Abstract
This prospective study evaluated the plasma and intrapulmonary pharmacokinetics and pharmacodynamics (PKPD) of posaconazole (POS) in lung transplant recipients. Twenty adult lung transplant patients were instructed to take a 400-mg POS oral suspension twice daily (BID) with a high-fat meal for a total of 14 doses. Pulmonary epithelial lining fluid (ELF) and alveolar cell (AC) samples were obtained via bronchoalveolar lavage, and blood samples were collected at the approximate time of bronchoscopy. POS concentrations were assayed using liquid chromatography with tandem mass spectrometry. The maximum concentrations (Cmax) (mean ± standard deviation [SD]) in plasma, ELF, and AC were 1.3 ± 0.4, 1.3 ± 1.7, and 55.4 ± 44.0 μg/ml. POS concentrations in plasma, ELF, and AC did not decrease significantly, indicating slow elimination after multiple dosing. Mean concentrations of POS in plasma, ELF, and AC were above the MIC90 (0.5 μg/ml) for Aspergillus species over the 12-h dosing interval and for 24 h following the last dose. Area under the concentration-time curve from 0 to 12 h (AUC0-12)/MIC90 ratios in plasma, ELF, and AC were 21.98, 22.42, and 1,060. We concluded that a dose of 400 mg BID resulted in sustained plasma, ELF, and AC concentrations above the MIC90 for Aspergillus spp. during the dosing interval. Confirmation of the therapeutic value of these observations requires further investigation. The intrapulmonary PKPD of POS may be favorable for treatment or prevention of aspergillosis, although further research on the relevant PKPD parameters and the effect of POS protein binding is required.
Posaconazole (POS) is a new antifungal agent with activity against Aspergillus, Cryptococcus, Candida, Histoplasma, and Blastomyces spp. and others (9, 15, 17, 19, 27). POS is approved for prophylaxis of invasive aspergillosis and candidiasis in immunocompromised patients and for the treatment of refractory oropharyngeal candidiasis. Recent reports suggest that it may also be effective in the treatment of refractory invasive aspergillosis (16, 26), zygomycosis (23), and other fungal infections (3, 18).
The oral bioavailability of POS is increased by ingestion of a high-fat meal and by the use of divided dosing (8, 10). There are no clinically or kinetically important metabolites of POS. POS has a high apparent volume of distribution, 1,774 liters, and a prolonged half-life, 35 h, at steady state (G. Krishna and A. Sansone-Parsons, presented at the 41st American Society of Health System Pharmacists Midyear Clinical Meeting and Exhibition, Anaheim, CA, 3 to 7 December 2006). Its pharmacokinetics (PK) are unaffected by age, gender, or race/ethnicity (21), and dose correction is not required for patients with impaired renal function (7); 66.3% of an oral POS dose is excreted unchanged in feces (12). Protein binding in human plasma is >98%. We recently reported on the intrapulmonary pharmacokinetics and pharmacodynamics (PKPD) of POS in healthy subjects (5). Maximum concentration of drug in serum (Cmax) values were 2.08, 1.86, and 87.7 μg/ml, and area under the concentration-time curve from 0 to 12 h (AUC0-12) values were 21.9, 18.3, and 715 μg·h/ml in plasma, pulmonary epithelial lining fluid (ELF), and alveolar cells (AC), respectively. POS concentrations did not decline significantly over 24 h in any compartment. The purpose of this study was to determine the intrapulmonary PKPD of POS in lung transplant recipients.
MATERIALS AND METHODS
Study design and subjects.
This prospective study assessed the steady-state plasma, ELF, and AC concentrations of POS. All subjects had undergone lung transplantation at the University of California, San Francisco, where the study was conducted. Participation was voluntary, and written informed consent was obtained from each subject. The enrolled subjects were divided into five groups of four subjects each, with bronchoscopy times at approximately 3, 5, 8, 12, and 24 h after the final dose. The subjects were scheduled to receive a bronchoscopy as part of normal posttransplantation care; their POS dose times were planned so that the elapsed time between the last dose and bronchoscopy corresponded to their time group assignment. The patients were instructed to take a total of 14 doses of POS, administered as an oral suspension of 400 mg every 12 h (BID).
The participants were at least 21 years of age. Patients were excluded if they had known hypersensitivity to or intolerance for posaconazole, any of its excipients, or other azole drugs; were receiving any drug for which coadministration with POS is contraindicated, according to the POS label; or were receiving another azole antifungal, or if the study doctor considered participation inadvisable upon review of the patient's medical record.
After the subject gave consent, the most recent set of clinical laboratory tests (complete blood count with differential, platelet count, blood urea nitrogen, serum creatinine, alkaline phosphatase, total bilirubin, aspartate aminotransferase [AST], and albumin) were recorded in the research record. Enrolled subjects received the study medication either in person or by mail and were given written and oral instructions to take the drug after a high-fat meal every 12 h, for a total of 14 doses. A study coordinator phoned each subject on the day of the first dose and on the day before bronchoscopy to receive reports of adverse events. For each dose, the subjects were advised to record in a study diary the time at which they completed the high-fat meal, the precise time that the dose of POS was taken, and comments about any symptoms they experienced after taking the study drug. The diary was collected immediately before the bronchoscopy, at which time blood was also drawn for POS assay and for a repeat of the clinical laboratory tests. The used POS vials were returned to study staff for weighing, and percent compliance was calculated using the formula [(weight of drug consumed)/(weight of drug in 14 400-mg doses)] × 100.
Bronchoscopy and BAL.
Bronchoscopy and bronchoalveolar lavage (BAL) were performed at the University of California, San Francisco, Medical Center at approximately 3, 5, 8, 12, or 24 h after the last POS dose. The exact time that the lavage was performed was recorded. Subjects received local anesthesia with topical lidocaine and systemic sedation with fentanyl and midazolam. Pulmonary ELF and AC were collected by bronchoscopy and BAL from a lobe of the transplanted lung. The locations of the lavages were the lingula (n = 11), the right middle lobe (n = 6), the right upper lobe (n = 1), and the left lower lobe (n = 1). The duration (mean ± standard deviation [SD]) of the lavage was 4.8 ± 2.9 min. Lavage was performed by infusing, and promptly aspirating, 30-ml aliquots of sterile 0.9% saline. In the 19 patients who underwent bronchoalveolar lavage, the mean volumes (±SD) of saline infused and aspirated were 124.2 ± 16.1 ml and 70.8 ± 10.7 ml, respectively. After the last approximately 50 ml of aspirate were pooled and mixed, 27.7 ± 2.5 ml was used for this study. A cell count and differential were performed on a 1.5-ml sample of the BAL fluid, and a measured volume of the remaining BAL fluid was centrifuged for 5 min at 400 × g at 4°C. The cell pellet and supernatant were frozen separately at −80°C until they were assayed for POS concentrations. The urea concentration in the supernatant was measured using a spectrophotometric assay (Infinity Urea Liquid Stable Reagent; Thermo Fisher Scientific, Middletown, VA).
Blood samples.
Blood was obtained for drug assay at the approximate time of BAL. The exact time of the blood draw was recorded, and the samples were collected into sodium heparin-containing tubes and placed in ice until centrifugation. Plasma was frozen at −80°C until it was assayed.
POS assay.
The POS concentrations in plasma, BAL fluid, and AC were assayed at PPD Inc. (Richmond, VA) using a liquid chromatography with tandem mass spectrometry (LC-MS/MS) method that was modified from the one we previously published (5). A human plasma calibration curve was used to quantify the drug concentrations in all three media. The samples were analyzed using the human plasma method, except that the extraction was different. Cell pellets were prepared for assay by adding 200 μl water to each sample. The samples were then vortexed for 2 min, sonicated for 10 min, centrifuged at 14,000 rpm for 10 min, and transferred to snap cap vials. The AC supernatant samples thus prepared, as well as the BAL fluid and plasma samples, were then diluted 1:1 with blank plasma to a total volume of 25 μl. To each 25-μl sample was added 100 μl of internal standard (50 ng/ml of SCH 56984, a standard supplied by Schering-Plough, in 1:1 acetonitrile-methanol), and the resulting mixture was vortexed and centrifuged for 5 min at 10,000 rpm. One hundred microliters of water was added to 100 μl of the supernatant before injection. The lower limit of quantitation for this assay was 0.5 ng/ml, and the calibration range was 0.5 to 5,000 ng/ml. For quality control runs (3 levels: 4, 200, and 4,000 ng/ml), the accuracy ranged from 81.0% to 125.8%.
The concentration of unbound POS in plasma was calculated using the following relationship: unbound concentration = 0.015 × total concentration. Because the amounts of protein binding in ELF and AC are unknown, the unbound concentrations in these compartments were not calculated.
The BAL fluid supernatant was assayed for the urea concentration using a modified enzymatic assay (Infinity Urea Liquid Stable Reagent; Thermo Fisher Scientific, Middletown, VA). The assay was linear (R = 0.9994; P = 0.0001) throughout the range of urea concentrations (0.317 to 1.760 mg/dl) present in the BAL fluid samples.
The volume of ELF in BAL fluid, the concentration of POS in the ELF, and the concentration of POS in AC were calculated using methods that we have previously reported (4-6).
PKPD and statistical analysis.
The statistical analysis was performed using Kinetica, version 4.4.1 (Adept Scientific), employing a model-independent method. For all compartments, the exact concentration-time data for all 20 individuals were used to calculate the area under the curve over the dosing interval (AUC0-12) and for the 24 h following the last dose (AUC0-24). The Cmax during the 12-hour dosing interval was taken as the maximum mean concentration that was observed among the time groups, and the time to maximum concentration of drug in serum (Tmax) was the mean time from the last dose to the BAL for the time group in which the Cmax occurred. The Cmin was taken as the lowest mean concentration observed among the time groups during the dosing interval. The AUC0-12 and AUC0-24 were calculated using the linear trapezoidal method. The terminal-phase half-life (t1/2) was not calculated for plasma, ELF, or AC because the concentrations did not decline significantly during the 12 or 24 h following the last dose.
The Cmax, AUC0-12, and AUC0-24 and the concentration-time data were used to derive the following PD parameters in plasma, ELF, and AC: Cmax/MIC90 ratio, AUC0-12/MIC90 ratio, AUC0-24/MIC90, and time above MIC90 (T > MIC90). The MIC90 value for Aspergillus spp. was taken from the literature (19).
RESULTS
Twenty subjects were enrolled in the study. The mean age (±SD) of the 25 subjects was 53.4 ± 11.2 years; 12 were men, and 8 were women; 13 were white, 3 were black, 3 were Hispanic, and 1 was Asian. The mean weight (±SD) of the subjects was 74.4 ± 15.6 kg. Nineteen subjects were recipients of bilateral lung transplants, and 1 had received a unilateral transplant. The mean transplant-to-study time (±SD) was 1,130 ± 689 days. The diagnoses responsible for lung transplantation were interstitial pulmonary fibrosis (n = 9), chronic obstructive pulmonary disease (n = 6), cystic fibrosis (n = 3), sarcoidosis (n = 1), and lymphangioleiomyomatosis (n = 1).
Of the 20 subjects enrolled in the study, 19 completed BAL. One subject completed the full course of POS, and her plasma sample was included in the analysis, but BAL was not clinically indicated for reasons unrelated to the study medication. Fifteen of the 20 patients (75%) recorded taking 14 doses, 1 (5%) recorded taking 13, 1 (5%) recorded taking 12, and 3 (15%) clearly recorded taking at least 12 doses and confirmed verbally that they had taken all 14. Based on the weight analysis of the residual drug in vials, the amount (mean ± SD) taken was 84.8% ± 21.1% of the amount prescribed.
Adverse events were reported by 12 (60%) of the 20 subjects; all were mild and self-limited. Seven subjects experienced adverse effects related to the gastrointestinal tract (nausea, “upset stomach,” acid reflux, gas, bloating, decreased appetite, and diarrhea). Other adverse effects included sleepiness, pruritus, joint pains, fatigue, and nosebleed. There was no significant difference between mean pre- and post-POS administration laboratory values for white blood cell count, hematocrit, hemoglobin, alkaline phosphatase, or total bilirubin. Mean values for platelets and AST were greater after POS administration but remained within the normal range (222/liter and 29.8 U/liter for platelets and AST, respectively). Serum creatinine values increased from (mean ± SD) 1.2 ± 0.2 to 1.6 ± 0.4 mg/dl (P < 0.5) and returned to normal with reduction of the tacrolimus dose (see Discussion).
The recovery of cells and ELF from BAL is summarized in Table 1. The number (mean ± SD) of AC recovered per liter of BAL fluid was 2.3 × 108 ± 1.2 × 108. There was no significant difference in AC recovery among the time groups (P > 0.05). In all time groups, the majority of the recovered cells were in the monocyte/macrophage class (range, 77.5% ± 11.3% to 95.0% ± 6.8%). The volume (mean ± SD) of ELF recovered was 0.85 ± 0.45 ml, and there was no significance difference in ELF recovery among the time groups (P > 0.05) (Table 1). The cell counts and differential cell counts obtained in the current study were similar to those we reported in our previous studies with healthy subjects (4, 5).
TABLE 1.
Recovery of cells and ELF from BAL fluid
| Parameter | Value for time (h) group: |
||||
|---|---|---|---|---|---|
| 3 (n = 4) | 5 (n = 3) | 8 (n = 4) | 12 (n = 4) | 24 (n = 4) | |
| No. of cells/liter (SD) | 2.8 × 108 (1.6 × 108) | 2.4 × 108 (1.7 × 108) | 2.1 × 108 (1.3 × 108) | 1.9 × 108 (9.8 × 107) | 2.3 × 108 (6.7 × 107) |
| No. of PMNsa (%) | 10.5 ± 16.1 | 4.0 ± 5.2 | 4.0 ± 5.5 | 9.0 ± 9.5 | 10.8 ± 7.6 |
| No. of lymphocytes (%) | 4.2 ± 1.5 | 6.0 ± 5.6 | 0.5 ± 1.0 | 2.0 ± 1.8 | 11.5 ± 12.1 |
| No. of monocytes/macrophages (%) | 82.2 ± 16.5 | 89.3 ± 11.0 | 95.0 ± 6.8 | 81.5 ± 11.1 | 77.5 ± 11.3 |
| No. of eosinophils (%) | 0.5 ± 0.6 | 0.3 ± 0.6 | 0.0 ± 0.0 | 0.2 ± 0.5 | 0.0 ± 0.0 |
| No. of other cellsb (%) | 0.2 ± 0.5 | 0.3 ± 0.6 | 0.5 ± 0.6 | 4.0 ± 6.7 | 0.2 ± 0.5 |
| No. of degenerated cells (%) | 2.2 ± 2.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.2 ± 5.3 | 0.0 ± 0.0 |
| ELF vol (ml) | 0.9 ± 0.6 | 0.8 ± 0.5 | 1.2 ± 0.6 | 0.7 ± 0.3 | 0.7 ± 0.2 |
PMNs, polymorphonuclear leukocytes.
Other, unrecognizable cells.
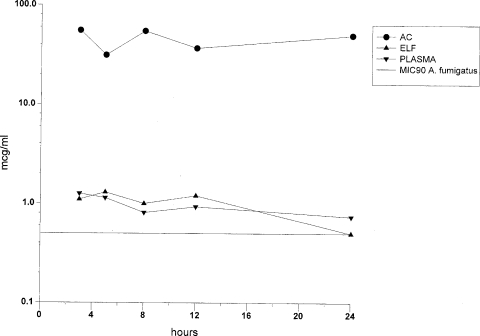
The concentrations of POS in plasma, AC, and ELF and the AC/plasma and ELF/plasma ratios at the time of BAL are summarized in Fig. 1 and Table 2. The PD parameters in all compartments are summarized in Table 3. The concentrations of POS in plasma, ELF, and ACs did not decrease during the 24 h following the last dose, consistent with a long half-life and slow elimination from these compartments.
FIG. 1.
Concentrations of POS in plasma, AC, and ELF. Standard deviations from the values shown are given in Table 2.
TABLE 2.
Posaconazole concentrations in plasma, ELF, and AC
| Parameter | Value for time (h) group: |
||||
|---|---|---|---|---|---|
| 3 (n = 4) | 5 (n = 5) | 8 (n = 4) | 12 (n = 4) | 24 (n = 4) | |
| Time to blood draw (h) | 2.8 ± 0.6 | 4.5 ± 1.0 | 8.7 ± 0.9 | 11.8 ± 0.4 | 21.1 ± 3.2 |
| Time to BAL (h) | 3.2 ± 0.7 | 5.3 ± 1.0a | 9.1 ± 0.7 | 12.1 ± 0.9 | 22.4 ± 3.0 |
| Plasma (μg/ml) | 1.3 ± 0.4 | 1.1 ± 1.4 | 0.81 ± 0.49 | 0.93 ± 0.56 | 0.74 ± 0.61 |
| ELF (μg/ml) | 1.1 ± 0.7 | 1.3 ± 1.7b | 1.0 ± 0.8c | 1.2 ± 1.2 | 0.5 ± 0.3 |
| ELF/plasma ratio | 0.94 | 0.57 | 0.90 | 1.0 | 0.68 |
| AC (μg/ml) ratio | 55.4 ± 44.0 | 31.3 ± 25.4b | 54.7 ± 58.8 | 36.8 ± 30.8 | 49.6 ± 62.1 |
| AC/plasma ratio | 40.7 | 27.3 | 63.6 | 53.3 | 56.4 |
One subject in the 5-h group underwent bronchoscopy but did not complete the BAL.
The ELF and AC calculations in this group are based on an n of 3.
The ELF sample from one subject was lost in transit; this ELF calculation is based on an n of 3.
TABLE 3.
Model independent pharmacodynamic parameters in plasma, ELF, and AC
| Parameter | Value |
||
|---|---|---|---|
| Plasma | ELF | AC | |
| AUC0-12 (μg/ml·h−1) | 10.99 | 11.21 | 530.2 |
| AUC0-12/MIC90 | 19.95 | 18.42 | 1,162 |
| AUC0-24 (μg/ml·h−1) | 21.98 | 22.42 | 1,060 |
| AUC0-24/MIC90 | 39.90 | 36.84 | 2,324 |
| 12-h Cmax (μg/ml) | 1.3 ± 0.4 | 1.3 ± 1.7 | 55.4 ± 44.0 |
| 12-h Cmax/MIC90 | 2.5 | 2.6 | 110.8 |
| 12-h Tmax | 2.8 ± 0.6 | 5.3 ± 1.0 | 3.2 ± 0.7 |
| 12-h Cmin (μg/ml) | 0.81 ± 0.49 | 1.0 ± 0.8 | 31.3 ± 25.4 |
| T > MIC90 (h) | 24 | 24 | 24 |
The free and total Cmax/MIC90 ratios in plasma were 0.04 and 2.5, respectively. The free AUC0-12 and AUC0-24 were 0.16 μg·h/ml and 0.30 μg·h/ml, respectively. The free AUC0-12/MIC90 ratio was 0.33, and the free AUC0-24/MIC90 ratio was 0.60.
DISCUSSION
In our previous report of the intrapulmonary PKPD of POS in healthy human subjects (5), we concluded that the PKPD profile of POS was favorable for the treatment of aspergillosis. That study found that the Cmax values (mean ± SD) in plasma, ELF, and AC were 2.08 ± 0.93, 1.86 ± 1.30, and 87.7 ± 65.0 μg/ml, respectively. The AUC0-12 in plasma, ELF, and AC were 21.9, 18.3, and 715 μg·h/ml. These values are similar to those we are currently reporting for the transplanted lung, although the values for healthy subjects are somewhat greater in all three compartments. It has been reported that coadministration with proton pump inhibitors (PPIs) decreases POS plasma concentrations (13, 14). Seventeen of the 20 lung transplant patients (85%) and none of the healthy subjects were taking proton pump inhibitors for the purpose of reducing acid reflux. Furthermore, our compliance analysis demonstrated that subjects in this study took 84.8% of the drug prescribed, compared with 96.6% for the healthy subjects. The combination of the effect of the PPIs and the compliance may explain the lower drug concentrations in this study. Despite these factors, the Cmax and AUC0-12 for the transplant recipients were well in excess of the MIC90 in all compartments in both studies.
The pharmacokinetics of another triazole, voriconazole, have also been studied in lung transplant recipients (2). In a study of 12 subjects, the mean ELF/plasma ratio was 11, with a range of 2 to 28; alveolar cell concentrations were not measured. In the current study, the mean ELF/plasma ratio was 0.82, with a range of 0.57 to 1.0, and the AC/plasma ratios ranged from 27.3 to 67.6. Methodological differences may partially explain the disparity between their findings and ours: subjects in the voriconazole study did not receive a set number of doses, may not all have been at steady state, and were studied, on average, much closer to the time of transplantation. In the current study and in our previous study in healthy patients, ELF recoveries were (mean ± SD) 0.85 ± 0.45 ml and 0.81 ± 0.29 ml, respectively. However, in the voriconazole study, the volume of ELF recovered was 1.4 ± 0.7 ml. Finally, some of the difference may reflect a true biological difference between voriconazole and posaconazole with respect to ELF penetration.
The high AC concentrations observed in this study may be clinically significant, because it has been reported that alveolar macrophages ingest the conidia of Aspergillus spp. early in infection (22, 24, 25). POS has concentration-dependent activity against Candida spp., and treatment efficacy is best correlated with the AUC/MIC ratio (1, 11); however, the PKPD parameter that best predicts efficacy in treating Aspergillus spp. has not been well established. A study of neutropenic rabbits with invasive aspergillosis reported that the time above MIC was the best pharmacodynamic indicator of efficacy (A. H. Groll, D. Mickiene, R. Petraitiene, V. Petraitis, T. Sein, J. Roach, K. Roth, S. C. Piscitelli, and T. J. Walsh, presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 17 to 20 December 2000), but this has not been demonstrated in humans. In our study, the time above MIC90 was 24 h in all compartments, indicating that concentrations remained therapeutic throughout the dosing interval, and AUC0-12 and Cmax values in all compartments were high in comparison with the MIC90. These pharmacodynamic parameters therefore suggest that 400 mg BID of POS is may be effective in treating or preventing Aspergillus infection in lung transplant recipients, although further study of the effect of protein binding is required.
In the current study, the 14 400-mg doses of POS had no clinically relevant effect on any blood chemistry or hematology value other than serum creatinine. The observed increase in the serum creatinine over the course of the study was likely due to the fact that concomitant administration of azoles and tacrolimus results in a substantial increase in tacrolimus blood levels (20). This effect is usually countered by reducing the tacrolimus dose, but such reduction was originally felt not to be necessary because of the short duration of this study. After elevated creatinine and tacrolimus were observed in the first 5 study subjects, a tacrolimus dose reduction of 50 to 67% was instituted for the week of the study. This dose reduction resulted in tacrolimus blood levels that were within the normal range for the majority of subjects. The increased concentrations of tacrolimus and serum creatinine noted in the first 5 subjects were transient and without clinical effects.
A limitation of the current study is that, since concentrations did not decline in any compartment during the 24-hour sampling interval, the half-life of POS in AC, plasma, and ELF could not be calculated. An additional limitation is that all of the transplant recipients enrolled in the study were on multiple concomitant medications, and it was impossible to determine what effect, if any, these medications may have had on POS concentrations. However, since such complicated medication regimens are typical of lung transplant recipients, our results are probably a good approximation of the levels that would be observed in other patients in this population.
We conclude that (i) an oral dosing regimen of 400 mg POS twice daily resulted in sustained plasma, ELF, and AC concentrations above the MIC90 for Aspergillus spp. during the entire 12-h dosing interval and for 24 h after the last dose, although further study will be required to confirm the therapeutic value of this finding; (ii) the high intrapulmonary AUC0-12/MIC90 ratio observed in this study is similar to that observed in healthy subjects and may be favorable for the treatment or prevention of aspergillosis; (iii) cell counts, differential cell counts, and ELF recovery from transplanted lungs are similar to those obtained from native lungs; and (iv) oral POS was well tolerated in lung transplant recipients.
Acknowledgments
Financial support for this study was provided by Schering-Plough, Kenilworth, NJ.
Footnotes
Published ahead of print on 1 June 2010.
REFERENCES
- 1.Andes, D., K. Marchillo, R. Conklin, G. Krishna, F. Ezzet, A. Cacciapuoti, and D. Loebenberg. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capitano, B., B. A. Potoski, S. Husain, S. Zhang, D. L. Paterson, S. M. Studer, K. R. McCurry, and R. Venkataramanan. 2006. Intrapulmonary penetration of voriconazole in patients receiving an oral prophylactic regimen. Antimicrob. Agents Chemother. 50:1878-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catanzaro, A., G. A. Cloud, D. A. Stevens, B. E. Levine, P. L. Williams, R. H. Johnson, A. Rendon, L. F. Mirels, J. E. Lutz, M. Holloway, and J. N. Galgiani. 2007. Safety, tolerance, and efficacy of posaconazole therapy in patients with nonmeningeal disseminated or chronic pulmonary coccidioidomycosis. Clin. Infect. Dis. 45:562-568. [DOI] [PubMed] [Google Scholar]
- 4.Conte, J. E., Jr., J. A. Golden, J. Kipps, M. McIver, and E. Zurlinden. 2004. Intrapulmonary pharmacokinetics and pharmacodynamics of itraconazole and 14-hydroxyitraconazole at steady state. Antimicrob. Agents Chemother. 48:3823-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conte, J. E., Jr., J. A. Golden, G. Krishna, M. McIver, E. Little, and E. Zurlinden. 2009. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob. Agents Chemother. 53:703-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conte, J. E., Jr., J. A. Golden, M. McIver, E. Little, and E. Zurlinden. 2007. Intrapulmonary pharmacodynamics of high-dose levofloxacin in subjects with chronic bronchitis or chronic obstructive pulmonary disease. Int. J. Antimicrob. Agents 30:422-427. [DOI] [PubMed] [Google Scholar]
- 7.Courtney, R., A. Sansone, W. Smith, T. Marbury, P. Statkevich, M. Martinho, M. Laughlin, and S. Swan. 2005. Posaconazole pharmacokinetics, safety, and tolerability in subjects with varying degrees of chronic renal disease. J. Clin. Pharmacol. 45:185-192. [DOI] [PubMed] [Google Scholar]
- 8.Courtney, R., D. Wexler, E. Radwanski, J. Lim, and M. Laughlin. 2004. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharmacol. 57:218-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diekema, D. J., S. A. Messer, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 2003. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 41:3623-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezzet, F., D. Wexler, R. Courtney, G. Krishna, J. Lim, and M. Laughlin. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211-220. [DOI] [PubMed] [Google Scholar]
- 11.Groll, A. H., and T. J. Walsh. 2006. Antifungal efficacy and pharmacodynamics of posaconazole in experimental models of invasive fungal infections. Mycoses 49(Suppl. 1):7-16. [DOI] [PubMed] [Google Scholar]
- 12.Krieter, P., B. Flannery, T. Musick, M. Gohdes, M. Martinho, and R. Courtney. 2004. Disposition of posaconazole following single-dose oral administration in healthy subjects. Antimicrob. Agents Chemother. 48:3543-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishna, G., M. AbuTarif, F. Xuan, M. Martinho, D. Angulo, and O. A. Cornely. 2008. Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy 28:1223-1232. [DOI] [PubMed] [Google Scholar]
- 14.Krishna, G., A. Moton, L. Ma, M. M. Medlock, and J. McLeod. 2009. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob. Agents Chemother. 53:958-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 16.Raad, I. I., H. A. Hanna, M. Boktour, Y. Jiang, H. A. Torres, C. Afif, D. P. Kontoyiannis, and R. Y. Hachem. 2008. Novel antifungal agents as salvage therapy for invasive aspergillosis in patients with hematologic malignancies: posaconazole compared with high-dose lipid formulations of amphotericin B alone or in combination with caspofungin. Leukemia 22:496-503. [DOI] [PubMed] [Google Scholar]
- 17.Ramani, R., and V. Chaturvedi. 2007. Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non-endemic areas. Mycopathologia 163:315-319. [DOI] [PubMed] [Google Scholar]
- 18.Saag, M. S., R. J. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, and W. E. Dismukes. 2000. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 19.Sabatelli, F., R. Patel, P. A. Mann, C. A. Mendrick, C. C. Norris, R. Hare, D. Loebenberg, T. A. Black, and P. M. McNicholas. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansone-Parsons, A., G. Krishna, M. Martinho, B. Kantesaria, S. Gelone, and T. G. Mant. 2007. Effect of oral posaconazole on the pharmacokinetics of cyclosporine and tacrolimus. Pharmacotherapy 27:825-834. [DOI] [PubMed] [Google Scholar]
- 21.Sansone-Parsons, A., G. Krishna, J. Simon, P. Soni, B. Kantesaria, J. Herron, and R. Stoltz. 2007. Effects of age, gender, and race/ethnicity on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob. Agents Chemother. 51:495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segal, B. H. 2007. Role of macrophages in host defense against aspergillosis and strategies for immune augmentation. Oncologist 12(Suppl. 2):7-13. [DOI] [PubMed] [Google Scholar]
- 23.van Burik, J. A., R. S. Hare, H. F. Solomon, M. L. Corrado, and D. P. Kontoyiannis. 2006. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin. Infect. Dis. 42:e61-e65. [DOI] [PubMed] [Google Scholar]
- 24.Waldorf, A. R. 1989. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol. Ser. 47:243-271. [PubMed] [Google Scholar]
- 25.Waldorf, A. R., S. M. Levitz, and R. D. Diamond. 1984. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J. Infect. Dis. 150:752-760. [DOI] [PubMed] [Google Scholar]
- 26.Walsh, T. J., I. Raad, T. F. Patterson, P. Chandrasekar, G. R. Donowitz, R. Graybill, R. E. Greene, R. Hachem, S. Hadley, R. Herbrecht, A. Langston, A. Louie, P. Ribaud, B. H. Segal, D. A. Stevens, J. A. van Burik, C. S. White, G. Corcoran, J. Gogate, G. Krishna, L. Pedicone, C. Hardalo, and J. R. Perfect. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2-12. [DOI] [PubMed] [Google Scholar]
- 27.Wheat, L. J., P. Connolly, M. Smedema, M. Durkin, E. Brizendine, P. Mann, R. Patel, P. M. McNicholas, and M. Goldman. 2006. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J. Antimicrob. Chemother. 57:1235-1239. [DOI] [PubMed] [Google Scholar]