Abstract
In a randomized controlled trial in Ghana, treatment of Mycobacterium ulcerans infection with streptomycin (SM)-rifampin (RIF) for 8 weeks was compared with treatment with SM-RIF for 4 weeks followed by treatment with RIF-clarithromycin (CLA) for 4 weeks. The extent of the interaction of RIF and CLA combined on the pharmacokinetics of the two compounds is unknown in this population and was therefore studied in a subset of patients. Patients received CLA at a dose of 7.5 mg/kg of body weight once daily, rounded to the nearest 125 mg. RIF was administered at a dose of 10 mg/kg, rounded to the nearest 150 mg. SM was given at a dose of 15 mg/kg once daily as an intramuscular injection. Plasma samples were drawn at steady state and analyzed by liquid chromatography-tandem mass spectroscopy. Pharmacokinetic parameters were calculated with the MW/Pharm (version 3.60) program. Comedication with CLA resulted in a 60% statistically nonsignificant increase in the area under the plasma concentration-time curve (AUC) for RIF of 25.8 mg·h/liter (interquartile ratio [IQR], 21.7 to 31.5 mg·h/liter), whereas the AUC of RIF was 15.2 mg·h/liter (IQR, 15.0 to 17.5 mg·h/liter) in patients comedicated with SM (P = 0.09). The median AUCs of CLA and 14-hydroxyclarithromycin (14OH-CLA) were 2.9 mg·h/liter (IQR, 1.5 to 3.8 mg·h/liter) and 8.0 mg·h/liter (IQR, 6.7 to 8.6 mg·h/liter), respectively. The median concentration of CLA was above the MIC of M. ulcerans, but that of 14OH-CLA was not. In further clinical studies, a dose of CLA of 7.5 mg/kg twice daily should be used (or with an extended-release formulation, 15 mg/kg should be used) to ensure higher levels of exposure to CLA and an increase in the time above the MIC compared to those achieved with the currently used dose of 7.5 mg/kg once daily.
Although many antimycobacterial agents appeared to be effective against Mycobacterium ulcerans infections in in vitro and in animal models (4, 11, 27, 30), clinical evidence of the effectiveness of antimicrobial treatment was predominantly based on a small study conducted with patients in Ghana (15). Conceivably, using antimycobacterial agents results not only in preventing bacilli from replicating and killing microorganisms but also in halting the production of the toxin mycolactone (36). This toxin that causes the tissue damage is produced by enzymes encoded by the pMUM001 plasmid (33). Current WHO recommendations suggest 8 or more weeks of treatment with rifampin (RIF) plus streptomycin (SM) for all clinical forms of active Buruli ulcer disease (BUD). Daily injections with streptomycin are problematic, as most patients live in remote areas with limited health care facilities. Proper hygiene with these injections, as well as intrinsic ototoxicity and renal toxicity, is a concern. Therefore, an oral treatment schedule is urgently needed to reduce the number of injections and to improve the tolerability and safety of the proposed regimen. Pregnant women might also benefit from treatment without aminoglycosides. This problem was addressed by comparing 8 weeks of SM (15 mg/kg of body weight) and RIF (10 mg/kg) treatment (8SR arm) and 4 weeks of streptomycin and rifampin treatment followed by 4 weeks of RIF plus clarithromycin (CLA; 7.5 mg/kg) treatment (4SR/4CR arm) in a randomized controlled trial (the BURULICO trial) (26). CLA was chosen for inclusion in the treatment regimen because of in vitro data suggesting that this drug is active against M. ulcerans, for which the MICs range from <0.125 to 2.0 mg/liter (30). In a pharmacokinetic (PK) study with adults who received doses of 500 mg twice daily, plasma CLA concentrations (5) were well above the MIC for most M. ulcerans isolates.
The clinical effectiveness of macrolides is only partly explained by pharmacokinetics, because these drugs typically accumulate in inflammatory cells, especially macrophages, at the site of infection (1). Although M. ulcerans infection has long been regarded a predominantly extracellular infection (20), evidence has emerged from animal models that M. ulcerans infection has an intracellular stage in which it multiplies inside macrophages (10, 34). These data taken together suggest that the intramacrophage CLA concentration might add to the beneficial effect of the drug to fight M. ulcerans infection and that CLA might exert its effect inside these immune cells without reaching inhibitory drug concentrations in the bloodstream. On the other hand, if plasma CLA concentrations do not reach inhibiting or mutant-inhibiting concentrations, at least in the extracellular space where bacilli are present during later stages of the disease, inadvertent monotherapy with RIF alone would result. At the time that the present study was designed, the activity of the 14-hydroxyclarithromycin (14OH-CLA) metabolite against M. ulcerans was unknown, and sensitivity to 14OH-CLA therefore had to be determined. In mycobacterial infections, monotherapy has invariably resulted in the failure of treatment, as drug-resistant pathogens within the total microbial load might escape and repopulate the diseased lesions in the host (9).
RIF is known to induce cytochrome P450 isoenzymes (e.g., CYP 3A4, involved in the elimination of CLA), while CLA is also known to inhibit the enzyme activity of CYP 3A4 (3, 18). The P-glycoprotein (Pgp) efflux transporter is also affected, as RIF induces and is the substrate of Pgp and CLA inhibits Pgp (7, 14).
Earlier studies with patients with M. avium infections showed an induction of metabolism of clarithromycin, but the data on 14OH-CLA were not consistent between the two studies (28, 37). The purpose of the present study was to assess the influence of this CLA-RIF interaction on the areas under the plasma concentration-time curves (AUCs) for the 4SR/4CR study arm and to compare those AUCs to the AUC for RIF in patients who were treated in the 8SR study arm. In addition, the average time that plasma drug concentrations were maintained in excess of the MIC was studied.
MATERIALS AND METHODS
The open-label prospective pharmacokinetic study described here evaluated the pharmacokinetics of RIF and RIF combined with CLA in the treatment of Mycobacterium ulcerans disease in patients already enrolled in the BURULICO trial. The study was conducted at the Nkawie-Toase Governmental Hospital and at the Agogo Presbyterian Hospital, both in the Ashanti region of Ghana.
Study subjects.
Patients ≥10 years of age (male and female) were eligible for inclusion in this side study to assess the pharmacokinetics of clarithromycin and rifampin when they were allocated to the 4SR/4CR arm and the pharmacokinetics of rifampin when they were allocated to the 8SR arm. Exclusion criteria for the BURULICO trial were treatment with macrolide or quinolone antibiotics, antituberculosis medication, or immunomodulatory drugs (including corticosteroids) within the previous 1 month; current treatment with any drugs likely to interact with the study medication, e.g., anticoagulants, cyclosporine, phenytoin, oral contraceptives, and phenobarbitone; a history of hypersensitivity to rifampin, streptomycin, and/or clarithromycin; and an inability to take oral medication or the presence of a gastrointestinal disease likely to interfere with drug absorption.
Drug administration.
Patients received CLA at a dose of 7.5 mg/kg once daily, which was rounded to the nearest 125 mg. RIF was administered at a dose of 10 mg/kg, which was rounded to the nearest 150 mg. The drugs were administered on an empty stomach, but the participants were allowed to take a light standardized breakfast after drug ingestion. Although food intake does not influence the AUC of CLA (8), it influences the AUC of RIF (39). By offering a standardized light breakfast approximately 30 min after drug ingestion, the effect on drug absorption would be minimized and equally distributed over both groups. The adherence to the treatment regimen was 100%, as the participants were in a guided patient program.
Pharmacokinetic assessment.
On the day of this PK study, a full pharmacokinetic curve was recorded at steady state after a minimum of 7 days of consecutive treatment with the same dose. Blood samples (2 ml, EDTA anticoagulant) were obtained before a dose of CLA-RIF or RIF was administered (time zero) and at 0.5,1, 1.5, 2, 2.5, 3, 5, 7.5, and 10 h after administration of the dose. This schedule was based on earlier data (17, 29), with the restriction that the participants in the present study had to be able to return to their homes (often, in remote rural villages) on the evening of the sampling day. Plasma was separated and frozen at −20°C until it was processed.
Analytical methods.
The plasma concentrations of RIF and 25-desacetylrifampicin (25DA-RIF) and of CLA and 14OH-CLA were determined at the Laboratory for Clinical Toxicology and Drugs Analysis of the Department of Hospital and Clinical Pharmacy of the University Medical Center Groningen, Groningen, Netherlands, using a validated liquid chromatography-tandem mass spectrometry (LC/MS/MS) assay (13).
Pharmacokinetic analysis.
The values of the pharmacokinetic parameters were calculated using the KINFIT program (MW/Pharm, version 3.60; Mediware, Netherlands) (31). Cmax was defined as the highest observed serum concentration, and Tmax was the time corresponding to Cmax. Cmin was the serum concentration before intake of the dose. The AUC from time zero to 24 h (AUC0-24) was calculated using the log-linear trapezoidal rule. The elimination half-life (t1/2) was calculated by 0.693/kel. The apparent clearance of the drug (CL/F) was calculated as the dose/AUC0-24, and the apparent volume of distribution (V/F) was calculated as the dose/concentration at steady state.
For both treatment groups, a population one-compartment model with first-order absorption pharmacokinetics with lag time was generated using the RIF dose, the body surface area of the participants, and the observed RIF serum concentrations in an iterative two-stage Bayesian procedure (31). Pharmacokinetic parameters were assumed to be log-normally distributed. Residual error was assumed to be distributed normally with a standard deviation (SD) of 0.1 + (0.25 × C), where C is the serum rifampin concentration. Since the patients received RIF only orally, bioavailability could not be assessed and was fixed at a value of 1, as the bioavailability of RIF is nearly complete.
Bacteriologic assessment.
The MICs of CLA and 14OH-CLA were determined by the agar proportion method on Middlebrook 7H11 agar at pH 6.6 supplemented with 10% oleic acid-albumin-dextrose-catalase. For both drugs, 2-fold concentrations ranging from 0.125 to 8 μg/ml were tested using two different strains of M. ulcerans: strain 1059, a recent human isolate from Ghana (38), and strain 1615 (ATCC 35840), a well-characterized Malaysian human isolate (16). The strains were subsequently passaged in mice in a laboratory at Johns Hopkins University School of Medicine. These reference strains instead of test isolates from our patients were used, as isolation of M. ulcerans by culture has a very low yield (21). Bacillary suspensions adjusted to an optical density at 600 nm of 1.0 were diluted to 10−5 in phosphate-buffered saline; and 0.5 ml of each of the 10−1, 10−3, and 10−5 dilutions was inoculated onto plates with and without drugs. The plates were then incubated at 32°C, and the colony counts were read 2 and 3 months later. The MIC was defined as the lowest drug concentration inhibiting ≥99% of the colony counts on the control plates.
Statistical methods.
Data are presented as median values and interquartile ranges (IQRs). Differences in age, body mass index, and the values of the pharmacokinetic parameters between the patients groups were assessed with the Wilcoxon rank-sum test (Mann-Whitney U test) for unpaired data. A sample size of 10 was estimated to be needed to detect peak CLA concentrations of >0.5 mg/liter in 80% of the patients with a statistical power of 80% and a significance level of 5%. A sample size of five was estimated to be needed to detect a 20% increase in the RIF concentrations in the presence of CLA compared to the RIF concentrations in the group treated with RIF and streptomycin with a statistical power of 80% and a significance level of 5%.
Ethics.
The present study was a side study of a randomized trial (ClinicalTrials.gov identifier NCT00321178). As for the main study protocol, written and verbal informed consent was obtained from participants of 10 years and over and from their custodians if they were below 18 years of age. The protocol for the present study was also approved by the local institutional ethics committees of the Kwame Nkrumah University of Science and Technology and the Komfo Anokye Teaching Hospital. Only individuals over age 10 years were eligible for participation in the present study. All study participants gave informed consent after being given an appropriate amount of time to consider their participation; a small incentive in cash and kind was offered, and for participants aged between 10 and 18 years, their parents or caretakers also gave written informed consent.
RESULTS
Study subjects.
Thirteen patients (12 females and 1 male) were included in the present study: 8 were in the 4SR/4CR arm and 5 were in the 8SR arm. The baseline age, body weight, and body mass index characteristics were similar for the two groups. The patient characteristics are presented in Table 1. Twelve of 13 patients had Buruli ulcer lesions, as confirmed by IS2404-based PCR (21). The Buruli ulcer lesions of these 12 patients were completely reepithelialized within the time frame of the study (healing without a recurrence within 1 year after inclusion). The one unconfirmed lesion—a nodule—in a patient treated with 8SR was excised 2 weeks after the end of treatment because another diagnosis was suspected. After excision, that patient also tested IS2404-based PCR positive.
TABLE 1.
Baseline characteristics
| Characteristica | Result for each treatment arm |
P value | |
|---|---|---|---|
| 4SR/4CR (n = 8) | 8SR (n = 5) | ||
| Age (yr)b | 26.0 (13.5-41.0) | 26.5 (19.5-36.3) | 0.07 |
| Gender (no. of M/no. of F) | 0/8 | 1/4 | 0.4 |
| Wt (kg)b | 53.5 (51.5-56.5) | 45.0 (35.0-60.0) | 0.4 |
| BMI (kg/m2)b | 18.7 (15.2-22.0) | 20.5 (19.0-22.9) | 0.17 |
M, male; F, female; BMI; body mass index.
Data are medians (25th to 75th percentiles).
Bacteriologic assessment.
The MICs of CLA were 0.25 mg/liter and 0.50 mg/liter for M. ulcerans 1059 and M. ulcerans 1615, respectively. The MICs of 14OH-CLA were 4 mg/liter and 8 mg/liter for M. ulcerans 1059 and M. ulcerans 1615, respectively. For both strains, the MIC of 14OH-CLA was 16 times higher than that of CLA.
Pharmacokinetic study.
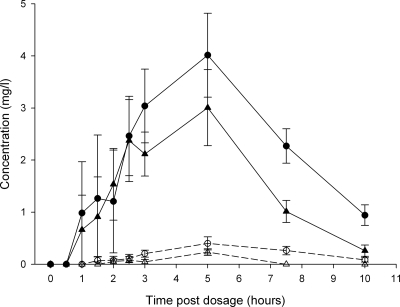
The patients in both study arms received the same dose of RIF per kg of body weight. The patients in the 8SR study arm received RIF at a dose of 8.8 mg/kg (IQR, 8.6 to 10.0 mg/kg) once daily, which was not significantly different from the RIF dose of 8.8 mg/kg (IQR, 8.4 to 10.8 mg/kg) administered in the 4SR/4CR study arm (P = 0.70). The patients had received the study medication for a median duration of 50 days (IQR, 29 to 55 days) before blood samples were drawn. The RIF concentration-time curves of both arms are displayed in Fig. 1. The CLA concentration-time curve is displayed in Fig. 2. The values of the pharmacokinetic parameters of the two treatment arms are summarized in Table 2. Although the difference was not statistically significantly (P = 0.09), the median increase in the AUC0-24 value for RIF was 60% if the AUC0-24 of 25.9 mg·h/liter (IQR, 21.9 to 31.5 mg·h/liter) for the patients in the 4SR/4CR arm was compared to the median AUC0-24 value of RIF of 16.2 mg·h/liter (IQR, 15.0 to 17.6 mg·h/liter) for patients in the 8SR arm. 25DA-RIF could be detected in only 5/13 patients. The geometric mean AUC ratio of CLA to 14OH-CLA was 0.33 (range, 0.22 to 0.39). Population pharmacokinetic analysis showed that apparent clearance of RIF was (not significantly) reduced in patients who also received CLA but was due to interpatient variability in both groups (P = 0.17) (Table 3). The median AUC/MIC ratio of RIF was 52 (IQR, 44 to 63) in patients treated with CLA as well, which was 63% higher (P = 0.09) than the median AUC/MIC ratio of 32 (IQR, 30 to 35) in patients treated with RIF and SM.
FIG. 1.
RIF/25DA-RIF concentration-time curve. Mean (standard error) plasma concentration-time curves for RIF (solid symbols) and 25DA-RIF (open symbols) from the 4SR/CR4 arm (circles) and SR8 arm (triangles) are shown.
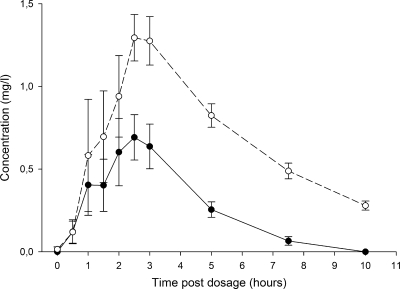
FIG. 2.
CLA/14OH-CLA concentration-time curve. Mean (standard error) plasma concentration-time curves for CLA (solid circles) and 14OH-CLA (open circles) from the 4SR/CR4 arm are shown.
TABLE 2.
Pharmacokinetics of rifampin and clarithromycin in both treatment arms
| Pharmacokinetic parameter | Result for each treatment arma |
P value for difference in RIF | |||||
|---|---|---|---|---|---|---|---|
| 4SR/4CR (n = 8) |
8SR (n = 5) |
||||||
| CLA | 14OH-CLA | RIF | 25DA-RIFb | RIF | 25DA-RIFc | ||
| AUC0-24 (mg·h/liter) | 2.9 (1.5-3.8) | 8.6 (6.8-9.0) | 25.8 (21.9-31.5) | 4.2 (3.2-5.1) | 16.2 (15.0-17.6) | 1.1 | 0.09 |
| Cmax (mg/liter) | 1.0 (0.5-1.3) | 1.5 (1.2-2.1) | 4.9 (3.3-6.4) | 0.6 (0.4-0.6) | 4.2 (4.0-4.3) | 0.4 | 0.35 |
| Tmax (h) | 2.3 (1.6-2.9) | 2.9 (2.4-3.5) | 3.6 (3.2-4.4) | 4.1 (3.3-4.4) | 3.5 (2.5-3.9) | 0.6 | 0.62 |
| t1/2 (h) | 1.3 (1.0-1.4) | 2.6 (2.3-2.9) | 2.1 (2.0-2.2) | 2.2 (1.3-3.1) | 2.3 (1.9-2.4) | 0.1 | 0.35 |
| CL/F (liter/h) | 89.2 (68.8-151) | 30.9 (19.7-41.3) | 11.9 (8.8-15.2) | 132 (109-156) | 18.5 (17.0-21.1) | 89.7 | 0.09 |
| V/F (liter) | 174 (144-214) | 141 (117-230) | 38.1 (26.4-50.8) | 425 (243-632) | 58.6 (57.4-59.8) | 13.5 | 0.27 |
The values are medians (IQRs).
n = 4.
n = 1.
TABLE 3.
Rifampin population pharmacokinetic model parameter valuesa
| Parameterb | Result (mean ± SD) for each treatment arm |
P value | |
|---|---|---|---|
| 4SR/4CR | 8SR | ||
| CL (liters/h/1.85 m2) | 24.1 ± 9.8 | 37.3 ± 5.5 | 0.17 |
| V (liters/kg LBMc) | 1.19 ± 0.21 | 1.43 ± 0.10 | 0.17 |
| ka (h−1) | 0.998 ± 1.35 | 0.807 ± 0.574 | 0.83 |
| Tlag (h) | 2.07 ± 0.55 | 1.52 ± 0.86 | 0.44 |
Oral bioavailability was fixed at a value of 1 for both treatment arms.
LBMc, lean body mass; ka, absorption rate constant; Tlag, lag time; the other abbreviations are defined in the text.
The times the concentrations remained above the MIC of CLA were 300, 210, 180, 285, 300, 30, 300, and 90 min above 0.25 mg/liter and 170, 105, 10, 135, 190, 0, 200, and 0 min above 0.5 mg/liter for each patient. This resulted in median times that the concentration was above the MIC of 0.25 mg/liter and 0.5 mg/liter of 248 min and 120 min, respectively. In two of the patients, the concentration never reached the MIC of 0.5 mg/liter. The concentration of 14OH-CLA was never above MICs of 4 mg/liter and 8 mg/liter.
DISCUSSION
The present study investigated the interaction between RIF and CLA in patients infected with M. ulcerans as part of a prospective randomized trial comparing two drug regimens. We observed that the median total RIF exposure was increased 70% in patients receiving CLA compared to that in patients receiving RIF combined with SM. This may be explained by the inhibitory effect of CLA on the Pgp-mediated efflux of RIF and not by the inhibitory effect of CLA on CYP 3A4, as RIF is not a CYP 3A4 substrate. Our study had a relatively small sample size, and due to the high degree of variability in the results, only a trend toward significance could be shown. Although the difference was not significant in our study, it might still be relevant for clinical practice, as the increased exposure to RIF could have contributed to the efficacy of the drug treatment because the RIF AUC/MIC ratio is one of the best parameters predicting the efficacy of treatment of infections caused by Mycobacterium tuberculosis (23). No differences in the concentration of 25DA-RIF were seen between the treatment arms. Due to the low of level exposure of 25DA-RIF in plasma, no additional bactericidal effect can be expected. The only effect of this metabolite may be expected in human bile, in which it tends to accumulate (22). The effect of RIF on CLA is more difficult to explain, as the present study lacked a study arm without RIF. We observed that the level of exposure to 14OH-CLA was significantly higher than that to CLA. The median 14OH-CLA concentration-time curve (Fig. 2) is higher than the median CLA concentration-time curve for the time period observed. This indicates that the metabolism of CLA to 14OH-CLA is induced by RIF, as the 14OH-CLA concentration-time curve is higher than the CLA concentration-time curve. This is consistent with the findings of earlier studies (28, 37). The ratio of the parent drug to metabolite is, however, less than that reported earlier (28).
The results of our study are in line with the observations of the interaction of CLA and rifabutin (RBN), which has been studied in volunteers infected with HIV. Rifabutin, like rifampin, induces Pgp and the CYP 450 system, but less profoundly than RIF does (2). In the present study, the CLA-RBN interaction resulted in a significantly decreased AUC for CLA compared to that before the introduction of RBN, but the 14OH-CLA concentration increased significantly along with a significant increase in RBN drug concentrations (19).
Our results are consistent with earlier data that the ratio of the Cmax of CLA to the Cmax of 14OH-CLA is reduced to a greater extent when CLA is combined with RIF (28) than when RBN is combined with RIF (37) and no inducer is coadministered. The major concern in the case of these drug-drug interactions is that the resulting antibiotic exposure is not efficacious due to the altered plasma concentrations. The decrease in CLA levels must therefore be compensated for by the increase in 14OH-CLA level and can be expressed as the ratio of the CLA level/14OH-CLA level. Whether the observed ratio is sufficient for efficacious treatment depends on the in vitro susceptibility of the targeted microorganism to both CLA and 14OH-CLA. In our study we observed that the concentration of CLA in plasma was above the MIC of 0.25 to 0.5 mg/liter for M. ulcerans for a median period of at least 120 to 248 min. As the MIC for 14OH-CLA was 4 to 8 mg/liter, it was presumed that only CLA contributed to the bactericidal activity of the agent, as the level of exposure to 14OH-CLA was always below the MIC. In other bacterial infections, CLA can be efficacious if the concentration is above the MIC for some part of the dosing interval (1). The time above the MIC for CLA therefore seems to be less important than it is for, e.g., beta-lactam antibiotics and is difficult to correlate with efficacy (1). In addition, the plasma concentration of CLA does not necessarily reflect its intracellular bactericidal efficacy, as efficacy depends on the distribution of the antibiotic into the tissue and cells (6). Although the CLA concentration in the interstitial fluid of the skin might be lower than that in plasma (35), the concentrations of CLA in cells are expected to be higher than those in plasma (32).
The present study was limited by the fact that neither the distribution of the antibiotic into the tissue and cells nor the concentration in the interstitial fluid of the skin was determined. On the basis of the results of the BURULICO trial, both CLA and RIF exposures appeared to be sufficient for the treatment of M. ulcerans infection, as no significant difference (one-sided Fisher's exact test, P = 0.16) in the healing rate was observed between the 8SR (96%) and 4SR/4CR (91%) arms (26). As 5 of the 75 subjects treated with CLA appeared to be positive for M. ulcerans as a result of the non-protocol-driven testing of samples by culture, there are still some doubts about the total antibiotic exposure. Although the coadministration of RIF resulted in a lower level of CLA exposure, it should be mentioned that CLA was given at a low dose of 7.5 mg/kg once daily. During the design of the randomized controlled trial, concerns about the safety of CLA at a dose of 7.5 mg/kg twice daily resulted in the use of this dosage scheme.
On the basis of these clinical results, it might be hypothesized that RIF was effective in reducing the M. ulcerans bacterial load or at least stopping mycolactone production, while CLA prevented the emergence of microorganisms resistant to RIF (25). The contribution of CLA is uncertain, however, and measuring the intracellular concentrations CLA would be the best method to assess the combined extra- and intracellular exposures of M. ulcerans to CLA. Animal (mouse) model studies of Mycobacterium ulcerans infection have evaluated only the correlates between dosages and the bacteriological response and clinical effects, especially in the footpad model (11, 12, 24). Future animal studies should address PK aspects, along with these clinical and bacteriological responses, to develop PK/pharmacodynamic models for the prediction of outcomes.
In further clinical studies, a regular CLA dose of 7.5 mg/kg twice daily or a CLA slow-release formulation at a dose of 15 mg/kg once daily should be used to ensure higher levels of exposure to CLA and increase the time above the MIC compared to those achieved with the currently used dose of 7.5 mg/kg once daily, which appeared to be safe and well tolerated.
Conclusion.
Combination of RIF and CLA resulted in a considerable but nonsignificant increase in plasma RIF levels compared to the RIF levels achieved with coadministration of RIF and SM. A decrease in plasma CLA levels and an increase in plasma 14OH-CLA levels were observed, but the time that the concentrations of CLA in plasma were above MIC of M. ulcerans was reached for at least a part of the day.
Acknowledgments
The purified clarithromycin drug substance and metabolite were kindly provided by Abbott, and the purified rifampin drug substance and metabolite were kindly provided by Sanofi Aventis (Germany). Both M. ulcerans strains were generously provided by Pamela Small, University of Tennessee, Knoxville, TN.
W. A. Nienhuis and T. S. van der Werf were supported by grant EU FP6 2003-INCO-Dev2-015476. A. T. Zuur was supported by Stichting BuG.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print on 28 June 2010.
REFERENCES
- 1.Amsden, G. W. 2001. Advanced-generation macrolides: tissue-directed antibiotics. Int. J. Antimicrob. Agents 18(Suppl. 1):S11-S15. [DOI] [PubMed] [Google Scholar]
- 2.Baciewicz, A. M., C. R. Chrisman, C. K. Finch, and T. H. Self. 2008. Update on rifampin and rifabutin drug interactions. Am. J. Med. Sci. 335:126-136. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti, M. S. 1995. Inducing properties of rifabutin and effects on the pharmacokinetics and metabolism of concomitant drugs. Pharmacol. Res. 32:177-187. [DOI] [PubMed] [Google Scholar]
- 4.Bentoucha, A., J. Robert, H. Dega, N. Lounis, V. Jarlier, and J. Grosset. 2001. Activities of new macrolides and fluoroquinolones against Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 45:3109-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkhardt, O., K. Borner, H. Stass, G. Beyer, M. Allewelt, C. E. Nord, and H. Lode. 2002. Single- and multiple-dose pharmacokinetics of oral moxifloxacin and clarithromycin, and concentrations in serum, saliva and faeces. Scand. J. Infect. Dis. 34:898-903. [DOI] [PubMed] [Google Scholar]
- 6.Carbon, C. 1995. Clinical relevance of intracellular and extracellular concentrations of macrolides. Infection 23(Suppl. 1):S10-S14. [DOI] [PubMed] [Google Scholar]
- 7.Chen, J., and K. Raymond. 2006. Roles of rifampicin in drug-drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann. Clin. Microbiol. Antimicrob. 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu, S., Y. Park, C. Locke, D. S. Wilson, and J. C. Cavanaugh. 1992. Drug-food interaction potential of clarithromycin, a new macrolide antimicrobial. J. Clin. Pharmacol. 32:32-36. [DOI] [PubMed] [Google Scholar]
- 9.Cole, S. T., and P. M. Alzari. 2005. Microbiology. TB—a new target, a new drug. Science 307:214-215. [DOI] [PubMed] [Google Scholar]
- 10.Coutanceau, E., L. Marsollier, R. Brosch, E. Perret, P. Goossens, M. Tanguy, S. T. Cole, P. L. Small, and C. Demangel. 2005. Modulation of the host immune response by a transient intracellular stage of Mycobacterium ulcerans: the contribution of endogenous mycolactone toxin. Cell. Microbiol. 7:1187-1196. [DOI] [PubMed] [Google Scholar]
- 11.Dega, H., A. Bentoucha, J. Robert, V. Jarlier, and J. Grosset. 2002. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob. Agents Chemother. 46:3193-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dega, H., J. Robert, P. Bonnafous, V. Jarlier, and J. Grosset. 2000. Activities of several antimicrobials against Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 44:2367-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Velde, F., J. W. Alffenaar, A. M. Wessels, B. Greijdanus, and D. R. Uges. 2009. Simultaneous determination of clarithromycin, rifampicin and their main metabolites in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877:1771-1777. [DOI] [PubMed] [Google Scholar]
- 14.Eberl, S., B. Renner, A. Neubert, M. Reisig, I. Bachmakov, J. Konig, F. Dorje, T. E. Murdter, A. Ackermann, H. Dormann, K. G. Gassmann, E. G. Hahn, S. Zierhut, K. Brune, and M. F. Fromm. 2007. Role of P-glycoprotein inhibition for drug interactions: evidence from in vitro and pharmacoepidemiological studies. Clin. Pharmacokinet. 46:1039-1049. [DOI] [PubMed] [Google Scholar]
- 15.Etuaful, S., B. Carbonnelle, J. Grosset, S. Lucas, C. Horsfield, R. Phillips, M. Evans, D. Ofori-Adjei, E. Klustse, J. Owusu-Boateng, G. K. Amedofu, P. Awuah, E. Ampadu, G. Amofah, K. Asiedu, and M. Wansbrough-Jones. 2005. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob. Agents Chemother. 49:3182-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. L. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854-857. [DOI] [PubMed] [Google Scholar]
- 17.Guay, D. R., L. E. Gustavson, K. J. Devcich, J. Zhang, G. Cao, and C. A. Olson. 2001. Pharmacokinetics and tolerability of extended-release clarithromycin. Clin. Ther. 23:566-577. [DOI] [PubMed] [Google Scholar]
- 18.Gurley, B., M. A. Hubbard, D. K. Williams, J. Thaden, Y. Tong, W. B. Gentry, P. Breen, D. J. Carrier, and S. Cheboyina. 2006. Assessing the clinical significance of botanical supplementation on human cytochrome P450 3A activity: comparison of a milk thistle and black cohosh product to rifampin and clarithromycin. J. Clin. Pharmacol. 46:201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafner, R., J. Bethel, M. Power, B. Landry, M. Banach, L. Mole, H. C. Standiford, S. Follansbee, P. Kumar, R. Raasch, D. Cohn, D. Mushatt, and G. Drusano. 1998. Tolerance and pharmacokinetic interactions of rifabutin and clarithromycin in human immunodeficiency virus-infected volunteers. Antimicrob. Agents Chemother. 42:631-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayman, J. 1993. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J. Clin. Pathol. 46:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbinger, K. H., O. Adjei, N. Y. wua-Boateng, W. A. Nienhuis, L. Kunaa, V. Siegmund, J. Nitschke, W. Thompson, E. Klutse, P. Agbenorku, A. Schipf, S. Reu, P. Racz, B. Fleischer, M. Beissner, E. Fleischmann, K. Helfrich, T. S. van der Werf, T. Loscher, and G. Bretzel. 2009. Comparative study of the sensitivity of different diagnostic methods for the laboratory diagnosis of Buruli ulcer disease. Clin. Infect. Dis. 48:1055-1064. [DOI] [PubMed] [Google Scholar]
- 22.Holdiness, M. R. 1984. Clinical pharmacokinetics of the antituberculosis drugs. Clin. Pharmacokinet. 9:511-544. [DOI] [PubMed] [Google Scholar]
- 23.Jayaram, R., S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharat, R. K. Shandil, E. Kantharaj, and V. Balasubramanian. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji, B., A. Chauffour, J. Robert, S. Lefrancois, and V. Jarlier. 2007. Orally administered combined regimens for treatment of Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 51:3737-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsollier, L., N. Honore, P. Legras, A. L. Manceau, H. Kouakou, B. Carbonnelle, and S. T. Cole. 2003. Isolation of three Mycobacterium ulcerans strains resistant to rifampin after experimental chemotherapy of mice. Antimicrob. Agents Chemother. 47:1228-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nienhuis, W. A., Y. Stienstra, W. A. Thompson, P. C. Awuah, K. M. Abass, W. Tuah, N. Y. Awua-Boateng, E. O. Ampadu, V. Siegmund, J. P. Schouten, O. Adjei, G. Bretzel, and T. S. van der Werf. 2010. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375:664-672. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira, M. S., A. G. Fraga, E. Torrado, A. G. Castro, J. P. Pereira, A. L. Filho, F. Milanezi, F. C. Schmitt, W. M. Meyers, F. Portaels, M. T. Silva, and J. Pedrosa. 2005. Infection with Mycobacterium ulcerans induces persistent inflammatory responses in mice. Infect. Immun. 73:6299-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peloquin, C. A., and S. E. Berning. 1997. Evaluation of the drug interaction between clarithromycin and rifampin. J. Infect. Dis. Pharmacother. 2:19-35. [Google Scholar]
- 29.Peloquin, C. A., R. Namdar, M. D. Singleton, and D. E. Nix. 1999. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 115:12-18. [DOI] [PubMed] [Google Scholar]
- 30.Portaels, F., H. Traore, K. De Ridder, and W. M. Meyers. 1998. In vitro susceptibility of Mycobacterium ulcerans to clarithromycin. Antimicrob. Agents Chemother. 42:2070-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proost, J. H., and D. K. Meijer. 1992. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput. Biol. Med. 22:155-163. [DOI] [PubMed] [Google Scholar]
- 32.Rodvold, K. A. 1999. Clinical pharmacokinetics of clarithromycin. Clin. Pharmacokinet. 37:385-398. [DOI] [PubMed] [Google Scholar]
- 33.Stinear, T. P., M. J. Pryor, J. L. Porter, and S. T. Cole. 2005. Functional analysis and annotation of the virulence plasmid pMUM001 from Mycobacterium ulcerans. Microbiology 151:683-692. [DOI] [PubMed] [Google Scholar]
- 34.Torrado, E., A. G. Fraga, A. G. Castro, P. Stragier, W. M. Meyers, F. Portaels, M. T. Silva, and J. Pedrosa. 2007. Evidence for an intramacrophage growth phase of Mycobacterium ulcerans. Infect. Immun. 75:977-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traunmuller, F., M. Zeitlinger, P. Zeleny, M. Muller, and C. Joukhadar. 2007. Pharmacokinetics of single- and multiple-dose oral clarithromycin in soft tissues determined by microdialysis. Antimicrob. Agents Chemother. 51:3185-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Werf, T. S., T. Stinear, Y. Stienstra, W. T. van der Graaf, and P. L. Small. 2003. Mycolactones and Mycobacterium ulcerans disease. Lancet 362:1062-1064. [DOI] [PubMed] [Google Scholar]
- 37.Wallace, R. J., Jr., B. A. Brown, D. E. Griffith, W. Girard, and K. Tanaka. 1995. Reduced serum levels of clarithromycin in patients treated with multidrug regimens including rifampin or rifabutin for Mycobacterium avium-M. intracellulare infection. J. Infect. Dis. 171:747-750. [DOI] [PubMed] [Google Scholar]
- 38.Williamson, H. R., M. E. Benbow, K. D. Nguyen, D. C. Beachboard, R. K. Kimbirauskas, M. D. McIntosh, C. Quaye, E. O. Ampadu, D. Boakye, R. W. Merritt, and P. L. Small. 2008. Distribution of Mycobacterium ulcerans in Buruli ulcer endemic and non-endemic aquatic sites in Ghana. PLoS Negl. Trop. Dis. 2:e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zent, C., and P. Smith. 1995. Study of the effect of concomitant food on the bioavailability of rifampicin, isoniazid and pyrazinamide. Tuber. Lung Dis. 76:109-113. [DOI] [PubMed] [Google Scholar]