Abstract
A new fixed-dose artesunate (AS)-mefloquine (MQ) was assessed in adults hospitalized for 28 days with uncomplicated drug-resistant falciparum malaria. The patients (n = 25/arm) were treated with (i) two fixed-dose tablets (AS-MQ arm; 100 mg AS-200 mg MQ/tablet) daily for 3 days (days 0, 1, and 2) or (ii) nonfixed AS (AS-plus-MQ arm; 4 mg/kg of body weight/day for 3 days) plus MQ (15 mg/kg on day 1 and 10 mg/kg on day 2), dosed by weight. Clinical laboratory electrocardiogram (ECG), adverse events (AEs), efficacy, and pharmacokinetic parameters were assessed over 28 days. Both regimens were well tolerated. No AEs were drug related. Two serious AEs of malaria-induced hypotension occurring in the AS-MQ arm necessitated rescue treatment. There were no significant changes in hematology, biochemistry, or PR and QRS intervals. For all patients, mean Fridericia-corrected QT intervals were significantly (P ≤ 0.0027) prolonged on day 3 (407 ms) and day 7 (399 ms) versus day 0 (389 ms), in parallel with significant (P ≤ 0.0003) falls in heart rates (67 [day 3], 73 [day 7], and 83 [day 0] beats/minute). Fixed-nonfixed formulations were bioequivalent for MQ, but not for AS and dihydroartemisinin (DHA). One AS-MQ patient developed a new infection on day 28; his day 28 plasma MQ concentration was 503.8 ng/ml. Fixed-dose AS-MQ was well tolerated, had pharmacokinetic (PK) profiles broadly similar to those of nonfixed AS plus MQ, and is a suitable replacement.
Multidrug-resistant Plasmodium falciparum on the Thai-Burmese border dates back to the mid 1970s and has been treated with artesunate (AS) and mefloquine (MQ) since 1994, 7 years before the WHO recommendation to use artemisinin-based combinations (ACTs) as first-line treatment for acute uncomplicated falciparum malaria (17, 23). AS plus MQ, dosed at 12 and 25 mg/kg of body weight over 3 and 2 days, respectively, has achieved sustained cure rates of 95%, reduced P. falciparum transmission, and reversed the progression of the median in vitro mefloquine 50% inhibitory concentration (IC50) (23). More recent data still show high cure rates despite a slowing of the parasite clearance time compared to earlier studies (8).
The adequacy of the artesunate dose was supported by a dose-ranging and pharmacodynamic (PD) study in P. falciparum-infected patients from the Thai-Burmese border, which found that 2 mg/kg of oral artesunate was the minimum dose that resulted in maximal parasite killing, the minimum parasiticidal concentration (MPC) (3). Allowing for the interindividual variation of AS absorption (7), 4 mg/kg was recommended. This dose has been adopted widely for other AS-based combinations (1, 2). Artesunate is tolerated remarkably well (25, 31). Serious toxicity is limited to acute anaphylaxis, which occurs at an estimated rate of 1 in 2,833 (15).
Mefloquine is associated with nausea, vomiting, and dizziness. When used at a stat dose of 25 mg/kg (MQ25), high rates of early (≤1 h) vomiting occurred, especially in children <7 and adults >50 years old (29, 34). Vomiting was reduced by 43% by giving MQ at 15 and 10 mg/kg (MQ15-10) 24 h later and by 2- and 3-fold in those who received an artemisinin derivative on the first day of treatment, followed by MQ25 on the second day of treatment or later (19, 34). The 15- to 10-mg/kg split dose alone also increased the mean area under the concentration-time curve from zero hour to infinity (AUC0-∞) by ∼50% to 51,020 ng·day/ml compared to MQ25 alone (34,106 ng·day/ml), and a 20% increase was seen when MQ was combined with artesunate: 24,343 (MQ15-10) versus 20,292 (MQ25) ng·day/ml (28). Similar AUC results were found in artesunate-treated children who received MQ25 on day 0 (21,196 ng·day/ml) or delayed to day 1 (28,196 ng·day/ml) (24). An increased AUC translates into more time that MQ concentrations are above the MPC and MIC, which are important pharmacokinetic (PK) parameters for parasite killing.
Serious dose-related acute psychiatric side effects occurred in 1/2,089 (15 mg/kg) and 1/1,217 (25 mg/kg) patients (18). Rates of sinus bradycardia and sinus arrhythmia were similar between MQ and other antimalarials and were probably due to fever resolution (9, 14). The cardiotoxicity (QT prolongation) of halofantrine is enhanced when halofantrine is used to treat mefloquine failures (16, 22).
A new, easy-to-use, fixed-dose combination of AS-MQ has been developed and registered. It has four age-based dosing regimens. The MQ dose has been split into 8 mg/kg daily for 3 days. AS-MQ underwent a phase III field trial in patients older than 6 months in Thailand (5). It was well tolerated, resulted in a lower rate of early vomiting than the conventional nonfixed AS-plus-MQ regimen, and achieved a day 63 PCR-corrected cure rate of almost 92%, a rate similar to that of the nonfixed combination. A population PK model of nonfixed MQ, delivered at 8 mg/kg daily for 3 days in 50 patients, resulted in a mean whole-blood maximum concentration of drug (Cmax) of 2,202 ng/ml and an AUC0-∞ of 31,395 ng·day/ml, a 40% increase compared to a previous population PK model of 24,343 ng·day/ml of AS plus MQ15-10 (6, 27).
As part of the development of AS-MQ, a phase IIb study was conducted focusing on tolerability, electrocardiogram (ECG) changes, and the PK profiles of the two different formulations and dosing regimens. The study results are reported here.
MATERIALS AND METHODS
Study design and site.
This was a randomized, open-label trial comparing the safety, PK, and efficacy of fixed-dose AS-MQ to those of the currently used, nonfixed AS and MQ. It took place from December 2004 to July 2005 at the Hospital for Tropical Diseases, Bangkok, Thailand, and received ethical approval from the Faculty of Tropical Medicine, Mahidol University, and the WHO. The trial was registered (http://www.controlled-trials.com/ISRCTN24192353/). A sample size of 25 patients per arm was considered sufficient for an intensive PK study.
Study conduct.
The study entry criteria were (i) age, 18 to 65 years; (ii) body weight, ≥40 kg; (iii) microscopically confirmed monoinfection with P. falciparum (parasitemia, ≥2/200 white blood cells [WBC]); (iv) history of fever or a documented fever (axillary temperature, ≥37.5°C); and (v) written informed consent. Exclusion criteria were (i) pregnancy (all women of childbearing age underwent a urine pregnancy test) or lactation; (ii) P. falciparum parasitemia of ≥4% of red blood cells (≥175,000/μl); (iii) clinical and/or laboratory features of severe malaria (4); (iv) other significant illnesses, e.g., liver disease or renal disease; (v) ingestion of mefloquine within the previous 60 days; (vi) contraindications to mefloquine, i.e., history of convulsions and/or psychiatric illnesses; (vii) known hypersensitivity to artemisinins or mefloquine; and (viii) splenectomy.
A randomization list was computer generated. Sealed envelopes in a locked cabinet contained the drug regimen and were opened only after informed consent was obtained. All study drugs were administered by study nurses.
AS-MQ dosing regimens.
One fixed-dose tablet (Far-Manguinhos, Rio de Janeiro, Brazil; batch number 070.008; expiration date, April 2006) contained 100 mg of AS and 200 mg of MQ base. The dose was two fixed-dose tablets per day for 3 days (total dose, 600 mg AS and 1,200 mg MQ).
The nonfixed AS tablets contained 50 mg of AS (Arsumax; Sanofi Aventis, France; batch number 031201; expiration date, December 2005) and were dosed at 4 mg/kg/day on days 0, 1, and 2. The nonfixed mefloquine tablets contained 250 mg of mefloquine base (Roche, Basel, Switzerland; batch number B1100; expiration date, March 2006) and were dosed at 15 (day 1) and 10 (day 2) mg/kg, rounded to the nearest 1/4 tablet (21). Drugs were administered every 12 hours, with ∼200 ml of water and independent of meals. Patients were observed for 1 h for vomiting. A full or half dose was readministered if vomiting occurred within 30 min or 60 min, respectively. Patients were rescued with parenteral artesunate.
Study evaluations.
Patients were hospitalized for 28 days, but short home leave was allowed. Clinical evaluations (symptoms, vital signs, and clinical examinations) were done on days 0, 1, 2, 3, 7, 14, 21, and 28. Giemsa-stained thick and thin blood films were examined at baseline; every 6 hours for the first 3 days; and on day 7, day 14, day 21, and day 28. Asexual parasites were reported as the number per μl. Patients with recurrent parasitemia were classed as having new or recrudescent infections by analysis by PCR and by comparing three polymorphic genetic markers: merozoite surface proteins 1 and 2 (MSP-1 and MSP-2) and the glutamate-rich protein (GLURP). A new infection was diagnosed if any of the genotypes of the three genes were different, based on their electrophoretic patterns (30). Standard 12-lead ECGs were done at baseline and days 3 and 7.
Blood sampling.
Full blood count and liver function tests were performed at baseline, day 7, and day 28. Urine dipstick examinations were done on days 0, 7, 14, 21, and 28. PK samples (5-ml heparinized tubes) for AS and MQ were taken at baseline, 0.25 h, 0.5 h, 0.75 h, 1 h, 1.5 h, 2 h, 3 h, 4 h, 6 h, 8 h, 12 h, 24 h, day 3, day 7, day 14, day 21, and day 28 and centrifuged immediately, and the plasma was stored at −70°C until it was analyzed at the Universiti Sains Malaysia. Plasma samples were analyzed within 6 months of collection. MQ, AS, and its metabolite dihydroartemisinin (DHA) were simultaneously extracted by solid-phase extraction (SPE) and measured separately by reversed-phase high-pressure liquid chromatography (RP-HPLC) with UV detection for MQ (lower limit of quantification [LLOQ], 25 ng/0.5 ml) and an electrochemical detector operating in the reductive mode for AS and DHA (13). The lower limit of quantification of mefloquine was 25 ng/0.5 ml, with an accuracy of 98.9% and a coefficient of variation (CV%) of 8%. The method was found to be accurate, with an average deviation of <5.2% from the true value. The within-day and day-to-day precision for mefloquine were less than 4%. The extraction recoveries of artesunate, dihydroartemisinin, and the internal standard artemisinin were above 86%, with a coefficient of variation of ≤13%. The lower limit of quantification for artesunate was 10 ng/0.5 ml, with an accuracy of 109.9% and a coefficient of variation of 7.2%. The lower limit of quantification for dihydroartemisinin was 10 ng/0.5 ml, with an accuracy of 99.1% and a coefficient of variation of 5.9%. The within-day and day-to-day precision for both artesunate and dihydroartemisinin was less than 8%.
The pharmacokinetic parameters Cmax, time to maximum concentration of drug in serum (Tmax), and AUC0-t were determined using model-independent formulae (26). Cmax and Tmax were obtained from a visual inspection of the plasma concentration-versus-time curve. AUC0-t was calculated using the linear trapezoidal rule, and AUC0-∞ was calculated by the formula AUC0-t + Ct/λz, where Ct is the concentration at the last quantifiable time. From the terminal log-linear (disposition) phase, a first-order elimination rate constant (λz) was estimated by linear regression, and the terminal half-life value (T1/2) was estimated using the equation T1/2 = ln2/λz. AS, DHA, and total DHA (AS converted stoichiometrically to DHA plus measured DHA) concentrations are presented.
An adverse event (AE) was defined as either the development of a new symptom, sign, clinical syndrome, or laboratory result or their exacerbation that was reported or detected after study drug administration (11). AEs were graded 1 to 4 using the common toxicity criteria (CTC) (National Cancer Institute, NIH, 1999). The relationship of the adverse event to the study drugs was determined to be unrelated, unlikely, possible, probable, or very probable. A serious AE was an AE with at least one of the following characteristics: (i) life threatening, (ii) resulted in death, (iii) caused residual significant disability, or (iv) resulted in prolongation of a patient's hospital stay. This study had an independent Drug Safety and Monitoring Board.
An early treatment failure was defined as (i) the development of severe malaria within the first 72 h, (ii) a day 2 parasite count greater than the day 0 parasite count, (iii) a day 3-day 0 parasite count of ≥25%, and (iv) parasitemia on day 3 with fever. A late treatment failure was (i) initial clearing of parasites by day 7, followed by a recurrence of parasitemia with the same genotype as the baseline or (ii) parasitemia on days 4 to 7 with fever. The parasite clearance time (PCT) was defined as the time required for malaria slides to become and remain parasite negative for at least two consecutive slides. The fever clearance time (FCT) was the time required to become afebrile (axillary temperature, <37.5°C) for at least 24 h within the first 7 days.
The study endpoints were (i) the occurrence of adverse events; (ii) pharmacokinetic parameters (AS, DHA, and MQ); (iii) the PCR-adjusted day 28 parasitological failure rate; (iv) PCT and FCT; (v) the proportions of patients without parasitemia on day 1, day 2, and day 3; (vi) gametocyte carriage; and (vii) the fractional change (day 28 to day 0) in the hemoglobin (Hb) concentrations.
Continuous parameters were compared by paired and unpaired t tests or their nonparametric tests, as indicated. Categorical data are presented as the number of patients and the percentage of patients and were analyzed using the Cochran-Mantel-Haenszel test or Fisher's exact test, as appropriate.
The data were recorded on case record forms by the research team. Double data entry (Clintrial 4.3), cleaning, and analysis using the SAS system (version 8.02; SAS Institute, Cary, NC), to a prospectively defined analytical plan, were done by a clinical research organization.
RESULTS
Patient characteristics.
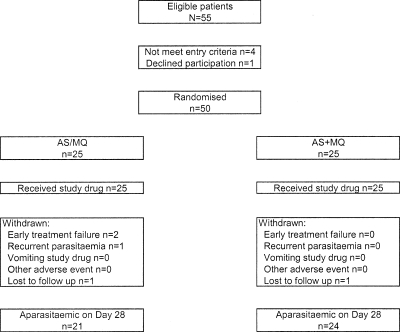
Of the 55 screened patients, 50 were recruited into the study. Patient disposition is presented in Fig. 1. Most were Burmese males (Table 1). Reported symptoms were of mild or moderate intensity and consisted of headache (n = 46 [92.0%]), weakness (n = 45 [90.0%]), myalgia (n = 39 [78.0%]), chills/rigors (n = 37 [74.0%]), anorexia (n = 34 [68.0%]), dizziness (n = 31 [62.0%]), nausea (n = 24 [48.0%]), vomiting (n = 20 [40.0%]), abdominal pain (n = 10 [20.0%]), insomnia (n = 7 [14.0%]), palpitations (n = 7 [14.0%]), coughing (n = 6 [12.0%]), and diarrhea (n = 6 [12.0%]). Two patients were lost to follow up (both arms), and there were two early and one late (day 28) treatment failures in the fixed group.
FIG. 1.
Trial profile.
TABLE 1.
Patient characteristics at baseline
| Variable | Valuea |
|
|---|---|---|
| AS/MQ fixed (n = 25) | AS + MQ nonfixed (n = 25) | |
| Age (yr) | 26.6 (17-50) | 28.9 (16-45) |
| Wht (kg)b | 50 (40-62) | 51.0 (40-65) |
| Male/Female [no. (%)] | 19 (76)/6 (24) | 22 (88)/3 (12) |
| Burmese (no.) | 13 | 14 |
| Karen (no.) | 7 | 8 |
| Mon (no.) | 4 | 3 |
| Thai (no.) | 1 | 0 |
| Tempb (°C) | 38.5 (37.3-40) | 38.1 (37-40) |
| Pulse beatsb/minute | 90 (72-118) | 90 (76-124) |
| Supine blood pressureb (mm Hg) | 110 (90-130)/70 (60-90) | 110 (90-130)/70 (60-80) |
| Palpable spleen [no. (%)] | 0 | 0 |
| Palpable liver [no. (%)] | 3 (12) | 4 (16) |
| Asexual parasitemiab/μl | 30,816 (69-170,800) | 28,231 (20-140,280) |
| Gametocyte carriage [no. (%)] | 3 (12.5) | 6 (25) |
| Hb (g/dl) | 12.58 (10.3-17) | 12.05 (8.7-15.5) |
| Total white cell count × 109/liter | 5.72 (1.5-9.2) | 5.04 (2-10.1) |
| Neutrophils × 109/liter | 3.67 (0.7-6.9) | 3.15 (1.1-6.1) |
| Lymphocytes × 109/liter | 1.45 (0.5-3.4) | 1.34 (0.4-4.0) |
| Eosinophils × 109/liter | 0.13 (0-0.4) | 0.15 (0-0.7) |
| Monocytes × 109/liter | 0.39 (0.1-0.8) | 0.34 (0.1-0.8) |
| Platelet count × 109/literb | 85 (19-334) | 99 (28-239) |
| Creatinine (mg/dl) | 0.87 (0.5-1.2) | 0.84 (0.4-1.3) |
| AST (IU/liter) | 24.7 (11-44) | 32.2 (12-111) |
| ALT (IU/liter) | 20.9 (9-48) | 30 (3-113) |
| Total bilirubin (mg/dl) | 1.36 (0.5-2.5) | 1.32 (0.4-2.8) |
| Dose of AS (mg/kg)b | 4 (3.2-5) | 4 |
| Dose of MQ (mg/kg)b | 8 (6.5-10) | 15 and 10 |
All continuous data are reported as mean (range) unless otherwise indicated.
Median (range).
The total doses of AS and MQ received by the fixed group ranged from 9.6 to 15 (median, 12) mg/kg/day and 19.5 to 30 (median, 24) mg/kg/day, respectively; corresponding doses for the nonfixed group were 9.3 to 15.9 (median, 12) and 20.1 to 32.7 (median, 25.2).
Adverse events and tolerability.
Both drug regimens were well tolerated. Over 28 days, a total of 181 clinical or laboratory AEs (irrespective of their causality) were reported or detected in 24 (96.0%; AS-MQ) and 25 (100%; AS plus MQ) patients (Table 2 ). Two AEs were serious AEs (AS-MQ arm) that were classed as CTC grade 3 hypotension due to the development of severe malaria; both patients were treated with intravenous (i.v.) artesunate, i.v. fluids, i.v. antibiotics, and dopamine and recovered. Of the remaining 22 AS-MQ patients, 21 had a mild (CTC grade 1) AE(s) and 1 had a moderate (grade 2) AE; all AS-plus-MQ patients had mild AEs. No AEs were considered drug related. There was no vomiting within 1 h of drug administration. Two patients from each arm reported mild late vomiting within the first 3 days.
TABLE 2.
Main clinical AEs reported by patients and main laboratory AEs detected at any time after the first dose of fixed or nonfixed AS-MQ until the end of follow-up at 28 daysa
| AE | No. (%) |
|
|---|---|---|
| AS-MQ fixed (n = 24) | AS + MQ nonfixed (n = 25) | |
| Gastrointestinal | ||
| Anorexia | 3 (12.5) | 0 (0.0) |
| Nausea | 2 (8.3) | 2 (8.0) |
| Vomiting | 2 (8.3) | 2 (8.0) |
| Flatulence | 1 (4.2) | 1 (4.0) |
| Abdominal pain | 4 (16.7) | 2 (8) |
| Diarrhea | 1 (4.2) | 1 (4.0) |
| Constipation | 0 (0.0) | 2 (8.0) |
| Respiratory | ||
| Nasal congestion | 2 (8.3) | 0 (0.0) |
| URTIb | 6 (25.0) | 3 (12.0) |
| Cough | 2 (8.3) | 1 (4.0) |
| Chest pain | 1 (4.2) | 0 (0.0) |
| Palpitations | 1 (4.2) | 0 (0.0) |
| Musculoskeletal | ||
| Myalgia | 1 (4.2) | 1 (4.0) |
| Neck pain | 0 (0.0) | 1 (4.0) |
| Pain in extremity | 0 (0.0) | 2 (8.0) |
| Central nervous system | ||
| Weakness | 0 (0.0) | 2 (8.0) |
| Dizziness | 0 (0.0) | 4 (16.0) |
| Headache | 3 (12.5) | 3 (12.0) |
| Insomnia | 4 (16.7) | 1 (4.0) |
| Vertigo | 0 (0.0) | 1 (4.0) |
| Skin | ||
| Itching | 1 (4.2) | 2 (8.0) |
| Rash | 1 (4.2) | 1 (4.0) |
| General symptoms | ||
| Fever | 4 (16.7) | 2 (8.0) |
| Dehydration | 0 (0.0) | 1 (4.0) |
| Epistaxis | 1 (4.2) | 0 (0.0) |
| Infections/infestations | ||
| Abscess | 0 (0.0) | 1 (4.0) |
| Hordeolum | 1 (4.2) | 0 (0.0) |
| Gut helminths | 15 (62.6) | 6 (24.0) |
| Tuberculosis | 1 (4.2) | 0 (0.0) |
| Laboratory findings | ||
| Anemia | 5 (20.8) | 2 (8.0) |
| Eosinophilia | 20 (83.3) | 21 (84.0) |
| ALT/AST increased | 2 (8.3) | 4 (16.0) |
| Hypoglycemiab | 0 (0.0) | 1 (4.0) |
| Microscopic hematuria | 3 (12.5) | 4 (16.0) |
| Proteinuria | 8 (33.3) | 3 (12.0) |
Patients may have had more than one adverse event.
URTI, upper respiratory tract infection.
Plasma glucose, ≤66 mg/dl; lower limit of normal for laboratory, 75 mg/dl.
Hematological changes over time.
Compared to day 0, the mean Hb fell significantly on day 7 to (i) 11.42 g/dl (difference = −1.25 ± 1.13; P < 0.0001) for AS-MQ and (ii) 11.25 g/dl (difference = −0.86 ± 1.12; P = 0.0001) for AS plus MQ. By day 28, the mean Hb values increased modestly to 13.05 and 12.8 g/dl, respectively, and were not significantly different from the day 0 values (P = 0.24 and P = 0.20, respectively).
The mean total white cell count increased at each time interval over time, and by day 28, the mean total white cell counts were 8.65 × 109/liter for AS-MQ and 7.5 × 109/liter for AS plus MQ, representing mean increases over baseline of 2.82 × 109/liter (P < 0.0001) and 2.41 × 109/liter (P = 0.0002), respectively. There was some fluctuation in the absolute neutrophil counts over time. One patient with a baseline neutrophil count of 2,170/μl developed a grade 3 neutropenia of 970/μl on day 21 which resolved by day 28 (2,930/μl). Compared to day 0, the median platelet counts rose significantly by day 28 to 238.5 × 109/μl for AS-MQ (P < 0.001) and 245 × 109/μl for AS plus MQ (P < 0.001). Three patients had mild and one had moderate day 28 thrombocytopenia.
Biochemistry.
The mean day 0 total bilirubin values in both arms were mildly raised: 1.36 (AS-MQ) and 1.32 (AS plus MQ) mg/dl. They normalized by day 7 (0.58 and 0.63 mg/dl, respectively) and remained stable to day 28 (0.6 and 0.63 mg/dl, respectively). The corresponding (day 0, day 7, and day 28) aspartate transaminase (AST), alanine aminotransferase (ALT), and creatinine values were (i) 24.7, 24.2, and 21.2 IU/liter (AST); 20.9, 35.8, and 26.1 IU/liter (ALT); and 0.87, 0.78, and 0.76 mg/dl (creatinine) for AS-MQ and (ii) 32.2, 31.5, and 24.5 IU/liter (AST); 30, 51.9, and 27 IU/liter (ALT); and 0.84, 0.78, and 0.76 mg/dl (creatinine) for AS plus MQ. Shift tables showed that most patients had biochemical values within the normal range at baseline and study end (data not shown). By day 28, a total of five and two patients with normal baseline liver enzymes had raised day 28 ALT and AST values, respectively. The highest ALT values were 67 IU/liter (day 0 value, 3 IU/liter) in the AS-plus-MQ arm (grade 1 CTC AE) and 109 IU/liter (day 0 value, 32 IU/liter) in the AS-MQ arm, a CTC grade 2 (>2.5 × upper limit of normal) AE. The AST values, 47 (AS-MQ) and 66 (AS plus MQ) IU/liter, were classed as CTC grade 1 AEs; their respective day 0 values were 18 and 20 IU/liter.
ECG findings.
A total of 145 ECGs were analyzed (day 0, 50; day 3, 47; day 7, 48). ECG interval and heart rate data were very similar in the fixed and nonfixed combination arms (data not shown) and were combined. There were no significant changes in the mean PR and QRS intervals over time (Table 3 ). The QTcF interval increased significantly on day 3 and day 7 compared to baseline, in parallel with a significant decline in the mean temperature and heart rates (Table 3). Using a backward stepwise manual modeling, only the heart rate was a significant variable (data not shown).
TABLE 3.
ECG intervals and temperature and heart rate data for all patients combined who were treated for uncomplicated falciparum malaria with either the fixed or nonfixed dose combinations of AS and MQ
| Parameter | Valuea for day: |
||||
|---|---|---|---|---|---|
| 0 | 3 | 7 | 3-0 | 7-0 | |
| Temp (°C) | 38.4 (37-40) | 37.1 (36.5-37.7) | 37.1 (36.5-39) | <0.000 | <0.000 |
| Heart rate/min | 83 (59-112) | 67 (44-84) | 73 (59-101) | <0.000 | 0.0003 |
| [MQ] ng/ml | 0 | 3,095 | 1,721 | 0.6908 | 0.1473 |
| PR ms | 148 (122-232) | 148 (111-190) | 145 (101-182) | 0.97 | 0.23 |
| QRSb (ms) | 91 (75-118) | 94 (63-135) | 91 (73-111) | 0.055 | 0.67 |
| QTcF (ms) | 389 (344-434) | 407 (319-504) | 399 (357-443) | <0.000 | 0.0027 |
All continuous data are reported as mean (range) unless otherwise indicated.
Median (range); QRS was not normally distributed. QRS comparisons used the sign test.
Pharmacokinetic findings.
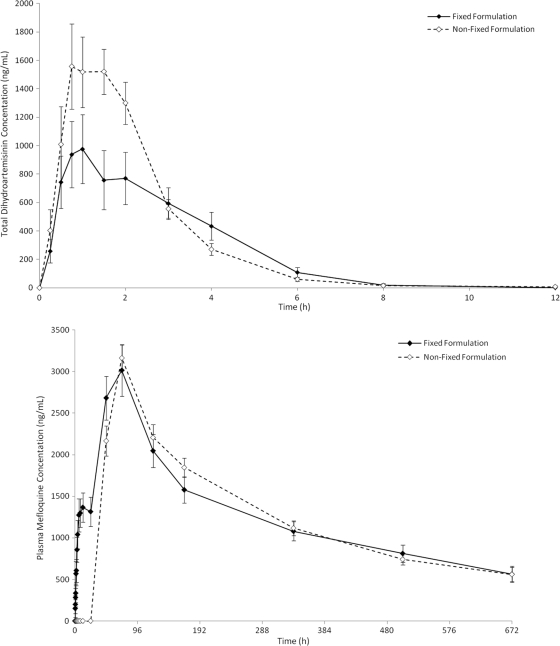
The interindividual variations in absorption (i.e., Cmax variation) were similar (Table 4) for MQ (3.2-fold [fix] versus 2.5-fold [nonfixed]) and AS (24.9-fold [fix] versus 29.4-fold [nonfixed]). However, the Cmax variations for DHA, reflecting absorption and metabolism, differed 13.1-fold (fixed) versus 21.5-fold (nonfixed). The coefficients of variation (CV%) for the all PK parameters were higher for the fixed-dose group, except for the AS Cmax and DHA AUC0-t. The clearance for the fixed and nonfixed MQ formulations were similar: 0.4 (±0.2) and 0.4 (±0.1) ml/min/kg. Based on the 90% confidence intervals of the ratios (fixed/nonfixed) of the geometric least squares means of the Cmax and AUC values, the formulations were not bioequivalent (i.e., outside the limits of 80 to 125%) for AS and DHA but were for MQ (Table 4). The Tmax values for AS, DHA, and MQ were not significantly different between the two arms. The PK profiles of total DHA and MQ are shown in Fig. 2. A more systematic comparison of the bioavailabilities and dispositions of the two products will be published separately.
TABLE 4.
Main disposition parameters of MQ, AS, and DHA when given as either fixed or nonfixed products for the treatment of uncomplicated falciparum malariaa
| Treatment | Drug | Cmax (ng/ml) | CV% | Tmax (h) | CV% | AUC0-t (ng·h/ml) | CV% | AUC0-∞ (ng·h/ml) | CV% | T1/2 (h) | CV% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed | MQ | 3,279 ± 1,252 (n = 20) (1,809-5,796) | 38.2 | 72.0 ± 19.1 (n = 20) (48-120) | 26.5 | 837,064 ± 378,271 (n = 20) (376,173-1,671,400) | 45.2 | 1145,977 ± 678,719 (n = 13) (519,164-3,103,075) | 59.2 | 285.6 ± 128.2 (n = 13) | 44.9 |
| DHA | 1,234 ± 857 (n = 20) (306.2-4,019.2) | 69.4 | 1.99 ± 1.12 (n = 20) (0.75-4) | 56.3 | 3,027 ± 2,491 (n = 20) (745-12,661) | 82.3 | 3,138 ± 2,491 (n = 14) (16-272) | 79.4 | 1.1 ± 0.5 (n = 14) | 45.5 | |
| AS | 255 ± 175 (n = 19) (50-1,248) | 68.6 | 0.833 ± 0.70 (n = 19) (0.5-1.5) | 84.0 | 310 ± 142 (n = 19) (61-1,075) | 45.8 | DNS | DNS | |||
| Nonfixed | MQ | 3,239 ± 734 (n = 23) (1,817-4,583) | 22.7 | 70.9 ± 13.5 (n = 23) (48.0-120.0) | 19.0 | 814,365 ± 232,116 (n = 23) (425,319-1,362,127) | 28.5 | 1095,421 ± 370,167 (n = 19) (571,397-2,127,627) | 33.8 | 321.7 ± 113.7 (n = 19) | 35.3 |
| DHA | 2,043 ± 949 (n = 23) (192-4,119) | 46.4 | 1.4 ± 0.71 (n = 23) (0.25-3) | 50.7 | 3,633 ± 1,367 (n = 23) (518-6,245) | 37.6 | 3,745 ± 1,371 (n = 18) (26-333) | 36.6 | 0.8 ± 0.2 (n = 18) | 25 | |
| AS | 451 ± 440 (n = 23) (75-2,206) | 97.6 | 0.925 ± 0.563 (n = 23) (0.25-2) | 60.9 | 419 ± 670 (n = 21) (57-1,124) | 159.9 | DNS | DNS |
The Tmax for total DHA was measured after the first dose and the Tmax of MQ after the full course was given. Parameters are expressed as means ± standard deviations. Ranges and patient numbers are given in parentheses. Geometric least squares mean ratios were (i) MQ Cmax, 100.8% (90% confidence interval, 84.4-120.3); AUC0-t, 100.9% (82.4-123.6); AUC0-∞, 100.8% (81.2-125.1); (ii) AS Cmax, 55% (36-83); AUC0-t, 74% (45-121); and (iii) DHA Cmax, 58% (42-82); AUC0-t, 75% (57-102). DNS, data not shown because of small patient numbers.
FIG. 2.
The mean (± standard errors) plasma concentrations of AS and DHA expressed as the total DHA equivalents (AS + DHA) (top graph) and MQ (bottom graph) over time for patients with uncomplicated falciparum malaria who were treated with fixed and nonfixed combinations of AS and MQ.
Efficacy.
The day 28 parasitological failure rates were 2/24 (8.3%) for AS-MQ and 0/24 (0%) for AS plus MQ (P = 0.49). Two AS-MQ patients (A and B) developed early treatment failure and one developed a new infection on return from a home visit. Patient A had a day 0 parasitemia of 3,680/μl and received 4.8 (AS) and 9.7 (MQ) mg/kg. Four hours postdose, her blood pressure fell from 90/50 to 80/50. A repeat blood film showed increased parasitemia of 7,360/μl, so she was rescued. Her AS PK values were (i) Cmax, 1,319.9 ng/ml; (ii) Tmax, 45 min; and (iii) AUC0-t, 407.4 ng·h/ml; the corresponding DHA values were 1,501.6 ng/ml, 4 h, and 2,752.23 ng·h/ml, and those for total DHA were 1,501.64 ng/ml, 4 h, and 3,059.34 ng·h/ml. These values confirmed adequate absorption.
Patient B had a day 0 parasitemia of 170,800/μl and blood pressure of 100/60. She received 4.3 (AS) and 8.7 (MQ) mg/kg. She reported palpitations and mild dyspnea soon after treatment that became worse some 6 h later. She was treated with oxygen and frusemide and transferred to the intensive-care unit (ICU). Two blood films showed declining parasitemia: 161,040/μl (∼8 h postadmission) and 9,760/μl (∼17 h postadmission). The following day, she was persistently hypotensive (92/55 supine and 81/48 sitting) and was rescued. Her AS PK data were (i) Cmax, 319.7 ng/ml; (ii) Tmax, 1 h; and (iii) AUC0-t, 674.3 ng·h/ml; the corresponding values for DHA were 1,283.1 ng/ml, 4 h, and 3,792.7 ng·h/ml, and for total DHA, they were 1,301.32 ng/ml, 3 h, and 2,840.97 ng·h/ml. These values confirmed adequate absorption.
For patient C, the day 0 parasite count was 20,960/μl, which fell to 110/μl (24 h) and cleared by 60 h after treatment with 3.7 (AS) and 7.4 (MQ) mg/kg/day. He became and remained afebrile after 48 h. He went home on day 21, and on day 28, he was afebrile and asymptomatic but had a P. falciparum parasitemia of 10,060/μl. The paired parasite-genotyping results were (i) MSP-1 genes, 471 on day 0 and day 28; (ii) MSP-2, day 0, 682 and 743 (indicating a double infection), and day 28, 616 and 665; and (iii) GLURP, 814 (day 0) and 900 (day 28), indicating a new infection. The MQ PK parameters were (i) Cmax, 1,835.8; (ii) AUC0-t, 16,416.8 ng·day/ml (AUC0-∞, not available); and (iii) day 28 MQ, 503.81 ng/ml. The AS and DHA parameters were (i) AS Cmax, 149.3 ng/ml, and AUC0-t, 96.22 ng·h/ml; (ii) DHA, 489.50 ng/ml and 1,329.76 ng·h/ml; and (iii) total DHA, 887.30 ng/ml and 1,408.17 ng·h/ml, respectively. All PK parameters were below the mean values for the AS-MQ group.
The proportions of patients with parasites or fever on days 1, 2, and 3 were similar in the two arms (data not shown). When the two arms were combined, these proportions for parasites were 36/48 (75%), 13/48 (27.1%), and 3/48 (6.3%), respectively, and for fever they were 37/48 (77.1%), 13/48 (27.1%), and 5/48 (10.4%). Accordingly, the mean PCTs (1.93 [fixed] versus 1.67 [nonfixed] days, P = 0.26) and FCTs (2.3 [fixed] versus 2.2 [nonfixed] days, P = 0.68) were similar. For both arms, gametocyte carriage declined over time; no patients carried gametocytes by days 14 (AS-MQ) and 21 (AS plus MQ).
DISCUSSION
This small study has shown that the new AS-MQ fixed-dose combination was well tolerated, with no drug-related clinical AEs, and did not produce any clinically significant ECG changes.
AS-MQ was well tolerated, but the small sample size precluded detecting the rare, well-known, and potentially serious neuropsychiatric side effects of mefloquine (31). There were two malaria-related serious AEs (hypotension), which necessitated rescue treatment. Over the course of the study, at least one AE was detected in virtually all patients, but none were considered drug related. Hematological findings were unremarkable. A small number of patients had day 28 thrombocytopenia, probably related to continuing bone marrow recovery (32). The high rate of posttreatment eosinophilia was related to resolution of malaria (10). Biochemical parameters were generally normal during the study, and there was a downward trend in the total bilirubin and liver enzymes consistent with disease resolution. There were a few patients with mildly abnormal liver enzymes at day 28, but they were of doubtful clinical significance.
The ECG findings in these predominantly fit young men were unremarkable and consistent with the good cardiac safety record of mefloquine (34, 35). The QTcF interval increase was best explained by the falling heart rate consequent to disease resolution (detailed results will be reported elsewhere).
The fixed-dose AS-MQ combination has been developed by using two well-known antimalarial drugs and choosing target doses that are known to be effective: 4 mg/kg/day for AS and 8 mg/kg/day for MQ. Patients in the fixed arm received 3.2 to 5 mg/kg/day of AS and 6.5 to 10 mg/kg/day of MQ. The MQ PK parameters were similar. The fixed group had lower mean AS and DHA exposures, but this did not result in lower mean parasite clearance times compared to the nonfixed group.
Our fixed and nonfixed AS and DHA PK data are similar to those of others regarding the short Tmax and half-life values (20, 33), but our interindividual fold differences in absorption and AUC were higher. The interindividual fold differences reported by Teja-Isavadharn et al. were 3.2 for the DHA Cmax and 3.6 for the DHA AUC0-12, respectively (33). The interindividual variations for the MQ Cmax (3.2 [fixed], 2.5 [nonfixed]) and AUC0-∞ (6 [fixed] and 3.7 [nonfixed]) were tight and similar to those of Bethell et al. and Price et al. (3.7 and 3, respectively) (7, 24). Our mean fixed MQ AUC0-∞ (☰47,749 ng·day/ml; n = 13) was higher than that reported in a study of nonfixed MQ dosed at 8 mg/kg/day for 3 days (31,395 ng·day/ml; n = 50) (6). The mean nonfixed MQ AUC0-∞ (☰45,643 ng·day/ml) was higher in 25 children (28,654 ng·day/ml) but lower in 12 adults (63,590 ng·day/ml) using the same commercial formulation (12, 24). More rapid MQ clearance (0.87 liter/kg/day [☰0.6 ml/min/kg] versus our 0.4 ml/min/kg) in the children is likely to explain their lower AUC0-∞.
This study was not powered to compare efficacy, and the follow-up extended to only 28 rather than the standard 63 days. Two AS-MQ patients with good AS and DHA absorption developed early treatment failure due to hypotension, a sign consistent with severe malaria. One patient had a rising parasite count 4 h post-AS, while the other had a declining parasite count. Patients with uncomplicated malaria may deteriorate because of the evolution of their disease and despite commencing highly effective ACT treatment; this was the probable cause in our patients. The AS-MQ patient with a day 28 new infection had a day 28 MQ concentration of just over 500 ng/ml, a value that is associated with resistant P. falciparum parasites from Thailand (27, 28).
In conclusion, fixed-dose AS-MQ was well tolerated and had PK profiles broadly similar to those of the nonfixed combination. These data support the use of this AS-MQ fixed-dose combination for treating drug-resistant falciparum malaria. Continuing assessment of the safety and effectiveness of AS-MQ should be done in large field trials.
Acknowledgments
We thank the patients and the ward nurses for their help with study execution. We are grateful to the DSMB members, N. Day, R. Price, S. Awasti, A. Babiker, and I. Ribeiro.
Financial support for this study came from the INCO-DEV Programme (Confirming the International Role of Community Research for Development), European Commission (proposal no. ICA4-2001-10193).
The authors' specific contributions were as follows: protocol development, W.R.J.T., S.K., S.L., and J.R.K.; study execution, S.K., S.L., P.W., W.P., W.L., N.T., and K.C.; study coordination and supervision, W.R.J.T. and J.R.K.; pharmacokinetic measurements, analyses, and interpretation, S.R., V.N., M.V., and P.O.; ECG analyses, M.V. and W.R.J.T.; first draft of the paper, W.R.J.T.; critical review and input to the manuscript, P.O., M.V., S.K., J.R.K., and V.N.
We dedicate this work and publication to the late S. Looareesuwan. He was a well-liked colleague who played a significant part in the AS-MQ development project.
P.O. is a staff member of the WHO. M.V. is a staff member of the CRP-Santé. None of us have a conflict of interest.
We are responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy, or views of the WHO or the CRP-Santé.
Footnotes
Published ahead of print on 14 June 2010.
REFERENCES
- 1.Adjuik, M., P. Agnamey, A. Babiker, S. Borrmann, P. Brasseur, M. Cisse, F. Cobelens, S. Diallo, J. F. Faucher, P. Garner, S. Gikunda, P. G. Kremsner, S. Krishna, B. Lell, M. Loolpapit, P. B. Matsiegui, M. A. Missinou, J. Mwanza, F. Ntoumi, P. Olliaro, P. Osimbo, P. Rezbach, E. Some, and W. R. Taylor. 2002. Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomised, multicentre trial. Lancet 359:1365-1372. [DOI] [PubMed] [Google Scholar]
- 2.Adjuik, M., A. Babiker, P. Garner, P. Olliaro, W. Taylor, and N. White. 2004. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363:9-17. [DOI] [PubMed] [Google Scholar]
- 3.Angus, B. J., I. Thaiaporn, K. Chanthapadith, Y. Suputtamongkol, and N. J. White. 2002. Oral artesunate dose-response relationship in acute falciparum malaria. Antimicrob. Agents Chemother. 46:778-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. 2000. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]
- 5.Ashley, E. A., K. M. Lwin, R. McGready, W. H. Simon, L. Phaiphun, S. Proux, N. Wangseang, W. Taylor, K. Stepniewska, W. Nawamaneerat, K. L. Thwai, M. Barends, W. Leowattana, P. Olliaro, P. Singhasivanon, N. J. White, and F. Nosten. 2006. An open label randomized comparison of mefloquine-artesunate as separate tablets vs. a new co-formulated combination for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. Trop. Med. Int. Health 11:1653-1660. [DOI] [PubMed] [Google Scholar]
- 6.Ashley, E. A., K. Stepniewska, N. Lindegardh, R. McGready, R. Hutagalung, R. Hae, P. Singhasivanon, N. J. White, and F. Nosten. 2006. Population pharmacokinetic assessment of a new regimen of mefloquine used in combination treatment of uncomplicated falciparum malaria. Antimicrob. Agents Chemother. 50:2281-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bethell, D. B., P. Teja-Isavadharm, X. T. Cao, T. T. Pham, T. T. Ta, T. N. Tran, T. T. Nguyen, T. P. Pham, D. Kyle, N. P. Day, and N. J. White. 1997. Pharmacokinetics of oral artesunate in children with moderately severe Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 91:195-198. [DOI] [PubMed] [Google Scholar]
- 8.Carrara, V. I., J. Zwang, E. A. Ashley, R. N. Price, K. Stepniewska, M. Barends, A. Brockman, T. Anderson, R. McGready, L. Phaiphun, S. Proux, M. van Vugt, R. Hutagalung, K. M. Lwin, A. P. Phyo, P. Preechapornkul, M. Imwong, S. Pukrittayakamee, P. Singhasivanon, N. J. White, and F. Nosten. 2009. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One 4:e4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chongsuphajaisiddhi, T., A. Sabchareon, P. Chantavanich, V. Singhasivanon, P. Attanath, W. H. Wernsdorfer, and U. K. Sheth. 1987. A phase-III clinical trial of mefloquine in children with chloroquine-resistant falciparum malaria in Thailand. Bull. World Health Organ. 65:223-226. [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, T. M., M. Ho, W. Supanaranond, S. Looareesuwan, S. Pukrittayakamee, and N. J. White. 1991. Changes in the peripheral blood eosinophil count in falciparum malaria. Acta Trop. 48:243-246. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, I. R., and J. K. Aronson. 2000. Adverse drug reactions: definitions, diagnosis, and management. Lancet 356:1255-1259. [DOI] [PubMed] [Google Scholar]
- 12.Gutman, J., M. Green, S. Durand, O. V. Rojas, B. Ganguly, W. M. Quezada, G. C. Utz, L. Slutsker, T. K. Ruebush II, and D. J. Bacon. 2009. Mefloquine pharmacokinetics and mefloquine-artesunate effectiveness in Peruvian patients with uncomplicated Plasmodium falciparum malaria. Malar. J. 8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai, C. S., N. K. Nair, S. M. Mansor, P. L. Olliaro, and V. Navaratnam. 2007. An analytical method with a single extraction procedure and two separate high performance liquid chromatographic systems for the determination of artesunate, dihydroartemisinin and mefloquine in human plasma for application in clinical pharmacological studies of the drug combination. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 857:308-314. [DOI] [PubMed] [Google Scholar]
- 14.Laothavorn, P., J. Karbwang, K. Na Bangchang, D. Bunnag, and T. Harinasuta. 1992. Effect of mefloquine on electrocardiographic changes in uncomplicated falciparum malaria patients. Southeast Asian J. Trop. Med. Public Health 23:51-54. [PubMed] [Google Scholar]
- 15.Leonardi, E., G. Gilvary, N. J. White, and F. Nosten. 2001. Severe allergic reactions to oral artesunate: a report of two cases. Trans. R. Soc. Trop. Med. Hyg. 95:182-183. [DOI] [PubMed] [Google Scholar]
- 16.Lightbown, I. D., J. P. Lambert, G. Edwards, and S. J. Coker. 2001. Potentiation of halofantrine-induced QTc prolongation by mefloquine: correlation with blood concentrations of halofantrine. Br. J. Pharmacol. 132:197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Looareesuwan, S., T. Harinasuta, and T. Chongsuphajaisiddhi. 1992. Drug resistant malaria, with special reference to Thailand. Southeast Asian J. Trop. Med. Public Health 23:621-634. [PubMed] [Google Scholar]
- 18.Luxemburger, C., F. Nosten, F. ter Kuiile, L. Frejacques, T. Chongsuphajaisiddhi, and N. J. White. 1991. Mefloquine for multidrug-resistant malaria. Lancet 338:1268. [DOI] [PubMed] [Google Scholar]
- 19.Luxemburger, C., R. N. Price, F. Nosten, F. O. Ter Kuile, T. Chongsuphajaisiddhi, and N. J. White. 1996. Mefloquine in infants and young children. Ann. Trop. Paediatr. 16:281-286. [DOI] [PubMed] [Google Scholar]
- 20.Newton, P., Y. Suputtamongkol, P. Teja-Isavadharm, S. Pukrittayakamee, V. Navaratnam, I. Bates, and N. White. 2000. Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob. Agents Chemother. 44:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nosten, F., C. Luxemburger, F. O. ter Kuile, C. Woodrow, J. P. Eh, T. Chongsuphajaisiddhi, and N. J. White. 1994. Treatment of multidrug-resistant Plasmodium falciparum malaria with 3-day artesunate-mefloquine combination. J. Infect. Dis. 170:971-977. [DOI] [PubMed] [Google Scholar]
- 22.Nosten, F., F. O. ter Kuile, C. Luxemburger, C. Woodrow, D. E. Kyle, T. Chongsuphajaisiddhi, and N. J. White. 1993. Cardiac effects of antimalarial treatment with halofantrine. Lancet 341:1054-1056. [DOI] [PubMed] [Google Scholar]
- 23.Nosten, F., M. van Vugt, R. Price, C. Luxemburger, K. L. Thway, A. Brockman, R. McGready, F. ter Kuile, S. Looareesuwan, and N. J. White. 2000. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 356:297-302. [DOI] [PubMed] [Google Scholar]
- 24.Price, R., J. A. Simpson, P. Teja-Isavatharm, M. M. Than, C. Luxemburger, D. G. Heppner, T. Chongsuphajaisiddhi, F. Nosten, and N. J. White. 1999. Pharmacokinetics of mefloquine combined with artesunate in children with acute falciparum malaria. Antimicrob. Agents Chemother. 43:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price, R., M. van Vugt, L. Phaipun, C. Luxemburger, J. Simpson, R. McGready, F. ter Kuile, A. Kham, T. Chongsuphajaisiddhi, N. J. White, and F. Nosten. 1999. Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am. J. Trop. Med. Hyg. 60:547-555. [DOI] [PubMed] [Google Scholar]
- 26.Rowland, M., and T. Tozer. 1989. Clinical pharmacokinetics: concepts and applications. Lea and Feiber, Philadelphia, PA.
- 27.Simpson, J. A., R. Price, F. ter Kuile, P. Teja-Isavatharm, F. Nosten, T. Chongsuphajaisiddhi, S. Looareesuwan, L. Aarons, and N. J. White. 1999. Population pharmacokinetics of mefloquine in patients with acute falciparum malaria. Clin. Pharmacol. Ther. 66:472-484. [DOI] [PubMed] [Google Scholar]
- 28.Simpson, J. A., E. R. Watkins, R. N. Price, L. Aarons, D. E. Kyle, and N. J. White. 2000. Mefloquine pharmacokinetic-pharmacodynamic models: implications for dosing and resistance. Antimicrob. Agents Chemother. 44:3414-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slutsker, L. M., C. O. Khoromana, D. Payne, C. R. Allen, J. J. Wirima, D. L. Heymann, L. Patchen, and R. W. Steketee. 1990. Mefloquine therapy for Plasmodium falciparum malaria in children under 5 years of age in Malawi: in vivo/in vitro efficacy and correlation of drug concentration with parasitological outcome. Bull. World Health Organ. 68:53-59. [PMC free article] [PubMed] [Google Scholar]
- 30.Snounou, G., and H. P. Beck. 1998. The use of PCR genotyping in the assessment of recrudescence or reinfection after antimalarial drug treatment. Parasitol. Today 14:462-467. [DOI] [PubMed] [Google Scholar]
- 31.Taylor, W. R., and N. J. White. 2004. Antimalarial drug toxicity: a review. Drug Saf. 27:25-61. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, W. R., H. Widjaja, H. Basri, C. Ohrt, T. Taufik, E. Tjitra, S. Baso, D. Fryauff, S. L. Hoffman, and T. L. Richie. 2008. Changes in the total leukocyte and platelet counts in Papuan and non-Papuan adults from north-east Papua infected with acute Plasmodium vivax or uncomplicated Plasmodium falciparum malaria. Malar. J. 7:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teja-Isavadharm, P., G. Watt, C. Eamsila, K. Jongsakul, Q. Li, G. Keeratithakul, N. Sirisopana, L. Luesutthiviboon, T. G. Brewer, and D. E. Kyle. 2001. Comparative pharmacokinetics and effect kinetics of orally administered artesunate in healthy volunteers and patients with uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 65:717-721. [DOI] [PubMed] [Google Scholar]
- 34.ter Kuile, F. O., F. Nosten, C. Luxemburger, D. Kyle, P. Teja-Isavatharm, L. Phaipun, R. Price, T. Chongsuphajaisiddhi, and N. J. White. 1995. Mefloquine treatment of acute falciparum malaria: a prospective study of non-serious adverse effects in 3673 patients. Bull. World Health Organ. 73:631-642. [PMC free article] [PubMed] [Google Scholar]
- 35.Touze, J. E., P. Heno, L. Fourcade, J. C. Deharo, G. Thomas, S. Bohan, P. Paule, P. Riviere, E. Kouassi, and A. Buguet. 2002. The effects of antimalarial drugs on ventricular repolarization. Am. J. Trop. Med. Hyg. 67:54-60. [DOI] [PubMed] [Google Scholar]