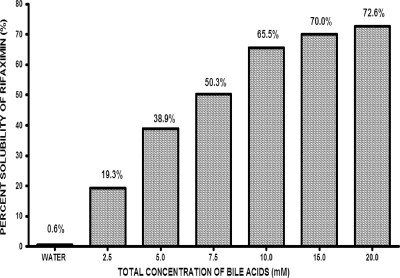
The effect of bile acids on the solubility of rifaximin was evaluated spectrophotometrically at 450 nm. Different dilutions of rifaximin in 100% acetone were initially evaluated over a concentration range of 0.01 mg/ml to 20 mg/ml. As shown in Fig. 1, rifaximin is markedly more soluble in an aqueous solution in the presence of bile acids. Accordingly, within the concentration range analyzed, the aqueous solubility of rifaximin increased 70- to 120-fold in the presence of bile acids.
FIG. 1.
Solubility of rifaximin (12 mg) in water and equimolar concentrations of the bile acids cholic, chenodeoxycholic, deoxycholic, glycocholic, lithocholic, and taurocholic acids in a mixture at pH 7.4. The total bile acid concentration at each reading is equal to the individual bile acid concentration multiplied by 6.
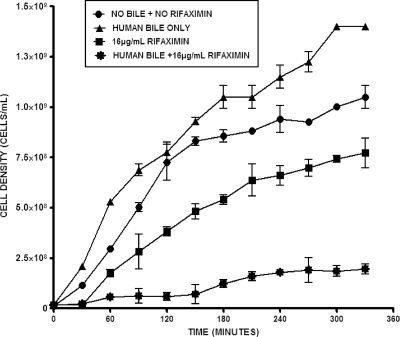
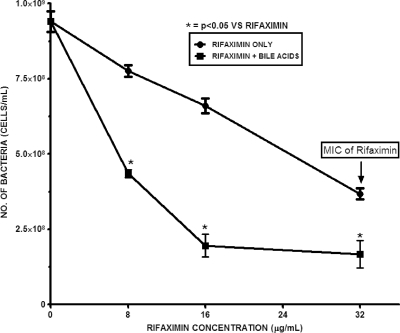
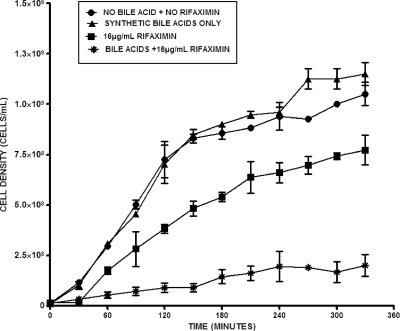
To confirm that the increased solubility of rifaximin in the presence of bile acid translates to improvement in its antimicrobial effect, the growth of E. coli H10407 in the presence of different concentrations of rifaximin and a constant physiologic total bile acid concentration was monitored. This was done by culturing the test ETEC strain in LB medium and measuring the OD600 at 30-min intervals during the incubation period. The results showed that at physiological pH and temperature, 4 mM total bile acids did not have any inhibitory or killing effect but, rather, tended to modestly enhance bacterial growth (Fig. 2 and 3). In sharp contrast, rifaximin (16 μg/ml) treatment in the absence of bile acids resulted in statistically significant (P = 0.026; n = 4) inhibition of bacterial growth during the incubation period. Strikingly, the effect of rifaximin became more remarkable and improved significantly when bile acid (P = 0.007 for synthetic bile acids and P = 0.012 for human bile; n = 4) was added. No significant difference (P = 0.686; n = 4) between the effect of synthetic bile acids and that of human bile was observed. The antimicrobial effect increased with increasing concentrations of rifaximin (8 μg/ml to 32 μg/ml). Conversely, this observation was not feasible with increasing concentrations of bile acids due to its deleterious effect on the cells at higher concentrations (data not shown). After 4 h of incubation, 8 μg/ml and 16 μg/ml of rifaximin containing synthetic bile acids resulted in 2-fold and 5.5-fold increases, respectively, in the bacteriostatic effect over that for the samples with no bile acids (Fig. 4). The effect of bile acids was more notable at lower concentrations of rifaximin.
FIG. 2.
Growth of ETEC strain H10407 in the presence of 16 μg/ml rifaximin in water and 4 mM human bile at pH 7.4. Cells were grown with rifaximin in the presence and absence of human bile, and the absorbance at 600 nm was measured at 30-min intervals. Mann-Whitney two-tailed nonparametric t test analysis showed statistically significant differences between treatments, as follows: no rifaximin plus no bile acids versus 16 μg/ml rifaximin, P = 0.026 (n = 4); no rifaximin plus no bile acids versus 16 μg/ml rifaximin plus bile acids, P = 0.002 (n = 4); and 16 μg/ml rifaximin versus 16 μg/ml rifaximin plus bile acids, P = 0.012 (n = 4). The error bars represent the standard deviations between four replicate experiments.
FIG. 4.
Growth of ETEC strain H10407 after 4 h incubation at 37°C in the presence of rifaximin (8 μg/ml, 16 μg/ml, and 32 μg/ml) in water and 4 mM total synthetic bile acids in a pooled mixture of cholic, deoxycholic, chenodeoxycholic, glycocholic, lithocholic and taurocholic acids at pH 7.4. The error bars represent the standard deviations between four replicate experiments.
To appraise the potential physiologic limitations due to the pooled equimolar concentrations of synthetic bile acids used, single bile acids (cholic, chenodeoxycholic, deoxycholic, glycocholic, lithocholic, or taurocholic acids) were evaluated (see Fig. S1A to F in the supplemental material). The E. coli H10407 cells were exposed to each of these bile acids in the presence and absence of a subinhibitory concentration of rifaximin (16 μg/ml). The antimicrobial effect of rifaximin did not improve to a significant level on addition of 1 mM each single bile acid (data not shown). However, 4 mM of each single bile acid except lithocholic acid increased the antimicrobial effect of rifaximin beyond that observed from both pooled synthetic bile acids and human bile (Table 1). In each case, the cell density underwent a further decrease in the presence of bile acid compared to that in cultures that contained only rifaximin. The percent decreases in cell density due to addition of cholic, deoxycholic, chenodeoxycholic, glycocholic, taurocholic, and lithocholic acids were 95.5%, 93.8%, 89.2%, 80.8%, 77.6%, and 43.9%, respectively. Together, the data illustrate that at physiologic temperature and pH, bile acids significantly increased the antimicrobial effect of rifaximin.
TABLE 1.
Densities of E. coli H10407 cells grown with rifaximin in the presence and absence of both synthetic acids and human bile
| Bile acid | Cell densitya (no. of bacteria/ml of culture) |
% decrease in cell density on addition of bile acids to rifaximin | |||
|---|---|---|---|---|---|
| No bile acid + no rifaximin (108) | 4 mM bile acids (108) | 16 μg/ml rifaximin (108) | 4 mM bile acids + 16 μg/ml rifaximin (108) | ||
| Human bile | 9.4 ± 0.69 | 12 ± 0.30 | 6.6 ± 0.50 | 1.8 ± 0.08 | 72.8 ± 0.84 |
| Pooled synthetic bile | 9.4 ± 0.69 | 9.6 ± 0.25 | 6.6 ± 0.50 | 2.0 ± 0.74 | 70.6 ± 0.48 |
| Cholic acid | 12 ± 0.16 | 11 ± 0.46 | 4.9 ± 1.0 | 0.22 ± 0.20 | 95.5 ± 0.80 |
| Deoxycholic acid | 12 ± 0.16 | 3.9 ± 0.34 | 4.9 ± 1.0 | 0.30 ± 0.49 | 93.8 ± 0.51 |
| Chenodeoxycholic acid | 12 ± 0.16 | 7.0 ± 0.06 | 4.9 ± 1.0 | 0.53 ± 0.51 | 89.2 ± 0.49 |
| Glycocholic acid | 12 ± 0.16 | 14 ± 0.32 | 4.9 ± 1.0 | 0.94 ± 0.60 | 80.8 ± 0.40 |
| Taurocholic acid | 12 ± 0.16 | 14 ± 0.51 | 4.9 ± 1.0 | 1.1 ± 0.23 | 77.6 ± 0.77 |
| Lithocholic acid | 12 ± 0.16 | 6.6 ± 0.25 | 4.9 ± 1.0 | 2.8 ± 0.27 | 43.9 ± 0.73 |
Data represent the cell densities after 4 h of incubation.
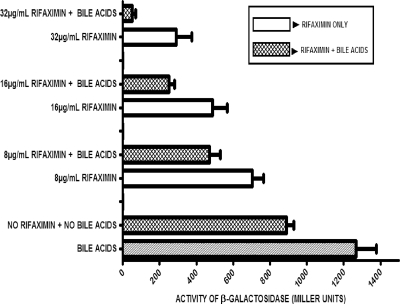
The extent of RNA synthesis inhibition in the test ETEC strain by rifaximin and bile acids was investigated. This was done by monitoring the level of β-galactosidase expressed by E. coli H10407 grown in medium containing rifaximin with and without bile acids. In the presence of bile acids and no rifaximin, the level of expression of β-galactosidase was higher (indicating a lack of organism inhibition) than that by the untreated cells (control), as shown in Fig. 5. On the other hand, the amount of the enzyme produced decreased in a dose-dependent fashion below the control values when rifaximin was added to the culture medium. The enzyme level was, in turn, decreased below these already depressed values when the cells were treated with both rifaximin and bile acids. The percent decreases in inhibition of enzyme expression in samples containing bile acids and rifaximin at concentrations of 8 μg/ml, 16 μg/ml, and 32 μg/ml were 33%, 49%, and 82%, respectively.
FIG. 5.
Effect of bile acids on the expression of β-galactosidase enzyme. Each treatment contained 4 mM total synthetic bile acids in a mixture of cholic, deoxycholic, chenodeoxycholic, glycocholic, lithocholic, and taurocholic acids at pH 7.4. One unit is the amount of enzyme required to convert a micromole of o-nitrophenyl-β-d-galactoside to o-nitrophenol and galactose per minute at pH 7.4 at 30°C. The error bars represent the standard deviations between three replicate experiments.
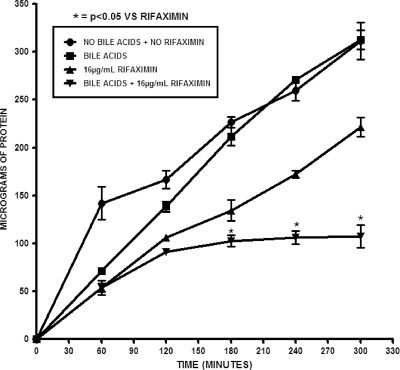
The total protein content of E. coli H10407 grown under different conditions of rifaximin and bile acids was monitored to further confirm the increased antimicrobial effect of rifaximin caused by addition of bile acids (as observed from the other methods). After 4 h of incubation, no significant difference (P = 0.873; n = 3) in the amount of total bacterial protein expressed between samples treated with only bile acids and the control was observed, as shown in Fig. 6. Bacteria grown in a medium containing both rifaximin and bile acids expressed a smaller amount of total protein than those grown without bile acids. The percent decrease in total bacterial protein expression after a 4-h incubation when bile acids were added to medium containing 16 μg/ml rifaximin was 59% compared to the level of bacterial protein expression for samples that contained an equivalent amount of rifaximin but no bile acids. These results indicate that addition of bile acids to rifaximin makes the nonabsorbable antibiotic more bioavailable to inhibit an essential enzyme and proteins required for bacterial growth and that this inhibition occurred in a dose-response manner at sublethal concentrations of rifaximin.
FIG. 6.
Evaluation of total protein content of bacteria treated with 16 μg/ml rifaximin and bile acids. Each treatment contained 4 mM total synthetic bile acids in a mixture of cholic, deoxycholic, chenodeoxycholic, glycocholic, lithocholic, and taurocholic acids at pH 7.4. Cells were grown with rifaximin in the presence and absence of bile acids, and aliquots were taken every hour for total protein concentration determination. The total protein concentration was determined using the Bradford assay. The error bars represent the standard deviations between three replicate experiments.
DISCUSSION
Rifaximin is used for the treatment of bacterial diarrhea due to ETEC (16, 44, 45) and EAEC (28), which are pathogens of the small bowel. The drug is not effective in treating colonic shigellosis (47) and is known to be poorly soluble in water, requiring dissolution in an organic solvent before in vitro susceptibility testing can be performed (29). The present study shows the aqueous insolubility of rifaximin, which appears to explain the lack of drug effect in the colon. Rifaximin's colonic bioavailability appears to be below the average MICs of most coliform flora (∼32 μg/ml), with the exception of colonic pathogens with lower MICs, such as Clostridium difficile (MICs = 0.025 μg/ml). Assays of rifaximin concentration in stools of treated subjects reveal large amounts of drug. However, due to the drug's low aqueous solubility, only low concentrations are bioavailable.
The study demonstrated an increase in rifaximin solubility with increasing concentrations of bile acids. This is consistent with the reported physical properties of bile acids that enable these natural detergents to dissolve other hydrophobic substances (11). Bile acids contain structural components that are hydrophilic on one side and hydrophobic on the other. The amphipathic nature of bile acids enables them to self-associate in water to form polymolecular aggregates (11). Each micelle contains 4 to 50 molecules, depending on the type and structure, which can solubilize other lipids as well as hydrophobic molecules in the form of mixed micelles.
We have provided data showing that the antimicrobial effect of rifaximin was markedly improved by the presence of bile acids. The positive antimicrobial effect of the rifaximin-bile acid mixture was shown to be dose related. Subinhibitory concentrations of rifaximin show inhibition of the tested ETEC strain when bile acids are added. ETEC grown in medium containing rifaximin and bile acids expressed smaller amounts of total proteins than ETEC grown in medium plus rifaximin without bile acids. It is likely that rifaximin could probably be solubilized by bile acids and delivered as mixed micelles to a wider area of the bowel. Rifaximin has to permeate the bacterial cell and enter the cytoplasm to exert its antimicrobial effects. The drug cannot enter the cell in its undissolved form, primarily due to steric constraint in the cytoplasmic membrane. Solubilization by bile acids reduces its particulate size and hence may allow it to enter the cell. Bile acids may also influence the cell membrane. Several factors determine the exact outcome of the action of bile on cell membranes. It is important to note that bile acids at high concentrations can rapidly dissolve membrane lipids and cause the dissociation of integral membrane proteins (12, 25), resulting in the breakdown of the cell membrane and subsequent leakage of cell contents and bacterial cell death.
It should be noted that the concentration of bile acids used did not kill the ETEC cells but, interestingly, had a modest growth-promoting effect on this enterotoxigenic E. coli strain. The literature on the bile tolerance of Gram-negative bacteria is scanty, but they are generally alleged to be inherently more resistant to bile acids than Gram-positive bacteria (3). In fact, bile salts are often used in selective and enrichment media, such as MacConkey agar, salmonella-shigella agar, and violet red bile agar, to select for Gram-negative bacteria. E. coli is also frequently isolated from the gallbladder and bile of animals and humans (8, 9, 19, 37). Gänzle et al. (1999) observed E. coli growth in the small intestine of a gastrointestinal tract model in the presence of high porcine bile extract concentrations where Gram-positive bacteria could not survive (20).
Bile acid absorption occurs mostly in the small bowel by diffusion and sodium-dependent secondary active transport mechanisms (15), which returns the bile to the liver via the portal vein and completes the enterohepatic circulation. This is an extremely efficient process and involves reabsorption of approximately 95 to 98% of the bile acids secreted in normal human bile (15, 22). The small quantity of bile acid that escapes reabsorption in the small bowel enters the colon and is excreted in the feces. Our findings of increased drug activity in the presence of bile acids may explain why rifaximin shows a dose-response efficacy in the treatment of bacterial overgrowth in the small bowel (31) but with a minimal effect in the colon. On the basis of a rifaximin dose of 400 mg given three times a day (1,200 mg/day), the usual dose used in gastroenterology, we estimate that in an individual with a bile acid concentration in the small bowel of 5 to 10 mM, between 468 mg and 792 mg (39% to 66%) of the drug would be solubilized in the small bowel each day, whereas about 46 mg (3.8%) of rifaximin would be solubilized in the colon with an average bile salt concentration of 0.1 to 0.5 mM.
While rifaximin is ineffective in the treatment of Shigella colitis, the drug effectively prevents the development of shigellosis when it is given prophylactically, before the ingestion of a virulent strain (46). This is likely due to the antibacterial effect of rifaximin in preventing infection at the level of the bile-rich small intestine, with the drug having a limited effect once the bacteria colonize the colon.
It is likely that the increased antimicrobial effect of adding bile acids to rifaximin relates to the drug's increase in solubility due to the presence of hydrophobic bile acids at concentrations that are normally present in the small bowel, as our data demonstrate. However, the contributions of other factors that could affect bacterial growth, such as the effect of bile acids on the structural integrity of bacterial membrane and cell components, cannot be excluded. Further investigation is necessary to elucidate the exact mechanism under which this may occur. Mechanistically, we propose that bile acids, in addition to solubilizing rifaximin, may also weaken the bacterial cell membrane and thus increase the drug's entry into the cytoplasm. Our findings have important implications for the potential use of hydrophobic detergents and/or biocompatible compounds to improve the efficacies and antimicrobial effects of hydrophobic antibiotics. Questions that would need to be answered using this approach include a potential increase in absorption of the antibiotic and the effect of the more solubilized drug on the normal gut flora. We assume that alterations of the flora and inhibition of colonic bacterial pathogens would be a consequence of this modification, which could be an advantage.
Supplementary Material
FIG. 3.
Growth of ETEC strain H10407 in the presence of rifaximin (16 μg/ml) in water and an equimolar mixture of the synthetic bile acids cholic, deoxycholic, chenodeoxycholic, glycocholic, lithocholic, and taurocholic acids at pH 7.4. The total bile acid concentration was 4 mM. Cells were grown with rifaximin in the presence and absence of bile acids, and the absorbance at 600 nm was measured at 30-min intervals. Mann-Whitney two-tailed nonparametric t test analysis showed a statistically significant difference between treatments, as follows: no rifaximin plus no bile versus 16 μg/ml rifaximin, P = 0.026 (n = 4); no rifaximin plus no bile acids versus 16 μg/ml rifaximin plus bile acids, P = 0.002 (n = 4); and 16 μg/ml rifaximin versus 16 μg/ml rifaximin plus bile acids, P = 0.007 (n = 4). The error bars represent the standard deviations between four replicate experiments.
Acknowledgments
This work was supported in part by discretionary funds from the University of Texas School of Public Health and Public Health Service (grant DK56338), which funds the Texas Medical Center Digestive Disease Center.
We acknowledge David Graham for providing the human bile used in this project. We also thank Claudia Kozinetz (Baylor College of Medicine and Study Design and Clinical Research Core, Texas Medical Center Digestive Disease Center) and Xiaoying Yu (Baylor College of Medicine) for helping us with the statistical analysis.
Footnotes
Published ahead of print on 14 June 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Adachi, J. A., Z. D. Jiang, J. J. Mathewson, M. P. Verenkar, S. Thompson, F. Martinez-Sandoval, R. Steffen, C. D. Ericsson, and H. L. DuPont. 2001. Enteroaggregative Escherichia coli as a major etiologic agent in traveler's diarrhea in 3 regions of the world. Clin. Infect. Dis. 32:1706-1709. [DOI] [PubMed] [Google Scholar]
- 2.Al-Abri, S. S., N. J. Beeching, and F. J. Nye. 2005. Traveler's diarrhoea. Lancet Infect. Dis. 5:349-360. [DOI] [PubMed] [Google Scholar]
- 3.Begley, M., G. M. Cormac, and C. Hill. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29:625-651. [DOI] [PubMed] [Google Scholar]
- 4.Black, R. E. 1990. Epidemiology of travelers' diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12(Suppl. 1):S73-S79. [DOI] [PubMed] [Google Scholar]
- 5.Black, R. E. 1993. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine 11:100-106. [DOI] [PubMed] [Google Scholar]
- 6.Bortolini, O., A. Medici, and S. Poli. 1997. Biotransformations on steroid nucleus of bile acids. Steroids 62:564-577. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Brook, I. 1989. Aerobic and anaerobic microbiology of biliary tract disease. J. Clin. Microbiol. 27:2373-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter, H. A. 1998. Bacterial and parasitic cholangastrointestinal tractis. Mayo Clin. Proc. 73:473-478. [DOI] [PubMed] [Google Scholar]
- 10.Christiaens, H., R. J. Leer, P. H. Pouwels, and W. Verstraete. 1992. Cloning and expression of a conjugated bile acid hydrolase gene from Lactobacillus plantarum by using a direct plate assay. Appl. Environ. Microbiol. 58:3792-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen, D. E., M. Angelico, and M. C. Carey. 1990. Structural alterations in lecithin-cholesterol vesicles following interactions with monomeric and micellar bile salts: physical-chemical basis for subselection of biliary lecithin species and aggregative states of biliary lipids during bile formation. J. Lipid Res. 31:55-70. [PubMed] [Google Scholar]
- 12.Coleman, R., P. J. Lowe, and D. Billington. 1980. Membrane lipid composition and susceptibility to bile salt damage. Biochim. Biophys. Acta 599:294-300. [DOI] [PubMed] [Google Scholar]
- 13.De Smet, I., L. Van Hoorde, M. V. Woestyne, H. Christiaens, and W. Verstraete. 1995. Significance of bile salt hydrolytic activities of lactobacilli. J. Appl. Bacteriol. 79:292-301. [DOI] [PubMed] [Google Scholar]
- 14.Doerner, K. C., F. Takamine, C. P. LaVoie, D. H. Mallonee, and P. B. Hylemon. 1997. Assessment of fecal bacteria with bile acid 7α-dehydroxylation activity for the presence of bai like genes. Appl. Environ. Microbiol. 63:1185-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowling, R. H. 1973. The enterohepatic circulation of bile acids as they relate to lipid disorders. J. Clin. Pathol. 5:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuPont, H. L., Z. D. Jiang, C. D. Ericsson, J. A. Adachi, J. J. Mathewson, and M. W. DuPont. 2001. Rifaximin versus ciprofloxacin for the treatment of traveler's diarrhea: a randomized, double-blind clinical trial. Clin. Infect. Dis. 33:1807-1815. [DOI] [PubMed] [Google Scholar]
- 17.DuPont, H. L., and Z. D. Jiang. 2004. Influence of rifaximin treatment on the susceptibility of intestinal Gram-negative flora and enterococci. Clin. Microbiol. Infect. 10:1009-1011. [DOI] [PubMed] [Google Scholar]
- 18.DuPont, H. L., Z. D. Jiang, P. C. Okhuysen, C. D. Ericsson, F. J. de la Cabada, and S. Ke. 2005. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers' diarrhea. Ann. Intern. Med. 142:805-812. [DOI] [PubMed] [Google Scholar]
- 19.Flores, C., I. Maguilnik, E. Hadlich, and L. Z. Goldani. 2003. Microbiology of choledochal bile in patients with choledocholithiasis admitted to a tertiary hospital. J. Gastroenterol. Hepatol. 18:333-336. [DOI] [PubMed] [Google Scholar]
- 20.Gänzle, M. G., C. Hertel, J. M. B. van der Vossen, and W. P. Hammes. 1999. Effect of bacteriocin-producing lactobacilli on the survival of Escherichia coli and Listeria in a dynamic model of the stomach and the small intestine. Int. J. Food Microbiol. 48:21-35. [DOI] [PubMed] [Google Scholar]
- 21.Gomi, H., Z. D. Jiang, J. A. Adachi, D. Ashley, B. Lowe, M. P. Verenkar, R. Steffen, and H. L. DuPont. 2001. In vitro antimicrobial susceptibility testing of bacterial enteropathogens causing traveler's diarrhea in four geographic regions. Antimicrob. Agents Chemother. 45:212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray, C. H., D. C. Nicholson, and R. V. Quincey. 1968. Fate of bile in the bowel, p. 2483-2505. In C. F. Code (ed.), Handbook of physiology, section 6, alimentary canal, vol. V. American Physiological Society, Washington, DC. [Google Scholar]
- 23.Grill, J. P., F. Schneider, J. Crociani, and J. Ballongue. 1995. Purification and characterization of conjugated bile salt hydrolase from Bifidobacterium longum BB536. Appl. Environ. Microbiol. 61:2577-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardison, W. G. 1978. Hepatic taurine concentration and dietary taurine as regulators of bile acid conjugation with taurine. Gastroenterology 75:71-75. [PubMed] [Google Scholar]
- 25.Heuman, D. M., R. S. Bajaj, and Q. Lin. 1996. Adsorption of mixtures of bile salt taurine conjugates to lecithin-cholesterol membranes: implications for bile salt toxicity and cytoprotection. J. Lipid Res. 37:562-573. [PubMed] [Google Scholar]
- 26.Hofmann, A. F. 1999. Bile acids: the good, the bad, and the ugly. News Physiol. Sci. 14:24-29. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann, A. F. 1994. Bile acids, p. 677-718. In I. M. Arias, J. L. Boyer, N. Fausto, D. A. Jackoby, D. A. Schachter, and D. A. Shafritz (ed.), The liver: biology and pathobiology. Raven Press Ltd., New York, NY.
- 28.Infante, R. M., C. D. Ericsson, Z. D. Jiang, S. Ke, R. Steffen, and L. Riopel. 2004. Enteroaggregative Escherichia coli diarrhea in travelers: response to rifaximin therapy. Clin. Gastroenterol. Hepatol. 2:135-138. [DOI] [PubMed] [Google Scholar]
- 29.Jiang, Z. D., and H. L. DuPont. 2005. Rifaximin: in vitro and in vivo antibacterial activity—a review. Chemotherapy 51(Suppl. 1):67-72. [DOI] [PubMed] [Google Scholar]
- 30.Jiang, Z. D., B. Lowe, M. P. Verenkar, D. Ashley, R. Steffen, and N. Tornieporth. 2002. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J. Infect. Dis. 185:497-502. [DOI] [PubMed] [Google Scholar]
- 31.Lauritano, E. C., M. Gabrielli, A. Lupascu, A. Santoliquido, G. Nucera, and E. Scarpellini. 2005. Rifaximin dose-finding study for the treatment of small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 22:31-35. [DOI] [PubMed] [Google Scholar]
- 32.Lundeen, S. G., and D. C. Savage. 1990. Characterization and purification of bile salt hydrolase from Lactobacillus sp. strain 100-100. J. Bacteriol. 172:4171-4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallonee, D. H., and P. B. Hylemon. 1996. Sequencing and expression of a gene encoding a bile acid transporter from Eubacterium sp. strain VPI 12708. J. Bacteriol. 178:7053-7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 35.Monte, M. J., J. J. G. Marin, A. Antelo, and J. Vazquez-Tato. 2009. Bile acids: chemistry, physiology, and pathophysiology. World J. Gastroenterol. 15:804-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Northfield, T. C., and I. McColl. 1973. Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. Gut 14:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onyekaba, C. O., and H. O. Njoku. 1986. Bacteria and helminth isolated from bile and faeces of zebu cattle slaughtered for human consumption in the Niger Delta areas of Nigeria. Ann. Trop. Med. Parasitol. 80:421-424. [DOI] [PubMed] [Google Scholar]
- 38.Owen, R. W. 1985. Biotransformation of bile acids by clostridia. J. Med. Microbiol. 20:233-238. [DOI] [PubMed] [Google Scholar]
- 39.Poley, J. R., and A. F. Hofmann. 1976. Role of fat maldigestion in pathogenesis of steatorrhea in ileal resection. Fat digestion after two sequential test meals with and without cholestyramine. Gastroenterology 70:1125-1129. [PubMed] [Google Scholar]
- 40.Ruiza, J., L. Mensa, C. O'Callaghan, M. J. Pons, A. González, J. Vila, and J. Gascón. 2007. In vitro antimicrobial activity of rifaximin against enteropathogens causing traveler's diarrhea. Diagn. Microbiol. Infect. Dis. 59:473-475. [DOI] [PubMed] [Google Scholar]
- 41.Sierra, J. M., J. Ruiz, M. M. Navia, M. Vargas, J. Gascon, and J. Vila. 2001. In vitro activity of rifaximin against enteropathogens producing traveler's diarrhea. Antimicrob. Agent Chemother. 45:643-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sjovall, J. 1959. Dietary glycine and taurine on bile acid conjugation on man. Bile acids and steroids. Proc. Soc. Exp. Biol. Med. 100:676-678. [DOI] [PubMed] [Google Scholar]
- 43.Steffen, R., F. Castelli, N. H. Dieter, L. Rombo, and J. N. Zuckerman. 2005. Vaccination against enterotoxigenic Escherichia coli, a cause of travelers' diarrhea. J. Travel Med. 12:102-107. [DOI] [PubMed] [Google Scholar]
- 44.Steffen, R., D. A. Sack, L. Riopel, Z. D. Jiang, M. Sturchler, and C. D. Ericsson. 2003. Therapy of travelers' diarrhea with rifaximin on various continents. Am. J. Gastroenterol. 98:1073-1078. [DOI] [PubMed] [Google Scholar]
- 45.Taylor, D. N., A. L. Bourgeois, C. D. Ericsson, R. Steffen, Z. D. Jiang, and J. Halpern. 2006. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers' diarrhea. Am. J. Trop. Med. Hyg. 74:1060-1066. [PubMed] [Google Scholar]
- 46.Taylor, D. N., R. McKenzie, A. Durbin, et al. 2006. Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clin. Infect. Dis. 42:1283-1288. [DOI] [PubMed] [Google Scholar]
- 47.Taylor, D. N., R. McKenzie, A. Durbin, C. Carpenter, R. Haake, and A. L. Bourgeois. 2008. Systemic pharmacokinetics of rifaximin in volunteers with shigellosis. Antimicrob. Agents Chemother. 52:1179-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wells, J. E., and P. B. Hylemon. 2000. Identification and characterization of a bile acid 7α-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7α-dehydroxylating strain isolated from human feces. Appl. Environ. Microbiol. 66:1107-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wenneras, C., and V. Erling. 2004. Prevalence of enterotoxigenic Escherichia coli-associated diarrhoea and carrier state in the developing world. J. Health Popul. Nutr. 22:370-382. [PubMed] [Google Scholar]
- 50.Zhang, X., and H. Bremer. 1995. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J. Biol. Chem. 270:11181-11189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.