Abstract
With this prospective observational follow-up study of 165 methicillin-resistant Staphylococcus aureus (MRSA)-positive individuals (23 health care workers and 142 patients), we determined that our MRSA eradication therapy protocol results in a high success rate (81%). Five or more negative culture sets give a predictive value for MRSA eradication therapy success of >90%. Furthermore, MRSA colonization, at least in the throat, and the presence of wounds just before the start of MRSA eradication therapy are associated with MRSA eradication therapy failure.
Carriage of methicillin-resistant Staphylococcus aureus (MRSA) precedes endogenous MRSA infections (4, 11, 24, 34). MRSA prevalence in the Netherlands is among the lowest in the world (13) because of an active “search-and-destroy” policy (35, 38). The “destroy” part of this policy is important, because it eliminates two out of the three known reservoirs: carriage in patients and health care workers (HCWs). The third reservoir is the environment.
Different MRSA eradication therapies have been studied (1, 2, 7, 8, 10, 15, 17-19, 22, 26, 29, 31, 33, 36, 37). Failure of MRSA eradication therapy has often been attributed to colonization of extranasal sites (2, 17, 20). No consensus exists regarding the number of follow-up cultures that should be obtained after MRSA eradication therapy to assess the success of treatment. The Dutch national policy (WIP) suggests a minimum of three follow-up culture sets to declare a former MRSA carrier negative. However, this culture rule is based not on solid evidence but on expert opinion (40).
The aim of this observational study was threefold: first, to assess the success rate of our MRSA eradication therapy; second, to analyze determinants predicting the outcome of eradication therapy; and third, to assess the minimum number of follow-up screenings after eradication therapy that are necessary in order to determine its success.
(This work was presented in part at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy—Infectious Diseases Society of America [ICAAC/IDSA], Washington, DC, 24 to 28 October 2008.)
We prospectively studied 165 newly detected MRSA carriers, either health care workers (n, 23) or patients (n, 142), at the Erasmus University Medical Center (Rotterdam, Netherlands) from 2005 until 2008. Of these carriers, 110 were eligible for MRSA eradication therapy and follow-up (Fig. 1). Baseline characteristics are given in Table 1. Statistical analyses were done using SPSS, version 15.0 (SPSS Inc., Chicago, IL).
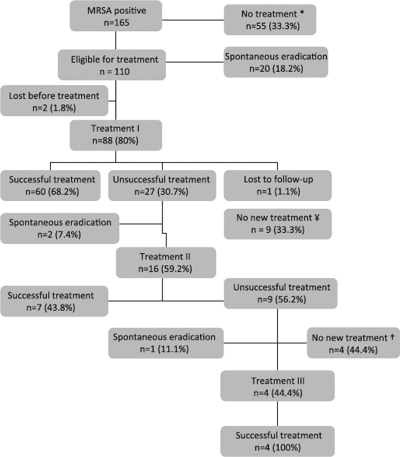
FIG. 1.
Flowchart demonstrating the outcomes for MRSA-positive individuals after the first detection of MRSA. Unsuccessful treatment was defined as one or more cultures positive in one or more out of six consecutive follow-up culture sets (e.g., nose, throat, perineum, and any wounds or skin lesions present). *, patients not included because either (i) there was no treatment in our hospital (n, 26), (ii) they were not eligible for treatment (due to relative contraindications for eradication therapy, loss to follow-up after the first MRSA detection, or a high risk of noncompliance with treatment) (n, 17), or (iii) they died before treatment could be offered (n, 12). ¥, no new MRSA eradication therapy because of newly arisen relative contraindications for eradication therapy (n, 7), unavailability of new therapy in our hospital (n, 1); or loss to follow-up (n, 1). †, no new MRSA eradication therapy because of newly arisen relative contraindications for eradication therapy (n, 4). ‡, not included in the intention-to-treat analysis.
TABLE 1.
General baseline characteristics of MRSA-infected individuals as measured at the time of detection of the first MRSA-positive culture
| Characteristic | Value for groupa |
P (patients with spontaneous clearance vs MRSA eradication therapy) | ||
|---|---|---|---|---|
| Health care workers (n = 22) | Patients undergoing MRSA eradication therapy (n = 68) | Patients with spontaneous MRSA clearance (n = 20) | ||
| Sex | 0.43 | |||
| Male | 6 (27) | 41 (60) | 14 (70) | |
| Female | 16 (73) | 27 (40) | 6 (30) | |
| Median (range) age (yr) | 29 (22-53) | 37.5 (1-82) | 48 (1-76) | 0.10b |
| Age category | ||||
| 0-10 yr | 0 (0) | 16 (24) | 2 (10) | 0.30 |
| 11-20 yr | 0 (0) | 3 (4) | 1 (5) | 0.48 |
| 21-60 yr | 22 (100) | 40 (59) | 11 (55) | 0.34 |
| >60 yr | 0 (0) | 9 (13) | 6 (30) | 0.07 |
| Μedian (range) no. of sites colonized | 1 (1-3) | 2 (1-4) | 1 (1-3) | 0.005 |
| MRSA colonization sites | ||||
| Nasal only | 9 (41) | 5 (7) | 3 (15) | 0.38 |
| Extranasal only | 7 (32) | 17 (25) | 11 (55) | 0.01 |
| Nasal and extranasal | 6 (27) | 46 (68) | 6 (30) | 0.003 |
| Hospital admission in preceding yr | 42 (62) | 16 (80) | 0.13 | |
| Nonintact skin | 8 (38)c | 44 (66)d | 15 (75) | 0.43 |
| Wounds | 4 (19)c | 34 (52)e | 14 (70) | 0.15 |
| Skin problems | 8 (38)c | 15 (22)d | 4 (20) | 0.82 |
| Invasive devices (total) | 23 (34)d | 11 (55) | 0.10 | |
| Catheter | 19 (29)f | 8 (40) | ||
| Drain | 11 (17)f | 4 (20) | ||
| Tracheostoma | 2 (3)e | 2 (10) | ||
| Implant | 9 (14)e | 6 (30) | ||
| Underlying disease (total)g | 2 (10)c | 11 (16)d | 6 (30) | 0.18 |
| Diabetes mellitus | 1 (5)c | 2 (3)d | 2 (10) | |
| COPD | 3 (5)d | 1 (5) | ||
| Renal insufficiency | 1 (5)c | 3 (5)d | 1 (5) | |
| Malignancy | 5 (8)d | 1 (5) | ||
| HIV | 1 (2)d | 1 (5) | ||
| Antibiotic use in the preceding mo | 1 (5)c | 20 (31)h | 8 (42)i | 0.38 |
Except where otherwise stated, the value is the number (percentage) of individuals with the characteristic.
Determined by the Mann-Whitney U test.
A total of 21 individuals were evaluated.
A total of 67 individuals were evaluated.
A total of 66 individuals were evaluated.
A total of 65 individuals were evaluated.
COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus.
A total of 64 individuals were evaluated.
A total of 19 individuals were evaluated.
At the time of first detection of MRSA (baseline measurement), as well as before the start of MRSA eradication therapy (second measurement), swabs were taken from all defined culture sites: the anterior nares, the throat, the perineum, and, if present, skin lesions, wounds, or indwelling devices. All cultures were tested by a PCR method (23).
All MRSA-positive individuals, whether they received eradication therapy or took the “wait-and-see” option (see below), were followed up with screening of all culture sites. Six follow-up culture sets were taken, with a median interval of 7 days (range, 2 to 230 days) between sets. For individuals undergoing MRSA eradication therapy, the first follow-up culture set was taken 1 week after the completion of therapy. The median duration of all follow-up cultures for the individuals who became MRSA negative (20 individuals with spontaneous MRSA clearance, 71 with successful MRSA eradication therapy, and 3 with spontaneous clearance after MRSA eradication therapy failure) was 43 days (range, 17 to 366 days). The median duration of follow-up for individuals whose first eradication therapy failed (n, 27) was shorter, because they did not complete the six follow-up culture sets (median, 12 days; range, 4 to 137 days).
Patients who had MRSA-negative cultures at all sites at the second measurement were offered the “wait-and-see” option (postponing eradication therapy and starting follow-up), and with this option, 20 patients (18%) spontaneously cleared their MRSA. The median number of colonized sites was significantly lower in patients with spontaneous MRSA clearance than in patients who needed MRSA eradication therapy (Table 1). Patients who received eradication therapy were more often colonized at both nasal and extranasal sites or at extranasal sites only than those with spontaneous MRSA clearance. This observation can be clarified by considering the findings of methicillin-susceptible Staphylococcus aureus (MSSA) studies showing that individuals with multiple-site colonization more often have high bacterial loads, which are associated with persistent carriage (8, 20, 27, 28, 39).
MRSA eradication therapies are listed in Table 2. These therapies were accompanied by hygienic instructions for daily life at home, such as washing bed linen, clothes, and towels. All health care workers (n, 20) received eradication therapy immediately after detection of MRSA (three HCWs were treated in another hospital). For MRSA-positive patients, the presence of indwelling devices, including drains, catheters, tracheostomas, and other implanted materials penetrating the skin, or nonintact skin was a relative contraindication for eradication therapy (14, 36), and MRSA eradication therapy was postponed if possible. Therefore, 68 patients received eradication therapy during the study period.
TABLE 2.
MRSA eradication therapies offered to MRSA-positive individualsa
| Therapy identification no. | Therapy description | No. (%) of MRSA-positive individuals receiving the therapyb |
|---|---|---|
| 1 | Mupirocin (2/day, 5 days) + chlorhexidine body wash (1/day, 5 days)c | 18 (21) |
| 2 | 1 + trimethoprim (2/day at 200 mg, 10 days) and rifampin (1/day at 600 mg, 10 days)d | 23 (26) |
| 3 | 2 + oral gentamicin solution (3/day at 80 mg, 10 days)e | 3 (3) |
| 4 | 1 + fusidic acid (3/day at 500 mg, 10 days) and rifampin (1/day at 600 mg, 10 days)d | 13 (15) |
| 5 | Otherd | 31 (35) |
There were no significant differences between the success rates of the different MRSA eradication therapies offered.
A total of 88 MRSA-positive individuals (20 health care workers and 68 patients) were studied.
In case of nasal MRSA colonization (including 6 persons with extranasal MRSA colonization).
In case of extranasal MRSA colonization with or without nasal MRSA colonization.
In case of perineal MRSA colonization with or without nasal MRSA colonization (5).
In total, 88 individuals received a mean of 1.5 MRSA eradication courses (range, 1 to 3 courses) per person. Seventy-one individuals (81% of those included in the intention-to-treat analysis) became MRSA negative (defined as six MRSA-negative follow-up culture sets). Eradication therapy has been studied in the past and has shown variable success rates (2, 6, 9, 12, 27, 28, 32, 36). Previous studies used definitions different from ours to ascertain the success of treatment; therefore, comparison of outcomes cannot yield robust conclusions.
Determinants for MRSA eradication therapy failure have been demonstrated in the past (6, 14, 16, 20, 21, 36), and our study confirmed several of these. Potential determinants of MRSA eradication therapy failure (MRSA-positive culture during follow-up) were colonization of the throat (74% compared to 53% with successful treatment; P, 0.06) and the presence of wounds (P, 0.05) at the second measurement (Table 3).
TABLE 3.
Determinants of MRSA eradication therapy failure for individuals receiving their first MRSA eradication therapy
| Determinanta | Value for groupb with: |
P | OR (95% CI) | |
|---|---|---|---|---|
| Successful treatment (n = 60) | Unsuccessful treatment (n = 27) | |||
| Sex | 0.36 | 1.53 (0.61-3.81) | ||
| Male | 33 (55) | 12 (44) | ||
| Female | 27 (45) | 15 (56) | ||
| Median (range) age (yr) | 39 (1-82) | 29 (1-73) | 0.19c | |
| Age category | ||||
| 0-10 yr | 12 (20) | 2 (7) | 0.33 | |
| 11-20 yr | 0 (0) | 3 (11) | 1.00 | |
| 21-60 yr | 40 (67) | 21 (78) | 0.16 | 3.15 (0.64-15.41) |
| >60 yr | 8 (13) | 1 (4) | 0.83 | 0.75 (0.06-9.72) |
| Median no. of colonized sites (range) | 2 (1-4) | 2 (1-4) | 0.10c | |
| MRSA colonization sitesd | ||||
| Nose (n = 87) | 42 (70) | 21 (78) | 0.45 | 1.50 (0.52-4.34) |
| Throat (n = 86) | 31 (53) | 20 (74) | 0.06 | 2.58 (0.95-7.02) |
| Perineum (n = 86) | 18 (31) | 12 (42) | 0.21 | 1.82 (0.71-4.66) |
| Other (n = 87)e | 21 (35) | 9 (33) | 0.88 | 0.93 (0.36-2.43) |
| Nasal only | 10 (17) | 4 (15) | 1.00c | |
| Extranasal only | 18 (30) | 6 (22) | 0.45 | 0.67 (0.23-1.93) |
| Nasal and extranasal | 32 (53) | 17 (63) | 0.40 | 1.49 (0.59-3.78) |
| Hospital admission in preceding yr | ||||
| At baseline measurement (n = 86) | 27 (46) | 15 (56) | 0.40 | 1.48 (0.59-3.76) |
| At second measurement (n = 86) | 11 (18) | 1 (4) | 0.10f | 0.18 (0.02-1.46) |
| Nonintact skin | ||||
| At baseline measurement (n = 85) | 35 (59) | 16 (62) | 0.40 | 1.10 (0.43-2.82) |
| At second measurement (n = 84) | 14 (24) | 6 (23) | 0.92 | 0.94 (0.32-2.81) |
| Wounds | ||||
| At baseline measurement (n = 84) | 24 (41) | 14 (56) | 0.20 | 1.86 (0.72-4.78) |
| At second measurement (n = 83) | 3 (5) | 5 (20) | 0.05f | 4.58 (1.00-20.96) |
| Skin problems | ||||
| At baseline measurement (n = 85) | 16 (27) | 7 (27) | 0.99 | 0.99 (0.35-2.80) |
| At second measurement (n = 84) | 7 (12) | 2 (8) | 0.71f | 0.61 (0.12-3.15) |
| Underlying disease | ||||
| At baseline measurement (n = 85) | 9 (15) | 4 (15) | 1.00f | |
| At second measurement (n = 85) | 8 (14) | 2 (8) | 0.72f | 0.53 (0.11-2.69) |
| Antibiotic use in preceding mo | ||||
| At baseline measurement (n = 82) | 12 (21) | 8 (33) | 0.23 | 1.92 (0.66-5.53) |
| At second measurement (n = 84) | 2 (3) | 0 (0) | 1.00f | |
| Indwelling device | ||||
| At baseline measurement (n = 85) | 15 (25) | 7 (27) | 0.88 | 1.08 (0.38-3.08) |
| At second measurement (n = 84) | 5 (9) | 3 (12) | 0.69f | 1.47 (0.32-6.70) |
n, number of individuals evaluated. The baseline measurement was taken at the time of MRSA detection. The second measurement was taken just before the start of MRSA eradication therapy.
Except where otherwise stated, the value is the number (percentage) of individuals with the determinant.
Determined by the Mann-Whitney U test.
A combination of more than one colonization site is possible.
Other colonization sites include sputum (4 individuals), urine (5 individuals), blood (2 individuals), wounds (16 individuals), and others (3 individuals).
Determined by Fisher's exact test.
As is known (3, 6, 25, 30, 39), MRSA can survive in the throat despite eradication therapy. For this reason, it is essential to include throat swabs during follow-up.
When patients and HCWs were analyzed separately, colonization of the throat became statistically more significant for patients (P, 0.02; odds ratio [OR], 4.18 [95% confidence interval {95% CI}, 1.21 to 14.74]) and was nonsignificant for HCWs. In addition, the presence of wounds became a stronger determinant of MRSA eradication therapy failure (P, 0.02; OR, 8.27 [95% CI, 1.43 to 47.74]) for patients and nonsignificant for HCWs. For this reason, it seems preferable to have no wounds present at the time MRSA eradication therapy is started.
Other potential determinants could not be defined, probably due to our policy of postponing treatment for patients with relative contraindications for MRSA eradication therapy, which leaves only novel determinants of treatment failure to be revealed.
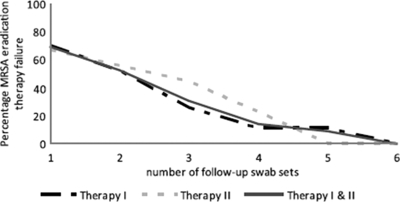
Seven (26%) of the individuals in our analysis whose first eradication therapy failed (n, 37) and 4 (44%) of the individuals whose second eradication therapy failed (n, 9) had MRSA-positive swabs after three consecutive negative culture sets taken more than 1 week after the end of treatment (Fig. 2). Eleven (31%) of the 36 individuals who failed to eradicate MRSA would, therefore, be incorrectly considered to have eradicated MRSA if only three follow-up culture sets had been obtained. When left untreated, this group can contribute to the spread of MRSA. Therefore, our study demonstrates that with five or more follow-up culture sets, the predictive value for eradication therapy success is >90%.
FIG. 2.
Number of MRSA-negative swabs needed to predict the success of MRSA eradication therapy as measured with 36 individuals whose MRSA eradication therapy failed.
We do not know whether individuals who became MRSA free according to our culture rule will remain so in the foreseeable future. In 2007 we started to follow up individuals from whom MRSA was eradicated with one culture set every 1 to 2 months for 1 year. To date, we have encountered recolonization in one case only.
Our study may have some limitations. First, we studied the overall effect of MRSA eradication therapies and spontaneous MRSA loss. We did not analyze one specific MRSA eradication therapy, because the therapy that was offered depended on the MRSA-positive sites (nares, throat, perineum or other sites), and in some cases (resistance to one or more antibiotics of the regimen, adverse effects, allergies), another antibiotic combination was used. Furthermore, when the different MRSA eradication therapies were analyzed separately, none of the regimens was demonstrated to be significantly superior with respect to the success rate of MRSA eradication. This is because our study was not designed for this purpose and probably also because of the relatively small population of individuals receiving MRSA eradication therapy (n, 88) that was studied. Second, infants, adults, and HCWs were included all together in this study, which assumes similar responses and epidemiology. As demonstrated in this study, patients have stronger determinants for MRSA eradication therapy failure than HCWs. In Table 1 we show that age (categorical or linear) is not significantly associated with the success of MRSA eradication therapy; this finding may be realistic or may be related to the presence of too small numbers per category. To study the effect of age on outcome, we should perform a large prospective study with balanced age categories.
In conclusion, the present study suggests that with our MRSA eradication treatment policy, a large proportion of MRSA carriers successfully returned to MRSA noncarriage either by MRSA eradication therapy (81%) or by spontaneous MRSA clearance (18%).
Furthermore, we recommend that five or more complete follow-up culture sets be taken to ascertain the MRSA status of an individual. The application of this rule may be a step forward in reducing the spread of MRSA.
Acknowledgments
We thank all infection control practitioners for their diligent efforts to isolate MRSA-positive patients and health care workers and for their work during the follow-up period for individuals receiving MRSA eradication therapy.
All authors declare no conflict of interest.
Footnotes
Published ahead of print on 14 June 2010.
REFERENCES
- 1.Adra, M., and K. R. Lawrence. 2004. Trimethoprim/sulfamethoxazole for treatment of severe Staphylococcus aureus infections. Ann. Pharmacother. 38:338-341. [DOI] [PubMed] [Google Scholar]
- 2.Ammerlaan, H. S., J. A. Kluytmans, H. F. Wertheim, J. L. Nouwen, and M. J. Bonten. 2009. Eradication of methicillin-resistant Staphylococcus aureus carriage: a systematic review. Clin. Infect. Dis. 48:922-930. [DOI] [PubMed] [Google Scholar]
- 3.Batra, R., A. C. Eziefula, D. Wyncoll, and J. Edgeworth. 2008. Throat and rectal swabs may have an important role in MRSA screening of critically ill patients. Intensive Care Med. 34:1703-1706. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, J. M. 1989. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect. Dis. Clin. North Am. 3:901-913. [PubMed] [Google Scholar]
- 5.Boyce, J. M., N. L. Havill, and B. Maria. 2005. Frequency and possible infection control implications of gastrointestinal colonization with methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5992-5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buehlmann, M., R. Frei, L. Fenner, M. Dangel, U. Fluckiger, and A. F. Widmer. 2008. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect. Control Hosp. Epidemiol. 29:510-516. [DOI] [PubMed] [Google Scholar]
- 7.Casewell, M. W., and R. L. Hill. 1986. Elimination of nasal carriage of Staphylococcus aureus with mupirocin (′pseudomonic acid')—a controlled trial. J. Antimicrob. Chemother. 17:365-372. [DOI] [PubMed] [Google Scholar]
- 8.Casewell, M. W., and R. L. Hill. 1985. In-vitro activity of mupirocin (′pseudomonic acid') against clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 15:523-531. [DOI] [PubMed] [Google Scholar]
- 9.Darouiche, R., C. Wright, R. Hamill, M. Koza, D. Lewis, and J. Markowski. 1991. Eradication of colonization by methicillin-resistant Staphylococcus aureus by using oral minocycline-rifampin and topical mupirocin. Antimicrob. Agents Chemother. 35:1612-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, E. A., A. M. Emmerson, G. M. Hogg, M. F. Patterson, and M. D. Shields. 1987. An outbreak of infection with a methicillin-resistant Staphylococcus aureus in a special care baby unit: value of topical mupirocin and of traditional methods of infection control. J. Hosp. Infect. 10:120-128. [DOI] [PubMed] [Google Scholar]
- 11.Davis, K. A., J. J. Stewart, H. K. Crouch, C. E. Florez, and D. R. Hospenthal. 2004. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin. Infect. Dis. 39:776-782. [DOI] [PubMed] [Google Scholar]
- 12.Dupeyron, C., B. Campillo, M. Bordes, E. Faubert, J. P. Richardet, and N. Mangeney. 2002. A clinical trial of mupirocin in the eradication of methicillin-resistant Staphylococcus aureus nasal carriage in a digestive disease unit. J. Hosp. Infect. 52:281-287. [DOI] [PubMed] [Google Scholar]
- 13.EARSS. 2007. Annual report EARSS—2006. RIVM, Bilthoven, Netherlands.
- 14.Ellison, R. T., III, F. N. Judson, L. C. Peterson, D. L. Cohn, and J. M. Ehret. 1984. Oral rifampin and trimethoprim/sulfamethoxazole therapy in asymptomatic carriers of methicillin-resistant Staphylococcus aureus infections. West. J. Med. 140:735-740. [PMC free article] [PubMed] [Google Scholar]
- 15.Frank, U., W. Lenz, E. Damrath, I. Kappstein, and F. D. Daschner. 1989. Nasal carriage of Staphylococcus aureus treated with topical mupirocin (pseudomonic acid) in a children's hospital. J. Hosp. Infect. 13:117-120. [DOI] [PubMed] [Google Scholar]
- 16.Frenay, H. M., C. M. Vandenbroucke-Grauls, M. J. Molkenboer, and J. Verhoef. 1992. Long-term carriage, and transmission of methicillin-resistant Staphylococcus aureus after discharge from hospital. J. Hosp. Infect. 22:207-215. [DOI] [PubMed] [Google Scholar]
- 17.Fung, S. K., M. Louie, and A. E. Simor. 2002. Combined topical and oral antimicrobial therapy for the eradication of methicillin-resistant Staphylococcus aureus (MRSA) colonization in hospitalized patients. Can. J. Infect. Dis. 13:287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gemmell, C. G., D. I. Edwards, A. P. Fraise, F. K. Gould, G. L. Ridgway, and R. E. Warren. 2006. Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J. Antimicrob. Chemother. 57:589-608. [DOI] [PubMed] [Google Scholar]
- 19.Grim, S. A., R. P. Rapp, C. A. Martin, and M. E. Evans. 2005. Trimethoprim-sulfamethoxazole as a viable treatment option for infections caused by methicillin-resistant Staphylococcus aureus. Pharmacotherapy 25:253-264. [DOI] [PubMed] [Google Scholar]
- 20.Harbarth, S., S. Dharan, N. Liassine, P. Herrault, R. Auckenthaler, and D. Pittet. 1999. Randomized, placebo-controlled, double-blind trial to evaluate the efficacy of mupirocin for eradicating carriage of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1412-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harbarth, S., N. Liassine, S. Dharan, P. Herrault, R. Auckenthaler, and D. Pittet. 2000. Risk factors for persistent carriage of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 31:1380-1385. [DOI] [PubMed] [Google Scholar]
- 22.Hill, R. L., G. J. Duckworth, and M. W. Casewell. 1988. Elimination of nasal carriage of methicillin-resistant Staphylococcus aureus with mupirocin during a hospital outbreak. J. Antimicrob. Chemother. 22:377-384. [DOI] [PubMed] [Google Scholar]
- 23.Kerremans, J. J., J. Maaskant, H. A. Verbrugh, W. B. van Leeuwen, and M. C. Vos. 2008. Detection of methicillin-resistant Staphylococcus aureus in a low-prevalence setting by polymerase chain reaction with a selective enrichment broth. Diagn. Microbiol. Infect. Dis. 61:396-401. [DOI] [PubMed] [Google Scholar]
- 24.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mertz, D., R. Frei, B. Jaussi, A. Tietz, C. Stebler, U. Fluckiger, and A. F. Widmer. 2007. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin. Infect. Dis. 45:475-477. [DOI] [PubMed] [Google Scholar]
- 26.Moran, G. J., A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDougal, R. B. Carey, and D. A. Talan. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666-674. [DOI] [PubMed] [Google Scholar]
- 27.Muder, R. R., M. Boldin, C. Brennen, M. Hsieh, R. M. Vickers, K. Mitchum, and Y. C. Yee. 1994. A controlled trial of rifampicin, minocycline, and rifampicin plus minocycline for eradication of methicillin-resistant Staphylococcus aureus in long-term care patients. J. Antimicrob. Chemother. 34:189-190. [DOI] [PubMed] [Google Scholar]
- 28.Peterson, L. R., J. N. Quick, B. Jensen, S. Homann, S. Johnson, J. Tenquist, C. Shanholtzer, R. A. Petzel, L. Sinn, and D. N. Gerding. 1990. Emergence of ciprofloxacin resistance in nosocomial methicillin-resistant Staphylococcus aureus isolates. Resistance during ciprofloxacin plus rifampin therapy for methicillin-resistant S aureus colonization. Arch. Intern. Med. 150:2151-2155. [PubMed] [Google Scholar]
- 29.Reagan, D. R., B. N. Doebbeling, M. A. Pfaller, C. T. Sheetz, A. K. Houston, R. J. Hollis, and R. P. Wenzel. 1991. Elimination of coincident Staphylococcus aureus nasal and hand carriage with intranasal application of mupirocin calcium ointment. Ann. Intern. Med. 114:101-106. [DOI] [PubMed] [Google Scholar]
- 30.Ringberg, H., A. C. Petersson, M. Walder, and P. J. H. Johansson. 2006. The throat: an important site for MRSA colonization. Scand. J. Infect. Dis. 38:888-893. [DOI] [PubMed] [Google Scholar]
- 31.Stevens, D. L., A. L. Bisno, H. F. Chambers, E. D. Everett, P. Dellinger, E. J. Goldstein, S. L. Gorbach, J. V. Hirschmann, E. L. Kaplan, J. G. Montoya, and J. C. Wade. 2005. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin. Infect. Dis. 41:1373-1406. [DOI] [PubMed] [Google Scholar]
- 32.Strausbaugh, L. J., C. Jacobson, D. L. Sewell, S. Potter, and T. T. Ward. 1992. Antimicrobial therapy for methicillin-resistant Staphylococcus aureus colonization in residents and staff of a Veterans Affairs nursing home care unit. Infect. Control Hosp. Epidemiol. 13:151-159. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland, R., R. J. Boon, K. E. Griffin, P. J. Masters, B. Slocombe, and A. R. White. 1985. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob. Agents Chemother. 27:495-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson, R. L., I. Cabezudo, and R. P. Wenzel. 1982. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann. Intern. Med. 97:309-317. [DOI] [PubMed] [Google Scholar]
- 35.Vos, M. C., M. D. Behrendt, D. C. Melles, F. P. N. Mollema, W. de Groot, G. Parlevliet, A. Ott, D. Horst-Kreft, A. van Belkum, and H. A. Verbrugh. 2009. MRSA Search and Destroy policy: 5-year experience in the largest University Medical Center of The Netherlands. Infect. Control Hosp. Epidemiol. 30:977-984. [DOI] [PubMed] [Google Scholar]
- 36.Walsh, T. J., H. C. Standiford, A. C. Reboli, J. F. John, M. E. Mulligan, B. S. Ribner, J. Z. Montgomerie, M. B. Goetz, C. G. Mayhall, D. Rimland, et al. 1993. Randomized double-blinded trial of rifampin with either novobiocin or trimethoprim-sulfamethoxazole against methicillin-resistant Staphylococcus aureus colonization: prevention of antimicrobial resistance and effect of host factors on outcome. Antimicrob. Agents Chemother. 37:1334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward, A., and D. M. Campoli-Richards. 1986. Mupirocin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 32:425-444. [DOI] [PubMed] [Google Scholar]
- 38.Wertheim, H. F., M. C. Vos, H. A. Boelens, A. Voss, C. M. Vandenbroucke-Grauls, M. H. Meester, J. A. Kluytmans, P. H. van Keulen, and H. A. Verbrugh. 2004. Low prevalence of methicillin-resistant Staphylococcus aureus (MRSA) at hospital admission in the Netherlands: the value of search and destroy and restrictive antibiotic use. J. Hosp. Infect. 56:321-325. [DOI] [PubMed] [Google Scholar]
- 39.Widmer, A. F., D. Mertz, and R. Frei. 2008. Necessity of screening of both the nose and the throat to detect methicillin-resistant Staphylococcus aureus colonization in patients upon admission to an intensive care unit. J. Clin. Microbiol. 46:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WIP. 2003. Policy for methicillin-resistant Staphylococcus aureus. Dutch Working Party on Infection Prevention, Leiden, Netherlands.