Abstract
The genus Listeria comprises food-borne pathogens associated with severe infections and a high mortality rate. Endolysins from bacteriophages infecting Listeria are promising tools for both their detection and control. These proteins feature a modular organization, consisting of an N-terminal enzymatically active domain (EAD), which contributes lytic activity, and a C-terminal cell wall binding domain (CBD), which targets the lysin to its substrate. Sequence comparison among 12 different endolysins revealed high diversity among the enzyme's functional domains and allowed classification of their CBDs into two major groups and five subclasses. This diversity is reflected in various binding properties, as determined by cell wall binding assays using CBDs fused to fluorescent marker proteins. Although some proteins exhibited a broad binding range and recognize Listeria strains representing all serovars, others target specific serovars only. The CBDs also differed with respect to the number and distribution of ligands recognized on the cells, as well as their binding affinities. Surface plasmon resonance analysis revealed equilibrium affinities in the pico- to nanomolar ranges for all proteins except CBD006, which is due to an internal truncation. Rapid multiplexed detection and differentiation of Listeria strains in mixed bacterial cultures was possible by combining CBDs of different binding specificities with fluorescent markers of various colors. In addition, cells of different Listeria strains could be recovered from artificially contaminated milk or cheese by CBD-based magnetic separation by using broad-range CBDP40 and subsequently identified after incubation with two differently colored CBD fusion proteins of higher specificity.
Listeria belong to the low G+C Gram-positive bacteria; are ubiquitously present in nature; and can be isolated from many sources such as soil, water, sewage effluents, and the feces of humans and animals (52). Within the genus Listeria, six species are recognized (Listeria monocytogenes, L. innocua, L. ivanovii, L. seeligeri, L. welshimeri, and L. grayi), in addition to the recently proposed new species L. marthii (12) and L. rocourtiae (24). Five major serovar (sv.) groups exist (1/2, 3, 4, 6, and 7), and at least 16 subserovars can be distinguished. Variations are mainly due to the structure and composition of cell wall-associated carbohydrates, wall teichoic acid (WTA), and lipoteichoic acid (LTA). However, serotyping does not necessarily correlate with the species (7, 8). Listeria monocytogenes is an opportunistic, intracellular pathogen causing an infection termed listeriosis and is exclusively transmitted via contaminated food such as raw meat, milk products, fish products, and vegetables. Listeriosis is a serious infection primarily affecting immunocompromised patients, pregnant women, the elderly, and newborns and is characterized by a high mortality rate up to >40% (5). Strains belonging to sv. groups 1/2 and 4b have been responsible for the majority of Listeria infections in humans (52).
Based on their host specificity, bacteriophages are useful tools for bacterial detection and differentiation (reviewed in references 40 and 43). To date, many phages infecting Listeria cells have been isolated. All of them are strictly genus specific. The few known virulent (obligately lytic) phages have a very broad host range (19, 29), whereas the majority are temperate and are restricted to a limited number of host strains within the individual serovar groups (32). Various applications based on Listeria phages have been developed, including phage typing (27) and the detection of viable Listeria cells by a recombinant luciferase reporter phage (31).
Bacteriophage endolysins are peptidoglycan hydrolases that mediate lysis of the host cell at the end of the lytic multiplication cycle. These enzymes represent powerful tools with many applications in molecular biology, biotechnology, and medicine (26). Listeria phage endolysins show a domain organization and belong to category 1 of modular enzymes, in which catalysis and substrate specificity are clearly separated (18). They feature an N-terminal enzymatically active domain (EAD) and a C-terminal cell wall binding domain (CBD) (30, 33). The EAD determines the catalytic activity of the enzyme, and the CBD is responsible for targeting the protein to the bacterial cell wall. Surprisingly, the CBDs feature high binding specificity; although they are able to lyse all Listeria cell walls, the individual endolysins display highest activity against cells or cell walls from specific serovars. Besides recognition specificity, the CBDs feature very strong, saturation-dependent binding to listerial cell walls, with equilibrium constants in the nanomolar ranges (30). Because of the absence of an outer membrane in Gram-positive bacteria, the cell wall can also be accessed from outside, enabling the CBD to attach to its ligand. These properties can be harnessed for rapid and efficient labeling and immobilization of bacterial cells (23, 30). While CBD118 recognizes cells of Listeria sv. groups 1/2 and 3 and predominantly binds at the polar and septal regions of these cells, CBD500 and CBD PSA (21, 30) exhibit binding over the entire cell surfaces of strains, belonging to sv. groups 4, 5, and 6. Although the ligands recognized by the various CBDs have not been conclusively identified, we have strong evidence for an involvement of cell wall-associated carbohydrates in recognition and binding. Furthermore, the proteins retain their lectinlike binding function in complex matrices and environments, such as infected eukaryotic cells (15) and homogenized food and enrichment cultures (23).
Fluorescent proteins (FP) such as the green fluorescent protein are very popular tools in molecular biology, medicine, and cell biology, based on their wide compatibility, lack of toxicity, incredible stability, and the fact that they do not require any cofactors other than oxygen for chromophore formation. Several FP derivatives with shifted spectral characteristics such as blue, cyan, and yellow fluorescent proteins have been developed (reviewed in references 45 and 49), which are useful for simultaneous staining applications. In addition to the green fluorescent protein (GFP) variants, several red fluorescent proteins have been described (14, 34). The RedStar protein (RS) (20) was derived from dsRed and features rapid development of high fluorescence intensity and a reduced tendency for oligomerization.
The aim of the present study was to develop a comprehensive toolbox consisting of different combinations of Listeria phage CBDs and FPs. Toward this goal, we first established a classification system for CBDs from all known Listeria phage endolysins, and characterized representative CBDs from each class regarding their binding range binding affinity, and spatial distribution and density of ligands on the cell surface. We then used fluorescent proteins for the construction of differently tagged reporter-CBDs and demonstrate the suitability of using these proteins in a single and simple assay for simultaneous detection and differentiation of Listeria strains in mixed cultures. We also provide proof of concept for application of this technique for differential staining and identification of different Listeria strains after recovery from contaminated food by magnetic separation with CBD-coated paramagnetic beads (CBD-MS).
MATERIALS AND METHODS
Bacteria, phages, plasmids, and culture conditions.
Escherichia coli JM109 and XL1-Blue MRF′ (Stratagene) served for cloning and overexpression of His-tagged fluorescent proteins and CBD fusion proteins. A total of 26 Listeria strains comprising the six major species and all serovars (selected from a laboratory collection) were used in binding assays, and a subset of these was used for the determination of ligand numbers and affinity studies. For binding assays with CBD025, 21 additional L. innocua and L. ivanovii strains (laboratory collection) were used. Furthermore, a collection of 20 other Gram-positive bacteria from the genera Bacillus, Enterococcus, Staphylococcus, Brochothrix, Bifidobacterium, Lactococcus, Lactobacillus, and Clostridium were used in CBD binding assays.
Purified DNA of phages A118, A006, A500, PSA, P35, A511, P40, and B025 were used as templates for PCR amplification of CBD coding regions (see below). Plasmids pRSET/BFP (Invitrogen), pECFP-C1, pEYFP-C1 (BD Biosciences/Clontech), and p415_Gal1_RedStar (20) served as templates for the amplification of the BFP, CFP, YFP, and RedStar coding sequences, respectively. Plasmid pQE-30 (Qiagen) and its derivative pHGFP (30) were used for cloning and production of N-terminally His6-tagged recombinant proteins in E. coli.
E. coli JM109 was cultured in LB medium at 30°C (with 100 μg of ampicillin/ml for plasmid selection), and XL1-Blue MRF′ in LB medium at 30°C with 100 μg of ampicillin/ml and 30 μg of tetracycline/ml. Listeria strains were grown in TB (tryptose broth) medium at 30°C. Lactococcus, Lactobacillus, and Bifidobacterium strains were incubated in MRS broth at 30°C. Clostridium was grown in TGY medium under anaerobic conditions at 37°C. For all other strains, TB at 30°C served as a growth medium.
In silico analyses.
Endolysins of the following Listeria phages were included into our analysis and classification: A118, A500, A511 (19, 22, 33), PSA (21, 55), A006, P35, B054, B025, P40 (4), and putative prophages or phagelike elements of L. monocytogenes strain EGDe and of L. innocua CLIP11262 (11) and WSLC 2438 (56). For identification of putative CBDs, pairwise alignments of Listeria phage endolysin amino acid sequences were created using AlignX (Vector NTI; Invitrogen). The best-fit locations of EADs and CBDs were then determined based on high sequence homologies of single domains from different endolysins. In the case of PlyPSA, the CBD moiety was deduced from the crystal structure of PlyPSA (21). In order to establish a classification system for the various CBDs, multiple alignments of the amino acid sequences and clustered similarity (phylogenetic) trees were created using CLUSTAL W (48) and neighbor-joining algorithms (41).
DNA techniques and cloning procedures.
Standard techniques (42) and a strategy previously described (30) were used for cloning and the creation of fusion proteins. Enzymes were purchased from New England Biolabs, MBI Fermentas, Roche, and Qiagen. All CBD and fluorescent protein coding fragments were amplified from phage DNA and plasmids, respectively, using Proof Start DNA polymerase (Qiagen) and the primers listed in Table S1 in the supplemental material. Restriction sites needed for insertion of the fragments into the plasmids were introduced via the primers. DNA concentrations were determined in a photometric assay (NanoDrop ND-1000 spectrophotometer).
All plasmids used and created in the present study are listed in Table 1. Vectors pHBFP, pHCFP, pHYFP, and pHRS were constructed as described earlier (30) by inserting the BFP, CFP, YFP, and RedStar coding sequences into the BamHI and SacI sites of pQE-30, omitting the native stop codons and thereby allowing in-frame fusion with sequences inserted downstream of the cloning sites.
TABLE 1.
Plasmids used and constructed in this study
| Plasmid | Relevant properties | Source or reference |
|---|---|---|
| pRSET/BFP | Source of bfp gene | Invitrogen |
| pECFP-C1 | Source of cfp gene | BD Biosciences |
| pEYFP-C1 | Source of yfp gene | BD Biosciences |
| p415_Gal1_RedStar | Source of redStar gene | 20 |
| pQE30 | Enables N-terminal fusion with 12-amino-acid leader containing a His6 tag; Ampr | Qiagen |
| pHGFP | gfp-mut2 cloned into BamHI-SacI sites of pQE30 | 30 |
| pHBFP | bfp cloned into BamHI-SacI sites of pQE30 | This study |
| pHCFP | cfp cloned into BamHI-SacI sites of pQE30 | This study |
| pHYFP | yfp cloned into BamHI-SacI sites of pQE30 | This study |
| pHRS | redStar cloned into BamHI-SacI sites of pQE30 | This study |
| pHGFP_CBD118-D | ply118 fragment encoding Asp90-Ile281 cloned into SacI-SalI sites of pHGFP | 30 |
| pHGFP_CBD500-A | ply500 fragment encoding His133-Lys289 cloned into SacI-SalI sites of pHGFP | 30 |
| pHGFP_CBDPSA | plyPSA fragment encoding Thr172-Lys314 cloned into SacI-SalI sites of pHGFP | 21 |
| pHGFP_CBD006-A | ply006 fragment encoding Glu135-Ile235 cloned into SacI-PstI sites of pHGFP | This study |
| pHGFP_CBD006-B | ply006 fragment encoding Lys115-Ile235 cloned into SacI-PstI sites of pHGFP | This study |
| pHGFP_CBD006-C | ply006 fragment encoding Leu90-Ile235 cloned into SacI-PstI sites of pHGFP | This study |
| pHGFP_CBDP35-A | plyP35 fragment encoding Phe167-Lys291 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBDP35-B | plyP35 fragment encoding Glu150-Lys291 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBDP35-C | plyP35 fragment encoding Pro130-Lys291 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBD511-A | ply511 fragment encoding Gly177-Lys341 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBD511-B | ply511 fragment encoding Ile161-Lys341 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBD511-C | ply511 fragment encoding Lys143-Lys341 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBDP40-A | plyP40 fragment encoding Pro291-Lys344 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBDP40-B | plyP40 fragment encoding Gly201-Lys344 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBDP40-C | plyP40 fragment encoding Asp187-Lys344 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBDP40-D | plyP40 fragment encoding Gly158-Lys344 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBD025-A | ply025 fragment encoding Lys144-Lys276 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBD025-B | ply025 fragment encoding Thr132-Lys276 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBD025-C | ply025 fragment encoding Met119-Lys276 cloned into SacI-SalI sites of pHGFP | This study |
| pHGFP_CBD025-D | ply025 fragment encoding Trp106-Lys276 cloned into SacI-SalI sites of pHGFP | This study |
| pHBFP_CBD118 | ply118 fragment encoding Asp90-Ile281 cloned into SacI-SalI sites of pHBFP | This study |
| pHBFP_CBD006 | ply006 fragment encoding Leu90-Ile235 cloned into SacI-PstI sites of pHBFP | This study |
| pHBFP_CBD500 | ply500 fragment encoding His133-Lys289 cloned into SacI-SalI sites of pHBFP | This study |
| pHBFP_CBDPSA | plyPSA fragment encoding Thr172-Lys314 cloned into SacI-SalI sites of pHBFP | This study |
| pHBFP_CBDP35 | plyP35 fragment encoding Pro130-Lys291 cloned into SacI-SalI sites of pHBFP | This study |
| pHBFP_CBD511 | ply511 fragment encoding Ile161-Lys341 cloned into SacI-SalI sites of pHBFP | This study |
| pHBFP_CBDP40 | plyP40 fragment encoding Gly158-Lys344 cloned into SacI-SalI sites of pHBFP | This study |
| pHBFP_CBD025 | ply025 fragment encoding Thr132-Lys276 cloned into SacI-SalI sites of pHBFP | This study |
| pHCFP_CBD118 | ply118 fragment encoding Asp90-Ile281 cloned into SacI-SalI sites of pHCFP | This study |
| pHCFP_CBD006 | ply006 fragment encoding Leu90-Ile235 cloned into SacI-PstI sites of pHCFP | This study |
| pHCFP_CBD500 | ply500 fragment encoding His133-Lys289 cloned into SacI-SalI sites of pHCFP | This study |
| pHCFP_CBDPSA | plyPSA fragment encoding Thr172-Lys314 cloned into SacI-SalI sites of pHCFP | This study |
| pHCFP_CBDP35 | plyP35 fragment encoding Pro130-Lys291 cloned into SacI-SalI sites of pHCFP | This study |
| pHCFP_CBD511 | ply511 fragment encoding Ile161-Lys341 cloned into SacI-SalI sites of pHCFP | This study |
| pHCFP_CBDP40 | plyP40 fragment encoding Gly158-Lys344 cloned into SacI-SalI sites of pHCFP | This study |
| pHCFP_CBD025 | ply025 fragment encoding Thr132-Lys276 cloned into SacI-SalI sites of pHCFP | This study |
| pHYFP_CBD118 | ply118 fragment encoding Asp90-Ile281 cloned into SacI-SalI sites of pHYFP | This study |
| pHYFP_CBD006 | ply006 fragment encoding Leu90-Ile235 cloned into SacI-PstI sites of pHYFP | This study |
| pHYFP_CBD500 | ply500 fragment encoding His133-Lys289 cloned into SacI-SalI sites of pHYFP | This study |
| pHYFP_CBDPSA | plyPSA fragment encoding Thr172-Lys314 cloned into SacI-SalI sites of pHYFP | This study |
| pHYFP_CBDP35 | plyP35 fragment encoding Pro130-Lys291 cloned into SacI-SalI sites of pHYFP | This study |
| pHYFP_CBD511 | ply511 fragment encoding Ile161-Lys341 cloned into SacI-SalI sites of pHYFP | This study |
| pHYFP_CBDP40 | plyP40 fragment encoding Gly158-Lys344 cloned into SacI-SalI sites of pHYFP | This study |
| pHYFP_CBD025 | ply025 fragment encoding Thr132-Lys276 cloned into SacI-SalI sites of pHYFP | This study |
| pHRS_CBD118 | ply118 fragment encoding Asp90-Ile281 cloned into SacI-SalI sites of pHRS | This study |
| pHRS_CBD006 | ply006 fragment encoding Leu90-Ile235 cloned into SacI-PstI sites of pHRS | This study |
| pHRS_CBD500 | ply500 fragment encoding His133-Lys289 cloned into SacI-SalI sites of pHRS | This study |
| pHRS_CBDPSA | plyPSA fragment encoding Thr172-Lys314 cloned into SacI-SalI sites of pHRS | This study |
| pHRS_CBDP35 | plyP35 fragment encoding Pro130-Lys291 cloned into SacI-SalI sites of pHRS | This study |
| pHRS_CBD511 | ply511 fragment encoding Ile161-Lys341 cloned into SacI-SalI sites of pHRS | This study |
| pHRS_CBDP40 | plyP40 fragment encoding Gly158-Lys344 cloned into SacI-SalI sites of pHRS | This study |
| pHRS_CBD025 | ply025 fragment encoding Thr132-Lys276 cloned into SacI-SalI sites of pHRS | This study |
The CBDPSA encoding fragment corresponding to Thr172-Lys314 (21) was inserted into SacI-SalI sites of pHBFP, pHCFP, pHYFP, and pHRS, yielding four different FP-CBDPSA constructs. For the construction of FP-CBD118 and FP-CBD500, the previously described fragments CBD118-D (Asp90-Ile281, subsequently termed CBD118) and CBD500-A (His133-Lys289, subsequently termed CBD500) were used (30). For all other CBDs (CBD006, CBDP35, CBD511, CBDP40, and CBD025), different variants of pHGFP-CBD constructs were created, featuring several different length variants of the putative CBD fragments inserted into the SacI-SalI (or SacI-PstI in the case of CBD006 fragments) sites of pHGFP (Table 1). After expression and purification of all HGFP-CBD proteins and determination of their cell wall binding properties, optimally suited versions of each CBD were chosen (CBD006-C, Leu90-Ile235; CBDP35-C, Pro130-Lys291; CBD511-B, Ile161-Lys341; CBDP40-D, Gly158-Lys344; CBD025-B, Thr132-Lys276) and subsequently termed CBD006, CBDP35, CBD511, CBDP40, and CBD025, respectively. These fragments served for creation of the full spectrum of reporter-CBD fusion constructs, as outlined above.
Expression and purification of FP-CBD proteins.
Overexpression of His-tagged fusion proteins in E. coli JM109 or XL1-Blue MRF′ was carried out in modified LB medium (tryptose, 15 g/liter; yeast extract, 8 g/liter; NaCl, 5 g/liter) with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) added as an inducer once an optical density at 600 nm of 0.5 was reached. After further incubation at 30°C for 4 h, cultures were transferred to 4°C and stored overnight before harvesting by centrifugation and resuspension in 5 ml of buffer A (500 mM NaCl, 50 mM Na2HPO4, 5 mM imidazole, 0.1% Tween 20 [pH 8.0]) per 250 ml of culture. Cultures producing RedStar fusions proteins were not refrigerated but further incubated overnight at 30°C before centrifugation. The cells were then disrupted by using a French press (SLM Aminco) at 100 MPa, and the raw extracts were centrifuged (20,000 × g, 40 min) and filtered (0.2-μm-pore-size PES membrane; Millipore). Proteins were purified by immobilized metal affinity chromatography (IMAC) by using MicroBiospin columns (Bio-Rad) packed with Ni-NTA Superflow resin (Qiagen). Buffer B (500 mM NaCl, 50 mM Na2HPO4, 250 mM imidazole, 0.1% Tween 20 [pH 8.0]) was used for the elution of His-tagged proteins. Fractions were dialyzed against two changes of dialysis buffer (100 mM NaCl, 50 mM NaH2PO4, 0.005% Tween 20 [pH 8.0]), and again filtered (0.2-μm-pore-size PES membrane; Millipore). Samples were assayed by SDS-PAGE, and protein concentrations were determined by using a spectrophotometer (NanoDrop ND-1000). All purified proteins were supplemented with 50% (vol/vol) glycerol and stored at −20°C until further use.
Cell wall binding assay and fluorescence microscopy.
Binding of the FP-CBD fusion proteins to Listeria cells and 20 other Gram-positive bacteria was examined as described earlier (30). For CBD025, an additional set of 21 L. innocua and L. ivanovii strains was tested. In brief, late-log-phase cells of the respective strains were harvested by centrifugation, resuspended in PBST buffer (50 mM NaH2PO4, 120 mM NaCl [pH 8.0], 0.01% Tween 20), and incubated with 10 to 20 ng of individual FP-CBD proteins for 5 min at room temperature. After two washing steps with buffer, cells were resuspended for fluorescence microscopy (Axioplan; Carl Zeiss AG), using appropriate filter sets and beam splitter mirrors (Carl Zeiss AG) for the different emission spectra of the various FPs. Images were recorded digitally (Leica DFC320; Leica Microsystems) and processed using image processing software (PhotoShop; Adobe). For multiplex (multicolour) binding assays, mixed suspensions containing cells of up to three different Listeria strains were simultaneously incubated with mixtures of different FP-CBD fusion proteins. Individual images were obtained using suitable filter settings, and color channels were processed and overlaid using image processing software.
Calculation of cell wall ligand numbers.
The numbers of binding ligands per average Listeria cell for CBD006, CBDPSA, CBDP35, CBD511, CBDP40, and CBD025 were estimated using FP-CBD fusion proteins as described previously (30), with strains WSLC 1042 for CBDPSA, WSLC 3009 for CBD025, and WSLC 1001 for all other CBDs. The fluorescence of the supernatants, the wash fractions (corresponding to unbound HGFP_CBD proteins), and the resuspended cells (corresponding to proteins bound to the cells) were measured in a fluorescence reader (Victor3; Perkin-Elmer). Because the scattering of light in fluorescence measurements of samples containing relatively large objects such as bacterial cells results in a decreased emission, the value for “protein bound to cells” was also calculated by subtracting the fluorescence from unbound protein (supernatant plus the wash fraction) from the total protein. For each CBD, these calculated values were plotted against the number of cells. From the resulting curves, the maximum number of cells, at which all cells were still completely saturated by the given number of CBDs could be estimated, making it possible to calculate the number of CBDs bound per average cell. All assays were carried out in triplicate.
Determination of binding affinities with SPR.
Real-time analysis of the interaction of HGFP-CBDPSA, HGFP-CBD500, HGFP-CBD118, HGFP-CBD006, HGFP-CBDP35, HGFP-CBD511, HGFP-CBDP40, and HGFP-CBD025 with Listeria cell walls (L. ivanovii WSLC 3009 for CBD025, L. monocytogenes WSLC 1042 for CBDPSA and CBD500, and L. monocytogenes WSLC 1001 for all other constructs) was performed by using surface plasmon resonance (SPR) analysis (BIAcore X; Uppsala, Sweden), on C1 sensor chips essentially as outlined earlier (30). First, purified GFP-CBD proteins in 10 mM sodium acetate buffer (pH 5.0) were covalently immobilized on the chip (70 μl of 0.5 mg/ml at a flow rate of 5 μl/min) in both flow channels (Fc-1, Fc-2). Then, heat-inactivated Listeria cells suspended in HEPES buffer were immobilized onto the primary lawn of CBD molecules in Fc-2 (∼3.0 × 1010 cells per ml at a flow rate of 3 μl/min). Finally, real-time interactions between immobilized cells and different concentrations of each analyte CBD protein could be measured (50 to 500 nM; flow rate, 10 μl/min; 25°C). The association phases were measured for 3 min each; the dissociation phases were measured for 12 min. The Fc-1 channel served as a reference. Evaluation of kinetic data was performed by using BIAcore software, using a predefined “1:1 binding with mass transfer” model. Because the CBD-coated sensor surfaces with associated Listeria cell layers could not be regenerated by complete removal of the cells, new sensor chips were required for every experiment. Therefore, local fittings had to be performed for the different CBD concentrations tested, and mean values were calculated.
Rapid and multiplexed differentiation of Listeria strains from foods.
Separation and recovery of Listeria cells from artificially contaminated food by CBD-MS was carried out as described by Kretzer et al. (23). In brief, Dynabeads M-270 (Dynal, Oslo, Norway) were coated with HGFP-CBDP40 (0.5 mg/ml). Performance of the beads for immobilization from Listeria suspensions was tested by a capture assay and calculation of recovery rates (23).
Two different foods (milk and camembert cheese) were included in the present study, and they were prepared as follows: 25-g portions each were placed in sterile plastic bags and spiked with 1 to 100 CFU of two different Listeria strains/g per experiment, in the following combinations: CNL 103/2005 (sv. 1/2a) (2) and ScottA (sv. 4b), or WSLC 1001 (sv. 1/2c), and WSLC 2012 (sv. 6b). As a negative control, buffer alone was used. Selective enrichment and subsequent CBD-MS with CBD-P40-coated beads was carried out as described earlier (23). After magnetic separation, washed beads were plated on Oxford agar (Oxoid), followed by incubation overnight at 37°C. Presumptive Listeria colonies were recovered from the plates and resuspended in 50 μl of PBST buffer. Finally, multiplexed FP-CBD labeling was carried out as described above, using mixtures of HRS_CBDP35/HGFP_CBD500 or HRS-CBDP35/HGFP-CBD025, respectively.
RESULTS
Listeria phage CBD domains belong to two major classes.
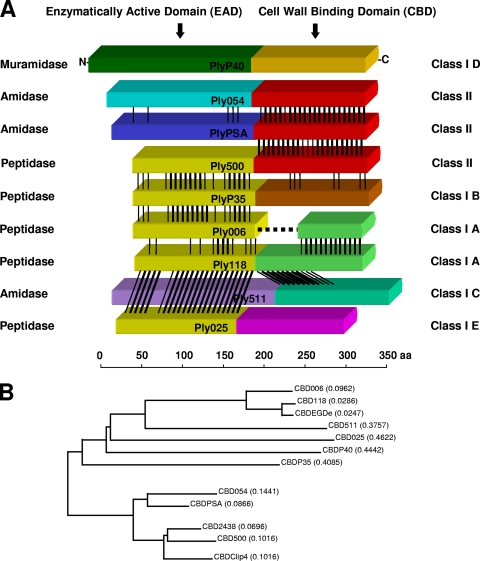
Pairwise alignments of amino acid sequences of all 12 endolysins investigated here revealed quite some diversity between the different enzymes' functional domains (Fig. 1A). The complex pattern of homologous sequences or modules permitted reasonably accurate localization of border regions between the functional domains. Considering what we have learned from determining the crystal structure of PlyPSA (21), putative linkers of the remaining endolysins were iteratively identified by in silico analyses (Fig. 2). Within the linkers, both conserved residues and repetitive sequences were found. Many of the linker sequences feature one or several Gly residues at their N termini, followed or separated by Glu and/or Lys residues. In their C-terminal regions, two Asn residues separated by one other amino acid are frequently present. In general, all putative linker sequences are rich in amino acids with polar or charged side chains, such as Lys, Ser, Asn, Thr, and Pro. A conspicuous proline-rich repetitive sequence was detected in the putative linker region of Ply511 (PAKPSTPAPKPSTPST), which corresponds to the respective region in PlyP40 (PAPKPTPSKPAPAKPAP).
FIG. 1.
Listeria bacteriophage endolysin domain structures and amino acid sequence relatedness. (A) Schematic alignment of nine different Listeria phage endolysins. The N-terminal EADs and C-terminal CBDs are marked according to the different homology groups and classes, respectively. Regions of sequence homology are indicated by black lines. Ply006 features a deletion of the N-terminal portion of its CBD, compared to Ply118. Catalytic activities are indicated. (B) Phylogenetic tree indicating the relationships between CBDs examined in the present study. For calculation of the tree, the respective amino acid sequences, including the putative linker regions, were used. The lengths of the branches correspond to evolutionary distances between the CBDs. The tree was used as basis for the classification of CBDs.
FIG. 2.
Pairwise amino acid sequence alignments of the border regions between EADs and CBDs of Listeria phage endolysins characterized in the present study. Identical and similar residues are marked in yellow and green, respectively. Putative linker regions between EADs and CBDs are indicated by black boxes. The linker of PlyPSA is known from the crystal structure (21).
Based on localization of likely linker sequence motifs and multiple alignments, an unrooted phylogenetic tree of the known CBD proteins was calculated (Fig. 1B), which corresponds to sorting the 12 different proteins into two major classes and subclasses (Table 2). Interestingly, class II contains CBDs from phages infecting Listeria strains of serovars 4, 5, and 6, while class I harbors CBDs from phages with diverse host ranges. A511 is a virulent phage with an extremely broad host range that infects strains from all serovars, B025 is specific for L. ivanovii (sv. 5) and L. innocua (sv. 6) strains only, and A118 and A006 infect sv. 1/2 strains (28). Based on sequence relatedness, the class I CBD polypeptides fall into five subclasses (A to E), indicating a higher degree of diversity. In contrast, the class II CBDs were found to be very similar (identity values for pairwise alignments of class II sequences ranged between 66.5 and 81.8%).
TABLE 2.
Classification of the Listeria phage endolysin CBDsa
| CBD class | CBD subclass | CBD protein(s) |
|---|---|---|
| I | A | CBD118, CBDEGDe, CBD006 |
| B | CBDP35 | |
| C | CBD511 | |
| D | CBDP40 | |
| E | CBD025 | |
| II | CBDPSA, CBD500, CBD054, CBDClip4, CBD2438 |
Compare to Fig. 1.
CBDs feature unique binding patterns to cell walls of different serovar groups.
The binding properties of CBD118 (class I A) and CBD500 (class II) (30), and CBDPSA (class II) (21) have been described earlier. Here, recombinant HGFP_CBD006_A/B/C, HGFP_CBDP35_A/B/C, HGFP_CBD511_A/B/C, HGFP_CBDP40_A/B/C/D, and HGFP_CBD025_A/B/C/D were produced and purified. For each protein, up to four different length variants were tested. The binding properties of all GFP_CBD fusions were determined using 26 different Listeria strains from the six main species and all serovars. As a standard and as a positive control, HGFP_CBDPSA (21) was used.
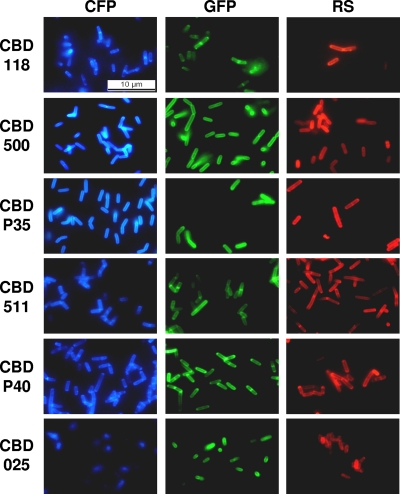
Generally speaking, for CBD006, CBDP35, and CBDP40 the longest variants (C or D) showed the best cell wall binding properties, while the shorter variants displayed a weaker binding and sometimes no function (e.g., CBDP35_A and CBDP40_A). With CBD511 and CBD025, the “B” variants displayed the strongest binding. The results of all binding assays with these products are summarized in Table 3, including CBDs investigated in previous studies; images of Listeria cells decorated by differently colored FP_CBD fusion proteins are presented in Fig. 3. Based on these assays, CBD006_C, CBDP35_C, CBD511_B, CBDP40_D, and CBD025_B were selected as the best variants. These proteins are further referred to as CBD006, CBDP35, CBD511, CBDP40, and CBD025, respectively. CBDP35 and CBD025 showed a similar binding pattern and decorated the entire cell surfaces very evenly, similar to CBD500 (30) and CBDPSA (21). In contrast, the relatively weak binding of CBD006 was primarily targeted to the septal regions and poles of the cells, similar to CBD118 (30; results not shown). CBD511 and CBDP40 showed a binding pattern somewhat in between those of CBD118 and CBD500, binding to the whole cell surface, but with an emphasis on the septal and polar regions. Similar to CBD118, CBD006 also mainly bound to sv. 1/2 and 3 strains, but much more weakly. Interestingly, the CBDP35 protein not only labeled most strains of sv. 1/2 and 3 but also bound to many strains of other serovar groups. However, no binding of CBDP35 occurred to WSLC 1442 and the sv. “7” strain, which both lack GlcNAc in their teichoic acids (53). CBD025 displayed a narrower binding range, restricted to strains from sv. groups 4 (L. monocytogenes), 5 (L. ivanovii), and 6 (L. innocua).
TABLE 3.
Binding ranges of GFP-tagged CBD proteins to Listeria cells from different species and serovars
| Species | Strain code | Source or origin | Sv. | Binding of CBDa |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 118 | 006 | 500 | PSA | P35 | 511 | P40 | 025 | ||||
| L. monocytogenes | EGDe | J. Kreft | 1/2a | ++ | + | - | - | ++ | ++ | ++ | - |
| L. monocytogenes | 10403S | D. Portnoy | 1/2a | ++ | + | - | - | ++ | + | ++ | - |
| L. monocytogenes | 1442 | No GlcNAc in TAb | 1/2a | ++ | ++ | - | - | - | ++ | ++ | - |
| L. monocytogenes | 1066 | SLCC 8800 | 1/2b | ++ | + | - | - | ++ | + | ++ | - |
| L. monocytogenes | 1001 | ATCC 19112 | 1/2c | ++ | + | - | - | ++ | + | ++ | - |
| L. seeligeri | 4007 | ATCC 35967 | 1/2b | ++ | + | - | - | ++ | + | ++ | - |
| L. welshimeri | 50149 | SLCC 5877 | 1/2b | ++ | ++ | - | - | + | ++ | ++ | - |
| L. monocytogenes | 1485 | Soft cheese | 3a | + | (+) | - | - | + | + | ++ | - |
| L. monocytogenes | 1031 | SLCC1694 | 3b | + | - | - | - | ++ | + | ++ | - |
| L. monocytogenes | 1032 | SLCC 2479 | 3c | + | (+) | - | - | ++ | + | ++ | - |
| L. seeligeri | 40127 | SLCC 8604 | 3b | + | - | - | - | ++ | ++ | ++ | - |
| L. monocytogenes | 1034 | SLCC 2482 | “7” | + | (+) | - | - | - | + | ++ | - |
| L. monocytogenes | 1020 | ATCC 19114 | 4a | - | + | ++ | ++ | ++ | ++ | ++ | ++ |
| L. monocytogenes | 1042 | ATCC 23074 | 4b | - | - | ++ | ++ | - | + | ++ | - |
| L. monocytogenes | ScottA | J. Jay | 4b | - | - | ++ | ++ | - | + | ++ | - |
| L. monocytogenes | 1019 | ATCC 19116 | 4c | - | - | ++ | ++ | ++ | + | + | - |
| L. monocytogenes | 1033 | ATCC 19117 | 4d | - | - | ++ | ++ | + | ++ | ++ | - |
| L. monocytogenes | 1018 | ATCC 19118 | 4e | - | - | ++ | + | (+) | ++ | ++ | - |
| L. ivanovii | 3009 | SLCC 4769 | 5 | - | - | ++ | ++ | ++ | + | ++ | ++ |
| L. ivanovii subsp. ivanovii | 3010 | ATCC 19119 | 5 | - | - | ++ | ++ | ++ | + | ++ | ++ |
| L. ivanovii subsp. londoniensis | 3060 | SLCC 3765 | 5 | - | - | ++ | (+) | - | + | ++ | - |
| L. innocua | 2011 | ATCC 33090 | 6a | - | - | ++ | (+) | - | + | + | - |
| L. innocua | 2012 | ATCC 33091 | 6b | - | - | ++ | ++ | ++ | + | ++ | ++ |
| L. welshimeri | 50146 | SLCC 7622 | 6a | - | - | ++ | ++ | + | + | (+) | - |
| L. grayi subsp. grayi | 6036 | ATCC 19120 | (+) | - | (+) | - | ++ | (+) | + | - | |
| L. grayi subsp. murrayi | 6037 | ATCC 25401 | (+) | - | (+) | + | ++ | (+) | + | - | |
++, strong binding; +, weak binding; (+), very weak binding; −, no binding.
Wendlinger et al. (53).
FIG. 3.
Listeria cells labeled with a set of 18 different fluorescent protein-CBD fusion proteins. Combinations of three different fluorescent reporters (CFP, cyan fluorescent protein; GFP, green fluorescent protein; RS, RedStar protein) and six different CBDs (one from each subclass) are shown. Listeria strains were selected according to the binding specificity of each CBD (see text). The samples were observed by epifluorescence microscopy using filter sets suitable for each fluorescent protein.
Listeria phage CBDs generally feature very high specificity toward binding to cells of this genus. CBDP40 and CBD511 were able to decorate cells of all Listeria strains tested, with stronger decoration by CBDP40. Interestingly, this CBD also bound to walls of several non-Listeria bacteria tested, including Bacillus, Enterococcus, Staphylococcus, and Bifidobacterium strains. CBDP35 also weakly decorated some Staphylococcus strains, and one Bacillus cereus strain was very weakly labeled by CBD006 and CBD511 (results not shown).
Quantification of surface ligands on cell walls.
Determination of the number of ligands recognized by CBD per average cell was done as described earlier (30), based on a direct relationship of the fluorescence signal (RFU) to the number of bound and free HGFP_CBD molecules. Different cell concentrations and a defined number of HGFP_CBD molecules (7.5 × 1012) were used in the assays. The measured RFU were plotted against the cell concentration, and the maximum number of cells at which the surfaces of all cells were still saturated by CBD proteins was determined from the resulting curves. This allowed the calculation of an average number of CBD molecules per cell, assumed to correspond to the number of accessible ligands per average single bacterium. These were found to range from 3.02 × 102 (CBD006) up to 2.35 × 105 (CBDP35) ligands per cell (Table 4).
TABLE 4.
Quantification of CBD ligands per average Listeria cell and equilibrium association constants of CBD binding to the cell wall determined by surface plasmon resonance
| CBDa | No. of ligands bound by CBD (avg/cell) | Equilibrium association constant KA (M−1) |
|---|---|---|
| CBD118* | 3.80 × 104 | (7.73 ± 1.55) × 107 |
| CBD006 | 3.02 × 102 | (9.58 ± 6.21) × 106 |
| CBD500* | 8.50 × 104 | (6.02 ± 0.45) × 108 |
| CBDPSA | 2.37 × 104 | (9.14 ± 1.39) × 108 |
| CBDP35 | 2.35 × 105 | (1.33 ± 0.71) × 109 |
| CBD511 | 7.63 × 103 | (1.41 ± 0.50) × 108 |
| CBDP40 | 1.18 × 105 | (2.40 ± 1.70) × 109 |
*, number of ligands for CBD118 and CBD500 from Loessner et al. (30).
CBD proteins feature rapid binding and high equilibrium affinity.
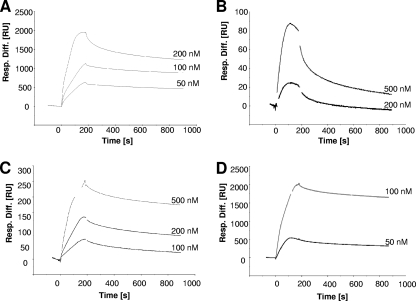
Kinetic data of the interaction of CBDs with the cell wall of Listeria cells were obtained by SPR analysis. First, CBD molecules were covalently immobilized on the gold-plated sensor surface, followed by attachment of intact bacterial cells on this lawn. Real-time CBD binding kinetics could then be measured after applying free HGFP-CBD molecules to the immobilized cells and monitoring their association and dissociation, resulting in characteristic sensorgrams (Fig. 4). After curve fitting, the equilibrium association and dissociation constants (KA and KD) for the cell wall-CBD interactions were calculated. These values are reciprocal constants and reflect the overall affinity of the CBD proteins to the cell surface. The KA for all CBDs tested (from independent local fittings for at least two different CBD concentrations) are listed in Table 4. In all but one case, overall values in the range of 108 to 109 (KD values in the pico- to nanomolar ranges) were determined. The affinity of CBD006 was found to be significantly lower, supposedly due to its truncated structure (see Discussion).
FIG. 4.
SPR analysis of the binding of CBD proteins to Listeria cell surfaces. The overlay plots show the binding kinetics of the indicated concentrations of HGFP-CBDP35 (A), HGFP-CBD006 (B), HGFP-CBD511 (C), and HGFP-CBDP40 (D) to L. monocytogenes cells immobilized on the sensor chip surface (see the text for explanation). The CBD association was monitored for 3 min; the subsequent dissociation was monitored for 12 min.
Fluorescent protein-CBD fusions allow rapid multiplex detection and discrimination of Listeria strains.
Besides fusions with GFP, we also used the differently colored fluorescent proteins BFP, CFP, YFP, and RedStar for the construction of reporter fusions with CBDs from Ply118, Ply006, Ply500, PlyPSA, PlyP35, Ply511, PlyP40, and Ply025. After synthesis in E. coli and IMAC purification, the proteins were tested for Listeria cell wall binding properties by fluorescence microscopy. Although the BFP constructs exhibited relatively weak fluorescence and were sensitive to photobleaching, all other proteins yielded bright fluorescent decoration of Listeria cells, corresponding to the binding ranges of the individual CBDs (Fig. 3). The YFP-tagged cells emitted a bright green color quite similar to that of GFP (Fig. 5B). The RedStar fusion proteins showed a slight tendency to aggregate and also sometimes caused slight agglomeration of bacterial cells. This undesirable effect was reduced by centrifugation of the CBD-RedStar solution immediately prior to use and by lowering the protein concentration in the binding experiments.
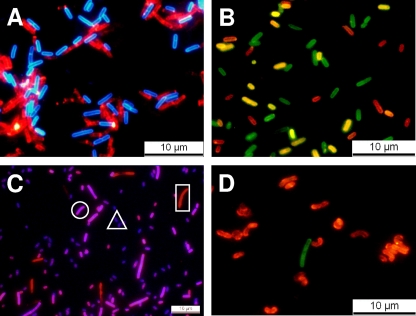
FIG. 5.
Differentiation of Listeria strains by multiplex fluorescent protein-CBD labeling (for details, see the text). (A) Cells of strains 1001 (sv. 1/2c) and 1042 (sv. 4b) incubated with HRS-CBD118 (red) and HCFP-CBD500 (blue), resulting in a serovar-specific cell wall decoration. (B) Strains 1066 (sv. 1/2b), 1020 (sv. 4a), and 1042 (sv. 4b) can be distinguished by using HRS-CBDP35 and HYFP-CBDPSA in a multiplex assay. Strain 1066 is labeled by CBD-P35 (red), strain 1042 is labeled by CBD-PSA (green), and strain WSLC 1020 is labeled by both CBDs (yellow). (C) Strains EGDe (sv. 1/2a), WSLC 1020 (sv. 4a), and WSLC 3010 (sv. 5) are distinguished by incubation with HRS-CBD511 and HBFP-CBD500. CBD511 strongly decorates WSLC 1020 and EGDe and only weakly binds to WSLC 3010, whereas CBD500 strongly binds to WSLC 1020 and 3010 and does not recognize EGDe cells. Therefore, strain EGDe appears red (square), WSLC 1020 is magenta (circle), and WSLC 3010 cells show up as purple (triangle). In panel D, detection and differentiation of Listeria strains CNL 103/2005 (1/2 a) and ScottA (4b) after recovery from contaminated milk and subsequent plating is shown, by a CBD binding assay. The green cells are strain ScottA, specifically tagged by HGFP-CBD500, while the red cells are CNL 103/2005 recognized by HRS-CBDP40. Note that deformation of the cells results from growth on drug-containing selective medium.
When mixtures of two reporter-CBD fusions (different color and CBD binding specificities) were used in multiplex assays, cells of up to three different Listeria strains from pooled cultures could be clearly distinguished. When combining different fluorescent markers, their excitation and emission spectra should be nonoverlapping to allow sufficiently clear separation of the signals by the filters (or laser wavelengths) used in the microscope. With our instrument setup, suitable combinations were RedStar/BFP, RedStar/CFP, RedStar/GFP, RedStar/YFP (Fig. 5), and CFP/GFP or CFP/YFP (data not shown).
CBDs enable both affinity separation and subsequent multiplex fluorescent labeling.
We wanted to test whether rapid identification and differentiation of Listeria cells by a multiplex decoration with CBD of different binding spectra and colors would also be possible after plate recovery of (mixed) Listeria cultures from foods. For this, the CBD magnetic separation assay (CBD-MS) (23) was used with CBDP40-coated beads, which combines the nonoverlapping binding ranges of CBD118 and CBD500 (23). Performance of the CBD-P40 coated beads was first evaluated in standardized assays with selected strains (23), and very satisfactory recovery rates of >90% were obtained (results not shown). Then, milk and camembert cheese samples were spiked with L. monocytogenes and, after sampling, homogenization and selective enrichment for 24 h, cells from 100 μl of the enrichment cultures were captured and separated by using the CBD-P40 beads, followed by surface plating on selective medium. After incubation for 16 h, typical brownish colonies with black halos appeared on the plates. Colonies were collectively harvested and resuspended in PBS buffer, followed by multiplex CBD decoration using a mixture of RS-CBDP35 and GFP-CBD500. This approach enabled rapid and easy detection and differentiation of the two contaminating strains both in the milk and cheese samples. As demonstrated in Fig. 5D, ScottA cells (sv. 4b) were decorated green, whereas CNL 103/2005 cells (sv. 1/2a) stained red. Interestingly, the ratio of both strains in the samples did not correspond to the 1:1 ratio used for artificial contamination. Instead, CNL 103/2005 cells were present in excess in both samples; they apparently had overgrown strain ScottA in the food or the enrichment culture. The same results were obtained when we changed the inoculation ratio to 1:100, i.e., adding 100-fold less CNL 103/2005 (only 1 CFU/mg of food). Similar observations were made with other strain combinations, e.g., WSLC 1001 (sv. 1/2c) and WSLC 2012 (sv. 6b) labeled with RS-CBDP35 and GFP-CBD025. Although both strains could be readily identified and distinguished by the differently colored cells, the L. innocua WSLC 2012 cells were clearly dominating (results not shown).
DISCUSSION
In view of the multitude of different techniques and procedures available for the detection and differentiation of bacterial pathogens such as Listeria monocytogenes in foods and clinical and environmental samples, any novel method has to be superior in at least one major aspect such as sensitivity, specificity, rapidity, cost, robustness, or ease of use. Although the conventional culture methods for Listeria (e.g., IDF 143A:1995; ISO 11290-1) still represents the gold standard, a major disadvantage is the lengthy enrichment and incubation periods required. Regarding differentiation of Listeria strains, one has to distinguish between the classical and historically developed serotyping, which is characterized by a low discrimination index, and various subtyping methods. Serotyping utilizes agglutination tests based on antibodies specifically reacting with somatic and flagellar antigens (44). Sera required for Listeria serotyping are commercially available only for Listeria monocytogenes, which has the highest relevance as the only human pathogenic species. Common subtyping methods that allow typing beyond the serovar border include phage typing (27, 28) and pulsed-field gel electrophoresis (PFGE) of DNA fragments (13). Comparison of these PFGE “fingerprints” is possible via the PulseNet network (http://www.cdc.gov/pulsenet/). Furthermore, a number of VNTR (variable number of tandem repeats) and MLVA (multilocus variable number of tandem repeat analysis) methods have recently been reported (3, 25, 36, 37, 46), as well as several other PCR- and DNA restriction-based methods for differentiation of Listeria (35, 39, 54), and as substitutes for serotyping (17). However, nucleic acid-based typing methods require the isolation of DNA, which in itself can be lengthy and, in the case of Listeria, sometimes is quite difficult.
The cell wall binding domains from phage endolysins as highly specific affinity molecules have recently been developed as a tool for the immobilization, separation, labeling, and detection of pathogens such as Listeria (23). Isolated CBDs tend to retain their full functionality, reflecting the modular organization and structure of the endolysins (21). CBDs offer high specificity coupled with high affinity, and fluorescent CBD fusion proteins allow direct microscopic detection of the target bacteria (30). Compared to antibodies, CBDs offer added functionality because they do not cause the agglutination of target cells (23). Moreover, antibodies directed against Listeria cells also frequently cross-react with other bacteria (16, 38, 51). CBD-based magnetic separation and recovery of bacteria from foods (23) provides higher sensitivity than standard methods within much less time. Based on characterization of the different CBD sequences of Listeria phage endolysins and evaluation of their properties, we sought to create a CBD-based toolbox useful for multiplex detection and differentiation of Listeria cells and strains, respectively.
The linker regions in between the EAD and CBD domains contain a high percentage of hydrophilic amino acids. This indicates that these domains are likely exposed in aqueous solvents, as observed for the PlyPSA linker (21). Some linkers contain conserved residues and, in the case of Ply511 and PlyP40, even proline-rich repetitive sequences. It may be speculated that the underlying (conserved) DNA sequences may function as homologous recombination loci, enabling horizontal exchange, as well as insertion or deletion of functional modules across the pool group of peptidoglycan hydrolase functional domains from phages and bacteria. The truncation observed in CBD006 may be explained by this mechanism.
In most of the CBDs examined, no conserved sequence motifs previously associated with cell wall recognition or binding could be identified. This indicates the uniqueness of these functional domains and their ligands, which might be based upon the serovar- and strain-specific structure of the cell wall and/or teichoic acid ligands. The exceptions are CBDP40 and CBD025, where in silico analyses demonstrated relatedness to bacterial SH3 domains. In addition, CBDP40 shares limited relatedness to a LysM domain, which has been described to possess a general peptidoglycan binding function (1).
In our attempt to classify the Listeria phage CBD domains, it became obvious that class II mainly contains CBDs from phages infecting strains of serovar group 4, 5, and 6, while the class I CBDs (with the exception of CBD025) are from phages infecting serovar 1/2 strains and from the polyvalent phages A511 and P40. This is also reflected by the CBD-cell wall binding ranges (Table 3). Whereas CBD500 and CBDPSA (class II) display very similar patterns and have sequences similar to those of the other class II CBDs, the sequences and binding ranges of class I CBDs are more heterogeneous. An explanation may be found in the structure and composition of the putative binding ligands, which are cell wall-associated carbohydrates. Listeria wall teichoic acids generally consist of a glycosylated polyribitolphosphate backbone (8). In serovar groups 1/2 and 3, the ribitol moiety can carry rhamnose or N-acetylglucosamine residues attached to the ribitol C2 or C4 positions. In sv. 4, 5, and 6, the N-acetylglucosamine is integrated into the polymer chain itself and can be further modified by attachment of glucose or galactose sugars (6, 7, 50). While all Listeria species and serovars feature the same directly cross-linked murein type (A1-gamma type; with mDAP as the diamino acid) (8), variations of secondary carbohydrates such as teichoic acids seem to determine the heterogeneity of the endolysin CBDs.
CBD006, CBD511, and CBDP40 mainly attach to the poles and septa of the cells, as does CBD118 (30). This pattern might be due to the presence of the respective ligands only in these regions, or steric hindrance/masking of the ligands on the cell surface, as demonstrated for LysM containing proteins on Lactococcus lactis cells (47). While CBDP40 is unique, CBD006 revealed strong homology to the CBD118 distal end. CBD118 also shows homology to CBD511, however, at the N-terminal region of the CBDs. Interestingly, this region also corresponds to the portion not present in CBD006 (see Fig. 1A). This directly implies that Ply006 may have been derived from a Ply118-like enzyme that has lost part of its cell wall binding domain. CBD006 resembles CBD118 not only in the spatial distribution of CBD binding over the cell surface but also in its binding range, although the cell decoration appeared much weaker.
Phages A511 and P40 are both virulent and feature broad host ranges (4, 53). This correlates with our observation that CBD511 and CBDP40 decorated all Listeria strains tested. Although their amino acid sequences are unrelated, the broad binding indicates recognition of serovar independent, conserved cell wall ligands, such as the peptidoglycan. Our finding that CBDP40 also bound to a several non-Listeria bacteria is in line with this assumption.
In contrast to CBD118, CBD006, CBD511, and CBDP40, which were primarily located at polar and septal regions, the ligands for CBDP35 and CBD025 seem to be evenly distributed or accessible over the whole cell surface, resembling CBD500 (30). Interestingly, CBDP35 labeled all sv. 1/2 and 3 strains (except WSLC 1442) and many strains of sv. groups 4, 5, and 6. Since phage P35 only infects sv. 1/2 strains (4), our finding supports previous evidence that the phage cellular receptors and the CBD ligands are not the same (30). An intriguing finding was the inability of CBDP35 to bind to strains WSLC 1442 and WSLC 1034, both of which possess rhamnose but lack N-acetylglucosamine in their wall teichoic acid molecules (53). In contrast, N-acetylglucosamine is the only substituent of sv. 3 teichoic acids (53), and CBDP35 displayed strong binding to all sv. 3 strains. Therefore, the N-acetylglucosamine residue appears to be required for binding of CBDP35.
Within the class I CBD domains, CBD025 is unusual since it does not bind to any of the sv. 1/2 and 3 strains. Its specific binding to strains of sv. 5 and 6 does, however, make perfect sense, since phage B025 can only infect the corresponding species L. ivanovii and L. innocua, respectively (53). However, CBD025 does not bind to sv. 6a strains, which contain galactose residues as teichoic acid substituents, while sv. 5 and 6b feature glucose instead (8). It is reasonable to assume that CBD025 requires galactose as a recognition element, although the exact nature of the ligands remains unknown. It should also be noted that such highly specific binding properties below the species levels can be very useful for rapid and easy serovar determination.
Quantification of CBD binding sites on the Listeria cells indicated that approximately 104 to 105 ligands are accessible for most of the CBDs, similar to earlier findings (30). However, ∼100-fold fewer binding sites were found for CBD511 and CBD006. This correlates well with the observation that the corresponding fluorescent proteins also exhibited less intense cell wall decoration compared to the other CBDs (Table 3). Interestingly, the ligand numbers of CBDs binding mainly to polar and septal regions of the cells were found to be similar to those of CBDs decorating the entire cell surface, including lateral walls. This suggests that the density of accessible ligands may be very high in the poles and septae, compensating for a lower density in the lateral surface.
The equilibrium binding affinity to the Listeria cell walls of the CBD domains tested here lies in the pico- to nanomolar range, similar to earlier findings (30). It has been postulated that the reason for the requirement of such high binding affinities in phage-encoded peptidoglycan hydrolases is the avoidance of collateral damage after cell lysis and the release of phage progeny (30). In fact, decreased cell wall-binding affinity resulted in better diffusion and more pronounced lysis of surrounding cells (10). Similarly, the observed much lower affinity of CBD006 seems to correlate to partial deletion of an N-terminal portion the CBD domain, compared to CBD118. Although this does not result in a different binding range, it causes a significant drop in binding affinity and a reduced binding efficiency. This indicates that CBDs may be made up from several functional submodules, which cooperate to achieve the full binding affinities. In fact, the affinity can be further increased or modulated to recognize additional ligands by adding more of the same or other binding modules, respectively (M. Schmelcher et al., unpublished data).
Fusion of functional CBD fragments to different fluorescent marker proteins and application of these tools in multiplexed binding assays provides an easy one step method for detection and differentiation of Listeria cells from different strains and serovars, even in mixed cultures. We also delivered proof-of-concept for the application of reporter-CBDs for distinction of Listeria strains following CBD-based recovery from contaminated foods. The performance of the CBD-MS is superior to the standard detection protocols (23). Instead of using two complementary types of CBD beads, the availability of the high affinity, broad binding CBDP40 protein for immobilization yielded excellent recovery rates for all Listeria serovars. The separation was followed by CBD differentiation using the fluorescently labeled, more specific reporter CBDs. For our experiments with milk and soft cheese we chose outbreak strains originally isolated from soft cheese (2) and pasteurized milk (9). Although in all our experiments one strain always overgrew the other independent of the initial inoculation ratio, cells of both strains could easily be detected after CBD staining of colony material and fluorescence microscopy, within less than 15 min. This simple assay appears to be a good alternative for other rapid methods for differentiation of strains, such as PCR or enzyme-linked immunosorbent assay. Moreover, the approach is generally applicable to other Gram-positive bacteria (23). Similar to the biological specificity offered by intact bacteriophages, the unique binding properties of CBD proteins render them ideal tools to develop rapid and easy differentiation procedures. In phage typing, a set of bacteriophages is used to distinguish bacterial strains (27). A comprehensive set of reporter-CBD fusion proteins could serve for the same purpose, offering advantages with respect to assay time, simplicity, and strains not typeable by using plaque formation. Although some molecular typing methods mentioned above may be superior with regard to discrimination, the addition of more CBD modules of different specificity to the existing toolbox will render a CBD-based differentiation system increasingly powerful. Since the reservoir of bacteriophages is virtually unlimited, this likely also holds true for the associated cell wall binding domains, opening up many possibilities for bacterial detection and differentiation.
Supplementary Material
Acknowledgments
We thank Michael Knop for the gift of plasmid DNA encoding dsRedStar, Fabian Raeber for his contribution to the work, and Jochen Klumpp and Julia Dorscht for sharing information. We are grateful to Susanne Guenther for critical comments and to Monique Herensperger and the late Ursula Schuler for excellent technical assistance.
This study was partly funded by the WWTF, Vienna, Austria.
Footnotes
Published ahead of print on 9 July 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bateman, A., and M. Bycroft. 2000. The structure of a LysM domain from Escherichia coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299:1113-1119. [DOI] [PubMed] [Google Scholar]
- 2.Bille, J., D. S. Blanc, H. Schmid, K. Boubaker, A. Baumgartner, H. H. Siegrist, M. L. Tritten, R. Lienhard, D. Berner, R. Anderau, M. Treboux, J. M. Ducommun, R. Malinverni, D. Genne, P. H. Erard, and U. Waespi. 2006. Outbreak of human listeriosis associated with Tomme cheese in northwest Switzerland, 2005. Euro Surveill. 11:91-93. [PubMed] [Google Scholar]
- 3.Call, D. R., L. Orfe, M. A. Davis, S. Lafrentz, and M. S. Kang. 2008. Impact of compounding error on strategies for subtyping pathogenic bacteria. Foodborne Pathog. Dis. 5:505-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorscht, J., J. Klumpp, R. Bielmann, M. Schmelcher, Y. Born, M. Zimmer, R. Calendar, and M. J. Loessner. 2009. Comparative genome analysis of Listeria bacteriophages reveals extensive mosaicism, programmed translational frameshifting, and a novel prophage insertion site. J. Bacteriol. 191:7206-7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiedler, F. 1988. Biochemistry of the cell surface of Listeria strains: a locating general view. Infection 16:S92-S97. [DOI] [PubMed] [Google Scholar]
- 7.Fiedler, F., and G. Ruhland. 1987. Structure of Listeria monocytogenes cell walls. Bull. Inst. Pasteur 85:287-300. [Google Scholar]
- 8.Fiedler, F., J. Seger, A. Schrettenbrunner, and H. P. R. Seeliger. 1984. The biochemistry of murein and cell wall teichoic acids in the genus Listeria. Syst. Appl. Microbiol. 5:360-376. [Google Scholar]
- 9.Fleming, D. W., S. L. Cochi, K. L. MacDonald, P. Brondum, S. J. Hayes, B. D. Plikaytis, M. B. Holmes, A. Audurier, C. V. Broome, and A. L. Reingold. 1985. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N. Engl. J. Med. 312:404-407. [DOI] [PubMed] [Google Scholar]
- 10.Gaeng, S., S. Scherer, H. Neve, and M. J. Loessner. 2000. Gene cloning and expression and secretion of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 66:2951-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 12.Graves, L. M., L. O. Helsel, A. G. Steigerwalt, R. E. Morey, M. I. Daneshvar, S. E. Roof, R. H. Orsi, E. D. Fortes, S. R. Milillo, H. C. den Bakker, M. Wiedmann, B. Swaminathan, and B. D. Saunders. 2010. Listeria marthii sp. nov., isolated from the natural environment, Finger Lakes National Forest. Int. J. Syst. Evol. Microbiol. 60:1280-1288. [DOI] [PubMed] [Google Scholar]
- 13.Graves, L. M., and B. Swaminathan. 2001. PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int. J. Food. Microbiol. 65:55-62. [DOI] [PubMed] [Google Scholar]
- 14.Gurskaya, N. G., A. F. Fradkov, A. Terskikh, M. V. Matz, Y. A. Labas, V. I. Martynov, Y. G. Yanushevich, K. A. Lukyanov, and S. A. Lukyanov. 2001. GFP-like chromoproteins as a source of far-red fluorescent proteins. FEBS Lett. 507:16-20. [DOI] [PubMed] [Google Scholar]
- 15.Henry, R., L. Shaughnessy, M. J. Loessner, C. Alberti-Segui, D. E. Higgins, and J. A. Swanson. 2006. Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell. Microbiol. 8:107-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaclikova, E., T. V. Kuchta, H. Kay, and D. Gray. 2001. Separation of Listeria from cheese and enrichment media using antibody-coated microbeads and centrifugation. J. Microbiol. Methods 46:63-67. [DOI] [PubMed] [Google Scholar]
- 17.Kerouanton, A., M. Marault, L. Petit, J. Grout, T. Tam Dao, and A. Brisabois. 2010. Evaluation of a multiplex PCR assay as an alternative method for Listeria monocytogenes serotyping. J. Microbiol. Methods 80:134-137. [DOI] [PubMed] [Google Scholar]
- 18.Khosla, C., and P. B. Harbury. 2001. Modular enzymes. Nature 409:247-252. [DOI] [PubMed] [Google Scholar]
- 19.Klumpp, J., R. J. Dorscht, Lurz, R. Bielmann, M. Wieland, M. Zimmer, R. Calendar, and M. J. Loessner. 2008. The terminally redundant, non-permuted genome of Listeria bacteriophage A511: a model for the SPO1-like myoviruses of Gram-positive bacteria. J. Bacteriol. 190:5753-5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knop, M., F. Barr, C. G. Riedel, T. Heckel, and C. Reichel. 2002. Improved version of the red fluorescent protein (drFP583/DsRed/RFP). Biotechniques 33:592-594. [DOI] [PubMed] [Google Scholar]
- 21.Korndoerfer, I. P., J. Danzer, M. Schmelcher, M. Zimmer, A. Skerra, and M. J. Loessner. 2006. The crystal structure of the bacteriophage PSA endolysin reveals a unique fold responsible for specific recognition of Listeria cell walls. J. Mol. Biol. 364:678-689. [DOI] [PubMed] [Google Scholar]
- 22.Korndoerfer, I. P., A. Kanitz, J. Danzer, M. Zimmer, M. J. Loessner, and A. Skerra. 2008. Structural analysis of the l-alanoyl-d-glutamate endopeptidase domain of Listeria bacteriophage endolysin Ply500 reveals a new member of the LAS peptidase family. Acta Crystallogr. D 64:644-650. [DOI] [PubMed] [Google Scholar]
- 23.Kretzer, J. W., R. Lehmann, M. Schmelcher, M. Banz, K. P. Kim, C. Korn, and M. J. Loessner. 2007. High affinity cell wall-binding domains of bacteriophage endolysins for immobilization and separation of bacterial cells. Appl. Environ. Microbiol. 73:1992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leclercq, A., D. Clermont, C. Bizet, P. A. Grimont, A. Le Flèche-Matéos, S. M. Roche, C. Buchrieser, V. Cadet-Daniel, A. Le Monnier, M. Lecuit, and F. Allerberger. 2009. Listeria rocourtiae sp. nov. Int. J. Syst. Evol. Microbiol. doi: 10.1099/ijs.0.017376-0. [DOI] [PubMed]
- 25.Lindstedt, B. A., W. Tham, M. L. Danielsson-Tham, T. Vardund, S. Helmersson, and G. Kapperud. 2008. Multiple-locus variable-number tandem-repeats analysis of Listeria monocytogenes using multicolour capillary electrophoresis and comparison with pulsed-field gel electrophoresis typing. J. Microbiol. Methods 72:141-148. [DOI] [PubMed] [Google Scholar]
- 26.Loessner, M. J. 2005. Bacteriophage endolysins: current state of research and applications. Curr. Opin. Microbiol. 8:480-487. [DOI] [PubMed] [Google Scholar]
- 27.Loessner, M. J., and M. Busse. 1990. Bacteriophage typing of Listeria species. Appl. Environ. Microbiol. 56:1912-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loessner, M. J. 1991. Improved procedure for bacteriophage typing of Listeria strains and evaluation of new phages. Appl. Environ. Microbiol. 57:882-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loessner, M. J., and R. Calendar. 2006. The Listeria bacteriophages, p. 593-601. In R. Calendar and S. T. Abedon (ed.), The bacteriophages. Oxford University Press, New York, NY.
- 30.Loessner, M. J., K. Kramer, F. Ebel, and S. Scherer. 2002. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 44:335-349. [DOI] [PubMed] [Google Scholar]
- 31.Loessner, M. J., C. E. Rees, G. S. Stewart, and S. Scherer. 1996. Construction of luciferase reporter bacteriophage A511::luxAB for rapid and sensitive detection of viable Listeria cells. Appl. Environ. Microbiol. 62:1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loessner, M. J., and C. E. D. Rees. 2005. Listeria phages: basics and applications, p. 362-379. In M. K. Waldor, D. I. Friedman, and S. L. Adhya (ed.), Phages: their role in bacterial pathogenesis and biotechnology. ASM Press, Washington, DC.
- 33.Loessner, M. J., G. Wendlinger, and S. Scherer. 1995. Heterogeneous endolysins in Listeria monocytogenes bacteriophages: a new class of enzymes and evidence for conserved holin genes within the siphoviral lysis cassettes. Mol. Microbiol. 16:1231-1241. [DOI] [PubMed] [Google Scholar]
- 34.Matz, M. V., A. F. Fradkov, Y. A. Labas, A. P. Savitsky, A. G. Zaraisky, M. L. Markelov, and S. A. Lukyanov. 1999. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 17:969-973. [DOI] [PubMed] [Google Scholar]
- 35.Mazurier, S. I., and K. Wernars. 1992. Typing of Listeria strains by random amplification of polymorphic DNA. Res. Microbiol. 143:499-505. [DOI] [PubMed] [Google Scholar]
- 36.Miya, S., B. Kimura, M. Sato, H. Takahashi, T. Ishikawa, T. Suda, C. Takakura, T. Fujii, and M. Wiedmann. 2008. Development of a multilocus variable-number of tandem repeat typing method for Listeria monocytogenes serotype 4b strains. Int. J. Food Microbiol. 124:239-249. [DOI] [PubMed] [Google Scholar]
- 37.Murphy, M., D. Corcoran, J. F. Buckley, M. O'Mahony, P. Whyte, and S. Fanning. 2007. Development and application of multiple-locus variable number of tandem repeat analysis (MLVA) to subtype a collection of Listeria monocytogenes. Int. J. Food Microbiol. 115:187-194. [DOI] [PubMed] [Google Scholar]
- 38.Nexmann Jacobsen, C., C. Fremming, and M. Jakobsen. 1997. Immunomagnetic separation of Listeria monocytogenes for flow cytometric determination of viable cells in liquid. J. Microbiol. Methods 31:75-81. [Google Scholar]
- 39.Nocera, D., E. Bannerman, J. Rocourt, K. Jaton-Ogay, and J. Bille. 1990. Characterization by DNA restriction endonuclease analysis of Listeria monocytogenes strains related to the Swiss epidemic of listeriosis. J. Clin. Microbiol. 28:2259-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rees, C. E., and C. E. Dodd. 2006. Phage for rapid detection and control of bacterial pathogens in food. Adv. Appl. Microbiol. 59:159-186. [DOI] [PubMed] [Google Scholar]
- 41.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., J. Maniatis, and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York, NY.
- 43.Schmelcher, M., and M. J. Loessner. 2008. Bacteriophage: powerful tools for the detection of bacterial pathogens, p. 731-754. In M. Zourob, S. Elwary, and A. Turner (ed.), Principles of bacterial detection: biosensors, recognition receptors, and microsystems. Springer, New York, NY.
- 44.Seeliger, H. R. P., and B. Langer. 1989. Serological analysis of the genus Listeria. Its values and limitations. Int. J. Food Microbiol. 8:245-248. [DOI] [PubMed] [Google Scholar]
- 45.Shaner, N. C., G. H. Patterson, and M. W. Davidson. 2007. Advances in fluorescent protein technology. J. Cell Sci. 120:4247-4260. [DOI] [PubMed] [Google Scholar]
- 46.Sperry, K. E., S. Kathariou, J. S. Edwards, and L. A. Wolf. 2008. Multiple-locus variable-number tandem-repeat analysis as a tool for subtyping Listeria monocytogenes strains. J. Clin. Microbiol. 46:1435-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steen, A., G. Buist, K. J. Leenhouts, M. El Khattabi, F. Grijpstra, A. L. Zomer, G. Venema, O. P. Kuipers, and J. Kok. 2003. Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J. Biol. Chem. 278:23874-23881. [DOI] [PubMed] [Google Scholar]
- 48.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsien, R. Y. 1998. The green fluorescent protein. Annu. Rev. Biochem. 67:509-544. [DOI] [PubMed] [Google Scholar]
- 50.Uchikawa, K., I. Sekikawa, and I. Azuma. 1986. Structural studies on teichoic acids in cell walls of several serotypes of Listeria monocytogenes. J. Biochem. 99:315-327. [DOI] [PubMed] [Google Scholar]
- 51.Uyttendaele, M., I. Van Hoorde, and J. Debevere. 2000. The use of immunomagnetic separation (IMS) as a tool in a sample preparation method for direct detection of L. monocytogenes in cheese. Int. J. Food. Microbiol. 54:205-212. [DOI] [PubMed] [Google Scholar]
- 52.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wendlinger, G., M. J. Loessner, and S. Scherer. 1996. Bacteriophage receptors on Listeria monocytogenes cells are the N-acetylglucosamine and rhamnose substituents of teichoic acids or the peptidoglycan itself. Microbiology 142:985-992. [DOI] [PubMed] [Google Scholar]
- 54.Wesley, I. V., and F. Ashton. 1991. Restriction enzyme analysis of Listeria monocytogenes strains associated with food-borne epidemics. Appl. Environ. Microbiol. 57:969-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmer, M., E. Sattelberger, R. B. Inman, R. Calendar, and M. J. Loessner. 2003. Genome and proteome of Listeria monocytogenes phage PSA: an unusual case for programmed +1 translational frameshifting in structural protein synthesis. Mol. Microbiol. 50:303-317. [DOI] [PubMed] [Google Scholar]
- 56.Zink, R., M. J. Loessner, and S. Scherer. 1995. Characterization of cryptic prophages (monocins) in Listeria and sequence analysis of a holin/endolysin gene. Microbiology 141:2577-2584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.