Abstract
TD-1792 is a glycopeptide-cephalosporin heterodimer antibiotic with activity against a broad spectrum of Gram-positive pathogens that includes methicillin-susceptible and -resistant Staphylococcus aureus. The objective of the present study was to evaluate the in vitro activity of TD-1792 against a collection of clinical isolates of vancomycin-intermediate Staphylococcus spp. (VISS), heteroresistant VISS (hVISS), and vancomycin-resistant S. aureus (VRSA). The TD-1792, vancomycin, daptomycin, linezolid, and quinupristin-dalfopristin MICs and minimum bactericidal concentrations (MBCs) were determined for 50 VISS/hVISS isolates and 3 VRSA isolates. Time-kill experiments (TKs) were then performed over 24 h with two vancomycin-intermediate S. aureus strains and two VRSA strains, using each agent at multiples of the MIC. TD-1792 and daptomycin were also evaluated in the presence and absence of 50% human serum to determine the effects of the proteins on their activities. Most of the VISS/hVISS isolates were susceptible to all agents except vancomycin. TD-1792 exhibited the lowest MIC values (MIC90 = 0.125 μg/ml), followed by quinupristin-dalfopristin and daptomycin (MIC90 = 1 μg/ml) and then linezolid (MIC90 = 2 μg/ml). The presence of serum resulted in a 2- to 8-fold increase in the TD-1792 and daptomycin MIC values. In TKs, QD demonstrated bactericidal activity at multiples of the MIC that simulated therapeutic levels, whereas linezolid was only bacteriostatic. Both TD-1792 and daptomycin demonstrated rapid bactericidal activities against all isolates tested. The presence of proteins had only a minimal impact on the activity of TD-1792 in TKs. TD-1792 exhibited significant in vitro activity against multidrug-resistant Staphylococcus isolates and represents a promising candidate for the treatment of infections caused by Gram-positive organisms.
Staphylococcus aureus is a well-recognized source of serious and recurrent bacterial infections. Over the years, this pathogen has developed and/or acquired mechanisms of resistance to numerous classes of antimicrobials, including penicillins, β-lactams, and more recently, lipopeptides and glycopeptides (6). As the primary treatment for methicillin-resistant S. aureus (MRSA) infections in the United States, the use of vancomycin has dramatically increased, paralleling the enhanced prevalence of MRSA infections (2, 15). These simultaneous phenomena have led to selective pressure on this pathogen, thus resulting in the emergence of clinical isolates of staphylococci with reduced susceptibility to glycopeptides (15).
The mechanism of vancomycin resistance has been clearly characterized in Staphylococcus spp. with the identification of the van genes acquired from Enterococcus spp. (21). To date, 10 isolates of vancomycin-resistant S. aureus (VRSA) have been described and display vancomycin MICs ranging from 32 to 1,024 μg/ml (2, 21). In addition to this problematic and well-known mechanism of resistance, Staphylococcus isolates have also developed reduced or intermediate susceptibility to glycopeptides. This phenotype represents a major issue for infectious disease clinicians and researchers, since these organisms are difficult to identify and have been associated with poor clinical outcomes, including increased lengths of stay and prolonged bacteremia (2, 19). Vancomycin-intermediate staphylococcal species (VISS) exhibit a vancomycin MIC value of 4 to 16 μg/ml (previously, 8 to 16 μg/ml) (19). In contrast, heterogeneous VISS (hVISS) have vancomycin MICs of ≤2 μg/ml by standard methods of MIC determination and are difficult to identify, requiring nonroutine quantitative tests such as the Etest macromethod or the expensive and time-consuming modified population analysis profile method (19, 23).
A number of new agents are currently under development to meet the challenge of the limited therapeutic alternatives to treat hVISS and VISS infections (9). These new drugs display multiple mechanisms of action that have the potential to lower the likelihood of the emergence of resistance (9). TD-1792 (Theravance Inc., South San Francisco, CA) is a new covalently linked glycopeptide-cephalosporin heterodimer antimicrobial agent being investigated for its substantial bactericidal activity against Gram-positive organisms, including multidrug-resistant (MDR) isolates (7). The spectrum of activity of TD-1792 covers the majority of clinically relevant Gram-positive pathogens and includes methicillin-susceptible and -resistant S. aureus, coagulase-negative staphylococci, Enterococcus spp., and Streptococcus spp. (7). Recent pharmacokinetic data from single- and multiple-dosing studies with healthy volunteers demonstrated achievable serum concentrations of 4.65 ± 0.64 μg/ml and a half-life of between 6.8 and 11.3 h. These studies also demonstrated that TD-1792 has time-independent linear pharmacokinetic properties and that the concentrations achieved in vivo support once-daily administration (8, 22).
Since limited data on TD-1792 are available, the primary objective of the present study was to compare its in vitro activity to the activities of vancomycin, linezolid, quinupristin-dalfopristin, and daptomycin against VISS/hVISS and three VRSA isolates. In addition, because any information regarding the effects of protein on the bactericidal properties of antimicrobials is essential to understand the data from in vitro and in vivo studies, we explored the activities of TD-1792 (49% protein binding in human plasma) (16) and daptomycin (92% protein binding in human plasma) in the presence of human serum (1).
(A portion of this work was presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 2007, poster E-1623.)
MATERIALS AND METHODS
Bacterial strains and antimicrobials.
Fifty clinical isolates of VISS/hVISS, including 37 VISA and 13 coagulase-negative Staphylococcus isolates (7 S. epidermidis [5 VISS and 2 hVISS], 5 S. haemolyticus isolates [3 VISS and 2 hVISS], and 1 unspecified strain [hVISS]), and 3 VRSA isolates (VRSA PA, VRSA MI, VRSA NY) were obtained from the Centers for Disease Control and Prevention (CDC); the Network on Antimicrobial Resistance in S. aureus (NARSA), Keiichi Hiramatsu (Japan); and the Detroit Medical Center, Detroit, MI.
Theravance, Inc., and Aventis Pharmaceuticals Inc. (Paris, France) generously provided TD-1792 and quinupristin-dalfopristin powders, respectively. Daptomycin and linezolid were commercially obtained, and vancomycin analytical powder was purchased from Sigma-Aldrich (St. Louis, MO). All antimicrobial solutions were prepared daily according to the Clinical and Laboratory Standards Institute (CLSI) and manufacturer's recommendations (maximum solubility of TD-1792 in sterile water, 10 mg/ml).
Medium.
Mueller-Hinton broth (MHB; Difco Laboratories, Detroit, MI) supplemented with 12.5 μg/ml magnesium and 25 μg/ml calcium (25 SMHB) was used for microdilution susceptibility testing and time-kill experiments (TKs) with vancomycin, linezolid, quinupristin-dalfopristin, and TD-1792. SMHB adjusted to contain the normal physiologic calcium concentration (SMHB-Ca2+; final concentration, 1.1 to 1.3 mM) was used for all experiments with daptomycin. Fifty percent (vol/vol) human serum (S-7023; Sigma-Aldrich, St. Louis, MO) was combined with 50% 50 SMHB (final concentration, 25 SMHB) for TD-1792 and 50% 100 SMHB (final concentration, 50 SMHB) for daptomycin susceptibility and time-kill experiments. Processing of the serum included heating to 56°C for 1 h and then passage through 0.8-, 0.5-, and 0.22-μm-pore-size filters. Brain heart infusion agar (BHI; Becton-Dickinson, Sparks, MD) was used for quantification of the bacteria in samples from the time-kill experiments.
Susceptibility testing.
MICs and minimum bactericidal concentrations (MBCs) were determined using a microdilution technique with an inoculum of 5 × 105 CFU/ml, according to CLSI guidelines (4). Microtiter plates were incubated for 24 h at 35°C prior to colony counting. Five-microliter samples from visually clear wells were plated onto BHI plates for the determination of MBCs, after 24 h of incubation at 35°C. MICs and MBCs were determined in duplicate to ensure reproducibility. The MIC and MBCs of TD-1792 and daptomycin were also determined in the presence of 50% human serum.
Time-kill curves.
TKs were performed in triplicate for all antibiotics against two VISA isolates (MU50, NJ992) and two VRSA isolates (VRSA MI, VRSA PA). TKs with TD-1792 and daptomycin were also conducted in the presence and absence of 50% human serum. The starting inoculum was prepared using a mid-exponential-phase bacterial culture (108 CFU/ml determined by obtaining spectrometer readings of the optical density at 600 nm of ∼0.3) diluted in SMHB to reach a final inoculum of 5 × 106 CFU/ml. All drugs were evaluated at 4×, 8×, 16×, and 32× their respective MICs. Briefly, aliquots (0.1 ml) were removed from cultures at 0, 1, 4, 8, and 24 h and diluted in cold normal saline for colony counting. Colony counts were performed on BHI plates using an automatic spiral plater (DW Scientific, Frederick, MD) when serial dilution prevented antibiotic carryover. To lower the limit of detection, samples were vacuum filtered before they were plated, therefore reducing the antibiotic concentration to below the MIC of the drug. We determined these methods to have a lower limit of reliable detection of 2 log10 CFU/ml and 1 log10 CFU/ml, respectively. The plates were incubated at 35°C for 24 h prior to colony counting, and TK curves were constructed by plotting the mean colony counts (log10 CFU/ml) versus time. Bactericidal activity was defined as a >3-log10-CFU/ml reduction in bacterial density (99.9% kill) from the starting inoculum, and the time to 99.9% killing (T99.9) was determined by linear regression of the sample points if r2 was ≥0.95 or by visual inspection.
Statistical analysis.
All statistical analyses were performed using PAWS statistical software (release 18.0; SPSS, Inc. Chicago, IL). The colony counts at 24 h were compared between groups using one-way analysis of variance, followed by the Bonferroni post hoc test for multiple comparisons. A P value of ≤0.05 indicated statistical significance.
RESULTS
Antimicrobial susceptibility.
With the exception of vancomycin (MIC value range, 4 to 1,024 μg/ml), most of the organisms were susceptible to all antimicrobials tested (Table 1). Five isolates of VISS (four S. aureus isolates and one S. haemolyticus isolate) were found to be nonsusceptible to daptomycin, and VRSA NY had an intermediate MIC for quinupristin-dalfopristin. Overall, TD-1792 exhibited the lowest MIC values against VISS/hVISS and VRSA isolates (MIC ranges, 0.015 to 0.125 and 0.03 to 0.25 μg/ml, respectively) (Tables 1 and 2). Daptomycin exhibited lower MIC values than quinupristin-dalfopristin for the VRSA group of isolates (ranges, 0.125 to 0.25 μg/ml and 0.25 to 2 μg/ml, respectively), whereas quinupristin-dalfopristin displayed lower MICs than daptomycin for the VISS group of isolates (Tables 1 and 2). Both TD-1792 and daptomycin were affected by the presence of human serum, showing increases in MIC values (from 2- to 8-fold and 2- to 4-fold, respectively). Finally, the linezolid MIC ranges were 0.5 to 4 μg/ml for the VISS/hVISA collection of isolates and 1 to 2 μg/ml for the VRSA group of isolates. MBCs were equal to or 1 dilution higher than the MIC values for all organisms and all antimicrobials tested except quinupristin-dalfopristin and linezolid, for which the MBCs were up to 8 and 16 times higher, respectively.
TABLE 1.
Susceptibility values for the collection of 50 VISS/hVISS isolatesa
| Antimicrobial | MIC (mg/ml) |
MBC (mg/ml) |
||||
|---|---|---|---|---|---|---|
| 50% | 90% | Range | 50% | 90% | Range | |
| TD-1792 | 0.06 | 0.125 | 0.015-0.125 | 0.06 | 0.125 | 0.015-0.125 |
| TD-1792 + S | 0.25 | 0.5 | 0.03-0.5 | 0.25 | 0.5 | 0.03-0.5 |
| DAP | 1 | 1 | 0.25-2 | 1 | 2 | 0.25-4 |
| DAP + S | 1 | 4 | 0.25-4 | 4 | 8 | 1-8 |
| VAN | 4 | 8 | 4-8 | 4 | 8 | 4-8 |
| LNZ | 2 | 2 | 0.5-4 | 8 | 32 | 1-64 |
| QD | 0.5 | 1 | 0.125-1 | 1 | 4 | 0.125-8 |
DAP, daptomycin; VAN, vancomycin; QD, quinupristin-dalfopristin; LNZ, linezolid; S, 50% human serum.
TABLE 2.
Susceptibility values for isolates used in time-kill assaysa
| Isolate | MIC (MBC) (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| TD-1792 | TD-1792 +S | DAP | DAP + S | VAN | LNZ | QD | |
| MU50 | 0.03 (0.03) | 0.25 (0.25) | 1 (1) | 2 (2) | 4 (4) | 1 (32) | 0.5 (0.5) |
| NJ992 | 0.06 (0.06) | 0.25 (0.25) | 1 (1) | 2 (2) | 8 (8) | 1 (2) | 0.5 (1) |
| VRSA MI | 0.25 (0.5) | 2 (8) | 0.25 (0.25) | 0.5 (1) | 1,024 (>2,048) | 1 (16) | 0.5 (4) |
| VRSA PA | 0.03 (0.125) | 0.125 (0.125) | 0.25 (0.25) | 0.25 (0.5) | 32 (64) | 2 (4) | 0.25 (0.5) |
| VRSA NY | 0.03 (0.06) | 0.25 (0.5) | 0.125 (0.125) | 0.25 (0.25) | 64 (128) | 0.25 (0.5) | 2 (4) |
DAP, daptomycin; VAN, vancomycin; QD, quinupristin-dalfopristin; LNZ, linezolid; S, 50% human serum.
Time-kill curves.
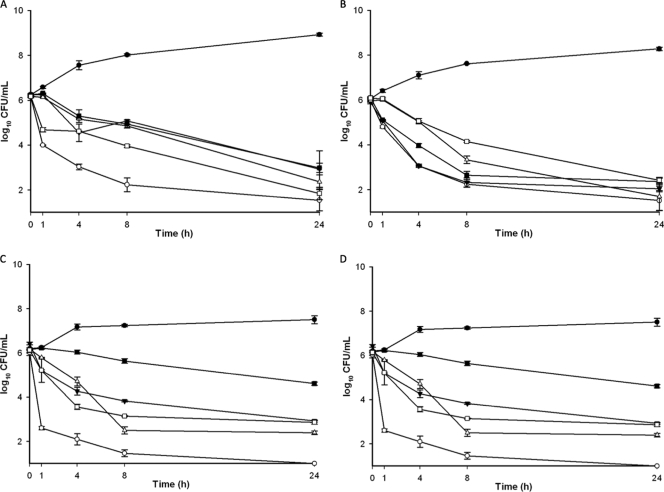
The susceptibility results and in vitro activities observed in TKs are reported in Tables 3 and 4 . In TKs, linezolid exhibited slow or no cidal activity (14.5 h < T99.9 < not defined) at concentrations of 4× to 8× the MICs, which are similar to therapeutic levels (Tables 2 and 4; Fig. 1). Vancomycin exhibited slow activity or was not cidal (10 h < T99.9 < not defined) at any of the concentrations tested (Tables 3 and 4; Fig. 1), including supratherapeutic levels (data not shown). Quinupristin-dalfopristin exhibited concentration-dependent activity, and a bactericidal effect was observed between 8 and 24 h at 8× MIC (Tables 3 and 4; Fig. 1). Daptomycin demonstrated rapid bactericidal activity, reaching the limit of detection between 1 and 8 h. The presence of proteins had little or no effect on the daptomycin activity. Differences between regimens with or without serum were significant only at 4× MIC against VRSA PA (P = 0.0001; 95% confidence interval [CI], 1.91 to 3.47), 4× to 8× MIC against VISA NJ992 (95% CIs, 0.04 to 2.19 [P = 0.038] and 0.39 to 2.23 [P = 0.002], respectively), and 8× MIC against VRSA MI (P = 0.045). Unlike daptomycin, TD-1792 demonstrated concentration-independent activity (Tables 3 and 4; Fig. 1). The time to achieve 99.9% of the kill varied from 1 to 24 h. Serum slightly affected the activity of TD-1792, but the differences between the regimens of TD-1792 with or without serum were not significant, except against MU50 at 4× MIC (95% CI, 0.03 to 1.41, P = 0.035) and VRSA MI at 8× and 16× MICs (95% CIs, 0.94 to 1.77 [P = 0.0001] and 0.05 to 1.20 [P = 0.026], respectively) (Tables 3 and 4; Fig. 1). The absence of a serum effect was confirmed by the fact that the time to achieve cidal activity did not vary in the presence of serum (data not shown).
TABLE 3.
Changes in VISA susceptibility from the baseline at 24 ha
| Multiple of MIC | Change in VISA susceptibility (log10 CFU/ml) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MU50 |
NJ992 |
|||||||||||||
| TD-1792 | TD-1792 + S | DAP | DAP + S | VAN | LIN | QD | TD-1792 | TD-1792 + S | DAP | DAP + S | VAN | LIN | QD | |
| 4 | 3.77b | 3.05b | 3.02b | 4.01b | 2.71 | 3.19 | 2.16 | 3.84 | 3.61 | 3.61b | 4.78b | 2.88 | 2.13 | 3.65 |
| 8 | 4.33 | 3.6 | 4.82 | 4.13 | 3.32 | 3.26 | 3.82 | 4.48 | 4.09 | 3.86b | 5.01b | 4.30 | 3.66 | 3.73 |
| 16 | 4.94 | 4.37 | 5.12 | 5.09 | 4.48 | 4.25 | 4.60 | 4.94 | 4.76 | 4.68 | 5.03 | 4.58 | 3.98 | 4.47 |
| 32 | 4.92 | 4.81 | 5.12 | 5.23 | 4.41 | 4.80 | 4.97 | 5.00 | 4.92 | 4.93 | 5.04 | 4.79 | 4.89 | 4.96 |
DAP, daptomycin; VAN, vancomycin; QD, quinupristin-dalfopristin; LNZ, linezolid; S, 50% human serum.
Significant differences (P < 0.05) between TD-1792 or daptomycin with and without serum.
TABLE 4.
Changes in VRSA susceptibility from the baseline at 24 ha
| Multiple of the MIC | Change in VRSA susceptibility (log10 CFU/ml) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI |
PA |
|||||||||||
| TD-1792 | TD-1792 + S | DAP | DAP + S | LIN | QD | TD-1792 | TD-1792 + S | DAP | DAP + S | LIN | QD | |
| 4 | 2.95 | 4.12 | 4.59b | 2.53b | 1.31 | 3.05 | 4.06 | 4.04b | 3.33b | 5.12 | 3.24 | 3.28 |
| 8 | 3.27b | 4.06b | 5.00 | 4.69 | 1.55 | 3.86 | 4.09 | 4.65 | 4.95 | 5.11 | 4.20 | 3.48 |
| 16 | 3.34 | 4.11 | 4.96 | 4.60 | 3.64 | 3.89 | 4.17b | 5.15 | 5.14 | 5.12 | 4.24 | 3.70 |
| 32 | 5.13 | 4.03 | 5.03 | 5.11 | 4.52 | 4.19 | 5.05 | 5.09 | 5.10 | 5.11 | 4.95 | 3.51 |
DAP, daptomycin; VAN, vancomycin; QD, quinupristin-dalfopristin; LNZ, linezolid; S, 50% human serum.
Significant differences (P < 0.05) between TD-1792 or daptomycin with and without serum.
FIG. 1.
Time-kill curves at 8× MIC. (A) MU50; (B) NJ992; (C) VRSA MI; (D) VRSA PA. Filled circles, growth control; open squares, TD-1792; open circles, daptomycin; inverted filled triangles, vancomycin; open triangles, QD; filled squares, linezolid. Of note, the results for the vancomycin regimen for the VRSA isolates are not presented since the concentrations simulated in TKs were supratherapeutic and are irrelevant.
DISCUSSION
TD-1792 is a new glycopeptide-cephalosporin heterodimer being primarily developed and investigated in the treatment of infections caused by Gram-positive organisms, including methicillin-susceptible and -resistant Staphylococcus spp. (7, 12). The particular interest in this new compound results from its unique chemical structure, designed using a multivalent approach to drug discovery (12). Typical approaches for the development of new antibiotics mainly focus on the modification of existing compounds. Unfortunately, this approach often appears to be unsatisfactory because of the rapid development of resistance. One of the best examples might be the β-lactam class of antibiotics, which was started by the discovery of penicillins in the late 1950s. The rapid emergence of β-lactamase-producing pathogens led to the development of penicillins resistant to β-lactamases, such as methicillin. However, the modifications made in the structures of these drugs were not sufficient, and MRSA emerged soon after the first use of methicillin (17). The multivalent approach to drug discovery is based on the design of molecules with enhanced activity and the probability of a reduced risk of antimicrobial resistance (3). TD-1792 was designed by combination of a glycopeptide nucleus and a cephalosporin ring. The rationale for this design was based on the apparent proximity of the biological targets of these two pharmacophores, potentially allowing a single compound to interact with both sites simultaneously (10). The synergy of the activities of the combination of those two classes of drugs has not been consistently reported (11, 13, 14). However, the apparent increased susceptibility to β-lactams observed in the VISA population may confirm the potential interest in such associations in order to enhance the antimicrobial activity and eventually prevent the emergence of glycopeptides and/or β-lactam resistance (20). Preliminary studies investigating the mechanism of action of TD-1792 against S. aureus have revealed a multivalent binding interaction of the novel heterodimer with the transpeptidase site of PBP 2 and the d-Ala-d-Ala components present in the peptidoglycan (7). Although the proposed mechanism has not been confirmed at this time, this dual mechanism of action may be favorable for the enhancement of activity against isolates exhibiting reduced susceptibility to either class of antibiotics.
Consistent with previous studies reporting potent activities in vitro and in vivo (7, 8, 16), we observed low MIC values for TD-1792 against Staphylococcus isolates with reduced susceptibility to vancomycin. The MIC90 of this compound was up to 8-fold lower than the MIC90 of the highly bactericidal agent, daptomycin, as well as quinupristin-dalfopristin and linezolid. We observed a 1-fold difference in the MIC values of coagulase-negative staphylococci and S. aureus isolates. However, the number of isolates was too low to make a significant conclusion. We demonstrated in this study that the MIC values of TD-1792 increased from 2- to 8-fold in the presence of human serum. Since only the free drug remains biologically active and able to interact with bacterial cells, protein binding represents an important characteristic of antimicrobial agents that needs to be clarified (5). Little information regarding TD-1792 pharmacokinetic parameters is available, and the effect of serum protein on the MICs of this new compound has not yet been reported (8, 16, 22). Despite the reduced activity recorded in the presence of serum, the MIC values of TD-1792 remained lower than those of the comparators against VISS/hVISS and VRSA and below the achievable concentrations observed in human volunteers (22). In addition, the presence of protein did not significantly affect of the activity of TD-1792 in time-kill experiments, in which we observed concentration-independent activity against the VISS and VRSA clinical isolates and times to achieve 99.9% of the kill ranging from 1 to 24 h. Further investigations are, however, warranted to clarify the effect of protein on the antimicrobial activity of this new compound.
In conclusion, TD-1792 was more effective in vitro than the other antimicrobial agents tested against a wide collection of MDR Gram-positive Staphylococcus spp. These preliminary results suggest the potential interest in this new compound for the treatment of infections due to staphylococci. A recent phase II clinical trial involving approximately 200 patients evaluated the safety and efficacy of TD-1792 in the treatment of complicated skin and skin structure infections caused by Gram-positive bacteria. TD-1792 demonstrated noninferiority to the comparator agent, vancomycin, with similar cure rates being achieved in the clinically evaluable population (91.7% and 90.7% in the TD-1792 and vancomycin groups, respectively). In microbiologically evaluable patients with MRSA, cure rates were 94.7% in the TD-1792 group and 91.9% in the vancomycin group, with similar tolerabilities between the groups being observed and fewer serious adverse events being reported for TD-1792 (none for TD-1792 versus two for vancomycin) (18). Further investigations are warranted to clarify the clinical utility of TD-1792 and this new class of antibiotics in the treatment of infections caused by MDR Gram-positive pathogens.
Acknowledgments
This work was sponsored by a grant from Theravance, Inc.
Footnotes
Published ahead of print on 28 June 2010.
REFERENCES
- 1.Anonymous. 2007. Cubicin package insert. Cubist Pharmaceuticals, Lexington, MA.
- 2.Appelbaum, P. C. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 12:16-23. [DOI] [PubMed] [Google Scholar]
- 3.Choi, S. 2004. Synthetic multivalent molecules. John Wiley & Sons, Inc., New York, NY.
- 4.Clinical and Laboratory Standards Institute. 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Drusano, G. L. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat. Rev. Microbiol. 2:289-300. [DOI] [PubMed] [Google Scholar]
- 6.Falcone, M., P. Serra, and M. Venditti. 2009. Serious infections due to methicillin-resistant Staphylococcus aureus: an evolving challenge for physicians. Eur. J. Int. Med. 20:343-347. [DOI] [PubMed] [Google Scholar]
- 7.Fritsche, T., J. Blais, and R. Jones. 2007. In vitro evaluation of TD-1792, a novel antimicrobic with potent Gram-positive activity, poster E-1627. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 8.Hedge, S., N. Reyes, J. Trumbull, R. Skinner, S. Difuntorum, D. Marquess, and S. Turner. 2007. Efficacy of TD-1792, a novel multivalent glycopeptide against Gram-positive pathogens in the neutropenic murine thigh model, poster A-42. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 9.Linden, P. K. 2008. Vancomycin resistance: are there better glycopeptides coming? Expert Rev. Anti Infect. Ther. 6:917-928. [DOI] [PubMed] [Google Scholar]
- 10.Long, D. D., J. B. Aggen, B. G. Christensen, J. K. Judice, S. S. Hegde, K. Kaniga, K. M. Krause, M. S. Linsell, E. J. Moran, and J. L. Pace. 2008. A multivalent approach to drug discovery for novel antibiotics. J. Antibiot. (Tokyo) 61:595-602. [DOI] [PubMed] [Google Scholar]
- 11.Lozniewski, A., C. Lion, F. Mory, and M. Weber. 2001. In vitro synergy between cefepime and vancomycin against methicillin-susceptible and -resistant Staphylococcus aureus and S. epidermidis. J. Antimicrob. Chemother. 47:83-86. [DOI] [PubMed] [Google Scholar]
- 12.Lunde, C., S. Lewis, B. Benton, M. Mammen, and J. Blais. 2009. TD-1792, mode of action of a multivalent glycopeptide-cephalosporin antibiotic, poster C1-1344. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 13.Miranda-Novales, G., B. E. Leanos-Miranda, M. Vilchis-Perez, and F. Solorzano-Santos. 2006. In vitro activity effects of combinations of cephalothin, dicloxacillin, imipenem, vancomycin and amikacin against methicillin-resistant Staphylococcus spp. strains. Ann. Clin. Microbiol. Antimicrob. 5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seibert, G., D. Isert, N. Klesel, M. Limbert, A. Markus, and E. Schrinner. 1992. The in-vitro antibacterial activity of a combination of cefpirome or cefoperazone with vancomycin against enterococci and Staphylococcus aureus. J. Antimicrob. Chemother. 29(Suppl. A):25-30. [DOI] [PubMed] [Google Scholar]
- 15.Septimus, E. J., and K. M. Kuper. 2009. Clinical challenges in addressing resistance to antimicrobial drugs in the twenty-first century. Clin. Pharmacol. Ther. 86:336-339. [DOI] [PubMed] [Google Scholar]
- 16.Shaw, J., G. Obedencio, J. Imperio, J. Shelton, S. Turner, J. Mccullough, D. Marquess, and S. Jaw Tsai. 2007. Pharmacokinetics of TD-1792, a novel multivalent glycopeptide, in preclinical animal species, abstr. A-43. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 17.Stapleton, P. D., and P. W. Taylor. 2002. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci. Prog. 85:57-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stryjewski, M. E., S. L. Barriere, M. M. Kitt, and G. R. Corey. 2007. TD-1792 vs vancomycin (VAN) for treatment of complicated gram-positive skin and skin structure infections (cSSSIs), poster L-1147a. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 19.Szabo, J. 2009. hVISA/VISA: diagnostic and therapeutic problems. Expert Rev. Anti Infect. Ther. 7:1-3. [DOI] [PubMed] [Google Scholar]
- 20.Vidaillac, C., K. Newton, and M. Rybak. 2009. Evaluation of oxacillin, daptomycin, and ceftaroline activity against clinical vancomycin heterovariant methicillin-resistant Staphylococcus aureus (MRSA), abstr. E205. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 21.Werner, G., B. Strommenger, and W. Witte. 2008. Acquired vancomycin resistance in clinically relevant pathogens. Future Microbiol. 3:547-562. [DOI] [PubMed] [Google Scholar]
- 22.Wong, S., J. Shaw, W. Kraft, C. Lanni, M. Kitt, and M. Goldberg. 2007. Pharmacokinetic (PK) disposition of a novel multivalent glycopeptide, TD-1792, abstr. E1623. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 23.Wootton, M., R. A. Howe, R. Hillman, T. R. Walsh, P. M. Bennett, and A. P. MacGowan. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47:399-403. [DOI] [PubMed] [Google Scholar]