Abstract
We have evaluated the clinical efficacy of the combination of oral rifampin at 10 mg/kg of body weight and intramuscular streptomycin at 15 mg/kg for 8 weeks (RS8), as recommended by the WHO, in 160 PCR-confirmed cases of Mycobacterium ulcerans disease. In 152 patients (95%) with all forms of disease from early nodules to large ulcers, with or without edema, the lesions healed without recourse to surgery. Eight patients whose ulcers were healing poorly had skin grafting after completion of antibiotics. There were no recurrences among 158 patients reviewed at the 1-year follow-up. The times to complete healing ranged from 2 to 48 weeks, according to the type and size of the lesion, but the average rate of healing (rate of reduction in ulcer diameter) varied widely. Thirteen subjects had positive cultures for M. ulcerans during or after treatment, but all the lesions healed without further antibiotic treatment. Adverse events were rare. These results confirm the efficacy of RS8 delivered in a community setting.
Mycobacterium ulcerans disease, known as Buruli ulcer, is a chronic subcutaneous infection which is common in humid rural tropical areas. The majority of patients are children aged less than 15 years living in rural areas remote from a hospital (29). Infection initially manifests as a painless nodule or plaque which breaks down centrally to form an ulcer with undermined edges (11). In a small proportion of lesions, there is edema around the ulcer which spreads rapidly, resulting in a very large ulcer. Significant morbidity results from Mycobacterium ulcerans disease when there is extensive scarring or functional limitation from contractures at joints, and there is occasionally a need for amputation of a limb. Ulcers or scars rarely undergo malignant transformation (12).
Until recently, the mainstay of treatment for Buruli ulcer was excision of lesions with a wide margin to ensure complete removal of infected tissue. Recurrence rates after surgery varied between 6 and 17%, depending on the type and extent of the lesion and on the experience and skill of the surgeon (1, 6, 22). Recent evidence that antibiotics are effective has shifted the balance between surgery and antibiotics. In vitro, M. ulcerans has been shown to be susceptible to rifampin (13), aminoglycosides (7), macrolides (21), and quinolones (26). Infection of mouse footpads has been used as a model for susceptibility testing, and after treatment with the same antibiotics, the lesions became smaller and the total number of M. ulcerans organisms in tissue was reduced (2, 8, 25). The combination of rifampin with amikacin or streptomycin for 8 weeks has been the most effective in reducing the bacterial load in a number of studies, and the low relapse rate after treatment suggested that this combination was bactericidal (2, 15). An important reason for using more than one drug is that resistant mutants were found after rifampin monotherapy in mice (16).
The bactericidal effect of rifampin at 10 mg/kg of body weight orally combined with streptomycin at 15 mg/kg intramuscularly daily was evaluated in humans with early M. ulcerans lesions which were excised for quantitative culture after treatment for 0, 2, 4, 8, or 12 weeks (10). Cultures for M. ulcerans were positive at the baseline and after treatment for 2 weeks but negative thereafter, and most lesions became smaller during treatment. On the basis of these observations, the WHO Advisory Group on Buruli ulcer issued interim guidelines recommending use of the above regimen for 8 weeks (RS8). Evidence from an observational study of 219 patients offered antibiotic treatment with or without surgery in Benin has subsequently been published (3). A total of 122 patients received RS8 without surgery, and 3 of these were lost to follow-up. Seventy-four percent healed after 8 weeks of treatment, and healing continued after treatment finished, with the overall success rate being 97.5% and the relapse rate being only 1.4%. More recently, a study comparing rifampin and streptomycin for 8 weeks with the same combination for 4 weeks followed by rifampin and clarithromycin for 4 weeks showed no difference between the two regimens and there were no recurrences (17).
We report on the clinical efficacy of RS8 in 160 patients with the full spectrum of M. ulcerans disease, most of whom were treated without surgical intervention in a community setting in Ghana. Post hoc analysis of the data was performed to determine the time to complete healing and the rate of healing of M. ulcerans disease while the patients were receiving this treatment regimen.
MATERIALS AND METHODS
Patients.
From September 2005 to December 2007, patients with a clinical diagnosis of M. ulcerans disease were actively recruited from villages in the Ahafo Ano North and Ashanti Akim Districts of the Ashanti Region of Ghana, where Buruli ulcer is endemic, as well as the Tano North and Asutifi Districts of the Brong Ahafo Region of Ghana. Patients were included if they met the WHO clinical case definition for M. ulcerans disease with a nodule, plaque, edema, or ulcer. Patients were excluded if they had tuberculosis, leprosy, or clinical and/or laboratory evidence of significant renal or hepatic impairment or auditory problems and if they were already receiving treatment with antibiotics or herbal preparations. Patients were not tested for HIV infection. Informed consent (the thumbprint or signature of the patient, depending on the patient's literacy) was sought before the patients were included in the study. The study protocol was approved by the Ethics Review Committees at the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, and St. George's, University of London, London, United Kingdom.
Treatment protocol.
A thorough history and clinical examination were performed by an experienced physician, and findings were recorded on the BU01 forms issued by the WHO. Lesions were categorized into (i) category I, which consisted of a lesion size of <5 cm at the widest diameter; (ii) category II, which consisted of a lesion size of between 5 and 15 cm at the widest diameter; and (iii) category III; which consisted of a lesion size >15 cm at the widest diameter. The presence and extent of edema were recorded. Lesions involving critical sites, such as the eyes, genitalia, and breasts, were included. Pregnancy tests were performed for all females above 15 years of age. Pregnant patients were treated with a regular wound dressing 5 to 7 days/week and kept under observation until after delivery, when they were offered standard treatment with RS8. Antibiotic treatment with oral rifampin at 10 mg/kg daily and intramuscular streptomycin at 15 mg/kg daily for 8 weeks was started on the basis of the clinical diagnosis, and treatment was continued only if the PCR result was positive (the result was available within 2 weeks). Within each of the four districts in which cases were recruited, treatment was administered 7 days a week at village health posts, situated less than a kilometer away from the patients, under the direct observation of an appropriately trained health care worker, and compliance was recorded by ticking on a treatment card. Patients who defaulted were rapidly traced by health workers. Dressings for ulcers were provided.
A simple assessment of limitation of joint movement adjacent to lesions was made, and if any limitation was detected, the patient and/or parent or guardian was taught some simple self-administered physiotherapy to mobilize the joint.
Diagnostic confirmation.
Three different sampling techniques were used to obtain specimens to confirm the presence of M. ulcerans in lesions. (i) Swabs were taken from the undermined edges of ulcerative lesions and analyzed for the presence of acid-fast bacilli (AFB), culture for M. ulcerans, and PCR for IS2404. (ii) Four-millimeter-diameter punch biopsy specimens were taken from nonulcerative lesions and from the margin of viable tissue in ulcerative lesions for analysis for the presence of AFB, semiquantitative culture, and PCR. (iii) Fine-needle aspirates (FNAs) were validated and used for PCR analysis, as described previously (20). This technique was introduced during the study period.
All specimens were processed at the Komfo Anokye Teaching Hospital in Kumasi, Ghana. Swabs and homogenized skin tissue specimens were stained for AFB by the Ziehl-Neelsen technique. For semiquantitative culture, 10 ml of skin homogenate was decontaminated using the N-acetyl cysteine-sodium hydroxide method (23) for 10 min. Tenfold dilutions of homogenized tissue up to 10−4 were inoculated on Lowenstein-Jensen slopes in triplicate, and a volume of 1 ml each dilution was used.
PCR targeting the IS2404 insertion sequence was performed as described previously (20). For lesions that enlarged or failed to show significant regression during antibiotic treatment, further biopsies were performed at 6 and 12 weeks to obtain specimens for quantitative culture to assess the in vivo killing of M. ulcerans.
Clinical response to antibiotic therapy.
Clinical response was assessed fortnightly during antibiotic treatment to monitor the time to complete healing and to detect recurrences. Serial photographs were taken until healing was complete, and when lesions could be traced onto acetate paper, the mean diameter was calculated by measuring the maximum diameter and that at right angles to it. The rate of reduction in diameter per week was calculated as the pretreatment mean diameter divided by the number of weeks to complete healing. After completion of antibiotic treatment, daily dressing with normal saline was continued until the ulcer healed and the patients were reviewed fortnightly. Thereafter, they were reviewed monthly for a year to detect recurrences. Patients defaulting the scheduled follow-up visits during the treatment period were traced by village health care workers, who assessed their compliance with therapy. If the patients had defaulted therapy for 14 or more consecutive days, they were retreated according to the WHO recommendations. New lesions occurring during the follow-up period were cultured to distinguish recurrent or new infection from sterile inflammatory lesions.
Patients whose lesions enlarged during or after treatment were offered excision surgery. Patients whose lesions had not healed after 8 weeks of antibiotic therapy were managed by daily dressing until healing was complete. Surgical excision and grafting were offered to patients whose lesions were not healing well. An assessment of functional limitations was performed before and after completion of antibiotic therapy. At each fortnightly clinician assessment, the patients were questioned about side effects from the antibiotic treatment and were asked to report any problems to the health center between periodic reviews. Further assessments were made as indicated by the history and examination. Side effects were judged to be mild (grade 1), moderate (grade 2), severe (grade 3), or life-threatening (grade 4), according to the NIH/NCI Common Toxicity Criteria.
Statistical analyses.
The median time to complete healing and the rate of healing of lesions were compared using the Mann-Whitney U test, and a P value of less than 0.05 was considered statistically significant.
RESULTS
Patient characteristics.
One hundred seventy-one patients with a clinical diagnosis of M. ulcerans disease were recruited, but diagnostic samples were not available from six patients and five patients had negative laboratory results. Sixty-seven percent, 27%, 5%, and 1% of the subjects were recruited from the Ahafo Ano North, Asutifi, Tano North, and Ashanti Akim Districts, respectively. Table 1 shows the clinical and demographic characteristics of the 160 evaluable patients before antibiotic treatment. The median age was 12 years (range, 1 to 75 years), and there was a preponderance of females over males at a ratio of 1.4:1. Fifty-four percent of the patients had ulcers, 22% had nodules, 9% had plaques, and 15% had edematous lesions (n = 160). Three percent of the lesions were localized on the head and neck, 45% on the upper limb, 8% on the trunk, and 44% on the lower limb. Before treatment, 30% of the lesions were classified as category I, 35% were category II, and 35% were category III. Three patients were pregnant at the time of recruitment. Nine lesions on the upper limbs and six on the lower limbs were associated with restricted joint movements before antibiotic therapy commenced.
TABLE 1.
Clinical and demographic data for study participants
| Characteristic | Form of M. ulcerans lesion |
||||
|---|---|---|---|---|---|
| Nodule (n = 36) | Plaque (n = 14) | Edema (n = 24) | Ulcer (n = 86a) | Total (n = 160) | |
| No. of males:no. of females | 13:23 | 8:6 | 12:12 | 33:53 | 66:94 |
| Age (yr) | |||||
| Median | 13 | 8 | 11 | 14 | 12 |
| Range | 2-54 | 2-60 | 3-42 | 1-75 | 1-75 |
| Site of lesionb | |||||
| Head and neck | 1 (3) | 1 (8) | 1 (4) | 2 (2) | 5 (3) |
| Upper limbs | 22 (61) | 9 (64) | 12 (50) | 29 (34) | 72 (45) |
| Trunk | 1 (3) | 2 (14) | 2 (8) | 7 (8) | 12 (8) |
| Lower limbs | 12 (33) | 2 (14) | 9 (38) | 48 (56) | 71 (44) |
| Lesion category (diam)b | |||||
| I (<5 cm) | 29 (81) | 2 (14) | 1 (4) | 16 (19) | 48 (30) |
| II (5-14.9 cm) | 7 (19) | 12 (86) | 4 (17) | 33 (38) | 56 (35) |
| III (>15 cm) | 0 (0) | 0 (0) | 19 (79) | 37 (43) | 56 (35) |
Eleven edematous lesions, one nodule, and two plaques had ulcerated at presentation.
Data represent the number (percent) of patients.
Laboratory diagnosis.
The diagnosis of M. ulcerans infection was confirmed by detection of AFB in 22% of patients tested (n = 120), by culture in 28% (n = 120), and by PCR in 97% (n = 160). The diagnosis was confirmed by one or more of these methods in all cases. An analysis of the laboratory results is shown in Table 2.
TABLE 2.
Laboratory data for confirmed cases of M. ulcerans disease
| Lesion form | Diagnostic investigation | Sampling technique |
Total no. positive/total no. tested (%) | ||
|---|---|---|---|---|---|
| FNA | Punch biopsy | Swab | |||
| Nodule (n = 36) | No. of samples | 21 | 29 | 1 | |
| No. ZNa +/no. ZN − | NDb | 6/18 | ND | 6/24 (25) | |
| No. culture +/no. culture − | ND | 11/14 | ND | 11/25 (44) | |
| No. PCR +/no. PCR− | 19/2 | 28/1 | 0 | 35/36 (97) | |
| Plaque (n = 14) | No. of samples | 4 | 14 | 0 | |
| No. ZN +/no. ZN − | ND | 4/4 | 0 | 4/8 (50) | |
| No. culture +/no. culture − | ND | 2/6 | 0 | 2/8 (25) | |
| No. PCR +/no. PCR− | 4/0 | 11/1 | 0 | 13/14 (93) | |
| Edema (n = 24) | No. of samples | 10 | 21 | 2 | |
| No. ZN +/no. ZN − | ND | 4/14 | 1/1 | 5/20 (25) | |
| No. culture +/no. culture − | ND | 6/15 | 0 | 6/21 (29) | |
| No. PCR +/no. PCR− | 9/1 | 20/0 | 1/1 | 23/24 (96) | |
| Ulcer (n = 86) | No. of samples | 39 | 57 | 28 | |
| No. ZN +/no. ZN − | ND | 6/44 | 5/13 | 11/68 (16) | |
| No. culture +/no. culture − | ND | 12/37 | 2/15 | 14/66 (21) | |
| No. PCR +/no. PCR− | 37/2 | 49/1 | 27/0 | 84/86 (98) | |
ZN, Ziehl-Neelsen staining.
ND, not determined.
Clinical response to antibiotic treatment.
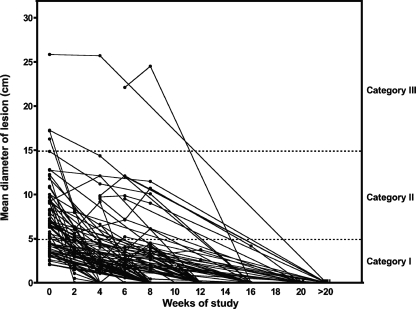
There was a progressive diminution in the sizes of most lesions during and after antibiotic treatment, although a few lesions enlarged before they started to heal (Fig. 1). The median diameter of the lesions which could be measured declined during antibiotic treatment from 6.4 cm (range, 2.1 to 25.9 cm; n = 72) before treatment to 2.1 cm (range, 0 to 25.7 cm; n = 41) at 4 weeks (P < 0.0001) and to 1.4 cm (range, 0.0 to 24.5 cm; n = 60) at 8 weeks (P < 0.0001 compared to the baseline).
FIG. 1.
Changes in the mean diameter of Buruli ulcer lesions in subjects whose lesions could be traced onto acetate sheets during and after treatment with rifampin and streptomycin for 8 weeks. The lines connect the data for individual subjects. Category I is lesions <5 cm in diameter, category II is lesions 5 to 15 cm, and category III is lesions >15 cm.
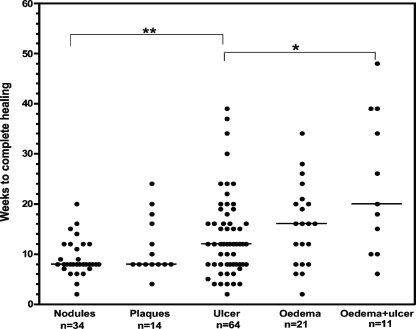
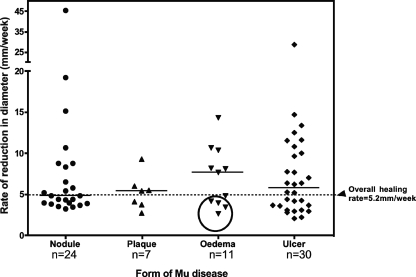
The median time to complete healing was measured in 144 patients who attended for regular follow-up after completing antibiotic treatment. The median time to complete healing of nodules (8 weeks; range, 2 to 20 weeks) was similar to that of plaques (8 weeks; range, 4 to 24 weeks) (Fig. 2). The median healing time for all ulcers was 12 weeks (range, 2 to 39 weeks); the times were 12 weeks (range, 4 to 30 weeks) for category I ulcers, 11 weeks (range, 4 to 20 weeks) for category II ulcers, and 15.5 weeks (range, 8 to 39 weeks) for category III ulcers (P < 0.05 compared with category II ulcers). There was a wide range in the healing times of edematous lesions, ranging from 2 to 48 weeks. When ulcers developed within an edematous area, they took significantly longer to heal than ulcers of a similar size without edema (Fig. 2). The rate of healing of each measurable lesion was calculated by dividing the mean diameter before treatment by the time in weeks to complete healing. The median rate of reduction in the diameter of all lesions was 5.2 mm/week and had a wide range of from 2.1 to 45.5 mm/week (n = 72). Nodules, plaques, edemas, and ulcers reduced at rates of 4.9 mm/week (range, 3.3 to 45.5 mm/week), 5.4 mm/week (range, 2.7 to 9.3 mm/week), 7.7 mm/week (range, 2.7 to 14.4 mm/week), and 5.8 mm/week (range, 2.1 to 28.7 mm/week), respectively, as shown in Fig. 3. In order to see whether all ulcers healed at the same rate after completion of antibiotic treatment, ulcers that could be measured reliably at 8 weeks or more were analyzed separately. The healing rates of this subgroup were 2.6 mm/week (range, 1.5 to 3.6 mm/week; n = 6) from 0 to 8 weeks and 3.7 mm/week (2.9 to 12.5 mm/week; n = 5) after completion of antibiotic treatment (P < 0.05).
FIG. 2.
Time to complete healing. Each dot represents one study subject treated with rifampin and streptomycin for 8 weeks. Horizontal lines represent the medians. The results for eight patients who had surgery, seven patients who defaulted from follow-up after completion of antibiotic treatment, and one patient who died are not included. **, P < 0.005; *, P < 0.01.
FIG. 3.
Rate of healing. The rate of healing was calculated by dividing the diameter of the lesions (in mm) before treatment by the time (in weeks) to complete healing. Horizontal lines represent the median rates of healing, and the broken line shows the overall median healing rate in 72 patients whose lesions were measurable before treatment. The circle highlights edematous lesions with ulceration. MU, M. ulcerans.
Culture for M. ulcerans.
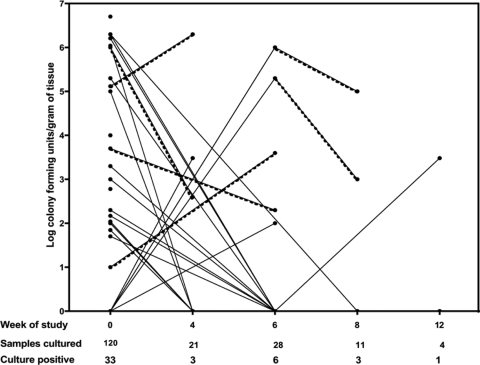
Prior to treatment, culture for M. ulcerans was positive for 33 (27.5%) out of 120 specimens, and the median was 3.3 log CFU/g of tissue (range, 1.0 to 6.7 log CFU/g) in positive cultures (Fig. 4). During treatment, samples were taken for culture after 4 or 6 weeks from 49 patients whose lesions were not healing satisfactorily. Of these, 9 (18%) were culture positive, and the median was 3.5 log CFU/g of tissue (range, 2.0 to 6.3 log CFU/g). Four of 15 (27%) samples taken either upon completion of antibiotics (n = 3) or 4 weeks later (n = 1) remained culture positive, and the median was 2.7 log CFU/g of tissue (range, 2 to 5.0 log CFU/g). All four of these lesions were category III ulcerative lesions that subsequently healed completely, with the median time to healing being 20 weeks (range, 12 to 48 weeks) and a diameter regression rate of 3.2 mm/week (range, 1.5 to 7.7 mm/week).
FIG. 4.
Semiquantitative culture for M. ulcerans. Samples were cultured from 120 lesions before treatment with RS for 8 weeks and from 64 selected cases responding poorly at 4, 6, 8, or 12 weeks. Negative cultures at each time point are shown as a single point on the baseline. Each line joins the data for samples taken from the same patient, and dotted lines highlight the results for six patients who had positive cultures at two different time points.
Paradoxical responses.
New inflammatory lesions developed close to or within the vicinity of healing lesions in three patients. They appeared 4, 6, and 12 weeks after the start of antibiotic treatment, respectively. The new lesions were fluctuant, erythematous, and nontender. Pus was aspirated from all lesions and was sterile after prolonged culture. All lesions healed after aspiration, and there was no need for further antibiotic therapy.
Surgical interventions.
Two patients with nodules and one with a plaque which progressively enlarged and ulcerated during treatment, five with ulcerated edematous lesions, and two with large ulcers were offered surgical debridement and grafting after 8 weeks antibiotic treatment because of unsatisfactory healing. Eight out of the 10 patients accepted surgery, and split skin grafting was carried out between weeks 8 and 12. All except one of the patients healed without complications within 8 to 13 weeks. In one patient, inflammation persisted in the graft site and healing was delayed until week 32. This was a 24-year-old farmer with a category III ulcerated edema who responded poorly to RS8, so a further 4 weeks of RS therapy was administered followed by surgery. A punch biopsy specimen taken for culture 8 weeks after the skin graft had broken down was positive. Daily dressings were continued until the ulcer healed at week 32.
One patient died 12 weeks after completing treatment. This 35-year-old farmer had multiple ulcerated edematous lesions (category III) on the left lower limb which did not improve during antibiotic therapy. He declined surgery and was lost to follow-up. A village health care worker from a different district reported that he died, possibly from sepsis.
Follow-up.
Seven patients failed to attend for review of healing after completing antibiotic treatment, so neither the time to complete healing nor the rate of healing could be calculated. However, of 160 patients treated with RS8, with or without surgery, 158 were reviewed 1 year after the completion of treatment. The only patient who received RS for 12 weeks was also reviewed a year later. All lesions had healed, and there had been no recurrences.
Functional limitations.
Fifteen patients had functional limitation of joint movement before antibiotic therapy, and all of them were advised to self-administer physiotherapy during antibiotic treatment. Eight had category III ulcers and six had category III edema; in three of the patients with edema, the lesions ulcerated at the time of presentation. One patient with a nodule on the left forearm had a contracture at the elbow of the affected limb secondary to a previous healed ulcer. This patient was excluded from the analysis of Buruli ulcer-associated functional limitation. Eight lesions were situated near the elbow joint, four near the knee joint, and two near the ankle joint. The median time to healing among this subgroup of patients was 25 weeks (range, 8 to 48 weeks), and upon healing the restriction of joint movements had resolved in 10 patients. The remaining four patients had persistent limitation of flexion that subsequently improved with physiotherapy, and only one patient still had a minor flexion deformity of the knee joint with a limp at the 1-year review.
Management of pregnant patients.
Three patients were pregnant at the time of presentation with M. ulcerans disease. Two, aged 20 and 26 years, who were in the third trimester had category III ulcers on their lower limbs, and the third, aged 38, was in the second trimester and had a category II ulcer on the upper limb. All three were managed conservatively with daily wound dressings until 8 weeks after delivery, following which antibiotic treatment with RS8 was administered, according to WHO recommendations.
Adverse events.
Three adverse events were attributed to antibiotic therapy. A woman aged 54 years developed dizziness (grade 1) 4 weeks into treatment and a woman aged 63 years had protracted vomiting and dizziness (grade 2) 2 weeks into treatment. These were attributed to streptomycin, which was stopped, and moxifloxacin at 400 mg daily was substituted. A 32-year-old woman with a category II ulcer developed a maculopapular rash and general malaise (grade 1) 4 weeks into treatment which were thought to be due to rifampin. Treatment was stopped, and the ulcer was managed by daily wound dressing until it healed 24 weeks after the start of treatment.
DISCUSSION
The findings from this study confirm the efficacy of rifampin combined with streptomycin in healing all forms of M. ulcerans disease. In contrast to the Benin study (3), 95% of patients in the present study were treated without surgery and all lesions healed within 2 to 48 weeks. There were no recurrences during a 12-month follow-up period, comparing favorably with the 1.4% recurrence rate observed in Benin and the 0% rate observed in a recent controlled trial in Ghana (17). Although the natural history of the disease is poorly documented, the recurrence rate after surgery has been reported to be between 6 and 17% in various studies (1, 6). Therefore, 8 weeks treatment with this combination of antibiotics has set a standard of treatment against which other antibiotic combinations can be tested in the future.
The aims of treatment can be defined clinically and bacteriologically. Clinically, the aims were to achieve full healing of lesions with minimal scarring, no functional limitation, and no recurrence. There was complete clinical resolution of all forms and categories of M. ulcerans disease within 1 year, with the median times to complete healing being 8, 10, and 20 weeks for category I, II, and III M. ulcerans disease, respectively (data not shown). This compares favorably with 18 weeks for category I and 30 weeks for categories II and III combined reported in both arms of the clinical trial in which RS8 was compared with RS for 4 weeks followed by rifampin and clarithromycin for 4 weeks (17). Natural history has not been documented except in a small placebo-controlled trial of clofazamine treatment, in which 10 patients out of a group of 34 with lesions less than 5 cm in diameter healed spontaneously in a median of 14 weeks. The other 24 required surgery. However, there was a high recurrence rate (29.4%) in the group of 34 patients (22). The present study did not include any measures of severity of scars. However, there were no scars after healing of nonulcerated lesions for a total of 72 patients (34 with nodules, 14 with plaques, 24 with edema without ulceration) who would otherwise have had a surgical scar, which may be larger than the lesion itself because surgeons leave a wide margin.
Another clinical aim is to achieve the maximum rate of healing. This is more difficult to measure than the time to complete healing, but the latter is dependent on the size of the initial lesion. When M. ulcerans infection has been fully treated, it is expected that Buruli ulcers will heal evenly from the outside inwards, provided they are kept clean and infection free. The rate of healing was calculated when lesions could be measured before treatment by tracing their margins onto acetate sheets and the time at which complete healing occurred was known. The rate of reduction in diameter was used as a measure of healing rather than the change in surface area, since this is expected to be linear. There was considerable variation in the healing rates overall (Fig. 2) and also in the small subset of ulcers for which the healing rates after treatment could be calculated. Further study of a larger group of patients is required to investigate the causes of slow healing.
Most nodules resolved within 8 weeks, emphasizing the benefit of early recognition of M. ulcerans lesions. Large edematous lesions could not be measured, but they regressed rapidly during treatment, sometimes without developing ulceration. However, when edematous lesions ulcerated they healed at the same rate or more slowly than ulcers without edema. The pathogenesis of edema, associated with 15% of Buruli lesions in the present study, is unknown. Its onset is usually within the first few weeks of the lesion appearing, and it sometimes spreads rapidly over a large area, giving rise to some of the most extensive lesions.
Samples taken from individuals whose ulcers were healing slowly showed that there was persistent infection with M. ulcerans in nine patients sampled at 4 or 6 weeks and in four patients who had completed treatment. These results were surprising, since all cultures were negative after treatment with SR for 4 weeks in another study, but only early lesions were included and the number of subjects was small (10). False-negative cultures are found in 40 to 60% of untreated ulcers (9, 14, 20) due to sampling error, so it is possible that more than four patients in the present study had persistent infection after completing RS8 treatment. We were not able to demonstrate a reduction in the quantity of M. ulcerans organisms isolated from tissue samples during antibiotic treatment, and semiquantitative culture of small punch biopsy specimens may not be a suitable way to compare the efficacies of new antibiotic regimens with the efficacy of RS8 in the future. However, the fact that some cultures remained positive is important, since it may explain why some lesions healed more slowly after completion of antibiotic therapy. Persisting viable organisms in these lesions may continue producing mycolactone at low concentrations, which would inhibit production of growth factors important for wound healing (4, 18, 27).
Despite having positive cultures during or after treatment, all the lesions healed without further antibiotic treatment and without recurrences, suggesting that the immune response to M. ulcerans was sufficient to contain the organism once mycolactone production had been inhibited. Other factors that affect the healing rate of wounds include the quality of wound dressing procedures and the nutritional status of the patient. The quality of wound dressing was uniform in this study, and there were no overt signs of malnutrition, although minor deficiencies cannot be ruled out.
Paradoxical reactions occurred in three patients at weeks 4, 6, and 12, respectively. Aspirated pus was sterile on prolonged incubation, suggesting that the reaction was caused by a vigorous inflammatory response rather than continuing infection. At low concentrations, mycolactone inhibits chemotaxis and some aspects of the immune response (5, 28), so as the antibiotic treatment kills the mycobacteria, an intense inflammatory response to high concentrations of organisms may be expected. The implication is that there is no indication to extend antibiotic treatment, and all the lesions in this series of patients with paradoxical reactions healed with RS8 treatment alone, with the median healing time being 12 weeks (range, 8 to 16 weeks).
When any limitation of joint flexion was detected at the time of diagnosis, patients were taught simple techniques for mobilizing the joint which they continued during antibiotic therapy and subsequently, if it was required. As a result, only 1 patient out of 15 in whom it was detected at diagnosis had a persistent functional limitation at the end of follow-up. This supports the WHO strategy for management of the functional disabilities associated with Buruli ulcer (24).
Administration of RS8 was accomplished in the present study through a network of village health care workers trained for the purpose. They achieved a high rate of compliance through a simple system of observation and documentation, and the treatment was popular with the patients. Ten patients were offered surgery, and two of those declined, possibly through fear of consequences such as amputation. In both cases, the lesion healed without further intervention. Patients in Western societies usually prefer early closure of wounds, but the perception of surgery is different in countries where Buruli ulcer is endemic, and in reality, it is not available to most patients.
The treatment was well tolerated overall, but two adults had side effects attributed to streptomycin, after treatment for 2 or 4 weeks. Moxifloxacin was substituted, and both lesions resolved after treatment for a total of 8 weeks. There is one report of a small series of patients treated successfully with rifampin and moxifloxacin for 8 weeks (19). Recently, a clinical trial has shown that clarithromycin can be substituted for streptomycin during the second 4 weeks of an 8-week regimen without any loss of benefit and with no major side effects (17). One patient in the present study had a reaction to rifampin after 4 weeks. Since the Buruli ulcer lesion was healing well, no further antibiotics were given and healing continued uneventfully. Treatment for 8 weeks was recommended by the WHO Technical Advisory Group when a study showed that M. ulcerans organisms were still viable in human tissue after treatment with RS for 2 weeks but not after treatment for 4, 8, or 12 weeks (10). It was judged that a safe margin should be allowed by recommending treatment for 8 weeks, but the above case shows that a shorter duration may be adequate in some instances.
In conclusion, RS8 was a highly successful and practical treatment for all forms of M. ulcerans disease. Its success was particularly notable in edematous disease, which has been associated with development of extensive ulcers. More work needs to be done to investigate the factors responsible for variations in the rate of healing and to define the optimal duration of therapy.
Footnotes
Published ahead of print on 21 June 2010.
REFERENCES
- 1.Amofah, G., S. Asamoah, and C. Afram-Gyening. 1998. Effectiveness of excision of pre-ulcerative Buruli lesions in field situations in a rural district in Ghana. Trop. Doct. 28:81-83. [DOI] [PubMed] [Google Scholar]
- 2.Bentoucha, A., J. Robert, H. Dega, N. Lounis, V. Jarlier, and J. Grosset. 2001. Activities of new macrolides and fluoroquinolones against Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 45:3109-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauty, A., M. F. Ardant, A. Adeye, H. Euverte, A. Guedenon, C. Johnson, J. Aubry, E. Nuermberger, and J. Grosset. 2007. Promising clinical efficacy of streptomycin-rifampin combination for treatment of Buruli ulcer (Mycobacterium ulcerans disease). Antimicrob. Agents Chemother. 51:4029-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutanceau, E., J. Decalf, A. Martino, A. Babon, N. Winter, S. T. Cole, M. L. Albert, and C. Demangel. 2007. Selective suppression of dendritic cell functions by Mycobacterium ulcerans toxin mycolactone. J. Exp. Med. 204:1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutanceau, E., L. Marsollier, R. Brosch, E. Perret, P. Goossens, M. Tanguy, S. T. Cole, P. L. Small, and C. Demangel. 2005. Modulation of the host immune response by a transient intracellular stage of Mycobacterium ulcerans: the contribution of endogenous mycolactone toxin. Cell. Microbiol. 7:1187-1196. [DOI] [PubMed] [Google Scholar]
- 6.Debacker, M., J. Aguiar, C. Steunou, C. Zinsou, W. M. Meyers, and F. Portaels. 2005. Buruli ulcer recurrence, Benin. Emerg. Infect. Dis. 11:584-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dega, H., A. Bentoucha, J. Robert, V. Jarlier, and J. Grosset. 2002. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob. Agents Chemother. 46:3193-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dega, H., J. Robert, P. Bonnafous, V. Jarlier, and J. Grosset. 2000. Activities of several antimicrobials against Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 44:2367-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddyani, M., M. Debacker, A. Martin, J. Aguiar, C. R. Johnson, C. Uwizeye, K. Fissette, and F. Portaels. 2008. Primary culture of Mycobacterium ulcerans from human tissue specimens after storage in semisolid transport medium. J. Clin. Microbiol. 46:69-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etuaful, S., B. Carbonnelle, J. Grosset, S. Lucas, C. Horsfield, R. Phillips, M. Evans, D. Ofori-Adjei, E. Klustse, J. Owusu-Boateng, G. K. Amedofu, P. Awuah, E. Ampadu, G. Amofah, K. Asiedu, and M. Wansbrough-Jones. 2005. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob. Agents Chemother. 49:3182-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, M. R., R. Phillips, S. N. Etuaful, G. Amofah, J. Adomako, O. Adjei, J. Dennis-Antwi, S. B. Lucas, and M. H. Wansbrough-Jones. 2003. An outreach education and treatment project in Ghana for the early stage of Mycobacterium ulcerans disease. Trans. R. Soc. Trop. Med. Hyg. 97:159-160. [DOI] [PubMed] [Google Scholar]
- 12.Evans, M. R. W., S. N. Etuaful, G. Amofah, O. Adjei, S. Lucas, and M. H. Wansbrough-Jones. 1999. Squamous cell carcinoma secondary to Buruli ulcer. Trans. R. Soc. Trop. Med. Hyg. 93:63-64. [DOI] [PubMed] [Google Scholar]
- 13.Havel, A., and S. R. Pattyn. 1975. Activity of rifampicin on Mycobacterium ulcerans. Ann. Soc. Belg. Med. Trop. 55:105-108. [PubMed] [Google Scholar]
- 14.Herbinger, K. H., O. Adjei, N. Y. Awua-Boateng, W. A. Nienhuis, L. Kunaa, V. Siegmund, J. Nitschke, W. Thompson, E. Klutse, P. Agbenorku, A. Schipf, S. Reu, P. Racz, B. Fleischer, M. Beissner, E. Fleischmann, K. Helfrich, T. S. van der Werf, T. Loscher, and G. Bretzel. 2009. Comparative study of the sensitivity of different diagnostic methods for the laboratory diagnosis of Buruli ulcer disease. Clin. Infect. Dis. 48:1055-1064. [DOI] [PubMed] [Google Scholar]
- 15.Lefrancois, S., J. Robert, A. Chauffour, B. Ji, and V. Jarlier. 2007. Curing Mycobacterium ulcerans infection in mice with a combination of rifampin-streptomycin or rifampin-amikacin. Antimicrob. Agents Chemother. 51:645-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsollier, L., N. Honore, P. Legras, A. L. Manceau, H. Kouakou, B. Carbonnelle, and S. T. Cole. 2003. Isolation of three Mycobacterium ulcerans strains resistant to rifampin after experimental chemotherapy of mice. Antimicrob. Agents Chemother. 47:1228-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nienhuis, W. A., Y. Stienstra, W. A. Thompson, P. C. Awuah, K. M. Abass, W. Tuah, N. Y. Awua-Boateng, E. O. Ampadu, V. Siegmund, J. P. Schouten, O. Adjei, G. Bretzel, and T. S. van der Werf. 2010. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375:664-672. [DOI] [PubMed] [Google Scholar]
- 18.Pahlevan, A. A., D. J. M. Wright, C. Andrews, K. M. George, P. L. C. Small, and B. M. Foxwell. 1999. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-kappa B function. J. Immunol. 163:3928-3935. [PubMed] [Google Scholar]
- 19.Phillips, R., F. S. Sarfo, F. Osei-Sarpong, and M. Wansbrough-Jones. 2006. Clinical response and bacterial killing during antibiotic treatment of Mycobacterium ulcerans disease, p. 60-61. http://www.who.int/buruli/information/en/Abstracts. [DOI] [PMC free article] [PubMed]
- 20.Phillips, R. O., F. S. Sarfo, F. Osei-Sarpong, A. Boateng, I. Tetteh, A. Lartey, E. Adentwe, W. Opare, K. B. Asiedu, and M. Wansbrough-Jones. 2009. Sensitivity of PCR targeting Mycobacterium ulcerans by use of fine-needle aspirates for diagnosis of Buruli ulcer. J. Clin. Microbiol. 47:924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portaels, F., H. Traore, K. De Ridder, and W. M. Meyers. 1998. In vitro susceptibility of Mycobacterium ulcerans to clarithromycin. Antimicrob. Agents Chemother. 42:2070-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Revill, W. D., R. H. Morrow, M. C. Pike, and J. Ateng. 1973. A controlled trial of the treatment of Mycobacterium ulcerans infection with clofazimine. Lancet ii:873-877. [DOI] [PubMed] [Google Scholar]
- 23.Shepard, C. C., and D. H. McRae. 1968. A method for counting acid-fast bacteria. Int. J. Lepr. Other Mycobact. Dis. 36:78-82. [PubMed] [Google Scholar]
- 24.Simonet, V. 2008. Prevention of disability in Buruli ulcer: basic rehabilitation. http://whqlibdoc.who.int/hq/2008/WHO_HTM_NTD_IDM_GBUI_2008.1_eng.pdf.
- 25.Stanford, J. L., and I. Phillips. 1972. Rifampicin in experimental Mycobacterium ulcerans infection. J. Med. Microbiol. 5:39-45. [DOI] [PubMed] [Google Scholar]
- 26.Thangaraj, H. S., O. Adjei, B. W. Allen, F. Portaels, M. R. W. Evans, D. K. Banerjee, and M. H. Wansbrough-Jones. 2000. In vitro activity of ciprofloxacin, sparfloxacin, ofloxacin, amikacin and rifampicin against Ghanaian isolates of Mycobacterium ulcerans. J. Antimicrob. Chemother. 45:231-233. [DOI] [PubMed] [Google Scholar]
- 27.Torrado, E., S. Adusumilli, A. G. Fraga, P. L. Small, A. G. Castro, and J. Pedrosa. 2007. Mycolactone-mediated inhibition of tumor necrosis factor production by macrophages infected with Mycobacterium ulcerans has implications for the control of infection. Infect. Immun. 75:3979-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torrado, E., A. G. Fraga, A. G. Castro, P. Stragier, W. M. Meyers, F. Portaels, M. T. Silva, and J. Pedrosa. 2007. Evidence for an intramacrophage growth phase of Mycobacterium ulcerans. Infect. Immun. 75:977-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wansbrough-Jones, M., and R. Phillips. 2006. Buruli ulcer: emerging from obscurity. Lancet 367:1849-1858. [DOI] [PubMed] [Google Scholar]