Abstract
Ceragenins are cholic acid-derived antimicrobial agents that mimic the activity of endogenous antimicrobial peptides. Ceragenins target bacterial membranes, yet the consequences of these interactions have not been fully elucidated. The role of the outer membrane in allowing access of the ceragenins to the cytoplasmic membrane of Gram-negative bacteria was studied using the ML-35p mutant strain of Escherichia coli that has been engineered to allow independent monitoring of small-molecule flux across the inner and outer membranes. The ceragenins CSA-8, CSA-13, and CSA-54 permeabilize the outer membrane of this bacterium, suggesting that the outer membrane does not play a major role in preventing the access of these agents to the cytoplasmic membrane. However, only the most potent of these ceragenins, CSA-13, was able to permeabilize the inner membrane. Interestingly, neither CSA-8 nor CSA-54 caused inner membrane permeabilization over a 30-min period, even at concentrations well above those required for bacterial toxicity. To further assess the role of membrane interactions, we measured membrane depolarization in Gram-positive bacteria with different membrane lipid compositions, as well as in Gram-negative bacteria. We found greatly increased membrane depolarization at the minimal bactericidal concentration of the ceragenins for bacterial species containing a high concentration of phosphatidylethanolamine or uncharged lipids in their cytoplasmic membranes. Although membrane lipid composition affected bactericidal efficiency, membrane depolarization was sufficient to cause lethality, providing that agents could access the cytoplasmic membrane. Consequently, we propose that in targeting bacterial cytoplasmic membranes, focus be placed on membrane depolarization as an indicator of potency.
Ceragenins are a family of bile acid derivatives that have been modified to yield an amphiphilic morphology similar to that of endogenous antimicrobial peptides (Fig. 1) (9, 13). The majority of antimicrobial peptides adopt amphiphilic secondary structures in which cationic amino acid side chains (i.e., arginine, lysine, and histidine) are oriented on one face of the molecule while hydrophilic side chains are on the opposing face. This morphology has been termed “facially amphiphilic.” The ceragenins effectively reproduce this morphology owing to their bile acid scaffolding.
FIG. 1.
Structures of ceragenins CSA-8, CSA-13, and CSA-54.
In a manner similar to antimicrobial peptides, ceragenins exhibit rapid bactericidal activity against a broad range of bacterial species. However, the ceragenins have several advantages over antimicrobial peptides (AMPs), including their resistance to proteolysis and their amenability to large-scale synthesis. Although some forms of ceragenins are effective against both Gram-negative and Gram-positive bacteria, they are generally more potent against Gram-positive organisms. The importance of these agents is exemplified by CSA-13, the most potent of the ceragenins with broad-spectrum antimicrobial activity. This compound has strong bactericidal activity against glycopeptide-resistant Staphylococcus aureus and has proven more effective than the human antimicrobial peptide LL-37 against a multidrug-resistant strain of Pseudomonas aeruginosa (3). In addition, CSA-13 is less susceptible to inactivation by negatively charged components in the sputa of cystic fibrosis (CF) patients, and it is potent even in cystic fibrosis sputum itself, suggesting its potential for therapeutic use in treating CF infections (2). The ceragenins are also highly active against cariogenic and periodontopathic bacteria (17). Ceragenins selectively associate with bacterial membranes and permeabilize the outer membranes (OMs) of Gram-negative bacteria. This permeabilization potentiates the antibacterial activity of other antibiotics (7, 13).
In the present work we have investigated the role of bacterial membrane composition in the bactericidal or bacteriostatic action of ceragenins by providing evidence of their ability to reach the inner membranes of Gram-negative bacteria and to induce their depolarization, as well as these agents' ability to cause depolarization in Gram-positive bacteria with different lipid compositions. These studies may prove useful in the design of more potent ceragenins.
MATERIALS AND METHODS
Materials.
ONPG (o-nitrophenyl-3-d-galactoside) was purchased from Sigma Chemical Co. Nitrocefin was kindly provided by R. Lehrer. The dye DiS-C2(5) (3,3′-diethylthiadicarbocyanine iodide) was from Fluka Chemical Co.
The Escherichia coli bacterial strain ML-35p was kindly provided by R. Lehrer. The following bacterial strains were purchased from ATCC (Manassas, VA): Klebsiella pneumoniae ATCC 13883, E. coli ATCC 25922, P. aeruginosa ATCC 27852, Bacillus cereus ATCC 11778, S. aureus (MRSA) ATCC BAA-41, Enterococcus faecalis ATCC 29212, Staphylococcus epidermidis ATCC 35984, Bacillus polymyxa ATCC 21830, and S. aureus ATCC 25923. Trypticase soy broth (TSB) and EDTA were purchased from Fisher Scientific (Pittsburgh, PA). Ceragenins CSA-13, CSA-8, and CSA-54 were prepared as described previously (16).
Permeabilization of inner membranes and OMs of E. coli ML-35p.
The E. coli bacterial strain ML-35p was engineered specifically to monitor permeation of both inner and outer membranes of the same bacterial strain (15). E. coli ML-35p is constitutive for cytoplasmic β-galactosidase, lacks lac permease, and expresses a plasmid-encoded periplasmic β-lactamase. Two chromogenic reporter molecules are used to monitor permeabilization of outer and inner membranes in a single assay (10, 14, 15). Nitrocefin, a chromogenic cephalosporin, cannot cross the OM and is excluded from the periplasmic space. However, permeabilization of the OM allows nitrocefin to enter the periplasm, where it is cleaved by a β-lactamase and produces a color change that can be monitored spectrophotometrically at 486 nm. Since there is no lactose permease in this strain of E. coli, ONPG cannot traverse its inner membrane to be cleaved by cytoplasmic β-galactosidase to o-nitrophenol unless permeabilization of the inner membrane occurs. ONPG cleavage produces a color change that can be measured spectrophotometrically at 420 nm.
Bacteria were grown in TSB from a single colony, overnight at 37°C. After three washings in phosphate buffer, pH 7.4 (10 mM phosphate buffer, 0.1 M NaCl), the culture was diluted to 106 CFU/ml in incubation buffer (phosphate buffer, pH 7.4, containing 300 μg/ml TSB) and added to all wells in a non-culture-treated polystyrene microplate, together with increasing concentrations of ceragenin or melittin (0 to 100 μg/ml), in duplicates. The solution of ceragenin was also made in phosphate buffer. Each well also contained 30 μM nitrocefin in phosphate buffer or 2.5 mM ONPG in phosphate buffer. Absorbance was followed simultaneously at 486 nm and at 420 nm for 60 min, with readings taken every 2 min at 37°C in a SpectraMax Pro microplate reader equipped with MaxSoft Plus software (Molecular Devices, Sunnyvale, CA), with shaking.
Spectroscopic measurements.
Optical density measurements were made using a Beckman Coulter DU 730 Life Science UV/Vis spectrophotometer. All fluorescence measurements were made using a Jobin Yvon Horiba FluoroMax-3 fluorimeter with a Fisher Scientific Isotemp 3028H bath to maintain the temperature at 37°C.
Membrane depolarization with Gram-positive strains.
Bacterial cultures were grown overnight in TSB at 37°C. Cells were harvested by centrifugation and washed in a buffer containing 250 mM sucrose, 5 mM MgSO4, and 10 mM potassium phosphate (pH 7.0). After three washings, pellets were resuspended in the same buffer. Fractions from each cell suspension were diluted in the same buffer in a cuvette to an optical density (A600) of 0.085 along with the dye DiS-C2(5) at a concentration of 1 μM (1). The dye was allowed to incorporate for 7 min at room temperature, followed by 7 min at 37°C, which gave a stable baseline. An excitation wavelength of 600 nm and an emission wavelength of 660 nm were used to monitor depolarization. Samples were stirred during the experiment at a constant temperature of 37°C. Fluorescence measurements were taken at 30-s intervals before and after addition of ceragenins (5).
Membrane depolarization with Gram-negative strains.
Bacterial cultures were grown overnight in TSB at 37°C. Cells were harvested by centrifugation, washed in TSB, and resuspended in TSB. After three such washings, pellets were resuspended in TSB. Cells were suspended in a buffer containing 20 mM glucose and 5 mM HEPES (pH 7.3) (18). Fractions from each cell suspension were diluted into a cuvette to an optical density (A600) of 0.085 along with the dye DiS-C2(5) at 1 μM in the buffer. The mixture was allowed to equilibrate to a stable baseline (ca. 60 min) at 37°C. An excitation wavelength of 600 nm and an emission wavelength of 660 nm were used to monitor depolarization. Samples were stirred during the measurement at a constant temperature of 37°C. Measurements were taken at 30-s intervals before and after addition of ceragenins CSA-13 and CSA-8.
Determination of MBC.
Minimum bactericidal concentration (MBC) determinations were performed in the buffers used in the depolarization studies and were based on a broth microdilution method described previously (19). Briefly, after incubation with incrementally varied concentrations of the ceragenins for 24 h, MBCs were determined by plating 100 μl of each sample on tryptic soy agar (TSA). The MBC was defined as the lowest concentration of compound that completely eradicated the bacterial population (to a detection limit of 10 CFU/ml).
RESULTS
Permeabilization of the inner membranes and OMs of E. coli ML-35p.
All three ceragenins, CSA-8, CSA-13, and CSA-54, rapidly permeabilized the OM of E. coli ML-35p in a concentration-dependent manner, as shown by the time-dependent increase in nitrocefin permeation (Fig. 2). The onset of permeabilization was more rapid with the ceragenins than with even the potent lytic peptide melittin, used as a positive control. However, the extent of nitrocefin permeation reached by higher concentrations of melittin was greater than that observed with the ceragenins. There were differences in the degrees of OM permeabilization among the ceragenins that correlate with their antimicrobial potencies. The most potent, CSA-13, at the lowest concentration exhibited an increase in permeabilization by nitrocefin while higher concentrations of CSA-8 and CSA-54 were required to achieve permeabilization. Interestingly, only ceragenin CSA-13 permeated the inner membrane of E. coli ML-35p to allow passage of ONPG (Fig. 2). For CSA-8 and CSA-54 no inner membrane permeabilization was observed even at high concentrations well above the MBC.
FIG. 2.
Outer and inner membrane permeabilization of E. coli ML-35p caused by one of the ceragenins or by melittin as a function of time at different concentrations (0 to 100 μg/ml) and at 37°C. Hydrolysis of nitrocefin by β-lactamase was used to monitor outer membrane permeabilization by absorbance at 486 nm (left-hand panel). Hydrolysis of ONPG by β-galactosidase was used to monitor inner membrane permeabilization by absorbance at 420 nm (right-hand panel).
Membrane depolarization.
Since results from the assays with E. coli ML-35p indicated that not all the ceragenins achieved lethality by increases in the rate of transport of molecules across the cytoplasmic membrane, we sought to investigate if there was a smaller breach of the permeability barrier of the cytoplasmic membrane that would allow passage of electrical current, perhaps as a result of proton flux, causing membrane depolarization without allowing passage of larger molecules. The assays for depolarization of the cytoplasmic membrane were done with both Gram-positive and Gram-negative bacteria.
A family of fluorescent cyanine dyes has been developed that can be used to monitor membrane depolarization (4). These dyes lose fluorescence intensity in polarized membranes and become highly fluorescent once polarization is lost. We used the dye DiS-C2(5) to test for the depolarization of the cytoplasmic membranes of both Gram-positive and Gram-negative bacteria.
Membrane depolarization of Gram-positive bacteria.
The ceragenins were tested with Gram-positive bacteria having different lipid compositions. Antibacterial activities of the ceragenins (measured as MBCs) were measured in the minimal medium used for the depolarization experiments, and the values varied among the strains tested (Table 1). E. faecalis proved to be the most resistant to the ceragenins, and this may be due the lipid composition of this organism compared to that of the other Gram-positive bacteria tested. This difference was particularly apparent with CSA-8 and CSA-54.
TABLE 1.
Proportions of major lipids in different Gram-positive bacteria and MBCs and depolarization percentages for ceragenins
| Bacterial species | Lipid composition (%) |
Antimicrobial activity of: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CSA-13 |
CSA-54 |
CSA-8 |
|||||||
| PE | PG | CL | MBC (μg/ml) | % Depolarization at MBC | MBC (μg/ml) | % Depolarization at MBC | MBC (μg/ml) | % Depolarization at MBC | |
| B. polymyxaa | 60 | 3 | 8 | 2.5 | 88.4 | 2.5 | 39.6 | 15 | 96.6 |
| B. cereusa | 43 | 40 | 17 | 1.0 | 86.7 | 7.0 | 79.7 | 7.0 | 84.7 |
| E. faecalisb | 0 | 27 | 19 | 5.5 | 74.0 | 280 | 77.2 | 50 | 80.6 |
| S. epidermisa | 0 | 90 | 1 | 1.0 | 25.0 | 2.5 | 26.3 | 12 | 60.1 |
| S. aureusc | 0 | 57 | 19 | 2.5 | 41.5 | 49 | 39.8 | 35 | 82.8 |
| MRSAd | 0 | 57 | 19 | 2.0 | 45.8 | 28 | 15.3 | 19 | 39.0 |
Contains small amounts of other components.
E. faecalis has in addition to 20% lysyl-PG, 26% phosphatidylkojibiosyl diacylglycerol attached to lipoteichoic acid, and 8% other lipids.
S. aureus has variable amounts of lysyl-PG, mostly in the inner leaflet of the membrane. Diglucosyl diacylglycerol attached to lipoteichoic acid is present at ∼24%.
Lipid composition was assumed to be similar to that of the sensitive strain.
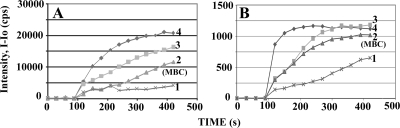
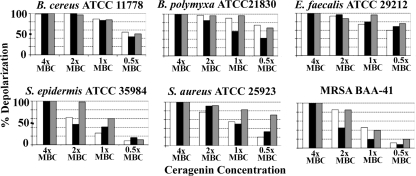
All the ceragenins were able to depolarize the cytoplasmic membrane in a concentration-dependent manner, as shown, for examples, in Fig. 3. At the highest concentration of ceragenins tested (i.e., 4× MBC), the level of depolarization appeared to reach a maximum at times from less than 1 min to 5 min. Comparisons were made between the maximum levels of depolarization indicated by the fluorophore to levels of depolarization reached at various time points and concentrations of ceragenins (Fig. 4). At concentrations of ceragenins comparable to the MBCs, depolarization of the membrane reached 50 to 90% (Fig. 3B).
FIG. 3.
Effects of CSA-13 on the fluorescence intensity of DiS-C2(5) in the presence of the indicated organisms. (A) S. aureus ATCC 25923. CSA-13 was added at 90 s at the following concentrations: line 1, 0.125 μg/ml; line 2, 0.2.5 μg/ml; line 3, 5.0 μg/ml; and line 4, 10.0 μg/ml. (B) B. cereus ATCC 11778. CSA-13 was added at 90 s at the following concentrations: line 1, 0.5 μg/ml; line 2, 1.0 μg/ml; line 3, 2.0 μg/ml; and line 4, 4.0 μg/ml. cps, counts per second.
FIG. 4.
Percentage of membrane depolarization with the indicated organisms at 330 s determined as an increase in fluorescence intensity of DiS-C2(5) and compared to the maximum fluorescence increase at 4× MBC for CSA-13. White bars, CSA-13; black bars, CSA-54; gray bars, CSA-8.
Membrane depolarization of Gram-negative bacteria.
With Gram-negative bacteria the depolarization assays were carried out in glucose-HEPES buffer to avoid interference in fluorescence measurements caused by the nutrient medium. Experiments were performed with three strains of pathogenic Gram-negative bacteria: E. coli, K. pneumoniae, and P. aeruginosa. The MBCs of the ceragenins were measured with each of these organisms (Table 2).
TABLE 2.
Lipid composition of different Gram-negative bacteria and MBCs for ceragenins
| Bacterial species | Lipid composition (%) |
CSA-13 MBC (μg/ml) | CSA-8 MBC (μg/ml) | ||
|---|---|---|---|---|---|
| PE | PG | CL | |||
| E. coli | 85 | 15 | 5 | 1.5 | 10.0 |
| K. pneumoniae | 82 | 5 | 6 | 2.5 | 12.5 |
| P. aeruginosa | 60 | 21 | 11 | 3.5 | 12.0 |
To measure depolarization of the cytoplasmic membrane, the fluorescent dye must traverse the OM. A function of the OM is to limit the access of hydrophobic compounds to bacteria, and consequently it was anticipated that permeabilization of the OM would be required to allow the fluorescent probe access to the cytoplasmic membrane. Various agents are known to permeabilize the OMs of Gram-negative bacteria without causing bactericidal effects. These agents include the metal chelator EDTA (6), derivatives of polymyxins (20), and the ceragenins. The structure of CSA-8 was optimized to permeabilize the OMs of Gram-negative bacteria, and it effectively sensitizes these organisms to hydrophobic antibiotics, including erythromycin, novobiocin, and rifampin (3, 16).
To view the impact of OM permeabilization on incorporation of the fluorescent probe, the probe was added to a suspension of bacteria in minimal medium, and the mixture was incubated until a steady fluorescence baseline was observed. EDTA was added at various concentrations, and a drop in the fluorescence of the probe was observed (Fig. 5 A). The fluorescence of the cyanine dye decreases as it incorporates into polarized membranes, and the decrease in fluorescence observed is consistent with EDTA-mediated permeabilization of the OM, allowing access of the probe to the polarized cytoplasmic membrane. At concentrations above 2 mg/ml EDTA, an initial loss of fluorescence intensity was followed by a large increase. This result suggests that at higher concentrations EDTA allows permeabilization but can also cause cell death.
FIG. 5.
Effects of EDTA and ceragenins CSA-8 and CSA-13 on the fluorescence intensity of DiS-C2(5) in the presence of the indicated organisms. The ceragenins and EDTA were added at 3,600 s. (A) EDTA, at the indicated concentrations, with K. pneumoniae ATCC 13883. (B) CSA-8, at the indicated concentrations, with K. pneumoniae ATCC 13883. (C) CSA-13, at the indicated concentrations, with K. pneumoniae ATCC 13883. (D) CSA-13, at the indicated concentrations, with E. coli ATCC 25922.
CSA-8 associates strongly with the OM and disrupts the permeability barrier; however, it is bactericidal at only relatively high concentrations. At a concentration well below its MBC, CSA-8 caused a decrease in fluorescence comparable to that observed with EDTA (Fig. 5B). At its MBC, a significant increase in fluorescence is observed, consistent with depolarization of the cytoplasmic membrane.
CSA-13 contains the same amphiphilic functionality found in CSA-8, and a lipid chain is appended that allows it to effectively traverse the OM. Consequently, the MBCs for CSA-13 are much lower than those for CSA-8 in Gram-negative bacteria. At the MBC of CSA-13, the fluorescence of the probe decreased initially but then rose to well above the baseline (Fig. 4 C), suggesting effective depolarization of the cytoplasmic membrane. Comparable behavior was observed in E. coli and P. aeruginosa.
DISCUSSION
Structures of the ceragenins used in this study are presented in Fig. 1, and among these compounds CSA-8 is the least cationic, with three positive charges, and CSA-54 is the most positively charged with six. The primary distinguishing feature of CSA-13 is the lipid chain at C-24. Tables 1 and 2 give data on the composition of the major lipids in the bacteria tested as well as the MBCs of the ceragenins for this collection of Gram-positive and Gram-negative bacteria, respectively. As reported previously, CSA-13 is a broad-spectrum bactericidal agent, while CSA-8 and CSA-54 are somewhat less active against Gram-positive organisms and much less active against Gram-negative bacteria. We have proposed that the lipid chain in CSA-13 is necessary for effective traversal of the OM of Gram-negative bacteria and that this process is essential for bactericidal activity at low concentrations.
A key step in the activity of the ceragenins is selective association with bacterial membranes. All three ceragenins tested possess the structural elements required for association with the membrane lipid components, including lipid A in the OM of Gram-negative bacteria. We have shown previously that association of ceragenins with the OM disrupts the permeability barrier generated by the lipid bilayer. The ML-35p strain of E. coli has been engineered to monitor permeation of both cytoplasmic and outer membranes (15). Results from experiments with this strain and the ceragenins demonstrate that all three compounds effectively permeabilize the OM to nitrocefin at concentrations comparable to those of melittin (Fig. 2). However, only the most potent ceragenin, CSA-13, displayed the capacity to permeabilize the cytoplasmic membrane to ONPG, a property CSA-13 has in common with melittin. Even at concentrations well above the MBC, neither CSA-8 nor CSA-54 caused sufficient permeabilization of the cytoplasmic membrane to allow entry of the probe (Fig. 2). These results suggest that the bactericidal activity of CSA-13 toward E. coli may be a consequence of its ability to promote unregulated passage of small polar molecules through the cytoplasmic membrane. Nevertheless, the fact that CSA-8 and CSA-54 display bactericidal activity argues that they function by mechanisms that do not involve such cytoplasmic membrane disruptions that would allow passage of small molecules like ONPG.
There is a large electrical potential across the cytoplasmic membrane of bacteria. This electrical barrier can be dissipated by a perturbation of the membrane that would allow the flow of charge without necessarily allowing the passage of small organic molecules such as ONPG. Dissipation of this potential may be the mechanism by which CSA-8 and CSA-54 cause cell death without permeabilizing the cytoplasmic membrane to small molecules.
Measuring the depolarization of the cytoplasmic membrane of Gram-positive bacteria is straightforward using a cyanine dye because the probe has direct access to the cytoplasmic membrane. Using a fluorescent dye, we found that the ceragenins, in a time- and concentration-dependent manner, depolarized the lipid bilayer of the Gram-positive bacteria tested (as shown in Fig. 3, for example). A comparison of the percentage of membrane depolarization after 5.5 min with MBCs is given in Fig. 4. With all of the strains, a significant amount of depolarization was observed at the MBC for all the ceragenins tested.
Of particular interest is the observation that the extent of depolarization by these agents depended on the composition of the cytoplasmic membrane of the bacteria (Table 1). The genus Staphylococcus lacks phosphatidylethanolamine (PE) in the cytoplasmic membrane, similar to most other Gram-positive bacteria (with some exceptions such as in the genus Bacillus or Clostridium), presenting instead mostly phospholipids with anionic head groups (phosphatidylglycerol [PG] or cardiolipin [CL]). These Gram-positive bacteria showed the least depolarization at the MBC or at 0.5× MBC (Fig. 4). In contrast, B. cereus and B. polymyxa have a high PE content in the cytoplasmic membrane (Table 1), and with these organisms the ceragenins are comparably more efficient in their depolarization abilities (Fig. 4). This phenomenon has been observed with antimicrobial peptides (11), and the possibility exists that ceragenins and antimicrobial peptides have secondary, intracellular targets that influence antimicrobial activity. Nevertheless, the fact that significant levels of membrane depolarization occur at the MBC suggests that membrane depolarization may be sufficient to cause cell death and may be the primary mode of action of the ceragenins against the Gram-positive bacteria.
The lipid composition in bacteria varies greatly, and, in addition, bacteria are able to modify their lipid composition in response to environmental conditions and to satisfy requirements for outer wall biosynthesis. For these reasons, comparative antimicrobial activity assays for viability with antimicrobial agents are always carried out under the same conditions of growth in our studies. In the Gram-positive bacterium E. faecalis, the cytoplasmic membrane, besides PG and CL, consists of 20% lysyl-PG, a cationic lipid that would neutralize some of the membrane's negative charge (Table 1), and 26% phosphatidylkojibiosyl diglycerol, an uncharged lipid covalently linked to the polyglycerol moieties of lipoteichoic acid through a phosphodiester linkage (12). This lipid acts as an anchor between the membrane and the cell wall, with its fatty acids embedded in the membrane and the lipoteichoic acid extending from the surface of the membrane. With this lipid profile, E. faecalis proved to be similar to the Bacillus strains tested, which contain substantial amounts of PE, in its susceptibility to membrane depolarization by the ceragenins. This contrasts with S. aureus, which has a much larger residual amount of negative charge in its membrane. The finding that E. faecalis behaves similarly to other Gram-positive bacteria with high PE content suggests that the property of enhancing membrane depolarization is not a consequence of the specific structure of PE but, rather, of having zwitterionic or uncharged lipids in the cytoplasmic membrane, with biophysical properties different from those of the major anionic lipids, PG and CL. A similar situation was found with Streptococcus pyogenes in its susceptibility to the action of AMPs (8).
Membrane depolarization experiments with Gram-negative bacteria are complicated by the permeability barrier of the OM, which impedes access of the fluorescent probe to the cytoplasmic membrane. Access to the cytoplasmic membrane can be facilitated by EDTA, which disrupts the ion-mediated cross-linking of lipid A in the OM, making the bilayer more permeable. As described above, binding to lipid A by ceragenins also results in permeabilization of the OM. This activity of EDTA and the ceragenins can be observed in the loss of fluorescence of the probe after addition of these agents, which is likely caused by incorporation of the dye in the polarized cytoplasmic membrane (Fig. 5). Notably, the concentrations of ceragenins necessary to achieve permeabilization are about 1,000-fold lower than those required for EDTA. Because CSA-8 and CSA-54 display similar abilities to permeabilize the OM, only CSA-8 and CSA-13 were used in membrane depolarization studies. These studies were performed with three different strains of Gram-negative bacteria (Table 2), and results shown in Fig. 5 with K. pneumoniae and E. coli are representative.
With relatively low concentrations of CSA-8 (0.5 μg/ml), a drop in the fluorescence of the dye was observed, consistent with permeabilization of the OM and incorporation of the dye in the cytoplasmic membrane (Fig. 5B). At the MBC and above, the fluorescence of the dye increased, and this result suggests that incorporation of the dye was competitive with membrane depolarization. The MBC of CSA-8 is relatively high, and the OM-permeabilizing and bactericidal activities were separated by a substantial difference in concentrations (ca. 12 μg/ml). In contrast, with CSA-13 the difference in concentrations between these two activities is approximately 2 μg/ml (Fig. 5C). Nevertheless, with K. pneumoniae, permeabilization of the OM could be achieved without apparent cytoplasmic membrane depolarization at 0.5 μg/ml (Fig. 5C). Similarly, with E. coli, at concentrations of CSA-13 at or above the MBC, an initial decrease in fluorescence was followed by a large increase (Fig. 5D). At concentrations below the MBC, only a drop in fluorescence was observed.
Elucidation of the respective roles of membrane permeabilization and depolarization is of importance for understanding the mechanisms of action of antimicrobial agents and for providing evidence of the role of membrane integrity in bacterial viability. In addition, for Gram-negative bacteria it is important to understand the role of the OM in preventing access of the antimicrobial agents to the cytoplasmic membrane. As mimics of antimicrobial peptides, ceragenins display both OM permeabilization and cytoplasmic membrane depolarization activities. These activities can be separated to some extent with CSA-8 in Gram-negative bacteria. Only the most potent compound, CSA-13, can efficiently depolarize the cytoplasmic membrane of Gram-negative bacteria and induce solute leakage, causing a bactericidal effect. Nevertheless, from studies with both Gram-negative and Gram-positive bacteria, it appears that membrane depolarization correlates to a large extent with the bactericidal activity of the ceragenins. Consequently, in continuing work to develop more potent and selective antimicrobials targeting bacterial membranes, focus should be placed on membrane depolarization as an indication of potency.
Acknowledgments
This research was supported by grant MOP 86608 from the Canadian Institutes of Health Research (R.M.E.) and funds from the NIH (U01 AI082209 to P.B.S.).
We are grateful to Robert Lehrer for generously providing us with E. coli strain ML-35p.
Footnotes
Published ahead of print on 28 June 2010.
REFERENCES
- 1.Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H. G. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. [DOI] [PubMed] [Google Scholar]
- 2.Bucki, R., A. G. Sostarecz, F. J. Byfield, P. B. Savage, and P. A. Janmey. 2007. Resistance of the antibacterial agent ceragenin CSA-13 to inactivation by DNA or F-actin and its activity in cystic fibrosis sputum. J. Antimicrob. Chemother. 60:535-545. [DOI] [PubMed] [Google Scholar]
- 3.Chin, J. N., R. N. Jones, H. S. Sader, P. B. Savage, and M. J. Rybak. 2008. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J. Antimicrob. Chemother. 61:365-370. [DOI] [PubMed] [Google Scholar]
- 4.Chongsiriwatana, N. P., and A. E. Barron. 2010. Comparing bacterial membrane interactions of antimicrobial peptides and their mimics. Methods Mol. Biol. 618:171-182. [DOI] [PubMed] [Google Scholar]
- 5.Ding, B., Q. Guan, J. P. Walsh, J. S. Boswell, T. W. Winter, E. S. Winter, S. S. Boyd, C. Li, and P. B. Savage. 2002. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 45:663-669. [DOI] [PubMed] [Google Scholar]
- 6.El-Kosasy, A. M. 2006. Potentiometric assessment of Gram-negative bacterial permeabilization of tobramycin. J. Pharm. Biomed. Anal. 42:389-394. [DOI] [PubMed] [Google Scholar]
- 7.Epand, R. F., C. M. Yip, L. V. Chernomordik, D. L. LeDuc, Y. K. Shin, and R. M. Epand. 2001. Self-assembly of influenza hemagglutinin: studies of ectodomain aggregation by in situ atomic force microscopy. Biochim. Biophys. Acta 1513:167-175. [DOI] [PubMed] [Google Scholar]
- 8.Epand, R. M., R. F. Epand, C. J. Arnusch, B. Papahadjopoulos-Sternberg, G. Wang, and Y. Shai.2010. Lipid clustering by three homologous arginine-rich antimicrobial peptides is insensitive to amino acid arrangement and induced secondary structure. Biochim. Biophys. Acta 1798:1272-1280. [DOI] [PubMed] [Google Scholar]
- 9.Epand, R. M., R. F. Epand, and P. B. Savage. 2008. Ceragenins (cationic steroid compounds), a novel class of antimicrobial agents. Drug News Perspect. 21:307-311. [DOI] [PubMed] [Google Scholar]
- 10.Ericksen, B., Z. Wu, W. Lu, and R. I. Lehrer. 2005. Antibacterial activity and specificity of the six human α-defensins. Antimicrob. Agents Chemother. 49:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich, C. L., D. Moyles, T. J. Beveridge, and R. E. Hancock. 2000. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 44:2086-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganfield, M. C., and R. A. Pieringer. 1975. Phosphatidylkojibiosyl diglyceride. The covalently linked lipid constituent of the membrane lipoteichoic acid from Streptococcus faecalis (faecium) ATCC 9790. J. Biol. Chem. 250:702-709. [PubMed] [Google Scholar]
- 13.Lai, X. Z., Y. Feng, J. Pollard, J. N. Chin, M. J. Rybak, R. Bucki, R. F. Epand, R. M. Epand, and P. B. Savage. 2008. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc. Chem. Res. 41:1233-1240. [DOI] [PubMed] [Google Scholar]
- 14.Lehrer, R. I., A. Barton, K. A. Daher, S. S. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Invest. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehrer, R. I., A. Barton, and T. Ganz. 1988. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J. Immunol. Methods 108:153-158. [DOI] [PubMed] [Google Scholar]
- 16.Li, C. H., L. P. Budge, C. D. Driscoll, B. M. Willardson, G. W. Allman, and P. B. Savage. 1999. Incremental conversion of outer-membrane permeabilizers into potent antibiotics for Gram-negative bacteria. J. Am. Chem. Soc. 121:931-940. [Google Scholar]
- 17.Ouhara, K., H. Komatsuzawa, S. Yamada, H. Shiba, T. Fujiwara, M. Ohara, K. Sayama, K. Hashimoto, H. Kurihara, and M. Sugai. 2005. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, β-defensins and LL37, produced by human epithelial cells. J. Antimicrob. Chemother. 55:888-896. [DOI] [PubMed] [Google Scholar]
- 18.Papo, N., and Y. Shai. 2005. A molecular mechanism for lipopolysaccharide protection of gram-negative bacteria from antimicrobial peptides. J. Biol. Chem. 280:10378-10387. [DOI] [PubMed] [Google Scholar]
- 19.Pollard, J., J. Wright, Y. Feng, D. Geng, C. Genberg, and P. B. Savage. 2009. Activities of ceragenin CSA-13 against established biofilms in an in vitro model of catheter decolonization. Anti. Infect. Agents Med. Chem. 8:290-294. [Google Scholar]
- 20.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2000. Structure-function studies of polymyxin B nonapeptide: implications to sensitization of gram-negative bacteria. J. Med. Chem. 43:3085-3092. [DOI] [PubMed] [Google Scholar]