Abstract
Foreign travel has been suggested to be a risk factor for the acquisition of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae. To our knowledge, this has not previously been demonstrated in a prospective study. Healthy volunteers traveling outside Northern Europe were enrolled. Rectal swabs and data on potential travel-associated risk factors were collected before and after traveling. A total of 105 volunteers were enrolled. Four of them did not complete the study, and one participant carried ESBL-producing Escherichia coli before travel. Twenty-four of 100 participants with negative pretravel samples were colonized with ESBL-producing Escherichia coli after the trip. All strains produced CTX-M enzymes, mostly CTX-M-15, and some coproduced TEM or SHV enzymes. Coresistance to several antibiotic subclasses was common. Travel to India was associated with the highest risk for the acquisition of ESBLs (88%; n = 7). Gastroenteritis during the trip was an additional risk factor (P = 0.003). Five of 21 volunteers who completed the follow-up after 6 months had persistent colonization with ESBLs. This is the first prospective study demonstrating that international travel is a major risk factor for colonization with ESBL-producing Enterobacteriaceae. Considering the high acquisition rate of 24%, it is obvious that global efforts are needed to meet the emergence and spread of CTX-M enzymes and other antimicrobial resistances.
The prevalence of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae is increasing globally, and community-onset infections with ESBL-producing Escherichia coli are a major clinical concern in many countries (5, 12, 16, 24). Known risk factors for infection with ESBL-producing bacteria include recent hospitalization or antibiotic use, urinary tract catheters, older age, and diabetes (5, 24, 25).
There are marked geographical differences in the proportions of ESBL production among clinical isolates of Klebsiella pneumoniae and E. coli. A high prevalence of ESBL production has been reported for South America (>40% of K. pneumoniae and 5 to 10% of E. coli isolates) and Asia (20 to 30% and 15 to 20%, respectively) (2, 11, 32). The highest rates so far were reported from a multicenter survey study in India (>55% and >60%, respectively) (18). Significantly lower prevalences of ESBL phenotypes have been reported for Europe (10 to 15% and 5 to 10%, respectively) and North America (5 to 10% and <5%, respectively) (2, 9, 20). In Europe, the highest rates of ESBL-producing clinical isolates have been reported for Southern and Eastern European countries (15 to 30%) (7). In contrast, the rate of infections by ESBL-producing K. pneumoniae and E. coli is still very low in Sweden (<3%) (27).
Data on fecal carriage with ESBLs in healthy individuals are lacking for most countries, including Sweden, but the rate of fecal carriage has been estimated to be 10% in Asia and was reported to be 5.5% and 13.2% in Spain and Saudi Arabia, respectively (10, 14, 30). Most likely, however, the geographical differences in prevalences of ESBL phenotypes in clinical cultures covary with the proportion of healthy individuals colonized with isolates producing ESBLs.
Based on these geographical differences and a few retrospective studies of patients infected with bacteria producing ESBLs, foreign travel to countries with higher prevalences of ESBL-producing Enterobacteriaceae was suggested to be a risk factor for the acquisition of ESBLs (8, 15). A recent Swedish study of patients with traveler's diarrhea reported an increased prevalence of fecal colonization with ESBL-producing bacteria for patients who had traveled outside Europe compared with the prevalence for those returning from European countries (29). However, no prospective study confirming this finding has previously been reported. The primary objective of our study was to prospectively study the fecal acquisition of ESBL-producing Enterobacteriaceae during foreign travel and the persistence rate of acquired ESBL-producing isolates after 6 months. Our secondary objective was to assess potential travel-associated risk factors for the acquisition of ESBL-producing bacteria, such as destination, gastroenteritis, and antibiotic use during travel.
(The results of this study were in part presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2008.)
MATERIALS AND METHODS
Study design.
Starting in November 2007, healthy Swedish travelers planning a trip outside Northern Europe were included in the study. Information and necessary materials to participate in the study were available at travel consultation clinics in Uppsala. After written consent, the participants were asked to collect a pretravel fecal sample and to fill out a questionnaire, including personal data, information on cotravelers, previous medical history, and details of the planned trip. Travelers who suffered from a severe chronic disease or needed daily medication were not included. Samples and questionnaires were sent to the Department of Clinical Microbiology and the Department of Infectious Diseases, Uppsala University Hospital. Upon return, the procedure was repeated. The second questionnaire included data on gastroenteritis and antibiotic use during the trip. Participants who did not provide the second sample and questionnaire, carried an ESBL-producing Enterobacteriaceae isolate before travel, or returned to Sweden later than 31 January 2009 were excluded at this point. The participants could choose to be informed about their microbiology findings or not.
Travelers who acquired bacteria producing ESBLs and who chose to be informed about their results were asked to fill out a third questionnaire after 6 months. This questionnaire contained questions regarding clinical infections and antibiotic treatment during the follow-up period. In addition, the travelers were asked to deliver a third fecal sample. This study was approved by the Regional Ethics Review Board in Uppsala.
Bacteria and media.
All fecal samples were collected with the Copan transport system (Copan Diagnostics, Corona, CA). The samples were inoculated in Luria-Bertani broth (Becton Dickinson, Sparks, MD) supplemented with cefotaxime (2.5 μg/ml) to select for cephalosporin-resistant strains. The broth was thereafter inoculated onto MacConkey agar (Acumedia Manufacturers, Inc., Lansing, MI) with cefotaxime (5-μg) and ceftazidime (10-μg) discs (Oxoid Ltd., Basingstoke, United Kingdom). Colonies growing within the expected zones of cefotaxime and ceftazidime or just cefotaxime (inhibition zones of <24 mm) were characterized to the species level by conventional methods or by use of a Vitek 2 instrument (bioMérieux, Lyon, France). Phenotypic confirmation of ESBL production was performed by a disc diffusion synergy test, as described previously by Jarlier and coworkers (13). All broths and plates were incubated overnight at 35°C in a room atmosphere.
ESBL typing.
Isolates with phenotypic ESBL production were screened for blaCTX-M, blaTEM, and blaSHV with PCR, as described previously (17, 22). PCR products from isolates carrying genes encoding CTX-M-type ESBLs were sequenced after using modified primers. The nucleotide sequences of both strands were determined by using a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) and an ABI 3130 instrument (Applied Biosystems) according to the manufacturers' instructions. The gene sequences were analyzed with SeqScape v2.5 and BioEdit v7.0 sequence alignment editors (Ibis Therapeutics, Carlsbad, CA) and compared with sequences in the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Antibiotic susceptibility.
The MICs of the following antibiotics were determined for all ESBL-producing isolates by the Etest method (AB Biodisk, Solna, Sweden): amdinocillin, ciprofloxacin, ertapenem, fosfomycin, gentamicin, imipenem, meropenem, nitrofurantoin, piperacillin-tazobactam, tobramycin, and trimethoprim-sulfamethoxazole. The susceptibility testing was carried out according to the manufacturer's instructions, and the results were interpreted by the MIC breakpoints recommended by EUCAST (the European Committee on Antimicrobial Susceptibility Testing) (www.eucast.org). The quality control strain included was E. coli ATCC 25922.
Identification of diarrheagenic Escherichia coli.
Due to the frequent episodes of gastroenteritis among travelers who acquired ESBL-producing E. coli strains, six specific virulence genes associated with enteropathogenic, enterohemorrhagic, enterotoxigenic, enteroinvasive (33), and enteroaggregative E. coli (28) were explored. The methods were modified to fit the GeneAmp PCR 9700 system (Applied Biosystems, Inc., Foster City, CA). In brief, DNA was prepared by boiling a single colony from a culture grown overnight in 100 μl sterile H2O at 95°C for 10 min. A Taq PCR Master Mix kit (Qiagen, Solna, Sweden) was used for the PCR. Forward and reverse primers (0.2 μM; Eurogentech S.A., Seraing, Belgium) and 2 μl of the DNA preparation were added to the mixture. The final reaction mixture volume was 25 μl. The following program was used: 94°C for 10 min followed by 35 cycles of 94°C for 45 s; 50°C (lt and st), 55°C (stx1, stx2, and ipaH), or 58°C (pCVD432 fragment) for 45 s; and 72°C for 1 min. The last cycle was followed by a final extension step at 72°C for 10 min. Positive and negative controls were added for each run. The amplified products were visualized on a 1% agarose gel stained with ethidium bromide. The sizes of the products were compared with an O'GeneRulerExpress DNA ladder (Fermentas Sweden, Helsingborg, Sweden). The positive-control strains were kindly provided by the Swedish National Food Administration, Uppsala, Sweden. Before the control strains were used, the amplified DNA fragments in the target genes were sequenced.
Statistical analysis.
McNemar's test, generalized previously by Durkalski et al. for clustered data, was used for comparison of ESBL colonization rates before traveling to colonization rates after traveling (6). For the risk factor analysis, the Pearson test was used for categorical data and the Wilcoxon test was used for continuous data. For all comparisons, a P value of <0.05 was considered to represent statistical significance. All statistical analyses were performed by using R software, version 2.8.0 (available at http://www.r-project.org).
RESULTS
Study population.
During the 15-month study period, 105 volunteers provided written consent, questionnaires, and rectal swabs and were enrolled in the study. Four of them did not complete the study, and one participant carried an ESBL-producing E. coli strain before leaving Sweden.
One hundred travelers (55 women and 45 men) with a median age of 43 years (range, 2 to 84 years) were included in the analysis. The median length of stay abroad was 2 weeks (range, 1 to 26 weeks), and the most common reason for the trip was vacation (n = 89). Fifteen travelers visited more than one country during their trip, and three visited more than one continent. In total, 35 different countries were visited (for continental distribution, see Table 1). In some cases cotravelers participated in groups of two (n = 13), three (n = 4), four (n = 4), five (n = 1), or six (n = 1).
TABLE 1.
Countries and continents visited by travelers who completed the studya
| Continent | Country | No. of travelers |
|---|---|---|
| Africa | Ethiopia | 2 |
| Gambia | 1 | |
| Ghana | 2 | |
| Kenya | 9 | |
| Malawi | 1 | |
| Morocco | 1 | |
| Mozambique | 4 | |
| Rwanda | 5 | |
| Senegal | 1 | |
| South Africa | 11 | |
| Tanzania | 2 | |
| Tunisia | 1 | |
| Asia | Bhutan | 1 |
| China | 4 | |
| India | 8 | |
| Cambodia | 3 | |
| Tajikistan | 1 | |
| Thailand | 25 | |
| Vietnam | 3 | |
| Central America | Mexico | 4 |
| West Indies | 1 | |
| Not specified | 1 | |
| Middle East | Egypt | 8 |
| Jordan | 4 | |
| North America | United States | 2 |
| South America | Ecuador | 1 |
| Southern Europe | Albany | 2 |
| Croatia | 2 | |
| France | 2 | |
| Greece | 4 | |
| Italy | 5 | |
| Slovenia | 1 | |
| Spain | 6 | |
| Turkey | 1 |
Note that some participants visited several countries and that the sum of travelers in this table exceeds the actual number of 100.
ESBL-producing Enterobacteriaceae.
For 24 of 100 travelers with initially negative samples, ESBL-producing E. coli strains were detected in posttravel rectal swabs. No other ESBL-producing Enterobacteriaceae strains were found. The difference in the colonization rate before the trip compared to that after the trip was statistically significant (P < 0.001). CTX-M enzymes were identified in all 24 ESBL-producing strains. Of the CTX-M enzymes detected, 14 belonged to CTX-M group I (CTX-M-1 and -15) and 10 belonged to group IV (CTX-M-9, -14, and -27) according to the classification reported previously by Pitout et al. (21). Some isolates coproduced the SHV (n = 3) and TEM (n = 11) enzymes. The distribution of the CTX-M, SHV, and TEM enzymes is displayed in Table 2. Overall, CTX-M-15 was predominant (13/24 strains). All travelers who acquired ESBL-producing strains after travel to India carried CTX-M-15 (7/7 strains), whereas CTX-M-14 was more common after travel to other Asian countries (5/9 strains).
TABLE 2.
Distribution of CTX-M genes detected in 24 strains of Escherichia coli isolated from rectal swabs after foreign travela
| Continent or region | No. of isolates |
||||
|---|---|---|---|---|---|
| Group I |
Group IV |
||||
| CTX-M-1 | CTX-M-15 | CTX-M-9 | CTX-M-14 | CTX-M-27 | |
| Africa | 1 | ||||
| Asia (India excluded) | 2 | 1 | 5 | 2 | |
| India | 7 | ||||
| Southern Europe | 1 | 1 | |||
| Middle East | 2 | 2 | |||
| Total | 1 | 13 | 3 | 5 | 2 |
PCR amplification with subtype-specific primers was used.
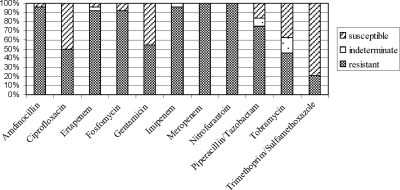
The in vitro antibiotic susceptibilities of the 24 ESBL-producing E. coli strains are shown in Fig. 1. Coresistance to antibiotics other than cephalosporins was common: 19 of 24 ESBL-producing E. coli strains displayed resistance to trimethoprim-sulfamethoxazole, and half of the isolates were resistant to fluoroquinolones and aminoglycosides, respectively. Nine strains displayed resistance to more than two antibiotic subclasses. High susceptibility rates were demonstrated for nitrofurantoin, amdinocillin, and the carbapenems.
FIG. 1.
In vitro antibiotic susceptibilities of 24 ESBL-producing Escherichia coli strains acquired after foreign travel. Susceptibilities were determined by the Etest method, and the results were interpreted according to EUCAST guidelines.
Travel-associated risk factors.
The rate of acquisition of an ESBL-producing strain was highest for travelers visiting India (7/8 isolates; P < 0.001) (Table 3). Travel to other destinations was associated with the following rates of posttravel ESBL colonization: 32% for Asia (India excluded), 29% for the Middle East, 13% for Southern Europe, and 0 to 4% for other parts of the world.
TABLE 3.
Travel destinations of travelers who were negative for ESBL-producing strains before the trip and rate of fecal colonization with ESBL-producing E. coli strains upon returna
| Continent or region | No. of travelers | No. (%) of travelers positive for ESBL-producing isolates |
|---|---|---|
| Africa | 25 | 1 (4) |
| Asia (India excluded) | 31 | 10 (32) |
| Central America | 6 | 0 (0) |
| India | 8 | 7 (88) |
| Middle East | 14 | 4 (29) |
| North America | 2 | 0 (0) |
| South America | 1 | 0 (0) |
| Southern Europe | 16 | 2 (13) |
The rate of acquisition of ESBL-producing strains was highest for travelers visiting India (P < 0.001). Three participants visited more than one continent, and therefore, the sum of travelers in this table exceeds the actual number of 100.
Participants who acquired ESBL-producing E. coli strains were more likely to have had gastroenteritis during the trip than others (P = 0.003). An analysis of six specific virulence genes associated with diarrheagenic E. coli revealed only one case of enteropathogenic E. coli (EPEC) infection, and this participant did not suffer from gastroenteritis. No other travel-associated risk factors were found (Table 4).
TABLE 4.
Descriptive statistics on 100 Swedish travelers with negative pretravel rectal swabs for ESBL-producing Enterobacteriaceaea
| Parameter | Value for group |
|
|---|---|---|
| ESBL negative (n = 76) | ESBL positive (n = 24) | |
| No. (%) of male travelers | 35 (46) | 10 (42) |
| Median age (yr) | 42 | 47 |
| No. (%) of vegetarians | 2 (3) | 0 (0) |
| Median length of stay (wk) (%) | 2.0 | 2.0 |
| No. (%) of travelers on vacation | 67 (88) | 22 (92) |
| No. (%) of business travelers | 10 (13) | 2 (8) |
| No. (%) of travelers visiting friends or relatives | 10 (13) | 1 (4) |
| No. (%) of travelers staying at a hotel | 61 (80) | 20 (83) |
| No. (%) of backpacking travelers | 6 (8) | 4 (17) |
| No. (%) of travelers staying with friends or relatives | 9 (12) | 5 (21) |
| No. (%) of travelers with gastroenteritis | 17 (22) | 13 (54) |
| No. (%) of travelers on antibiotic treatment | 7 (9) | 3 (12) |
Seventy-six travelers were negative for ESBL-producing strains after their trip, whereas 24 carried ESBL-producing Escherichia coli. The only statistically significant difference between the groups was gastroenteritis during travel (P = 0.003).
In total, 10 participants were treated with antibiotics during travel. Seven travelers treated for respiratory tract infections with cephalosporins (n = 3), penicillins (n = 2), tetracyclines (n = 1), or erythromycin (n = 1) were ESBL negative upon their return. In contrast, all three travelers treated with ciprofloxacin for gastroenteritis acquired ESBL-producing E. coli strains.
Persistence of colonization with ESBL-producing strains after 6 months.
Two of 24 travelers who acquired ESBL-producing strains had chosen not to be informed about their laboratory results and were therefore excluded from the 6-month follow-up, and one person did not provide the follow-up sample and questionnaire. Three participants had been treated for upper respiratory tract infections with penicillin, penicillin plus azithromycin, and doxycycline, respectively. Five of 21 participants who completed the follow-up had persistent colonization with ESBL-producing E. coli strains. None of these participants reported a clinical infection or antibiotic use during the follow-up period.
DISCUSSION
This is the first prospective study of foreign travel as a risk factor for colonization with ESBL-producing Enterobacteriaceae. Our results clearly demonstrate that travel to areas with a higher prevalence of strains producing ESBLs is a risk factor for the acquisition of ESBL-producing bacteria. Even when using a modified statistical model adjusting for participants in groups, the difference in colonization rates with ESBL-producing bacteria before travel versus those after travel was highly significant. The acquisition of ESBL-producing E. coli during foreign travel may be a significant source for increasing rates of colonization by bacteria producing ESBLs in Sweden and other countries with a low prevalence of ESBLs and a comparably low rate of consumption of antibiotics.
CTX-M genes were detected in all ESBL-producing strains in our study, which is consistent with the fact that these enzymes increasingly dominate the ESBL pandemic (26). However, some strains producing ESBLs other than the CTX-M type may have been lost, since cefotaxime was used as a selecting agent in the inoculation broth. The high prevalence of CTX-M-15 genes detected in our material reflects the worldwide spread of these enzymes (3, 4, 10, 17, 31). Although the number of isolates in our study was too small for statistical analysis, there seems to be some correlation between the CTX-M types acquired and travel destinations. All Indian ESBL-producing isolates carried CTX-M-15 genes, which is the only CTX-M type reported from India so far, and the majority of ESBL-producing isolates from other Asian countries carried CTX-M-14 genes, which is the dominating CTX-M type in this area (10, 19). These results support that the ESBL-producing isolates detected in our study were in fact acquired at the travel destinations. Similar findings were also reported by previous retrospective studies of travelers infected or colonized with ESBL-producing E. coli strains (21, 29).
A high rate of coresistance to potentially active drugs is a common feature of ESBL-producing E. coli strains (26) and was also seen in our material. Overall, nine strains were resistant to more than two antibiotic subclasses tested, which seriously limits therapeutic options in cases of a clinical infection. Although the level of carbapenem resistance among ESBL-producing E. coli and K. pneumoniae strains is still very low in most countries, it is worrisome that two isolates in our material displayed reduced susceptibility to these antibiotics. High susceptibility rates were demonstrated for nitrofurantoin and amdinocillin, which are currently recommended for lower urinary tract infections in Sweden, suggesting that these antibiotics can be used empirically even when infection with an imported ESBL-producing E. coli strain is suspected.
Participants visiting India were most likely to acquire ESBL-producing strains. Despite the small number of travelers to India, this was highly statistically significant, and previous studies also pointed out that visits to India in particular appear to be a risk factor for infection with ESBL-producing E. coli (8, 15). Although not statistically significant, travel to other countries in Asia and the Middle East was also associated with a higher risk of colonization by ESBL-producing strains (32 and 29%, respectively) in our study, which might well reflect the rates of ESBL production and fecal colonization in the countries visited (9, 11, 18, 32).
The risk of acquisition of ESBL-producing strains was strongly associated with gastroenteritis during travel. However, analyses of specific virulence genes associated with diarrheagenic E. coli revealed only one case of enteropathogenic E. coli (EPEC). These results suggest that the acquired ESBL-producing E. coli strains themselves did not cause gastroenteritis. Rather, we believe that participants suffering from gastroenteritis were exposed to contaminated food or water containing both diarrheagenic and ESBL-producing E. coli strains. However, person-to-person transmission of ESBL-producing strains is a known fact and might also have occurred, even within travel groups or families included in the study (23). Antibiotic treatment during travel was not a significant risk factor in this study, but notably, all three participants treated for gastroenteritis with ciprofloxacin acquired ESBL-producing strains.
We expected a far lower rate of acquisition of ESBL-producing strains than the 24% found, and our study was therefore not designed to enable a detailed analysis of the duration of colonization. However, the 6-month persistence rate of 24% is consistent with previous results for hospitalized patients (1). More prospective studies are needed in this area to assess the importance of foreign travel as a source of increasing rates of colonization with ESBL-producing Enterobacteriaceae and spread in the community.
The simple, prospective design of our study was a feasible means of evaluating foreign travel as a risk factor for the acquisition of ESBL-producing Enterobacteriaceae. By providing pretravel fecal samples, the participants constituted their own control group, thus adjusting for possible confounding factors in the traveling part of the population. The risk for statistical errors associated with retrospective studies and multivariate analysis, such as selection or recall bias and type 1 error, was reduced. Also, the prospective design enabled a detailed analysis of travel-associated risk factors that has not been possible to assess in previous retrospective studies (8, 15, 29).
In conclusion, considering the high rate of acquisition of ESBL-producing isolates and the extent of international travel in many countries, it is obvious that global efforts are needed to meet the emergence and spread of CTX-M enzymes and other antimicrobial resistances. Further international cooperation on rational antibiotic use and control is urgently needed.
Acknowledgments
This study was funded by grants from Strama (the Swedish Strategic Programme against Antibiotic Resistance) and the Investor-Initiated Studies Program of Merck.
Footnotes
Published ahead of print on 14 June 2010.
REFERENCES
- 1.Apisarnthanarak, A., T. C. Bailey, and V. J. Fraser. 2008. Duration of stool colonization in patients infected with extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Clin. Infect. Dis. 46:1322-1323. [DOI] [PubMed] [Google Scholar]
- 2.Baquero, F., P. R. Hsueh, D. L. Paterson, F. Rossi, G. V. Bochicchio, G. Gallagher, K. Lantz, J. B. Villasenor, K. McCarroll, M. A. Abramson, and J. W. Chow. 2009. In vitro susceptibilities of aerobic and facultatively anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2005 results from Study for Monitoring Antimicrobial Resistance Trends (SMART). Surg. Infect. (Larchmt.) 10:99-104. [DOI] [PubMed] [Google Scholar]
- 3.Bush, K. 2008. Extended-spectrum beta-lactamases in North America, 1987-2006. Clin. Microbiol. Infect. 14 (Suppl. 1):134-143. [DOI] [PubMed] [Google Scholar]
- 4.Canton, R., A. Novais, A. Valverde, E. Machado, L. Peixe, F. Baquero, and T. M. Coque. 2008. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 14(Suppl. 1):144-153. [DOI] [PubMed] [Google Scholar]
- 5.Colodner, R., W. Rock, B. Chazan, N. Keller, N. Guy, W. Sakran, and R. Raz. 2004. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. 23:163-167. [DOI] [PubMed] [Google Scholar]
- 6.Durkalski, V. L., Y. Y. Palesch, S. R. Lipsitz, and P. F. Rust. 2003. Analysis of clustered matched-pair data. Stat. Med. 22:2417-2428. [DOI] [PubMed] [Google Scholar]
- 7.European Antimicrobial Resistance Surveillance System. 2006. Annual report 2005. National Institute of Public Health and the Environment, Bilthoven, Netherlands.
- 8.Freeman, J. T., S. J. McBride, H. Heffernan, T. Bathgate, C. Pope, and R. B. Ellis-Pegler. 2008. Community-onset genitourinary tract infection due to CTX-M-15-producing Escherichia coli among travelers to the Indian subcontinent in New Zealand. Clin. Infect. Dis. 47:689-692. [DOI] [PubMed] [Google Scholar]
- 9.Goossens, H., and B. Grabein. 2005. Prevalence and antimicrobial susceptibility data for extended-spectrum beta-lactamase- and AmpC-producing Enterobacteriaceae from the MYSTIC Program in Europe and the United States (1997-2004). Diagn. Microbiol. Infect. Dis. 53:257-264. [DOI] [PubMed] [Google Scholar]
- 10.Hawkey, P. M. 2008. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin. Microbiol. Infect. 14(Suppl. 1):159-165. [DOI] [PubMed] [Google Scholar]
- 11.Hirakata, Y., J. Matsuda, Y. Miyazaki, S. Kamihira, S. Kawakami, Y. Miyazawa, Y. Ono, N. Nakazaki, Y. Hirata, M. Inoue, J. D. Turnidge, J. M. Bell, R. N. Jones, and S. Kohno. 2005. Regional variation in the prevalence of extended-spectrum beta-lactamase-producing clinical isolates in the Asia-Pacific region (SENTRY 1998-2002). Diagn. Microbiol. Infect. Dis. 52:323-329. [DOI] [PubMed] [Google Scholar]
- 12.Ho, P. L., W. W. Poon, S. L. Loke, M. S. Leung, K. H. Chow, R. C. Wong, K. S. Yip, E. L. Lai, and K. W. Tsang. 2007. Community emergence of CTX-M type extended-spectrum beta-lactamases among urinary Escherichia coli from women. J. Antimicrob. Chemother. 60:140-144. [DOI] [PubMed] [Google Scholar]
- 13.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 14.Kader, A. A., A. Kumar, and K. A. Kamath. 2007. Fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in patients and asymptomatic healthy individuals. Infect. Control Hosp. Epidemiol. 28:1114-1116. [DOI] [PubMed] [Google Scholar]
- 15.Laupland, K. B., D. L. Church, J. Vidakovich, M. Mucenski, and J. D. Pitout. 2008. Community-onset extended-spectrum beta-lactamase (ESBL) producing Escherichia coli: importance of international travel. J. Infect. 57:441-448. [DOI] [PubMed] [Google Scholar]
- 16.Laupland, K. B., D. B. Gregson, D. L. Church, T. Ross, and J. D. Pitout. 2008. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin. Microbiol. Infect. 14:1041-1047. [DOI] [PubMed] [Google Scholar]
- 17.Lytsy, B., L. Sandegren, E. Tano, E. Torell, D. I. Andersson, and A. Melhus. 2008. The first major extended-spectrum beta-lactamase outbreak in Scandinavia was caused by clonal spread of a multiresistant Klebsiella pneumoniae producing CTX-M-15. APMIS 116:302-308. [DOI] [PubMed] [Google Scholar]
- 18.Mathai, D., P. R. Rhomberg, D. J. Biedenbach, and R. N. Jones. 2002. Evaluation of the in vitro activity of six broad-spectrum beta-lactam antimicrobial agents tested against recent clinical isolates from India: a survey of ten medical center laboratories. Diagn. Microbiol. Infect. Dis. 44:367-377. [DOI] [PubMed] [Google Scholar]
- 19.Munday, C. J., J. Xiong, C. Li, D. Shen, and P. M. Hawkey. 2004. Dissemination of CTX-M type beta-lactamases in Enterobacteriaceae isolates in the People's Republic of China. Int. J. Antimicrob. Agents 23:175-180. [DOI] [PubMed] [Google Scholar]
- 20.Norskov-Lauritsen, N., H. Marchandin, and M. J. Dowzicky. 2009. Antimicrobial susceptibility of tigecycline and comparators against bacterial isolates collected as part of the TEST study in Europe (2004-2007). Int. J. Antimicrob. Agents 34:121-130. [DOI] [PubMed] [Google Scholar]
- 21.Pitout, J. D., L. Campbell, D. L. Church, D. B. Gregson, and K. B. Laupland. 2009. Molecular characteristics of travel-related extended-spectrum-beta-lactamase-producing Escherichia coli isolates from the Calgary Health Region. Antimicrob. Agents Chemother. 53:2539-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitout, J. D., A. Hossain, and N. D. Hanson. 2004. Phenotypic and molecular detection of CTX-M-beta-lactamases produced by Escherichia coli and Klebsiella spp. J. Clin. Microbiol. 42:5715-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Bano, J., L. Lopez-Cerero, M. D. Navarro, P. Diaz de Alba, and A. Pascual. 2008. Faecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J. Antimicrob. Chemother. 62:1142-1149. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Bano, J., M. D. Navarro, L. Romero, L. Martinez-Martinez, M. A. Muniain, E. J. Perea, R. Perez-Cano, and A. Pascual. 2004. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J. Clin. Microbiol. 42:1089-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Bano, J., M. D. Navarro, L. Romero, M. A. Muniain, M. Cueto, J. Galvez, E. J. Perea, and A. Pascual. 2008. Risk-factors for emerging bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Clin. Microbiol. Infect. 14:180-183. [DOI] [PubMed] [Google Scholar]
- 26.Rossolini, G. M., M. M. D'Andrea, and C. Mugnaioli. 2008. The spread of CTX-M-type extended-spectrum beta-lactamases. Clin. Microbiol. Infect. 14(Suppl. 1):33-41. [DOI] [PubMed] [Google Scholar]
- 27.Struwe, J., and B. Olsson-Liljequist. 2009. Short summary of Swedres 2008, a report on antimicrobial utilisation and resistance in humans in Sweden. Euro Surveill. 14(25):pii=19252. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19252. [PubMed] [Google Scholar]
- 28.Svenungsson, B., A. Lagergren, E. Ekwall, B. Evengard, K. O. Hedlund, A. Karnell, S. Lofdahl, L. Svensson, and A. Weintraub. 2000. Enteropathogens in adult patients with diarrhea and healthy control subjects: a 1-year prospective study in a Swedish clinic for infectious diseases. Clin. Infect. Dis. 30:770-778. [DOI] [PubMed] [Google Scholar]
- 29.Tham, J., I. Odenholt, M. Walder, A. Brolund, J. Ahl, and E. Melander. 2010. Extended-spectrum beta-lactamase-producing Escherichia coli in patients with travellers' diarrhoea. Scand. J. Infect. Dis. 42:275-280. [DOI] [PubMed] [Google Scholar]
- 30.Valverde, A., T. M. Coque, M. P. Sanchez-Moreno, A. Rollan, F. Baquero, and R. Canton. 2004. Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during nonoutbreak situations in Spain. J. Clin. Microbiol. 42:4769-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villegas, M. V., J. N. Kattan, M. G. Quinteros, and J. M. Casellas. 2008. Prevalence of extended-spectrum beta-lactamases in South America. Clin. Microbiol. Infect. 14(Suppl. 1):154-158. [DOI] [PubMed] [Google Scholar]
- 32.Winokur, P. L., R. Canton, J. M. Casellas, and N. Legakis. 2001. Variations in the prevalence of strains expressing an extended-spectrum beta-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin. Infect. Dis. 32(Suppl. 2):S94-S103. [DOI] [PubMed] [Google Scholar]
- 33.Yang, J. R., F. T. Wu, J. L. Tsai, J. J. Mu, L. F. Lin, K. L. Chen, S. H. Kuo, C. S. Chiang, and H. S. Wu. 2007. Comparison between O serotyping method and multiplex real-time PCR to identify diarrheagenic Escherichia coli in Taiwan. J. Clin. Microbiol. 45:3620-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]