Abstract
AmpC hyperproduction is the most frequent mechanism of resistance to penicillins and cephalosporins in Pseudomonas aeruginosa and is driven by ampD mutations or the recently described inactivation of dacB, which encodes the nonessential penicillin-binding protein (PBP) PBP 4. Recent work showed that nagZ inactivation attenuates β-lactam resistance in ampD mutants. Here we explored whether the same could be true for the dacB mutants with dacB mutations alone or in combination with ampD mutations. The inactivation of nagZ restored the wild-type β-lactam MICs and ampC expression of PAO1 dacB and ampD mutants and dramatically reduced the MICs (for example, the MIC for ceftazidime dropped from 96 to 4 μg/ml) and the level of ampC expression (from ca. 1,000-fold to ca. 50-fold higher than that for PAO1) in the dacB-ampD double mutant. On the other hand, nagZ inactivation had little effect on the inducibility of AmpC. The NagZ inhibitor O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate attenuated the β-lactam resistance of the AmpC-hyperproducing strains, showing a greater effect on the dacB mutant (reducing the ceftazidime MICs from 24 to 6 μg/ml) than the ampD mutant (reducing the MICs from 8 to 4 μg/ml). Additionally, nagZ inactivation in the dacB mutant blocked the overexpression of creD (blrD), which is a marker of the activation of the CreBC (BlrAB) regulator involved in the resistance phenotype. Finally, through population analysis, we show that the inactivation of nagZ dramatically reduces the capacity of P. aeruginosa to develop ceftazidime resistance, since spontaneous mutants were not obtained at concentrations ≥8 μg/ml (the susceptibility breakpoint) for the nagZ mutant but were obtained with wild-type PAO1. Therefore, NagZ is envisaged to be a candidate target for preventing and reverting β-lactam resistance in P. aeruginosa.
The increasing prevalence of nosocomial infections produced by multidrug-resistant (MDR) Pseudomonas aeruginosa strains severely compromises the selection of appropriate treatments and is therefore associated with significant morbidity and mortality (23, 33). Indeed, one of the most striking features of P. aeruginosa is its extraordinary capacity for developing resistance to almost any available antibiotic by the selection of mutations in chromosomal genes (26, 29). Among the mutation-mediated β-lactam resistance mechanisms, particularly noteworthy are those that lead to the repression or inactivation of the porin OprD, which confers resistance to carbapenems (12, 36), or those leading to the hyperexpression of the chromosomal cephalosporinase AmpC, which confers resistance to penicillins, cephalosporins, and monobactams (11, 17).
AmpC is a chromosomally encoded group I, class C cephalosporinase produced by P. aeruginosa, as well as many other nonfermenting Gram-negative bacilli and most Enterobacteriaceae family members (6). Although AmpC is produced at very low basal levels in wild-type strains, its expression is highly inducible in the presence of certain β-lactams (β-lactamase inducers), such as cefoxitin and imipenem (IMP) (28). In fact, the activities of the antipseudomonal penicillins (such as ticarcillin and piperacillin [PIP]), cephalosporins (such as ceftazidime [CAZ] and cefepime [FEP]), and monobactams (such as aztreonam [ATM]) rely on the fact that they are very weak AmpC inducers, since they, too, are hydrolytically inactivated by this enzyme (28). For this reason, during treatment with these weak inducers, mutants showing constitutive high-level AmpC production (AmpC derepressed mutants) are frequently selected, leading to the failure of antimicrobial therapy (11, 16, 17, 27).
There are several genes involved in the regulation of ampC expression, a process that was first investigated in the Enterobacteriaceae and found to be intimately linked to peptidoglycan recycling (32, 35). ampG encodes an inner membrane permease for GlcNAc-1,6-anhydromuropeptides, which are peptidoglycan catabolites that, upon entry into the cytosol, are processed by β-N-acetylglucosaminidase, known as NagZ, to generate 1,6-anhydromuropeptides. The 1,6-anhydromuropeptide products of NagZ are thought to induce AmpC production by interacting with the LysR-type transcriptional regulator AmpR (9, 14, 15, 21, 24). During regular bacterial growth, 1,6-anhydromuropeptides are processed by the N-acetyl-anhydromuramyl-l-alanine amidase AmpD, preventing ampC induction (13, 35). On the other hand, during growth in the presence of strong β-lactamase inducers, large amounts of muropeptides are generated and accumulate in the cytoplasm, which leads to the AmpR-mediated induction of ampC expression (9, 14, 15, 24). It is also well known that the mutational inactivation of AmpD leads to the accumulation of 1,6-anhydromuropeptides and high-level ampC expression, even in the absence of β-lactamase inducers, producing the classical constitutively derepressed phenotype of AmpC production (25). Additionally, given that NagZ removes GlcNAc to produce the 1,6-anhydromuropeptides (7, 40), inhibitors of this enzyme have been shown to mitigate AmpC-driven resistance (39).
In contrast to the classical fully derepressed phenotypes observed in the Enterobacteriaceae, the inactivation of ampD in P. aeruginosa was shown to lead to a partially derepressed phenotype, characterized by a moderately high level of basal ampC expression that is still further inducible (22). Further studies showed that the partial derepression of the P. aeruginosa ampD mutant is due to the presence of two additional ampD genes in this species, designated ampDh2 and ampDh3 (18). Furthermore, the sequential inactivation of these three ampD homologues was shown to lead to the stepwise upregulation of ampC expression, reaching full derepression with very high level basal ampC expression (over 1,000-fold compared to that of the wild type) in the triple mutant (18). While inactivation of ampD, producing moderate β-lactam resistance, is frequently observed in the clinical setting (5, 17), high-level resistance caused by inactivation of multiple ampD genes has not been observed (30, 38), most likely due to the high biological cost associated with inactivation of more than one of these genes (30).
Recent work (31) showed that one-step high-level (clinical) resistance in P. aeruginosa frequently results from the inactivation of dacB, encoding the nonessential penicillin-binding protein (PBP) PBP 4 (10, 20). The inactivation of PBP 4 was shown to give rise to a highly efficient and complex β-lactam resistance response, triggering the overproduction of the chromosomal β-lactamase AmpC and the specific activation of the CreBC (BlrAB) two-component regulator (1, 3, 4), which was demonstrated to play a major role in the resistance phenotype (31). Moreover, dacB (PBP 4) mutations were observed in a high proportion of AmpC-hyperproducing clinical isolates (31). Recent studies of the structure of PBP 4 have highlighted its potential role as a β-lactam sensor involved in regulating AmpC expression (19).
In a recent work, we showed that nagZ inactivation or direct inhibition of the NagZ enzyme by small molecules potentiates β-lactam susceptibility in a P. aeruginosa ampD background (2). In the present study, we explored whether the same could be true for the dacB (PBP 4) mutants, alone or in combination with ampD mutations. Moreover, since previous works have shown that in P. aeruginosa ampD or dacB mutations are the main mechanisms leading to penicillin and cephalosporin resistance, we investigated whether inactivation of NagZ prevents the emergence of resistant mutants in vitro.
MATERIALS AND METHODS
Strains, plasmids, and susceptibility testing.
The bacterial strains and plasmids used or constructed in this study are listed in Table 1. The MICs of CAZ, FEP, ATM, PIP, piperacillin-tazobactam (PTZ), IMP, and meropenem (MER) for all the strains were determined with Etest strips (AB Biodisk, Solna, Sweden) on Mueller-Hinton (MH) agar, according to the manufacturer's recommendations. Additionally, following previously established protocols (2), the MICs of CAZ, ATM, and PIP were determined by microdilution in 100 μl of cation-adjusted MH broth (8) in the presence or absence of 0.5 mM and 5 mM concentrations of the N-acetyl-β-glucosaminidase inhibitor O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc) (39). The phenotypic determination of AmpC inducibility was performed by assessing the presence of antagonism between IMP and CAZ disks (separated by 5 to 30 mm) in MH agar plates, as described previously (18).
TABLE 1.
Strains and plasmids used or constructed
| Strain or plasmid | Genotype/relevant characteristicsa | Reference or source |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Reference strain completely sequenced | Laboratory collection |
| PAΔD | PAO1 ΔampD::lox | 18 |
| PAΔdB | PAO1 ΔdacB::lox | 31 |
| PAdacB | 1A1 spontaneous dacB null mutant (W273X) of PAO1 | 31 |
| PAdacBΔD | PAdacB ΔampD::lox | 31 |
| PAΔDDh2Dh3 | PAO1 ΔampD::lox ΔampDh2::lox ΔampDh3::lox | 18 |
| PAΔR | PAO1 ΔampR::lox | 31 |
| PAΔC | PAO1 ΔampC::lox | 30 |
| PAΔnZ | PAO1 ΔnagZ::lox | This work |
| PAΔDnZ | PAO1 ΔampD::lox ΔnagZ::lox | This work |
| PAΔdBnZ | PAO1 ΔdacB::lox ΔnagZ::lox | This work |
| PAdacBΔDnZ | PAdacB ΔampD::lox ΔnagZ::lox | This work |
| PAΔDDh2Dh3Nz | PAO1 ΔampD::lox ΔampDh2::lox ΔampDh3::lox ΔnagZ::lox | This work |
| E. coli | ||
| XL1-Blue | F′::Tn10 proA+B+ lacIq Δ(lacZ)M15/recA1 endA1 gyrA96 (Nalr) thi hsdR17 (rk− mk−) mcrB1 | Laboratory collection |
| S17-1 | RecA pro (RP4-2Tet:: Mu Kan::Tn7) | Laboratory collection |
| Plasmids | ||
| pEX100Tlink | AprsacB, pUC19-based gene replacement vector with an MCS | 37 |
| PUCGmlox | Apr Gmr, pUC18-based vector containing the lox-flanked aacC1 gene | 37 |
| pCM157 | Tcr, cre expression vector | 37 |
| PEXnZ | pEX100Tlink containing 5′ and 3′ flanking sequences of nagZ | This work |
| PEXnZGm | pEX100Tlink containing 5′ and 3′ flanking sequences of nagZ::Gmlox | This work |
MCS, multiple cloning site; Apr, ampicillin resistant; Gmr, gentamicin resistant; Tcr, tetracycline resistant.
Construction of nagZ-knockout mutants.
nagZ-knockout mutants of strains PAO1, PAΔD, PAΔdB, PAdacBΔD, and PAΔDDh2Dh3 (Table 1) were constructed following well-established procedures (18, 31), based on the cre-lox system for gene deletion and antibiotic resistance marker recycling in P. aeruginosa (37). The PCR products of the upstream and downstream sequences (Table 2) of nagZ (using PAO1 DNA as the template) were digested with either BamHI or EcoRI and HindIII and cloned by a three-way ligation into pEX100Tlink deleted for the HindIII site and opened by EcoRI and BamHI. The resulting plasmid (pEXnZ) was transformed into the Escherichia coli XL1-Blue strain, and transformants were selected in 30 μg/ml ampicillin LB agar plates. The lox-flanked gentamicin resistance cassette (aac1) obtained by HindIII restriction of plasmid pUCGmlox was cloned into the single site for this enzyme formed by the ligation of the two flanking fragments. The resulting plasmid (pEXnZGm) was again transformed into E. coli XL1-Blue, and transformants were selected in 30 μg/ml ampicillin-5 μg/ml gentamicin LB agar plates. The plasmid was then transformed into the E. coli S17-1 helper strain. Knockout mutants were generated by conjugation, followed by selection of double recombinants using 5% sucrose-1 μg/ml cefotaxime-30 μg/ml gentamicin LB agar plates. Double recombinants were checked by first screening for carbenicillin (200 μg/ml) susceptibility and afterwards by PCR amplification and sequencing. For the recycling of the gentamicin resistance cassettes, plasmid pCM157 was electroporated into the different mutants. Transformants were selected in LB agar plates with 250 μg/ml tetracycline. One transformant for each mutant was grown overnight in 250 μg/ml tetracycline LB broth in order to allow the expression of the cre recombinase. Plasmid pCM157 was then cured from the strains by successive passages in LB broth. Selected colonies were then screened for their tetracycline (250 μg/ml) and gentamicin (30 μg/ml) susceptibilities and checked by PCR amplification and DNA sequencing.
TABLE 2.
Primers used for construction of nagZ-knockout mutants
| Primer | Sequence (5′-3′)a | Locationb | PCR product size (bp) |
|---|---|---|---|
| nZF1-BHI | TCGGATCCTCCGGGCGGAACTCCATG | −153 to −135 | 505 |
| nZR1-Hd3 | TCAAGCTTAGCACCGGCGCGAAACTCA | 335 to 353 | |
| nZF2-Hd3 | TCAAGCTTAGCGACCTGGTTCCGTTCG | 568 to 586 | 432 |
| nZR2-ERI | TCGAATTCACTCATCGACAGCTCCCTCA | 980 to 999 |
The restriction sites for endonucleases are underlined.
Location of primers with respect to the nagZ start codon.
Quantification of gene expression.
The relative mRNA levels of ampC (18) and creD (blrD) (31) were determined by real-time reverse transcription-PCR (RT-PCR) following previously described protocols. Briefly, total RNA from logarithmic-phase-grown cultures was obtained with an RNeasy minikit (Qiagen, Hilden, Germany). Fifty nanograms of purified RNA was then used for one-step reverse transcription and real-time PCR, using a QuantiTect SYBR green reverse transcription-PCR kit (Qiagen, Hilden, Germany), in a SmartCycler II apparatus (Cepheid, Sunnyvale). Previously described conditions and primers were used (18, 31, 34). rpsL housekeeping gene was used to normalize the expression levels, and the results were always referred to the basal level of expression for PAO1. All RT-PCRs were performed in duplicate, and the mean values of mRNA expression resulting from three independent experiments were considered in all cases.
Population analysis of PAO1 and PAΔnZ CAZ susceptibility.
Serial dilutions of 10-ml overnight cultures (MH broth) of PAO1 or PAΔnZ were seeded in MH agar plates containing 0, 0.5, 1, 2, 4, 8, or 16 μg/ml of CAZ. Colonies growing after 24 h of incubation were counted, to plot the numbers of CFU at each antibiotic concentration. All experiments were performed in triplicate, and the results shown are the mean values ± standard deviations. CAZ MICs were determined, after passage in antibiotic-free medium, for selected colonies from MH agar plates containing the concentrations indicated above MIC (2, 4, 8, and 16 μg/ml), to confirm that growth was the consequence of the selection of spontaneous CAZ-resistant mutants and to evaluate their resistance levels. Moreover, the level of ampC expression was quantified in these mutants as described above to investigate the resistance mechanism involved.
RESULTS AND DISCUSSION
Inactivation of nagZ restores β-lactam susceptibility of PBP 4-knockout mutants.
Previous work showed that nagZ inactivation markedly increases the β-lactam susceptibilities of PAO1 ampD mutants (2). In the present study we explored whether the same could be true for the recently described dacB (PBP 4) mutants. Indeed, as shown in Table 3, the deletion of nagZ fully restored the susceptibility of the PAΔdB mutant to CAZ, FEP, PTZ, and ATM; a 2-fold reduction of the IMP MICs was also noted. Moreover, the β-lactam MICs against all the nagZ mutants of PAO1 (PAΔnZ), PAΔD (PAΔDnZ), and PAΔdB (PAΔdBnZ) were nearly identical or similar to the levels seen for PAO1, despite the important differences in the susceptibilities of the parent strains: wild-type, moderate, and high-level resistance, respectively. Further, the CAZ, FEP, PTZ, and ATM MICs were also notably reduced after nagZ inactivation in the ampD-dacB double mutant (PAdacBΔD), which exhibits extremely high level β-lactam resistance (Table 3). Although wild-type MICs were not fully restored through nagZ inactivation, the MICs obtained for PAdacBΔDnZ remained within the CLSI susceptibility breakpoints. Similar results were obtained for the triple ampD mutant (PAΔDDh2Dh3) (Table 3).
TABLE 3.
MICs and basal and induced levels of ampC and creD expression in the studied strains
| Strain | MIC (μg/ml) |
Relative mRNA levela |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
ampC |
creD (blrD) |
|||||||||
| CAZ | FEP | IMP | MER | PTZ | ATM | Basal | Inducedb | Basal | Induced | |
| PAO1 | 1.5 | 1.5 | 2 | 0.38 | 2 | 4 | 1 | 78 ± 34 | 1 | 24 ± 9.1 |
| PA ΔnZ | 1 | 1.5 | 1 | 0.38 | 2 | 4 | −2.1 ± 1.5 | 469 ± 230 | 1.1 ± 0.3 | 54 ± 18 |
| PAΔD | 8 | 4 | 2 | 1 | 32 | 12 | 47 ± 9.5 | 134 ± 11 | 1.1 ± 0.3 | 2.7 ± 0.7 |
| PAΔDnZ | 2 | 1.5 | 1 | 0.5 | 3 | 4 | 1.0 ± 0.4 | 483 ± 26 | 2.8 ± 1.6 | 99 ± 15 |
| PAΔdB | 24 | 12 | 2 | 0.5 | 64 | 24 | 51 ± 16 | 232 ± 67 | 83 ± 7 | 120 ± 29 |
| PAΔdBnZ | 1.5 | 2 | 1 | 0.38 | 3 | 4 | 3.0 ± 1.9 | 661 ± 374 | 4.0 ± 0.8 | 95 ± 45 |
| PAdacBΔD | 96 | 48 | 2 | 1.5 | >256 | 64 | 1,770 ± 414 | 1,950 ± 480 | 51 ± 24 | 81 ± 40 |
| PAdacBΔDnZ | 4 | 3 | 1 | 0.5 | 16 | 8 | 40 ± 17 | 906 ± 80 | 1.9 ± 1.3 | 418 ± 170 |
| PAΔDDh2Dh3 | 32 | 16 | 2 | 1 | >256 | 32 | 1,020 ± 87 | 1,105 ± 88 | 1.3 ± 0.3 | 1.6 ± 0.7 |
| PAΔDDh2Dh3nZ | 3 | 3 | 1.5 | 1 | 8 | 8 | 57 ± 31 | 905 ± 407 | −1.4 ± 0.1 | 1.0 ± 0.3 |
| PAΔR | 2 | 2 | 0.5 | 0.25 | 4 | 4 | 3.8 ± 0.4 | 3.3 ± 0.8 | 1.6 ± 0.1 | 189 ± 8 |
| PAΔC | 1 | 1 | 0.5 | 0.25 | 2 | 2 | NA | NA | ND | ND |
Relative amount of ampC or creD (blrD) mRNA compared to the basal levels ± standard deviation in strain PAO1. NA, not applicable; ND, not determined.
Induction experiments were carried out with 50 μg/ml of cefoxitin.
Inhibition of NagZ with PUGNAc potentiates β-lactam susceptibility of PBP 4-knockout mutants.
Previous work has shown that blocking NagZ activity with small-molecule inhibitors based on the PUGNAc scaffold can attenuate β-lactam resistance in the PAO1 ampD mutant (2) and in Escherichia coli harboring a plasmid-borne ampR-ampC operon from Citrobacter freundii (39). As expected, PUGNAc did not exhibit antimicrobial properties even when it was tested at a concentration of 5 mM (data not shown). However, as shown in Table 4, we show that when it was coadministered with β-lactams at a concentration of 0.5 mM, PUGNAc sharply attenuates β-lactam resistance in the PAO1 dacB mutant and does so to a greater extent, in fact, than that documented for the ampD mutant. For example, 0.5 mM PUGNAc reduced the CAZ MICs from 24 to 8 μg/ml in the dacB mutant, whereas the reduction was from 8 to 4 μg/ml in the ampD mutant (Table 4). The β-lactam MICs of the dacB mutant were slightly further decreased in the presence of the higher 5 mM PUGNAc concentration (Table 4). The effect of PUGNAc on the dacB-ampD double mutant was much smaller, but it still reduced the β-lactam MICs (Table 4), particularly at a concentration of 5 mM. While NagZ appears to be the only enzyme within PAO1 that exhibits N-acetyl-β-glucosaminidase activity (2), a comparison of the MIC results obtained in the presence of PUGNAc (Table 4) with the MICs of PAO1 mutants in which nagZ was inactivated genetically (Table 3) suggests that PUGNAc does not inhibit all endogenous NagZ activity, possibly due to the limited permeation of the inhibitor into the cytosol. Further ongoing research will elucidate the effect of efflux pump expression on PUGNAc NagZ-inhibitory activity, as well the potential utility of this compound against clinical multidrug-resistant P. aeruginosa strains.
TABLE 4.
Susceptibilities of PAO1 and ampD and dacB null mutants to PIP, ATM, and CAZ in the presence or absence of PUGNAca
| Strain | MICb (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PIP |
ATM |
CAZ |
|||||||
| −I | +I (0.5 mM) | +I (5 mM) | −I | +I (0.5 mM) | +I (5 mM) | −I | +I (0.5 mM) | +I (5 mM) | |
| PAO1 | 4 | 4 | 4 | 3 | 3 | 3 | 2 | 2 | 2 |
| PAΔD | 48 | 24 | 24 | 6 | 3 | 3 | 8 | 4 | 4 |
| PAΔdB | 64 | 32 | 24 | 12 | 4 | 3 | 24 | 8 | 6 |
| PAdacBΔD | 384 | 256 | 256 | 32 | 32 | 24 | 96 | 64 | 64 |
Effect of nagZ inactivation on ampC basal expression and ampC inducibility.
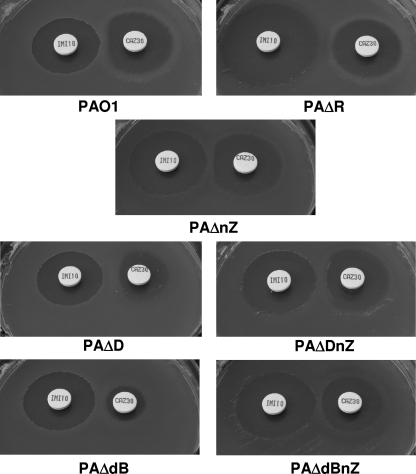
The results for basal and cefoxitin-induced ampC expression in the studied strains is shown in Table 3. In agreement with the susceptibility data, the inactivation of nagZ in the dacB or ampD mutant dramatically reduced the level of ampC expression, yielding values close to the basal levels of wild-type strain PAO1. Similarly, a marked decrease (from approximately 1,000-fold to approximately 50-fold) was also observed for the ampD-dacB double mutant and the ampD triple mutant, although consistent with the results of the antibiotic susceptibility assays, the wild-type basal expression levels were not fully restored. On the other hand, nagZ inactivation did not impair the ampC inducibility of wild-type PAO1 or the ampD and dacB mutants. These results are consistent with previous findings showing that nagZ inactivation has little effect on the cefoxitin-induced β-lactamase activity of PAO1 or its ampD mutants (2). Moreover, we show that inducible AmpC-mediated β-lactam resistance still occurs in the nagZ background (PAΔnZ, PAΔDnZ, and PAΔbBnZ), as demonstrated by the antagonism (Fig. 1) between IMP (a classical potent AmpC inducer) and CAZ (a poor AmpC inducer). In contrast, ampR inactivation completely abolished ampC inducibility (Table 1) and inducible resistance (Fig. 1). All together, these results strongly suggest that NagZ plays a major role in determining the basal level of ampC expression (and is therefore necessary for constitutive ampC overexpression) but not in cases wherein ampC is induced by classical β-lactam inducers, such as cefoxitin and imipenem. While it is clear that AmpR is necessary for both the constitutive overexpression and induction of ampC, there is still controversy over the true identity of the molecules that activate this transcriptional regulator in these two situations (35). Our results suggest that the product of NagZ, 1,6-anhydro-acetylmuramoyl peptides, is necessary for the activation of AmpR in the absence of classical β-lactam inducers and is therefore necessary for resistance to antispseudomonal penicillins, cephalosporins, and monobactams that is driven by constitutive AmpC overexpression (as caused by AmpD and/or PBP 4 inactivation). On the other hand, the absence of a significant effect on the inducibility of AmpC arising from the loss of nagZ suggests that a different molecule, not necessarily one produced by NagZ, activates AmpR in the presence of classical AmpC inducers. These results are consistent with previous data obtained with the Enterobacteriaceae model showing that the constitutive overexpression of AmpC resulted from the cytosolic accumulation of 1,6-anhydro-acetylmuramoyl tripeptide (15), a NagZ product, whereas the molecules that accumulated during induction when the classical β-lactam inducers were used were mainly free pentapeptides and a small amount of 1,6-anhydro-acetylmuramoyl pentapeptide (35). These observations therefore raise the interesting possibility that the pentapeptide itself may act as an inducer (35), a scenario we are currently investigating.
FIG. 1.
Double-disk (IMP-CAZ) AmpC induction test with strains PAO1, PAΔD (ampD), and PAΔdB (dacB) and their respective nagZ mutants, PAΔnZ, PAΔDnZ, and PAΔdBnZ. The results for the ampR mutant of PAO1 (PAΔR) are also included for comparison.
Effect of nagZ inactivation on basal level of creD (blrD) expression and inducibility.
In a previous study, we have shown that the inactivation of dacB in PAO1 leads to the activation of the CreBC (BlrAB) two-component regulator, evidenced by the overexpression of creD (blrD) mRNA (31). Moreover, we showed that creD is highly inducible by a classical AmpC inducer (cefoxitin) in wild-type PAO1, but the inducibility is abolished in an ampD background. Finally, we showed that the activation of the CreBC system caused by mutation of dacB (but not by mutation of the ampD genes) dramatically increases the β-lactam MICs. Therefore, the inactivation of dacB apparently leads to the specific activation of the CreBC (BlrAB) two-component regulator, which in turn gives rise to a high-level β-lactam-resistant phenotype. This information obtained in a PAO1 background was recently validated in genetically diverse P. aeruginosa clinical strains (41). In the present work, we evaluated whether the inactivation of nagZ (in wild-type PAO1 and its ampD and dacB mutants) affects creD expression or its inducibility. Remarkably, as shown in Table 3, the inactivation of nagZ attenuated creD overexpression in the dacB mutant. Also noteworthy is that the inactivation of nagZ restored the creD inducibility in the single ampD mutant, although, intriguingly, not in the triple ampD mutant. Therefore, through unknown mechanisms, NagZ products and/or substrates appear to be connected to the CreBC (BlrAB) regulatory pathway, governing the differential response driven by dacB and ampD inactivation. Collectively, these results suggest that nagZ inactivation in the dacB mutant reverts the two factors responsible for the high-level β-lactam resistance response, constitutive AmpC overexpression and constitutive activation of the CreBC two-component regulator.
Inactivation of nagZ prevents the emergence of high-level CAZ-resistant mutants.
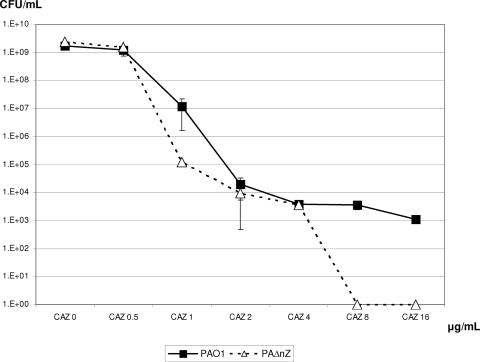
The results presented so far suggest that NagZ is necessary for resistance acquired through mutations (AmpD and/or PBP 4) that lead to constitutive AmpC overexpression. Since previous studies have shown that in P. aeruginosa AmpC overexpression is the main mechanism leading to resistance to antipseudomonal β-lactams such as CAZ in vitro, in vivo, and in the clinical setting, we hypothesized that blocking the NagZ function should prevent the selection of CAZ-resistant mutants. To test this hypothesis we performed a population analysis of PAO1 and PAΔnZ CAZ susceptibilities, and the results are shown in Fig. 2. Four differentiated features could be observed in the population analysis curves. First, no differences between PAO1 and PAΔnZ population sizes (approximately 109 CFU/ml) were observed in the absence or in the presence of subinhibitory concentrations of antibiotic (0.5 μg/ml). Second, at perinhibitory concentrations (1 μg/ml), the number of PAΔnZ cells (approximately 105 CFU/ml) was significantly less than the number of PAO1 cells (approximately 107 CFU/ml), likely reflecting the slightly increased CAZ susceptibility of the nagZ mutant, as already documented in Table 3 through MIC determination. Third, the numbers of PAO1 and PAΔnZ cells were again similar (approximately 104 CFU/ml) for low suprainhibitory concentrations (2 and 4 μg/ml), suggesting that low-level resistance occurs (through spontaneous mutation) at similar rates for both strains. Fourth and most relevant, while a significant number (approximately 103 CFU/ml) of high-level-resistant mutants (growing at 8 and 16 μg/ml, concentrations defining CLSI clinical resistance breakpoints) were still observed for PAO1, not a single CFU was recovered from PAΔnZ cultures at concentrations of ≥8 μg/ml. Moreover, the PAΔnZ mutants recovered at 2 to 4 μg/ml showed MICs (Etest) between 2 and 6 μg/ml, and none of them showed AmpC hyperproduction, as demonstrated through quantification of ampC expression. On the other hand, all PAO1 mutants growing at concentrations above 2 μg/ml had MICs >8 μg/ml. Data from our previous works had already shown that all spontaneous PAO1 CAZ-resistant mutants, selected at concentrations ranging from 4 to 16 μg/ml, are hyperproducers of AmpC, mainly through PBP 4 inactivation (31). In summary, loss of nagZ results in impairment of the ability of P. aeruginosa to acquire resistance to β-lactams arising through mechanisms leading to AmpC hyperproduction.
FIG. 2.
Population analysis of PAO1 and PAΔnZ CAZ susceptibility. Overnight cultures of PAO1 or PAΔnZ were plated in MH agar containing 0, 0.5, 1, 2, 4, 8, and 16 μg/ml of CAZ; and the numbers of CFU were enumerated after 24 h of incubation. The results shown here are the mean values of three experiments ± standard deviations.
Concluding remarks.
Collectively, these results show that blocking NagZ restores β-lactam susceptibility in ampD and dacB mutants, which is the most frequent mechanism leading to AmpC hyperproduction and penicillin and cephalosporin resistance in P. aeruginosa. Moreover, we show that the inactivation of nagZ dramatically reduces the capacity of P. aeruginosa to develop CAZ resistance through the selection of chromosomal mutations. Therefore, NagZ is envisaged to be a candidate target for preventing and reverting β-lactam resistance in P. aeruginosa.
Acknowledgments
This work was supported by grants from the Ministerio de Ciencia e Innovación of Spain and Instituto de Salud Carlos III, through the Spanish Network for the Research in Infectious Diseases (grants REIPI C03/14 and RD06/0008) and grant PS09/00033, as well as by the Canadian Institutes of Health Research and the Canadian Cystic Fibrosis Foundation. D.J.V. is a Tier II Canada Research Chair in Chemical Glycobiology and a scholar of the Michael Smith Foundation for Health Research.
We thank V. Larmour for technical assistance.
Footnotes
Published ahead of print on 21 June 2010.
REFERENCES
- 1.Alksne, L. E., and B. A. Rasmussen. 1996. Expression of the AsbA1, OXA-12, and AsbM1 beta-lactamases in Aeromonas jandaei AER 14 is coordinated by a two-component regulon. J. Bacteriol. 179:2006-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asgarali, A., K. A. Stubbs, A. Oliver, D. J. Vocadlo, and B. J. Mark. 2009. Inactivation of the glycoside hydrolase NagZ attenuates antipseudomonal β-lactam resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:2274-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avison, M. B., R. E. Horton, T. R. Walsh, and P. M. Bennett. 2001. Escherichia coli CreBC is a global regulator of gene expression that responds to growth in minimal media. J. Biol. Chem. 276:26955-26961. [DOI] [PubMed] [Google Scholar]
- 4.Avison, M. B., P. Niumpsup, K. Nurmahomed, T. R. Walsh, and P. M. Bennett. 2004. Role of the ‘cre/blr-tag’ DNA sequence in regulation of gene expression by the Aeromonas hydrophila beta-lactamase regulator, BlrA. J. Antimicrob. Chemother. 53:197-202. [DOI] [PubMed] [Google Scholar]
- 5.Bagge, N., O. Ciofu, M. Hentzer, J. I. A. Campbell, M. Givskov, and N. Hoiby. 2002. Constitutive high expression of chromosomal β-lactamase in Pseudomonas aeruginosa caused by a new insertion sequence (IS1669) located in ampD. Antimicrob. Agents Chemother. 46:3406-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, Q., H. Li, K. Merdek, and J. T. Park. 2000. Molecular characterization of the β-N-acetylglucosaminidase of Escherichia coli and its role in cell wall recycling. J. Bacteriol. 182:4836-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, sixth edition (M7-A6). Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Dietz, H., D. Pfeifle, and B. Wiedemann. 1997. The signal molecule for β-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob. Agents Chemother. 41:2113-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh, A. S., C. Chowdhury, and D. E. Nelson. 2008. Physiological functions of d-alanine carboxipeptidases in Escherichia coli. Trends Microbiol. 16:309-317. [DOI] [PubMed] [Google Scholar]
- 11.Giwercman, B., P. A. Lambert, V. T. Rosdahl, G. H. Shand, and N. Hoiby. 1990. Rapid emergence of resistance in Pseudomonas aeruginosa in cystic fibrosis patients due to in-vivo selection of stable partially derepessed β-lactamase producing strains. J. Antimicrob. Chemother. 26:247-259. [DOI] [PubMed] [Google Scholar]
- 12.Gutiérrez, O., C. Juan, E. Cercenado, F. Navarro, E. Bouza, P. Coll, J. L. Pérez, and A. Oliver. 2007. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa isolates from Spanish hospitals. Antimicrob. Agents Chemother. 51:4329-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Höltje, J. V., U. Kopp, A. Ursinus, and B. Wiedemann. 1994. The negative regulator of β-lactamase induction AmpD is a N-acetyl-anhydromuramyl-l-alanine amidase. FEMS Microbiol. Lett. 122:159-164. [DOI] [PubMed] [Google Scholar]
- 14.Honore, N., M. H. Nicolas, and S. T. Cole. 1986. Inducible cephalosporinase production in clinical isolates of Enterobacter cloacae is controlled by a regulatory gene that has been deleted from Escherichia coli. EMBO J. 5:3709-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, C., L. Huang, E. Bartowsky, S. Normark, and J. T. Park. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 13:4684-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juan, C., O. Gutiérrez, A. Oliver, J. I. Ayestarán, N. Borrell, and J. L. Pérez. 2005. Contribution of clonal dissemination and selection of mutants during therapy to Pseudomonas aeruginosa antimicrobial resistance in an intensive care unit setting. Clin. Microbiol. Infect. 11:887-892. [DOI] [PubMed] [Google Scholar]
- 17.Juan, C., M. D. Maciá, O. Gutiérrez, C. Vidal, J. L. Pérez, and A. Oliver. 2005. Molecular mechanisms of β-lactam resistance mediated by AmpC hyperproduction in Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 49:4733-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juan, C., B. Moyá, J. L. Pérez, and A. Oliver. 2006. Stepwise upregulation of the Pseudomonas aeruginosa chromosomal cephalosporinase conferring high level beta-lactam resistance involves three AmpD homologues. Antimicrob. Agents Chemother. 50:1780-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai, F., T. B. Clarke, D. I. Roper, G. J. Han, K. Y. Hwang, S. Unzai, E. Obayashi, S. Y. Park, and J. R. Tame. 2010. Crystal structures of penicillin-binding proteins 4 and 5 from Haemophilus influenzae. J. Mol. Biol. 396:634-645. [DOI] [PubMed] [Google Scholar]
- 20.Kishida, H., S. Unzai, D. I. Roper, A. Lloyd, S. Y. Park, and J. R. Tame. 2005. Crystal structure of penicillin binding protein 4 (dacB) from Escherichia coli, both in the native form and covalently linked to various antibiotics Biochemistry 45:783-792. [DOI] [PubMed] [Google Scholar]
- 21.Korfmann, G., and C. C. Sanders. 1989. ampG is essential for high level expression of AmpC beta-lactamase in Enterobacter cloacae. Antimicrob. Agents Chemother. 33:1946-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langaee, T. Y., L. Cagnon, and A. Huletsky. 2000. Inactivation of the ampD gene in Pseudomonas aeruginosa leads to moderate-basal-level and hyperinducible AmpC β-lactamase expression. Antimicrob. Agents Chemother. 44:583-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leibovici, L., I. Shraga, M. Drucker, H. Konigsberger, Z. Samra, and S. D. Pitliks. 1998. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 244:379-386. [DOI] [PubMed] [Google Scholar]
- 24.Lindberg, F., L. Westman, and S. Normark. 1985. Regulatory components in Citrobacter freundii ampC β-lactamase induction. Proc. Natl. Acad. Sci. U. S. A. 82:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindberg, F., S. Lindquist, and S. Normark. 1987. Inactivation of the ampD gene causes semiconstitutive overproduction of the inducible Citrobacter freundii β-lactamase. J. Bacteriol. 169:1923-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lister, P. D., D. J. Wolter, and N. D. Hanson. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22:582-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livermore, D. M. 1987. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. Eur. J. Clin. Microbiol. 6:439-445. [DOI] [PubMed] [Google Scholar]
- 28.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livermore, D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634-640. [DOI] [PubMed] [Google Scholar]
- 30.Moya, B., C. Juan, S. Alberti, J. L. Perez, and A. Oliver. 2008. Benefit of having multiple ampD genes for acquiring β-lactam resistance without losing fitness and virulence in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:3694-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moya, B., A. Döstch, C. Juan, J. Blázquez, L. Zamorano, S. Haussler, and A. Oliver. 2009. β-Lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PloS Pathog. 5:e1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Normark, S. 1995. β-Lactamase induction in Gram-negative bacteria is intimately linked to peptidoglycan recycling. Microb. Drug Resist. 1:111-114. [DOI] [PubMed] [Google Scholar]
- 33.Obritsch, M. D., D. N. Fish, R. MacLaren, and R. Jung. 2004. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob. Agents Chemother. 48:4606-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh, H., S. Stenhoff, S. Jalal, and B. Wretlind. 2003. Role of efflux pumps and mutations in genes for topoisomerases II and IV in fluoroquinolone-resistant Pseudomonas aeruginosa strains. Microb. Drug Res. 8:323-328. [DOI] [PubMed] [Google Scholar]
- 35.Park, J. T., and T. Uehara. 2008. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan). Microbiol. Mol. Biol. Rev. 72:211-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quale, J., S. Bratu, J. Gupta, and D. Landman. 2006. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 50:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quénée, L., D. Lamotte, and B. Polack. 2005. Combined sacB-based negative selection and cre-lox antibiotic marker recycling for efficient gene deletion in Pseudomonas aeruginosa. Biotechniques 38:63-67. [DOI] [PubMed] [Google Scholar]
- 38.Schmidtke, A. J., and N. D. Hanson. 2008. Role of ampD homologs in overproduction of AmpC in clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:3922-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stubbs, K. A., M. Balcewich, B. L. Mark, and D. J. Vocadlo. 2007. Small molecule inhibitors of a glycoside hydrolase attenuate inducible AmpC-mediated β-lactam resistance. J. Biol. Chem. 282:21382-21391. [DOI] [PubMed] [Google Scholar]
- 40.Vöstch, W., and M. F. Templin. 2000. Characterization of a β-N-acetylglucosaminidase of Escherichia coli and elucidation of its role in muropeptide recycling and β-lactamase induction. J. Biol. Chem. 275:39032-39038. [DOI] [PubMed] [Google Scholar]
- 41.Zamorano, L., B. Moya, C. Juan, and A. Oliver. 2010. Differential β-lactam resistance response driven by ampD or dacB (PBP4) inactivation in genetically-diverse Pseudomonas aeruginosa strains. J. Antimicrob. Chemother. 65:1540-1542. [DOI] [PubMed] [Google Scholar]