Abstract
T-2307, a novel arylamidine, has been shown to exhibit broad-spectrum in vitro and in vivo antifungal activities against clinically significant pathogens. In our preliminary studies, Candida glabrata exhibited significant trailing growth (partial inhibition of growth over an extended range of antifungal concentrations) in the presence of T-2307 when it was tested using the Clinical and Laboratory Standards Institute (CLSI) guidelines with 0.2% glucose and 48 h of incubation, making reading of the MIC difficult. In the present study, we attempted to attenuate trailing growth to avoid misreading of the MIC. On the basis of the hypothesis that T-2307 may inhibit the mitochondrial functions of cells, the carbon source or the glucose concentration in the medium was changed. The trailing growth of C. glabrata ATCC 90030 in the presence of T-2307 was attenuated as the concentration of glucose in the medium decreased to 0.1% or lower, and trailing growth was completely inhibited when glycerol was used. A susceptibility test using Alamar blue was performed to facilitate reading of the MIC without changing the composition of the medium and provided a clear MIC endpoint at 24 h. To investigate if T-2307 shows efficacy against trailing isolates in vivo, we evaluated the efficacy of T-2307 in a murine model of disseminated candidiasis caused by C. glabrata. T-2307 at 0.05 mg/kg of body weight/day significantly decreased the viable count in the kidneys compared to that for the control group (P < 0.05). It would be better to test the susceptibility of C. glabrata to T-2307 using modified media or Alamar blue to avoid misreading of the MIC due to the significant trailing growth.
Invasive mycoses are serious life-threatening infections for immunocompromised patients. Fungal infections are primarily treated with azole derivatives, candin derivatives, or amphotericin B. Azole antifungal agents, such as fluconazole, itraconazole, and voriconazole, are now widely used for the treatment of fungal infections due to their broad-spectrum activities and improved safety profiles. However, the increased prevalence of Candida glabrata isolates that exhibit reduced susceptibility to triazole antifungals and Candida krusei isolates that have developed intrinsic resistance to fluconazole and itraconazole has heightened concerns regarding the empirical use of triazole-based drugs, especially in the case of patients at risk of systemic invasion (1, 8, 14, 22, 23, 24).
We have previously shown that T-2307 (Fig. 1), a novel arylamidine, exhibits broad-spectrum in vitro and in vivo activities against clinically significant pathogens, including Candida species, Cryptococcus neoformans, and Aspergillus species (13). In our preliminary studies, C. glabrata exhibited significant trailing growth (partial inhibition of growth over an extended range of antifungal concentrations) in the presence of T-2307 when it was tested according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI; formerly the National Committee for Clinical Laboratory Standards) (4), making reading of the MIC difficult. Isolates with trailing growth in the presence of azoles appear to be susceptible after 24 h but resistant at 48 h; and testing by other methods (including changes in pH or temperature), animal models, and clinical experience also revealed trailing isolates to be susceptible (3, 12, 16, 17). The reference method recommends reading of the MIC at 24 h for trailing isolates (4). In the present study, we attempted to attenuate trailing growth to avoid misreading of the MIC, and we investigated in vivo activity of T-2307 against C. glabrata.
FIG. 1.
Chemical structure of T-2307.
(This study was presented at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 27 to 30 September 2006.)
MATERIALS AND METHODS
Antifungal agents.
T-2307 was synthesized by Toyama Chemical Co., Ltd. (Toyama, Japan). Fluconazole solution for injection (2 mg/ml) was provided by Toyama Chemical Co., Ltd. Micafungin and amphotericin B were commercially obtained from Astellas Pharma Inc. (Tokyo, Japan) and Bristol-Myers KK (Tokyo, Japan), respectively.
Organisms.
C. glabrata ATCC 90030 was obtained from the American Type Culture Collection (ATCC).
Antifungal susceptibility testing.
The in vitro susceptibility of C. glabrata ATCC 90030 was determined by the broth microdilution method in accordance with CLSI guideline M27-A3 (4). RPMI 1640 medium (Sigma-Aldrich Co., St. Louis, MO) was buffered to pH 7.0 with 0.165 M 3-(N-morpholino)propanesulfonic acid (MOPS) buffer (Dojindo Laboratories, Kumamoto, Japan). A stock inoculum suspension was obtained from a 24-h-old culture grown on a Sabouraud dextrose agar (SDA; Eiken Chemical Co., Ltd., Tokyo, Japan) plate at 35°C. The final inoculum concentration was 1 × 103 cells/ml. The MICs for T-2307, fluconazole, and micafungin were defined as the lowest concentration in which a prominent decrease in turbidity was observed; and the MIC for amphotericin B was defined as the lowest concentration in which no visible growth was observed. Percent inhibition was calculated on the basis of the growth in the control well after the optical density at 600 nm was measured using a microplate spectrophotometer to yield an inhibition curve. In an experiment where the carbon source was modified, RPMI 1640 medium without glucose was used; and it was supplemented with glucose or glycerol to yield final concentrations of 2%, 0.2% (equivalent to the concentration in the CLSI guidelines), 0.1%, 0.05%, and 0.02% glucose or 0.2% glycerol. The MICs were read after 48 h for all agents except micafungin; the MICs of micafungin were read after 24 h. The MIC of micafungin in the presence of 0.2% glycerol was read after 48 h because the level of growth was not enough at 24 h. In a colorimetric assay, 10% Alamar blue (Trek Diagnostic Systems, Inc., Westlake, OH) was added to RPMI 1640 medium (with 0.2% glucose and without phenol red). The MICs were read after 24 h and 48 h of incubation. The colorimetric MICs for T-2307, fluconazole, and micafungin were defined as the lowest concentration in which the color either changed from blue to purple or remained blue; the MIC for amphotericin B was defined as the lowest concentration in which the color remained blue (6).
In vivo study.
Four-week-old male specific-pathogen-free (SPF) ICR-strain mice (Japan SLC Inc., Shizuoka, Japan) were used for the in vivo study. The mice were provided with food and water ad libitum. All the experimental procedures with animals were conducted at Toyama Chemical Co., Ltd., in accordance with the guidelines for the care and use of laboratory animals. C. glabrata ATCC 90030 cells obtained from cultures grown overnight on SDA plates at 35°C were suspended in sterile saline. Disseminated candidiasis was induced in neutropenic mice by the intravenous inoculation of 0.2 ml of the cell suspension via the lateral tail vein. The immunosuppressive conditions were in accordance with those used in the method described by Ikeda et al., with slight modifications (7). Briefly, transient immunosuppression was induced by intraperitoneal treatment with 200 mg/kg of body weight cyclophosphamide (CY; Shionogi & Co., Ltd., Osaka, Japan) 3 days before the infection and with 100 mg/kg CY 1 day after the infection. The challenge dose was 7.68 × 106 CFU/mouse. Each group comprised five mice, and sterile saline was administered to the control mice. T-2307 and the reference agents were administered subcutaneously once a day for 8 days, beginning at 2 h after the infection. On day 8 postinfection, the kidneys of the euthanized mice were extirpated and homogenized in 2 ml saline using a 5-ml glass homogenizer. The homogenate was diluted 100- and 10,000-fold with saline, and 100 μl of each homogenate were spread on SDA plates containing 0.1 mg/ml of imipenem (Banyu Pharmaceutical Co., Ltd., Tokyo, Japan). After incubation at 35°C for 5 days, the colonies were counted, and the viable count in the kidneys (log number of CFU/kidneys) was calculated. The viable counts in the kidneys of the drug-treated groups and that of the control group were compared by the parametric Dunnett multiple-comparison test using SAS analytical software, release 8.2 (SAS Institute Japan Ltd., Tokyo, Japan). P values of <0.05 (two-tailed) were considered significant.
RESULTS
Antifungal susceptibility testing.
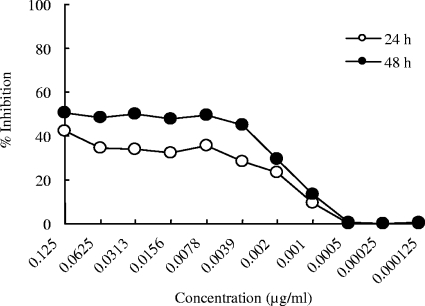
Figure 2 shows the inhibitory activity, which was determined spectrophotometrically, of T-2307 against C. glabrata ATCC 90030 at 24 h and 48 h of incubation. At 48 h, the standard incubation time in the CLSI guidelines (4), C. glabrata exhibited significant trailing growth in the presence of T-2307 at concentrations between 0.0039 and 0.125 μg/ml. The trailing growth of C. glabrata in the presence of T-2307 at 24 h of incubation was similar to that at 48 h.
FIG. 2.
Inhibitory effect of T-2307 against C. glabrata ATCC 90030 at 24 h and 48 h of incubation. The susceptibility of C. glabrata to T-2307 was tested in RPMI 1640 medium containing 0.2% glucose. Percent inhibition was calculated on the basis of the growth in the control well after the optical density at 600 nm was measured using a microplate spectrophotometer.
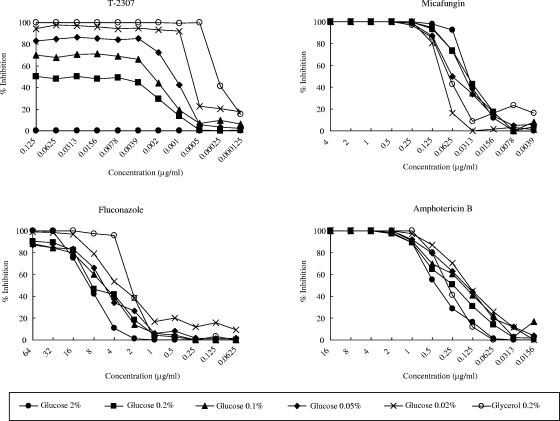
Figure 3 shows the effects of the glucose concentration and carbon source in the medium on the MIC determination, and Table 1 summarizes the MICs. The trailing growth of C. glabrata in the presence of T-2307 was attenuated as the concentration of glucose in the medium decreased to 0.1% or lower, and trailing growth was completely inhibited when glycerol was used. Similar behavior was observed for fluconazole. The inhibitory behavior of micafungin and amphotericin B was not affected by the glucose concentration or the carbon source. The optical densities at 600 nm of the growth control wells, measured using a microplate spectrophotometer, at 24 h/48 h were 0.65/0.73, 0.29/0.30, 0.17/0.19, 0.11/0.16, and 0.07/0.15 in the presence of 2%, 0.2%, 0.1%, 0.05%, and 0.02% glucose, respectively. The optical density of the growth control wells in the presence of 0.2% glycerol was 0.11 at 48 h. These values indicate that the turbidity in the growth control wells was enough for visual reading of the MIC.
FIG. 3.
Inhibitory effects of T-2307 and other agents against C. glabrata ATCC 90030 in modified media. The susceptibility of C. glabrata to T-2307 and the other agents was tested in RPMI 1640 medium containing 2%, 0.2%, 0.1%, 0.05%, and 0.02% glucose or 0.2% glycerol. Percent inhibition was calculated on the basis of the growth in the control well after the optical density at 600 nm was measured using a microplate spectrophotometer at 48 h of incubation. The percent inhibition by micafungin in the presence of 2%, 0.2%, 0.1%, 0.05%, and 0.02% glucose was calculated at 24 h of incubation.
TABLE 1.
Comparison of MICs of T-2307 and other antifungal agents against C. glabrata ATCC 90030 determined in various media
| Antifungal agent | MIC (μg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Glucoseb |
0.2% glycerolc | 0.2% Glucose + Alamar blue |
||||||
| 2% | 0.2%a | 0.1% | 0.05% | 0.02% | 24 h | 48 h | ||
| T-2307 | >0.125 | 0.0039 | 0.0039 | 0.001 | 0.001 | 0.0005 | 0.0156 | >0.125 |
| Fluconazole | 16 | 8 | 8 | 8 | 4 | 4 | 8 | 16 |
| Micafungin | 0.0625 | 0.0625 | 0.0625 | 0.0625 | 0.125 | 0.125 | 0.0625 | 0.0625 |
| Amphotericin B | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 |
Equivalent to the amount used in the CLSI method.
Incubation for 48 h except with micafungin (incubation for 24 h).
Incubation for 48 h.
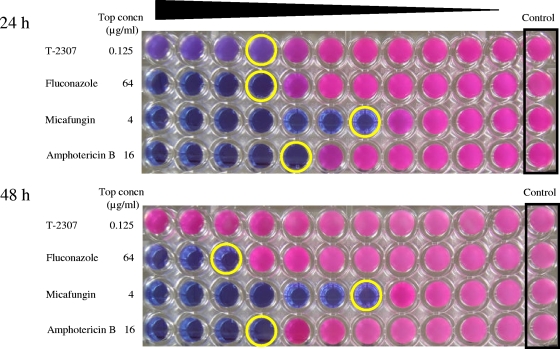
In a colorimetric assay, the susceptibility test using Alamar blue provided a clear MIC endpoint for T-2307 at 24 h; however, the MIC of T-2307 was above 0.125 μg/ml at 48 h (Fig. 4).
FIG. 4.
Colorimetric MIC determination of T-2307 and other agents against C. glabrata ATCC 90030 using Alamar blue at 24 h and 48 h of incubation. The susceptibility of C. glabrata to T-2307 and the other agents was tested in RPMI 1640 medium containing 0.2% glucose. Yellow circles, MICs.
Similar results were obtained for 25 clinical isolates of C. glabrata under the same conditions described above (data not shown).
In vivo efficacy.
The therapeutic effect of T-2307 on disseminated candidiasis caused by C. glabrata is shown in Table 2. T-2307 at 0.05 mg/kg/day and micafungin and amphotericin B at 0.5 mg/kg/day each significantly reduced the viable count in the kidneys compared to that for the control group, whereas fluconazole at 10 mg/kg/day did not.
TABLE 2.
Viable counts in kidneys in disseminated candidiasis caused by C. glabrata ATCC 90030
| Antifungal agent | Dose (mg/kg/day) | Log CFU/kidneys | SE |
|---|---|---|---|
| Control | 5.26 | 0.06 | |
| T-2307 | 0.025 | 4.30 | 0.20 |
| 0.05 | 4.00a | 0.26 | |
| 0.1 | 3.86a | 0.19 | |
| Fluconazole | 2.5 | 4.96 | 0.36 |
| 5 | 5.33 | 0.27 | |
| 10 | 4.62 | 0.20 | |
| Micafungin | 0.125 | 5.13 | 0.35 |
| 0.25 | 5.21 | 0.30 | |
| 0.5 | 3.59b | 0.17 | |
| Amphotericin B | 0.125 | 4.61 | 0.40 |
| 0.25 | 4.53 | 0.29 | |
| 0.5 | 2.93c | 0.45 |
P < 0.05 compared with the results for the control.
P < 0.01 compared with the results for the control.
P < 0.001 compared with the results for the control.
DISCUSSION
T-2307 is an aromatic diamidine derivative related to pentamidine, which is used to treat pneumocystosis, leishmaniasis, and trypanosomiasis. Recently, pafuramidine (DB289), an aromatic diamidine derivative and a prodrug of furamidine (DB75), has been demonstrated to have efficacy against African trypanosomiasis, Pneumocystis jirovecii pneumonia, and malaria (25). It also has been reported that Saccharomyces cerevisiae cells grown in a medium containing glycerol as the nonfermentative carbon source were more sensitive to the growth-inhibitory effects of DB75 or pentamidine than cells grown in a medium containing glucose, thus suggesting that DB75 and pentamidine inhibit the mitochondrial functions of cells (9, 11). This finding led us to believe that T-2307 may also inhibit the mitochondrial functions of cells and that the trailing growth of C. glabrata in the presence of T-2307 may be influenced by the carbon source or glucose concentration in the medium. The current study shows that the trailing growth of C. glabrata in the presence of T-2307 was attenuated as the concentration of glucose in the medium decreased to 0.1% or lower and that the trailing growth was completely inhibited when glycerol was used (Fig. 3).
The phenomenon of trailing growth for Candida spp. was originally reported with azole antifungal agents (18). It has been shown that the in vivo response to fluconazole in murine invasive candidiasis caused by Candida albicans isolates with the trailing growth phenomenon matched the MIC obtained at 24 h of incubation rather than that obtained at 48 h (3, 17). In addition, the clinical responses of three patients with trailing C. albicans isolates matched the MICs obtained at 24 h of incubation rather than those obtained at 48 h (16). Therefore, the reference guidelines suggest that the results obtained at 24 h of incubation may be more appropriate for such isolates (4). Several studies have attempted to resolve the difficulty of trailing growth. In one study, the trailing growth of Candida spp. was attenuated in media adjusted to a pH of ≤5.0 (12). In another study, Candida spp. lost the trailing phenotype at 25°C and 42°C (2). It has also been reported that RPMI 1640 medium containing 2% glucose facilitates reading of the fluconazole MIC for C. albicans (5, 19).
In the current study, several differences between the trailing growth of T-2307 and that reported for fluconazole were observed. First, the trailing growth of C. glabrata in the presence of T-2307 at 24 h of incubation was similar to that at 48 h, determined using standard CLSI methods (Fig. 2). Second, C. glabrata exhibited more significant trailing growth in the presence of T-2307 and 2% glucose (Fig. 3). Third, the trailing growth of C. glabrata in the presence of T-2307 was not attenuated when the pH of the medium was lowered to 5.4 (data not shown). These differences in behavior may be derived from a difference in the mechanism of trailing growth. The mechanisms proposed to explain the trailing growth for azoles include activation of calcineurin and the altered regulation of genes that mediate resistance, such as ERG11, CDR1, and MDR1 (10, 21). On the other hand, T-2307, like pentamidine and DB75, may be considered an inhibitor of the mitochondrial function; therefore, the cause of trailing growth may be fermentation.
Another approach to overcoming the difficulty of the MIC reading for azoles in C. albicans is the addition of an oxidation-reduction indicator such as Alamar blue (15). In the current study, the colorimetric method using Alamar blue provided a clear MIC endpoint for T-2307 at 24 h of incubation with a standard (0.2%) glucose concentration. However, at 48 h of incubation, all the wells with T-2307 changed to pink, indicating that 24 h of incubation was appropriate with this method. Modifying the glucose concentration or carbon source may be a better option because the MIC is easily read at up to 48 h of incubation.
To investigate if T-2307 shows efficacy against trailing isolates in vivo, we evaluated the in vivo efficacy of T-2307 in a murine model of disseminated candidiasis caused by C. glabrata, and T-2307 had an excellent therapeutic effect (Table 2). This result seems reasonable because the normal blood glucose level of mice is approximately 0.1% (20), at which the trailing growth of C. glabrata in the presence of T-2307 is less than that observed using the standard CLSI method. Since the normal blood glucose level in humans is also approximately 0.1% (20), T-2307 may show efficacy against candidiasis caused by C. glabrata.
In conclusion, susceptibility testing of C. glabrata using modified media or Alamar blue facilitates the MIC endpoint determination for T-2307, as compared to the standard CLSI method. Although C. glabrata exhibits trailing growth in the presence of T-2307 with the CLSI method, T-2307 has an excellent therapeutic effect in the murine model of disseminated candidiasis caused by C. glabrata. It would be better to test the susceptibility of C. glabrata to T-2307 using modified media or Alamar blue to avoid misreading of the MIC endpoint due to the significant trailing growth seen with the standard CLSI method. Further studies on the correlation between the trailing growth of C. glabrata in the presence of T-2307 and the clinical response are needed.
Footnotes
Published ahead of print on 14 June 2010.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal, D., T. F. Patterson, M. G. Rinaldi, and S. G. Revankar. 2007. Trailing end-point phenotype of Candida spp. in antifungal susceptibility testing to fluconazole is eliminated by altering incubation temperature. J. Med. Microbiol. 56:1003-1004. [DOI] [PubMed] [Google Scholar]
- 3.Arthington-Skaggs, B. A., D. W. Warnock, and C. J. Morrison. 2000. Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 44:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3rd ed. M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Coenye, T., M. De Vos, D. Vandenbosch, and H. Nelis. 2007. Factors influencing the trailing endpoint observed in Candida albicans susceptibility testing using the CLSI procedure. Clin. Microbiol. Infect. 14:495-497. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A., M. Pfaller, S. A. Messer, C. C. Knapp, N. Holliday, and S. B. Killian. 2004. Multicenter comparison of the Sensititre YeastOne colorimetric antifungal panel with the NCCLS M27-A2 reference method for testing new antifungal agents against clinical isolates of Candida spp. J. Clin. Microbiol. 42:718-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeda, F., Y. Wakai, S. S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwen, P. C., D. M. Kelly, E. C. Reed, and S. H. Hinrichs. 1995. Invasive infection due to Candida krusei in immunocompromised patients not treated with fluconazole. Clin. Infect. Dis. 20:342-347. [DOI] [PubMed] [Google Scholar]
- 9.Lanteri, C. A., B. L. Trumpower, R. R. Tidwell, and S. R. Meshnick. 2004. DB75, a novel trypanocidal agent, disrupts mitochondrial function in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 48:3968-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, M. K., L. E. Williams, D. W. Warnock, and B. A. Arthington-Skaggs. 2004. Drug resistance genes and trailing growth in Candida albicans isolates. J. Antimicrob. Chemother. 53:217-224. [DOI] [PubMed] [Google Scholar]
- 11.Ludewig, G., J. M. Williams, Y. Li, and C. Staben. 1994. Effects of pentamidine isethionate on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 38:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marr, K. A., T. R. Rustad, J. H. Rex, and T. C. White. 1999. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob. Agents Chemother. 43:1383-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsuyama, J., N. Nomura, K. Hashimoto, E. Yamada, H. Nishikawa, M. Kaeriyama, A. Kimura, Y. Todo, and H. Narita. 2008. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob. Agents Chemother. 52:1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, and S. A. Messer. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 36:1886-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., C. Grant, V. Morthland, and J. Rhine-Chalberg. 1994. Comparative evaluation of alternative methods for broth dilution susceptibility testing of fluconazole against Candida albicans. J. Clin. Microbiol. 32:506-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1998. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J. Clin. Microbiol. 36:153-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rex, J. H., P. W. Nelson, V. L. Paetznick, M. Lozano-Chiu, A. Espinel-Ingroff, and E. J. Anaissie. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 42:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rex, J. H., M. A. Pfaller, M. G. Rinaldi, A. Polak, and J. N. Galgiani. 1993. Antifungal susceptibility testing. Clin. Microbiol. Rev. 6:367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Tudela, J. L., and J. V. Martinez-Suarez. 1994. Improved medium for fluconazole susceptibility testing of Candida albicans. Antimicrob. Agents Chemother. 38:45-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rybak, B. 1968. Regulation of glycemia, p. 467-473. Principles of zoophysiology, vol. 1. Pergamon Press, London, United Kingdom. [Google Scholar]
- 21.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959-976. [DOI] [PubMed] [Google Scholar]
- 22.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmary, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1979. [DOI] [PubMed] [Google Scholar]
- 23.Wingard, J. R., W. G. Merz, M. G. Rinaldi, T. R. Johnson, J. E. Karp, and R. Saral. 1991. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 325:1274-1277. [DOI] [PubMed] [Google Scholar]
- 24.Wingard, J. R., W. G. Merz, M. G. Rinaldi, C. B. Miller, J. E. Karp, and R. Saral. 1993. Association of Torulopsis glabrata infections with fluconazole prophylaxis in neutropenic bone marrow transplant patients. Antimicrob. Agents Chemother. 37:1847-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeates, C. 2003. DB-289 Immtech International. IDrugs 6:1086-1093. [PubMed] [Google Scholar]