Abstract
This is a substudy of a larger randomized controlled trial on HIV-infected Zambian children, which revealed that cotrimoxazole prophylaxis reduced morbidity and mortality despite a background of high cotrimoxazole resistance. The impact of cotrimoxazole on the carriage and antibiotic resistance of Streptococcus pneumoniae and Haemophilus influenzae as major causes of childhood mortality in HIV-infected children was investigated since these are unclear. Representative nasopharyngeal swabs were taken prior to randomization for 181 of 534 children (92 on cotrimoxazole and 89 on placebo). Bacterial identification and antibiotic susceptibility were performed by routine methods. Due to reduced mortality, prophylactic cotrimoxazole increased the median time from randomization to the last specimen from 48 to 56 months (P = 0.001). The carriage of H. influenzae was unaltered by cotrimoxazole. Carriage of S. pneumoniae increased slightly in both arms but was not statistically significant in the placebo arm. In S. pneumoniae switching between carriage and no carriage in consecutive pairs of samples was unaffected by cotrimoxazole (P = 0.18) with a suggestion that the probability of remaining carriage free was lower (P = 0.10). In H. influenzae cotrimoxazole decreased switching from carriage to no carriage (P = 0.02). Cotrimoxazole resistance levels were higher in postbaseline samples in the cotrimoxazole arm than in the placebo arm (S. pneumoniae, P < 0.0001; H. influenzae, P = 0.005). Cotrimoxazole decreased switching from cotrimoxazole resistance to cotrimoxazole sensitivity in S. pneumoniae (P = 0.002) and reduced the chance of H. influenzae remaining cotrimoxazole sensitive (P = 0.05). No associations were observed between the percentage of CD4 (CD4%), the change in CD4% from baseline, child age at date of specimen, child gender, or sampling month with carriage of either pathogen.
Streptococcus pneumoniae and Haemophilus influenzae are major causes of respiratory tract infections and bacteremia worldwide (5, 29, 34). Children younger than 5 years of age, the elderly, and immunocompromised individuals are most at risk for respiratory infections (3). HIV-infected children younger than 2 years have a 40-fold greater risk of lower respiratory tract infections (34) with a 12-fold greater risk of pneumococcal invasive disease (11, 15, 37), increasing to a 100-fold-higher risk (31) compared to HIV-uninfected children. The prognosis for HIV-infected children developing pneumococcal disease is much worse than that for uninfected children developing pneumococcal disease (7, 21, 35, 54).
Invasive infections with S. pneumoniae and H. influenzae are usually preceded by nasopharyngeal colonization (10, 22). Colonizing bacteria are frequently exposed to antibiotics, particularly in regions where there are high burdens of disease. This can lead to the selection of antibiotic-resistant bacteria that can be transmitted and cause invasive disease. Children are the primary reservoir for these bacterial pathogens due to their immature immune systems (24, 33). Bacterial acquisition and carriage are highly variable and depend on age, geographic area, genetic background, and socioeconomic conditions (2, 4, 9, 32, 43). As healthy children age, their immunological responses mature. This results in an increase in the frequency of pneumococcal recolonization and colonization by different serotypes (new acquisitions), with a concomitant decrease in the duration of carriage (23). Furthermore, there is considerable interspecies competition during colonization with constant variation in the nasopharyngeal microbiota (16, 48, 49). The colonizing microbiota may also be altered by antibiotics (17). HIV infection causes a reduction in immunologic responsiveness and a decrease in the secretion of IgA that may affect nasopharyngeal colonization (17, 30). Studies in Africa (45) and in the United States (42) indicate that the increased burden of disease in pediatric HIV infection is not explained by higher rates of nasopharyngeal colonization with the bacterium.
Cotrimoxazole (trimethoprim-sulfamethoxazole [CoT]) prophylaxis in children and adults infected with HIV reduces significantly morbidity and mortality, even in regions with high resistance to CoT (7, 25, 38, 50). As a consequence, the World Health Organization (WHO) updated their guidelines for the care of HIV-infected patients in 2006, recommending prophylaxis for HIV-infected children, adolescents, and adults in resource-limited settings (52).
Studies undertaken in Zambia and other African countries have shown an increase over recent years in the proportion of resistant isolates to several antimicrobial agents (39). In particular, resistance to CoT has increased in S. pneumoniae and H. influenzae (26, 27, 51). However, these were mainly short-term studies.
In light of the recent WHO guidelines and the increasing threat of antibiotic resistance, we monitored over 2 years the impact of CoT prophylaxis on the carriage and resistance on S. pneumoniae and H. influenzae colonization in a double-blind study of a pediatric HIV-infected cohort and compared to children on placebo. This was a planned substudy of the Children with HIV Antibiotic Prophylaxis (CHAP) study (7).
MATERIALS AND METHODS
Study population.
Consent for the trial and for the nasopharyngeal carriage substudy was obtained from parents or care-givers prior to enrollment, and the study was approved by the Ethics Committees at University Teaching Hospital, Lusaka, Zambia (UTH), and University College London, London, England. A total of 534 eligible HIV-infected children between the ages of 6 months and 14 years, and living in suburbs around Lusaka, were recruited at UTH between 2001 and 2003 and randomized to prophylactic cotrimoxazole (n = 265), or placebo (n = 269) in the CHAP trial (51). Children were not vaccinated against pneumococci or H. influenzae type b, and very few children (4%) ever took antiretrovirals during the whole trial period with <1% of follow-up time spent on antiretrovirals. Children in the treatment arm under 5 years of age received a once-daily dose of 200 mg of sulfamethoxazole/40 mg of trimethoprim (5 ml) of CoT prophylaxis, or a matching placebo. Those over 5 years received a once-daily dose of 400 mg of sulfamethoxazole and 80 mg of trimethoprim (10 ml) CoT, or a matching placebo. The first patient was randomized on 4 March 2001. In October 2003, the trial was stopped prematurely on recommendation of the data and safety monitoring committee of the trial because of substantial benefit on the CoT arm. After this point, all children on placebo were switched to once-daily CoT prophylaxis, and children in both arms of the trial were then monitored for a further 12 months. The data after the switch were not included in this analysis. Starting on 7 February 2002, nasopharyngeal swabs were collected from all children at randomization and from any postbaseline scheduled visits occurring in children thereafter. All of these samples were included in this substudy.
Specimen collection and isolation.
For nasopharyngeal carriage, nasopharyngeal (nose) swabs were taken via the nostrils by using rayon swabs (Medical Wire and Equipment Limited, Wiltshire, United Kingdom) on thin flexible wires. The swabs were inserted into the nostrils and pushed all of the way to the back of the nose until resistance was met, withdrawn about 1 to 2 cm, and rotated to collect the specimen. The swab was withdrawn from the nostrils, and inserted into 3 ml of STGG transport medium (skim milk, tryptone soy broth, glucose, and glycerol), which was transported to the laboratory immediately.
Identification of S. pneumoniae and H. influenzae and antibiotic susceptibility were determined by routine methods. At each clinic visit at baseline and at 2, 4, 8, 12, 16, 24, and every 8 weeks thereafter, antibiotic use within the previous 2 weeks, information on current and recent illnesses and hospitalizations, the trial drug received, adherence, and clinical status was documented by study nurses or clinicians using standard clinic report forms. The percentage of CD4 (CD4%) was measured by standard flow cytometry every 24 weeks. Nose swabs were taken at baseline and at weeks 4, 8, 12, 16, and 24 and every 8 weeks thereafter. Specimens obtained after 24 October 2003 were not included in this analysis. Thus, all specimens included in this analysis were taken before the trial ended and the children in the placebo arm were switched to CoT. Rayon swabs on thin flexible wires (Medical Wire and Equipment, Ltd., Wiltshire, United Kingdom) were used and immediately transported to the laboratory in 1.5 ml of STGG transport and storage medium (6).
Upon receipt in the laboratory, the cryovials were vortexed for a few seconds to resuspend the organisms from the swab into STGG before inoculation. A portion (10 μl) of the swab suspension was inoculated onto 5% (vol/vol) blood agar plates made selective for S. pneumoniae by supplementation with 5 μg of gentamicin/ml and onto chocolate agar plates supplemented with 5 μg of vancomycin/ml for H. influenzae. The plates were incubated overnight at 37°C in 5% CO2. S. pneumoniae and H. influenzae were identified by using standard methods. Briefly, for pneumococci, samples were plated onto blood agar plates made selective by addition of 5 μg of gentamicin/ml. Presumptive identification was made from morphology (draftsman-like colonies) and α-hemolysis. Identification was confirmed by optichin sensitivity and/or bile salt solubility. For Haemophilus, identification was based on Gram staining, oxidase test, X factor (hemin), V factor (NAD), and combined X&V factor nutritional requirements.
Antimicrobial susceptibility testing.
Antimicrobial susceptibility tests for S. pneumoniae and H. influenzae were performed by the Kirby-Bauer disk diffusion method and interpreted according to CLSI criteria (53). Both S. pneumoniae and H. influenzae were tested against the following antimicrobial agents: cotrimoxazole (CoT), chloramphenicol, ampicillin, and tetracycline. S. pneumoniae was also tested against oxacillin (for penicillin) and erythromycin.
Statistical analysis.
Data were entered in real-time onto a database (Epi-info 6.04d; Centers for Disease Control and Prevention, Atlanta, GA; WHO, Geneva, Switzerland), using double data entry after validation and were sent each month to the MRC Clinical Trials Unit, London, United Kingdom. The data from all specimens collected before 24 October 2003 were included in the analysis, irrespective of whether the date of specimen coincided with a scheduled visit date.
Nasopharyngeal carriage and antibiotic resistance over time were analyzed by splitting the longitudinal patterns into pairs of specimens. For example, a child with four specimens taken during the trial will contribute three pairs to the analysis. Cause-specific hazards for subsequent test results being carriage versus no carriage were used to compare rates between placebo and CoT using a global test for difference between randomized arms across all possible combinations of carriage/no carriage pairs, and pairwise tests for each individual combination (e.g., carriage to no carriage). The Wilcoxon rank-sum test was used to test for differences in time between specimens in pairs between randomized groups. Due to limited resources, serotyping of pneumococci was not performed. Hence, new acquisitions by different serotypes were not detected. The same methods were used to analyze sensitive/resistant pairs and to compare randomized groups. Specimens with missing antibiotic resistance data were not included in the analysis of resistance.
To estimate the change in carriage over time mixed-effect logistic regression models were used that included the child as a random effect. Separate slopes and intercepts were included for placebo and CoT treatment arms. No random effect for time was included since this resulted in model instability.
Other factors were also included individually in the model to estimate any univariable effects on carriage. These factors were as follows: age of child at date of specimen, sex of child, CD4% within 7 days either side of date of specimen, change in CD4% from baseline, and calendar month at which specimen was taken. A χ2 test for independence was used to compare rates of resistance in postbaseline samples between treatment arms. Stata version 10.1 was used to perform these analyses.
RESULTS
Baseline characteristics.
A total of 534 children were randomized; from 439 of these (225 on the CoT arm and 214 on the placebo arm) nasal swabs were collected, and these were included in the present study. Baseline swabs were obtained from a total of 181 children (92 CoT and 89 placebo recipients) on or prior to randomization. The baseline characteristics are shown in Table 1. Baseline specimens were collected a median of 3 days (interquartile range [IQR], 10 to 0 days) prior to randomization. All baseline specimens were taken prior to the initiation of study treatment. At baseline, 93 (51%) children carried S. pneumoniae, 53 (29%) carried H. influenzae, and 37 (20%) carried both.
TABLE 1.
Characteristics of children with nose swabs taken at baseline
| Baseline characteristic | No. of children (%) |
||
|---|---|---|---|
| Placebo treated (n = 89) | CoT treated (n = 92) | Total (n = 181) | |
| Sex | |||
| Female | 39 (44) | 49 (53) | 88 (49) |
| Male | 50 (56) | 43 (47) | 93 (51) |
| Age (yr) at baseline specimen | |||
| <3 | 22 (25) | 30 (33) | 52 (29) |
| ≥3 | 67 (75) | 62 (67) | 129 (71) |
| Any antibiotics taken in last 2 wks | 20 (22) | 16 (17) | 36 (20) |
| S. pneumoniae positive | 49 (55) | 44 (48) | 93 (51) |
| H. influenzae positive | 27 (30) | 26 (28) | 53 (29) |
| Carriage of both | 20 (22) | 17 (18) | 37 (20) |
| Period (mo) that specimen was taken | |||
| Dec-Feb | 7 (8) | 11 (12) | 18 (10) |
| Mar-May | 19 (21) | 24 (26) | 43 (24) |
| Jun-Aug | 25 (28) | 21 (23) | 46 (25) |
| Sep-Nov | 38 (43) | 36 (39) | 74 (41) |
| S. pneumoniae resistance | |||
| Cotrimoxazole | 26/43 (60) | 22/38 (58) | 48/81 (59) |
| Penicillina | 14/48 (29) | 10/42 (24) | 24/90 (27) |
| Tetracycline | 4/49 (8) | 6/44 (14) | 10/93 (11) |
| Chloramphenicol | 4/49 (8) | 1/43 (2) | 5/92 (5) |
| Erythromycin | 0/49 (0) | 1/43 (2) | 1/92 (1) |
| H. influenzae resistance | |||
| Cotrimoxazole | 12/19 (63) | 11/22 (50) | 23/41 (56) |
| Ampicillin | 5/27 (19) | 6/25 (24) | 11/52 (21) |
| Tetracycline | 3/26 (12) | 6/25 (24) | 9/51 (18) |
| Chloramphenicol | 2/27 (7) | 4/26 (15) | 6/53 (11) |
| CD4%b | |||
| 0-4 | 18 (20) | 10 (11) | 28 (15) |
| 5-9 | 15 (17) | 22 (24) | 37 (20) |
| 10-14 | 18 (20) | 17 (18) | 35 (19) |
| 15-19 | 11 (12) | 11 (12) | 22 (15) |
| ≥20 | 9 (10) | 11 (12) | 20 (11) |
| Missing | 18 (20) | 21 (23) | 39 (22) |
Oxacillin was used to test for penicillin resistance.
CD4% is expressed as a percentage of the total lymphocyte count measured within 1 week before or after the date of the specimen.
Factors influencing nasopharyngeal carriage.
Specimens from children who had received antibiotics known to be active against S. pneumoniae or H. influenzae within the 2 weeks prior to specimen collection were less likely to be carrying these organisms. A total of 61% (104/170) of the nose swabs from children who had received antibiotics known to be active against S. pneumoniae within the 2 weeks prior to specimen collection were negative for S. pneumoniae compared to 45% (536/1,182) specimens from children who had not received such antibiotics within the 2 weeks prior to specimen collection (P < 0.001).
A total of 86% (148/173) of the nose swabs from children who had received antibiotics known to be active against H. influenzae within the 2 weeks prior to specimen collection were negative for H. influenzae compared to 72% (850/1,179) of the specimens from children who had not received such antibiotics within the 2 weeks prior to specimen collection (P < 0.001). Thus, the specimens from children who had received antibiotics known to be active against S. pneumoniae or H. influenzae within the 2 weeks prior to specimen collection were excluded from the analyses of carriage of S. pneumoniae or H. influenzae. There may be some under-reporting of antibiotic use, but we would expect this to be distributed approximately equally between placebo and CoT groups as a result of randomization.
S. pneumoniae.
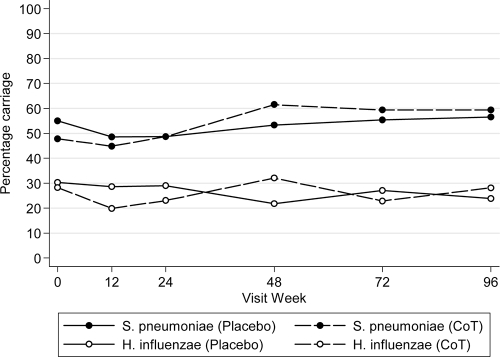
The level of carriage was similar in both arms of the trial (Fig. 1). There was no change in the carriage of S. pneumoniae over time in the placebo arm (odds ratio [OR] = 1.09 per 52 weeks, 95% confidence interval [CI] = 0.80 to 1.48). However, there was an apparent increase in carriage in the CoT arm, from 48% at baseline to 59% at week 96 (Fig. 1; OR =1.63 per 52 weeks, 95% CI = 1.23 to 2.16, P = 0.001). None of the criteria CD4%, change in CD4% from baseline, age of child at date of specimen, sex of child, or calendar month in which specimen was taken were associated with the carriage of S. pneumoniae (Table 2 ).
FIG. 1.
Percent carriage of S. pneumoniae and H. influenzae over time. Carriage in the placebo arm is indicated by a continuous line, and carriage in the CoT arm is indicated by a dashed line. The numbers of children at each visit for weeks 0, 12, 24, 48, 72, and 96 were and 89, 140, 148, 133, 74, and 46, respectively, for the placebo arm and 92, 136, 156, 156, 118, and 64, respectively, for the CoT arm.
TABLE 2.
ORs for nasopharyngeal carriage of S. pneumoniae and H. influenzae from mixed-effects logistic regression models with the child as a random effecta
| Characteristic | Carriage of S. pneumoniae |
Carriage of H. influenzae |
||
|---|---|---|---|---|
| OR (95% CI)c | P | OR (95% CI) | P | |
| Unadjusted ORs for probability of carriage over timeb | ||||
| CoT arm vs placebo arm at randomization | 0.78 (0.53-1.16) | 0.22 | 0.72 (0.46-1.13) | 0.16 |
| Change per 52 wks in placebo arm | 1.09 (0.80-1.48) | 0.58 | 0.82 (0.57-1.17) | 0.27 |
| Change per 52 wks in CoT arm | 1.63 (1.23-2.16) | 0.001 | 1.09 (0.80-1.50) | 0.59 |
| Test for comparison of change in carriage over time between CoT and placebo | 0.06 | 0.24 | ||
| Univariable ORs for other factors adjusted for time and treatment arm | ||||
| Per 10% higher CD4% at time of specimen | 0.96 (0.78-1.16)* | 0.65 | 1.14 (0.89-1.47) | 0.29 |
| Per 10% greater decrease CD4% from baseline at time of specimen | 0.79 (0.40-1.54)* | 0.49 | 0.97 (0.45-2.08) | 0.93 |
| Age (yr) of child at specimen | ||||
| ≤3 | 1.00 | 1.00 | ||
| >3 | 1.13 (0.83-1.55) | 0.44 | 1.28 (0.88-1.87) | 0.19 |
| Sex of child | ||||
| Female | 1.00 | 1.00 | ||
| Male | 0.87 (0.67-1.11) | 0.26 | 0.84 (0.63-1.12) | 0.24 |
| Period (mo) during which specimen was taken | ||||
| Dec-Feb | 1.00 | 1.00 | ||
| Mar-May | 1.17 (0.83-1.65) | 0.36 | 1.96 (1.30-2.98) | 0.001 |
| Jun-Aug | 1.42 (0.99-2.05) | 0.06 | 1.57 (1.01-2.45) | 0.05 |
| Sep-Nov | 1.12 (0.78-1.60) | 0.54 | 2.26 (1.48-3.48) | <0.001 |
All ORs are adjusted for time from randomization and treatment arm.
Changes in the probability of carriage over time estimated separately for placebo and CoT treatment arms, without adjustment for any other factors.
*, Estimated from fixed-effects logistic regression since mixed models estimating the effect of CD4% and CD4% change from the baseline on the carriage of S. pneumoniae were unstable.
H. influenzae.
There was no change in carriage of H. influenzae over time in either the placebo arm (OR = 0.82, 95% CI = 0.57 to 1.17) or the CoT arm (OR = 1.09, 95% CI = 0.80 to 1.50). Again, there was no association of CD4%, change in CD4% from baseline, age of child at date of specimen, or sex of child with the carriage of H. influenzae. Carriage was found to be less frequent in the rainy season (December to February) compared to other seasons (Table 2).
Longitudinal patterns of nasopharyngeal carriage.
Table 3 summarizes the longitudinal patterns of carriage of S. pneumoniae and H. influenzae during the trial by looking at consecutive pairs of specimens taken from an individual child during the trial. There was no statistical evidence for a difference in patterns of switching in carriage of S. pneumoniae between placebo and CoT overall (P = 0.18; Table 3), but there was a suggestion that the chance of remaining carriage free was higher in the placebo arm compared to the CoT arm (P = 0.10). Patterns of switching in carriage of H. influenzae (Table 3) differed between the CoT and placebo arms (P = 0.04), with higher rates of switching from carriage to no carriage observed in the placebo arm (P = 0.02).
TABLE 3.
Rate of carriage switching of S. pneumoniae and H. influenzae between two consecutive samples
| Sequence of test results | Trial arm | No. of occurrences | Pa | Total person-years from first to second tests | Rateb | P (placebo vs CoT)c |
|---|---|---|---|---|---|---|
| S. pneumoniae | ||||||
| No carriage→no carriage | Placebo | 77 | 0.53 | 52.0 | 1.48 | 0.10 |
| CoT | 94 | 0.47 | 77.9 | 1.21 | ||
| No carriage→carriage | Placebo | 68 | 0.47 | 52.0 | 1.31 | 0.61 |
| CoT | 108 | 0.53 | 77.9 | 1.39 | ||
| Carriage→no carriage | Placebo | 78 | 0.43 | 67.7 | 1.15 | 0.44 |
| CoT | 94 | 0.42 | 90.0 | 1.04 | ||
| Carriage→carriage | Placebo | 105 | 0.57 | 67.7 | 1.55 | 0.50 |
| CoT | 131 | 0.58 | 90.0 | 1.46 | ||
| H. influenzae | ||||||
| No carriage→no carriage | Placebo | 168 | 0.73 | 82.4 | 2.04 | 0.67 |
| CoT | 237 | 0.77 | 119.5 | 1.98 | ||
| No carriage→carriage | Placebo | 62 | 0.27 | 82.4 | 0.75 | 0.12 |
| CoT | 71 | 0.23 | 119.5 | 0.59 | ||
| Carriage→no carriage | Placebo | 71 | 0.73 | 36.8 | 1.93 | 0.02 |
| CoT | 71 | 0.60 | 47.2 | 1.51 | ||
| Carriage→carriage | Placebo | 26 | 0.27 | 36.8 | 0.71 | 0.11 |
| CoT | 47 | 0.40 | 47.2 | 1.00 |
That is, the probability of a subsequent test result being carriage versus no carriage conditional on the first test result.
The rate column normalizes probabilities to total person-time at risk between tests, which differs between groups because of higher survival rates associated with CoT treatment.
Calculated using cause-specific hazards for subsequent test result being carriage versus no carriage. A global test for differences between placebo and CoT across all four sequences for S. pneumoniae was performed (P = 0.18). A global test for differences between placebo and CoT across all four sequences for H. influenzae was performed (P = 0.04).
The median time from randomization to the last specimen was significantly greater in the CoT arm (56 months) than in the placebo arm (48 months) (P = 0.001 calculated using the Wilcoxon rank-sum test of difference between randomized groups in time from randomization to last specimen).
The number of pairs of specimens per person-year were similar in the placebo and CoT arms (P = 0.14), 2.74 and 2.54, respectively, for the S. pneumoniae analysis (with very similar figures for the H. influenzae analysis).
Longitudinal patterns of antibiotic resistance.
There were 591 postbaseline specimens with carriage of S. pneumoniae with results of resistance to CoT. Of the 330 specimens on the CoT arm, 288 (87%) had resistance to CoT, but only 190 specimens (72%) of the 261 on the placebo arm demonstrated resistance to CoT (P < 0.0001). There were 270 postbaseline specimens with carriage of H. influenzae with results of resistance to CoT. Of the 140 specimens on the CoT arm, 78 (56%) showed resistance to CoT, but only 50 (38%) specimens of the 130 on the placebo arm showed resistance to CoT (P = 0.005).
Table 4 summarizes the longitudinal patterns of resistance to CoT of S. pneumoniae and H. influenzae during the trial by looking at consecutive pairs of specimens taken from an individual child during the trial. Resistance to other antibiotics was considered too infrequent for any formal analysis. Patterns of switching of resistance of S. pneumoniae to CoT (Table 4) differed between the CoT and placebo arms (P = 0.005) with a 3-fold-higher rate of loss of CoT resistance in the placebo arm. There was no statistical evidence for a difference in patterns of switching of resistance of H. influenzae to CoT (P = 0.29; Table 4), but there was a suggestion that the chance of remaining CoT sensitive was higher in the placebo arm.
TABLE 4.
Rate of switching of resistance of S. pneumoniae and H. influenzae to CoT between two consecutive samplesa
| Sequence of test results | Trial arm | No. of occurrences | Pb | Total person-years from first to second tests | Ratec | P (placebo vs CoT)d |
|---|---|---|---|---|---|---|
| S. pneumoniae | ||||||
| Sensitive→sensitive | Placebo | 9 | 0.21 | 25.2 | 0.36 | 0.85 |
| CoT | 6 | 0.19 | 15.3 | 0.39 | ||
| Sensitive→resistant | Placebo | 33 | 0.79 | 25.2 | 1.31 | 0.18 |
| CoT | 25 | 0.81 | 15.3 | 1.63 | ||
| Resistant→sensitive | Placebo | 18 | 0.23 | 30.7 | 0.59 | 0.002 |
| CoT | 12 | 0.10 | 61.9 | 0.19 | ||
| Resistant→resistant | Placebo | 59 | 0.77 | 30.7 | 1.92 | 0.67 |
| CoT | 114 | 0.90 | 61.9 | 1.84 | ||
| H. influenzae | ||||||
| Sensitive→sensitive | Placebo | 11 | 0.61 | 9.3 | 1.19 | 0.05 |
| CoT | 7 | 0.28 | 12.1 | 0.58 | ||
| Sensitive→resistant | Placebo | 7 | 0.39 | 9.3 | 0.75 | 0.11 |
| CoT | 18 | 0.72 | 12.1 | 1.48 | ||
| Resistant→sensitive | Placebo | 6 | 0.46 | 7.1 | 0.84 | 0.63 |
| CoT | 9 | 0.39 | 13.4 | 0.67 | ||
| Resistant→resistant | Placebo | 7 | 0.54 | 7.1 | 0.98 | 0.86 |
| CoT | 14 | 0.61 | 13.4 | 1.04 |
Specimens with missing resistance results were excluded.
That is, the probability of a subsequent test result being resistant versus sensitive conditional on the first test result.
The rate column normalizes probabilities to total person-time at risk between tests, which differs between groups because of higher survival rates associated with CoT treatment.
Calculated using cause-specific hazards for subsequent test result being resistance versus sensitive. A global test for differences between placebo and CoT across all four sequences for S. pneumoniae was performed (P = 0.005). A global test for differences between placebo and CoT across all four sequences for H. influenzae was performed (P = 0.29).
The numbers of pairs of specimens per 100 person-years were similar in the placebo and CoT arms: 2.13 and 2.03, respectively, for the resistance of S. pneumoniae analysis (P = 0.46) and 1.89 and 1.88, respectively, for the resistance of H. influenzae (P = 0.99).
DISCUSSION
To our knowledge this is the first study to measure the nasopharyngeal carriage and antibiotic resistance of two important pathogens, S. pneumoniae and H. influenzae, in HIV-infected children on CoT prophylaxis versus placebo treatment over 2 years. The present study revealed that the pneumococcal level of carriage is high (51% at baseline) in agreement with other studies undertaken in Africa. Once-daily CoT prophylaxis increased the risk of carrying pneumococci (OR = 1.63 per 52 weeks, 95% CI = 1.23 to 2.16, P = 0.001). CoT resistance levels were higher in postbaseline samples in the CoT arm than in the placebo arm (P < 0.0001 for S. pneumoniae and P = 0.005 for H. influenzae), and CoT decreased the rate of switching from CoT-resistant to CoT-sensitive S. pneumoniae (P = 0.002); moreover, there was also a suggestion with H. influenzae that the chance of remaining sensitive was lower in the CoT arm (P = 0.05). These findings did not alter the ability of CoT prophylaxis to reduce morbidity and mortality in HIV-infected children. There were no associations observed between CD4 levels and the carriage of either S. pneumoniae or H. influenzae.
Nasopharyngeal carriage.
The pneumococcal carriage rates observed in the present study were similar to (8, 28) or lower than (27, 51) the findings in other African studies. The higher carriage levels observed in the latter two studies may be due to the younger median age of the children (1.7 years) compared to the present study of 5.1 years (IQR = 2.8 to 8.7 years) at baseline.
A gradual rise in the pneumococcal carriage rate was observed in the CoT prophylaxis arm, and a similar trend was seen in the placebo arm, although not statistically significant. This may have been due to an improvement in sampling efficiency over time.
Children on the CoT arm were more likely to maintain carriage of H. influenzae compared to placebo (P = 0.02). There was also a suggestion that the chance of remaining free of S. pneumoniae carriage was higher in the placebo arm than the CoT arm (P = 0.10). An increasing number of new acquisitions have been observed in healthy children as they get older and acquire immunity to an increasing number of serotypes (23). Paradoxically, by 48 weeks the children in the treatment arm have a slightly higher level of pneumococcal carriage compared to children in the placebo arm. This maybe due to a difference in behavior such that the healthier children on CoT prophylaxis are more likely to attend school or community centers and play together and thus increase the level of transmission and therefore carriage.
Antibiotic resistance.
At baseline, 59% of the colonizing S. pneumoniae specimens and 56% of the colonizing H. influenzae specimens were resistant to CoT. A study on pediatric population of children (who were not necessarily HIV infected) performed in 1994 at the same hospital as our study found a lower carriage rate of CoT-resistant pneumococci (13%) (51). However, one cannot exclude the possibility that this difference is due to differences in methodology. S. pneumoniae nonsusceptibility to penicillin was lower (14%) in 1994 compared to our data (27%). This emphasizes the pressing threat of antibiotic resistance. In contrast, tetracycline resistance levels of S. pneumoniae were lower in this (11% at baseline) compared to previous studies (23%) (51). This observation is consistent with a reduction of tetracycline prescriptions in children from 1996 (James C. L. Mwansa, unpublished data). In agreement with other studies, a low level of resistance to chloramphenicol was observed (51).
The high levels of CoT-resistant microorganisms carried in the nasopharynx, whereas CoT prophylaxis remains efficacious, suggests that the relationship between carriage and invasive organisms is not straightforward (7, 21, 38, 52). Studies from other developing countries indicate that most of the mortality among children is due to respiratory tract infections with S. pneumoniae, and this is likely to also be true in the present study (21, 36). Some invasive pneumococcal serotypes, for example, serotype 1, are difficult to capture and are probably carried for short durations. This would limit their exposure to antibiotics, and hence the acquisition of resistance is reduced despite the relatively high rate of CoT resistance observed in other serotypes that are frequently carried (46). Further studies are required to resolve this interesting paradox by comparing invasive and carriage isolates at both the serotype and the genotype level.
Other studies on the effects of CoT prophylaxis on pneumococcal carriage and CoT resistance have shown variable outcomes. A study in Malawi reported an increase in the carriage of CoT-resistant pneumococci by the end of the first week that returned to baseline levels by the end of the first month (12). In another study conducted in America, no difference was reported in either the pneumococcal carriage rate or the level of CoT-resistant pneumococci (42). A further study in America showed that although there was no effect on pneumococcal carriage, higher levels of resistance to penicillin and CoT were observed (1). A more recent study in Zambia indicated that CoT prophylaxis reduced colonization by ca. 7% and increased the risk of colonization with CoT-resistant pneumococci within 6 weeks of starting prophylaxis (20). There are few data on the effects of CoT prophylaxis on the nasopharyngeal carriage of H. influenzae.
CD4 level.
The immune status of the host is also an important variable in the carriage and therefore risk of invasive disease (14, 16). S. pneumoniae is a frequent and serious infection in HIV-1-infected adults in Africa (18, 19), North America (47), and Europe (13). In our study on HIV-infected children, no association was found between CD4 levels and the carriage of both S. pneumoniae and H. influenzae. This observation was similar to a study of 175 HIV-infected adult males vaccinated with a 23-valent pneumococcal vaccine and consequently with a low carriage rate (∼3%); the authors of that study found no association between CD4 levels and pneumococcal carriage (41). A similar observation was described by Nicoletti et al., who found that neither the HIV viral load nor the CD4 level was significantly associated with pneumococcal carriage (40). However, extended use of the same antiretroviral regimen for a year or more was associated with a lower risk of colonization, suggesting that prolonged use of highly effective antiretroviral therapy lowers pneumococcal carriage and may lower the risk of infection. In contrast, Rodriguez-Barradas et al. reported that HIV-infected unvaccinated men were more likely to be colonized with pneumococci than were HIV-uninfected controls (44).
Limitations.
Although the absence of detected pathogens may not reflect a true loss of carriage, both arms of the study were treated identically. Since one of the primary aims of the present study was to assess the impact of CoT on the level of carriage and not serotype distribution, expensive and labor-intensive serotyping could not be justified. The absence of pneumococcal serotyping meant that new acquisitions by different serotypes were not detected, and thus sequential colonization was recorded.
In conclusion, our data indicate that the pneumococcal level of carriage and resistance to CoT observed here are high and in agreement with the findings of other studies undertaken in Africa. CoT prophylaxis increased the risk of carrying pneumococci and the frequency of pneumococcal switching from no carriage to carriage. CoT resistance levels were higher in postbaseline samples in the CoT arm than in the placebo arm, and CoT prophylaxis reduced the rate of switching from CoT-resistant to CoT-sensitive organisms. Critically, these findings did not impact on the efficacy of CoT prophylaxis to reduce morbidity and mortality in HIV-infected children. In addition, in our cohort of HIV-infected children there was no association between CD4 levels and the carriage of either S. pneumoniae or H. influenzae.
Footnotes
Published ahead of print on 28 June 2010.
REFERENCES
- 1.Abdel-Haq, N., W. Abuhammour, B. Asmar, R. Thomas, S. Dabbagh, and R. Gonzalez. 1999. Nasopharyngeal colonization with Streptococcus pneumoniae in children receiving trimethoprim-sulfamethoxazole prophylaxis. Pediatr. Infect. Dis. J. 18:647-649. [DOI] [PubMed] [Google Scholar]
- 2.Bakir, M., A. Yagci, N. Ulger, C. Akbenlioglu, A. Ilki, and G. Soyletir. 2001. Asymptomatic carriage of Neisseria meningitidis and Neisseria lactamica in relation to Streptococcus pneumoniae and Haemophilus influenzae colonization in healthy children: apropos of 1,400 children sampled. Eur. J. Epidemiol. 17:1015-1018. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert, D., R. de Groot, and P. Hermans. 2004. Streptococcus pneumoniae colonization: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 4.Bogaert, D., M. N. Engelen, A. J. Timmers-Reker, et al. 2001. Pneumococcal carriage in children in The Netherlands: a molecular epidemiological study. J. Clin. Microbiol. 39:3316-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos, J., M. Hernando, F. Roman, et al. 2004. Analysis of invasive Haemophilus influenzae infections after extensive vaccination against H. influenzae type b. J. Clin. Microbiol. 42:524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charalambous, B. M., S. L. Batt, A. C. Peek, H. Mwerinde, N. Sam, and S. H. Gillespie. 2003. Quantitative validation of media for the transportation and storage of Streptococcus pneumoniae. J. Clin. Microbiol. 41:5551-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chintu, C., G. J. Bhat, A. S. Walker, et al. 2004. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet 364:1865-1871. [DOI] [PubMed] [Google Scholar]
- 8.Denno, D. M., E. Frimpong, M. Gregory, and R. W. Steele. 2002. Nasopharyngeal carriage and susceptibility patterns of Streptococcus pneumoniae in Kumasi, Ghana. West Afr. J. Med. 21:233-236. [PubMed] [Google Scholar]
- 9.El Ahmer, O. R., S. D. Essery, A. T. Saadi, et al. 1999. The effect of cigarette smoke on adherence of respiratory pathogens to buccal epithelial cells. FEMS Immunol. Med. Microbiol. 23:27-36. [DOI] [PubMed] [Google Scholar]
- 10.Faden, H., L. Duffy, R. Wasielewski, J. Wolf, D. Krystofik, and Y. Tung.1997. Relationship between nasopharyngeal colonization and the development of otitis media in children: Tonawanda/Williamsville Pediatrics. J. Infect. Dis. 175:1440-1445. [DOI] [PubMed] [Google Scholar]
- 11.Farley, J. J., J. C. King, Jr., P. Nair, S. E. Hines, R. L. Tressler, and P. E. Vink. 1994. Invasive pneumococcal disease among infected and uninfected children of mothers with human immunodeficiency virus infection. J. Pediatr. 124:853-858. [DOI] [PubMed] [Google Scholar]
- 12.Feikin, D. R., S. F. Dowell, O. C. Nwanyanwu, et al. 2000. Increased carriage of trimethoprim/sulfamethoxazole-resistant Streptococcus pneumoniae in Malawian children after treatment for malaria with sulfadoxine/ pyrimethamine. J. Infect. Dis. 181:1501-1505. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Leoni, M. E., S. Moreno, P. Rodeno, E. Cercenado, T. Vicente, and E. Bouza. 1992. Pneumococcal pneumonia in adult hospitalized patients infected with the human immunodeficiency virus. Arch. Intern. Med. 152:1808-1812. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Rodriguez, J. A., and M. J. Fresnadillo-Martinez. 2002. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J. Antimicrob. Chemother. 50:59-74. [DOI] [PubMed] [Google Scholar]
- 15.Gesner, M., D. Desiderio, M. Kim, et al. 1994. Streptococcus pneumoniae in human immunodeficiency virus type 1-infected children. Pediatr. Infect. Dis. J. 13:697-703. [DOI] [PubMed] [Google Scholar]
- 16.Ghaffar, F., I. R. Friedland, and G. H. McCracken, Jr. 1999. Dynamics of nasopharyngeal colonization by Streptococcus pneumoniae. Pediatr. Infect. Dis. J. 18:638-646. [DOI] [PubMed] [Google Scholar]
- 17.Ghaffar, F., L. S. Muniz, K. Katz, et al. 2002. Effects of large dosages of amoxicillin/clavulanate or azithromycin on nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, nonpneumococcal alpha- hemolytic streptococci, and Staphylococcus aureus in children with acute otitis media. Clin. Infect. Dis. 34:1301-1309. [DOI] [PubMed] [Google Scholar]
- 18.Gilks, C. F., R. J. Brindle, L. S. Otieno, et al. 1990. Life-threatening bacteraemia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet 336:545-549. [DOI] [PubMed] [Google Scholar]
- 19.Gilks, C. F., S. A. Ojoo, J. C. Ojoo, et al. 1996. Invasive pneumococcal disease in a cohort of predominantly HIV-1-infected female sex-workers in Nairobi, Kenya. Lancet 347:718-723. [DOI] [PubMed] [Google Scholar]
- 20.Gill, C. J., V. Mwanakasale, M. P. Fox, et al. 2008. Effect of presumptive co-trimoxazole prophylaxis on pneumococcal colonization rates, seroepidemiology and antibiotic resistance in Zambian infants: a longitudinal cohort study. Bull. World Health Organ. 86:929-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon, S. B., M. Chaponda, A. L. Walsh, et al. 2002. Pneumococcal disease in HIV-infected Malawian adults: acute mortality and long-term survival. AIDS 16:1409-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray, B. M., G. M. Converse III, and H. C. Dillon. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 23.Gray, B. M., M. E. Turner, and H. C. Dillon. 1982. Epidemiologic studies of Streptococcus pneumoniae in infants: the effects of season and age on pneumococcal acquisition and carriage in the first 24 months of life. Am. J. Epidemiol. 116:692-703. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg, D., A. Broides, I. Blancovich, N. Peled, N. Givon-Lavi, and R. Dagan. 2004. Relative importance of nasopharyngeal versus oropharyngeal sampling for isolation of Streptococcus pneumoniae and Haemophilus influenzae from healthy and sick individuals varies with age. J. Clin. Microbiol. 42:4604-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimwade, K., A. W. Sturm, A. J. Nunn, D. Mbatha, D. Zungu, and C. F. Gilks. 2005. Effectiveness of cotrimoxazole prophylaxis on mortality in adults with tuberculosis in rural South Africa. AIDS 19:163-168. [DOI] [PubMed] [Google Scholar]
- 26.Gwanzura, L., C. Pasi, K. J. Nathoo, et al. 2003. Rapid emergence of resistance to penicillin and trimethoprim-sulphamethoxazole in invasive Streptococcus pneumoniae in Zimbabwe. Int. J. Antimicrob. Agents 21:557-561. [DOI] [PubMed] [Google Scholar]
- 27.Huebner, R. E., A. Wasas, A. Mushi, L. Mazhani, and K. Klugman. 1998. Nasopharyngeal carriage and antimicrobial resistance in isolates of Streptococcus pneumoniae and Haemophilus influenzae type b in children under 5 years of age in Botswana. Int. J. Infect. Dis. 3:18-25. [DOI] [PubMed] [Google Scholar]
- 28.Huebner, R. E., A. D. Wasas, and K. P. Klugman. 2000. Prevalence of nasopharyngeal antibiotic-resistant pneumococcal carriage in children attending private paediatric practices in Johannesburg. S. Afr. Med. J. 90:1116-1121. [PubMed] [Google Scholar]
- 29.Jacobs, M. R. 2004. Streptococcus pneumoniae: epidemiology and patterns of resistance. Am. J. Med. 117:3S-15S. [DOI] [PubMed] [Google Scholar]
- 30.Janoff, E. N., R. F. Breiman, C. L. Daley, and P. C. Hopewell. 1992. Pneumococcal disease during HIV infection: epidemiologic, clinical, and immunologic perspectives. Ann. Intern. Med. 117:314-324. [DOI] [PubMed] [Google Scholar]
- 31.Jordano, Q., V. Falco, B. Almirante, et al. 2004. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 38:1623-1628. [DOI] [PubMed] [Google Scholar]
- 32.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leiberman, A., R. Dagan, E. Leibovitz, P. Yagupsky, and D. M. Fliss. 1999. The bacteriology of the nasopharynx in childhood. Int. J. Pediatr. Otorhinolaryngol. 49(Suppl. 1):S151-S153. [DOI] [PubMed] [Google Scholar]
- 34.Madhi, S. A., K. Petersen, A. Madhi, M. Khoosal, and K. P. Klugman. 2000. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin. Infect. Dis. 31:170-176. [DOI] [PubMed] [Google Scholar]
- 35.Madhi, S. A., K. Petersen, A. Madhi, A. Wasas, and K. P. Klugman. 2000. Impact of human immunodeficiency virus type 1 on the disease spectrum of Streptococcus pneumoniae in South African children. Pediatr. Infect. Dis. J. 19:1141-1147. [DOI] [PubMed] [Google Scholar]
- 36.Madhi, S. A., B. Schoub, K. Simmank, N. Blackburn, and K. P. Klugman. 2000. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J. Pediatr. 137:78-84. [DOI] [PubMed] [Google Scholar]
- 37.Mao, C., M. Harper, K. McIntosh, et al. 1996. Invasive pneumococcal infections in human immunodeficiency virus-infected children. J. Infect. Dis. 173:870-876. [DOI] [PubMed] [Google Scholar]
- 38.Mermin, J., J. Lule, J. P. Ekwaru, et al. 2004. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4 cell count, and viral load in HIV infection in rural Uganda. Lancet 364:1428-1434. [DOI] [PubMed] [Google Scholar]
- 39.Mwansa, J., K. Mutela, I. Zulu, B. Amadi, and P. Kelly. 2002. Antimicrobial sensitivity in enterobacteria from AIDS patients, Zambia. Emerg. Infect. Dis. 8:92-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicoletti, C., M. C. Brandileone, M. L. Guerra, and A. S. Levin. 2007. Prevalence, serotypes, and risk factors for pneumococcal carriage among HIV-infected adults. Diagn. Microbiol. Infect. Dis. 57:259-265. [DOI] [PubMed] [Google Scholar]
- 41.Onwubiko, C., E. Swiatlo, and L. S. McDaniel. 2008. Cross-sectional study of nasopharyngeal carriage of Streptococcus pneumoniae in human immunodeficiency virus-infected adults in the conjugate vaccine era. J. Clin. Microbiol. 46:3621-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polack, F. P., D. C. Flayhart, M. L. Zahurak, J. D. Dick, and R. E. Willoughby. 2000. Colonization by Streptococcus pneumoniae in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 19:608-612. [DOI] [PubMed] [Google Scholar]
- 43.Principi, N., P. Marchisio, G. C. Schito, and S. Mannelli. 1999. Risk factors for carriage of respiratory pathogens in the nasopharynx of healthy children. Ascanius Project Collaborative Group. Pediatr. Infect. Dis. J. 18:517-523. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Barradas, M. C., R. A. Tharapel, J. E. Groover, et al. 1997. Colonization by Streptococcus pneumoniae among human immunodeficiency virus-infected adults: prevalence of antibiotic resistance, impact of immunization, and characterization by polymerase chain reaction with BOX primers of isolates from persistent S. pneumoniae carriers. J. Infect. Dis. 175:590-597. [DOI] [PubMed] [Google Scholar]
- 45.Rusen, I. D., L. Fraser-Roberts, L. Slaney, et al. 1997. Nasopharyngeal pneumococcal colonization among Kenyan children: antibiotic resistance, strain types and associations with human immunodeficiency virus type 1 infection. Pediatr. Infect. Dis. J. 16:656-662. [DOI] [PubMed] [Google Scholar]
- 46.Scott, J. A., A. J. Hall, A. Hannington, et al. 1998. Serotype distribution and prevalence of resistance to benzylpenicillin in three representative populations of Streptococcus pneumoniae isolates from the coast of Kenya. Clin. Infect. Dis. 27:1442-1450. [DOI] [PubMed] [Google Scholar]
- 47.Simberkoff, M. S., W. El Sadr, G. Schiffman, and J. J. Rahal, Jr. 1984. Streptococcus pneumoniae infections and bacteremia in patients with acquired immune deficiency syndrome, with report of a pneumococcal vaccine failure. Am. Rev. Respir. Dis. 130:1174-1176. [DOI] [PubMed] [Google Scholar]
- 48.Tano, K., E. Grahn-Hakansson, S. E. Holm, and S. Hellstrom. 2000. Inhibition of OM pathogens by alpha-hemolytic streptococci from healthy children, children with SOM and children with rAOM. Int. J. Pediatr. Otorhinolaryngol. 56:185-190. [DOI] [PubMed] [Google Scholar]
- 49.Uehara, Y., K. Kikuchi, T. Nakamura, et al. 2001. Inhibition of methicillin-resistant Staphylococcus aureus colonization of oral cavities in newborns by viridans group streptococci. Clin. Infect. Dis. 32:1399-1407. [DOI] [PubMed] [Google Scholar]
- 50.Walker, A. S., V. Mulenga, D. Ford, et al. 2007. The impact of daily cotrimoxazole prophylaxis and antiretroviral therapy on mortality and hospital admissions in HIV-infected Zambian children. Clin. Infect. Dis. 44:1361-1367. [DOI] [PubMed] [Google Scholar]
- 51.Woolfson, A., R. Huebner, A. Wasas, S. Chola, P. Godfrey-Faussett, and K. Klugman. 1997. Nasopharyngeal carriage of community-acquired, antibiotic-resistant Streptococcus pneumoniae in a Zambian paediatric population. Bull. World Health Organ. 75:453-462. [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization. 2006. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents, and adults in resource-limited settings: recommendations for a public health approach. World Health Organization, Geneva, Switzerland.
- 53.World Health Organization. 2003. Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world. WHO/CDS/CSR/RMD/2003.6. World Health Organization, Geneva, Switzerland.
- 54.Zar, H. J., P. Apolles, A. Argent, et al. 2001. The etiology and outcome of pneumonia in human immunodeficiency virus-infected children admitted to intensive care in a developing country. Pediatr. Crit. Care Med. 2:108-112. [DOI] [PubMed] [Google Scholar]