Abstract
Obligately intracellular Wolbachia bacteria infect numerous invertebrates and often manipulate host reproduction to facilitate the spread of infection. An example of reproductive manipulation is Wolbachia-induced cytoplasmic incompatibility (CI), which occurs commonly in insects. This CI has been the focus both of basic scientific studies of naturally occurring invasion events and of applied investigations on the use of Wolbachia as a vehicle to drive desired genotypes into insect populations (“gene drive” or “population replacement” strategies). The latter application requires an ability to generate artificial infections that cause a pattern of unidirectional incompatibility with the targeted host population. A suggested target of population replacement strategies is the mosquito Aedes albopictus (Asian tiger mosquito), an important invasive pest and disease vector. Aedes albopictus individuals are naturally “superinfected” with two Wolbachia types: wAlbA and wAlbB. Thus, generating a strain that is unidirectionally incompatible with field populations requires the introduction of an additional infection into the preexisting superinfection. Although prior reports demonstrate an ability to transfer Wolbachia infections to A. albopictus artificially, including both intra- and interspecific Wolbachia transfers, previous efforts have not generated a strain capable of invading natural populations. Here we describe the generation of a stable triple infection by introducing Wolbachia wRi from Drosophila simulans into a naturally superinfected A. albopictus strain. The triple-infected strain displays a pattern of unidirectional incompatibility with the naturally infected strain. This unidirectional CI, combined with a high fidelity of maternal inheritance and low fecundity effects, suggests that the artificial cytotype could serve as an appropriate vehicle for gene drive.
Wolbachia spp. are maternally inherited, obligately intracellular bacteria that commonly infect invertebrates, including ∼20% of insect species (2). A hypothesized explanation for the evolutionary success of Wolbachia is its ability to affect host reproduction; cytoplasmic incompatibility (CI) is one of the most widely reported effects (25). Unidirectional CI can occur when the Wolbachia infection type present in a male differs from that in his mate. Although the precise mechanism is unknown, a lock/key model has been proposed in which the Wolbachia infection modifies the sperm during spermatogenesis (27). If the male inseminates a female lacking a compatible Wolbachia type, the modified sperm fail to achieve karyogamy. In contrast, “rescue” of the modified sperm occurs in embryos from females infected with compatible Wolbachia types. Thus, in populations that include both infected and uninfected individuals, Wolbachia-infected females can mate successfully with all males in the population. In contrast, uninfected females can reproduce successfully only with uninfected males. This pattern of unidirectional CI allows Wolbachia to spread rapidly through host populations.
Previous studies of insects with multiple Wolbachia types have demonstrated that unidirectional CI can be additive (4, 5). Multiple Wolbachia infection types within an individual male may independently modify sperm, requiring a similar combination of infection types in female mates for compatibility. Additive unidirectional CI can result in repeated population replacement events, in which single- or double-infection cytotypes are replaced by a Wolbachia “superinfection” (i.e., individuals harboring two or more infections).
The concept of population replacement has attracted attention for its potential applications. A frequently referenced strategy is based on the replacement of natural populations with modified populations that are refractory to disease transmission (1, 4, 8, 12, 22). A Wolbachia-based population replacement strategy requires the generation of artificial infection types that differ from those of the targeted populations.
Aedes albopictus (Skuse) (Diptera: Culicidae), the Asian tiger mosquito, is native to Asia and is a globally invasive insect. Examples of introduction and establishment include North and South America (11), and recent invasions have extended to Africa, Australia, and Europe (9). In addition to being an invasive pest, this mosquito is an aggressive daytime human biter and has been implicated as a vector of animal (20) and human (11) disease. Recent reports have highlighted its role as a primary vector during recent chikungunya virus epidemics (17, 21).
Aedes albopictus populations are naturally infected with two Wolbachia types: wAlbA and wAlbB (13, 24). Therefore, to employ Wolbachia as a vehicle for population replacement, an additional, incompatible infection must be introduced into the natural infection types. Previously, Wolbachia strain wRi was successfully established in A. albopictus by microinjecting the cytoplasm of Drosophila simulans (Riverside) into the embryos of aposymbiotic (i.e., without Wolbachia) A. albopictus mosquitoes (28). As hypothesized, the resulting artificial infection displayed a pattern of bidirectional CI when these mosquitoes were crossed with the naturally double infected strain. Thus, the modification/rescue mechanism(s) of the wRi infection is known to differ from those of the naturally occurring infection types. Therefore, we hypothesized that individuals harboring the combined wRi, wAlbA, and wAlbB infections would be unidirectionally incompatible with the naturally infected mosquitoes.
To develop a strain appropriate for an applied population replacement strategy, we have performed experiments to generate an artificial triple infection. Following embryonic microinjection, experiments were designed to examine individuals across generations for the hypothesized unidirectional CI pattern, to determine the stability and segregation of the different infection types, and to characterize the relative fitness of triple-infected individuals.
MATERIALS AND METHODS
Insect strains.
Two mosquito strains were used in experiments: the wild-type A. albopictus “Hou” strain double infected with Wolbachia types wAlbA and wAlbB (24) and the aposymbiotic HT1 strain, generated by treatment of the Hou strain with tetracycline (6). Mosquitoes were maintained in 30- by 30- by 30-cm cages at 28 ± 2°C and 75% ± 10% relative humidity with a photoperiod of 18 h of light and 6 h of darkness. Adult mosquitoes were continuously provided 10% sucrose as a carbohydrate source, and a blood meal was given once a week with anesthetized mice (IACUC no. 00905A2005). Drosophila simulans Riverside embryos, naturally infected with Wolbachia strain wRi (31), were used as the Wolbachia donor. Fly rearing and egg collection were performed as previously described (30).
Embryonic microinjection.
Embryos were collected, prepared, and microinjected as previously described (29, 30). Injection was performed using an IM 300 microinjector (Narishige Scientific, Tokyo, Japan). Injection needles were prepared using quartz glass capillaries with an outer diameter (OD) of 1.00 mm, an inner diameter (ID) of 0.70 mm, and a length of 7.5 cm (QF100-70-7.5; Sutter Instrument Co., Novato, CA) and a P-2000 micropipette puller (Sutter Instrument Co., Novato, CA). Needles were beveled at a 15° angle using a micropipette beveler, model BV-10 (Sutter Instrument Co., Novato, CA). Microinjection was done using an Olympus IX70 inverted microscope (Olympus Co., Tokyo, Japan) at ×200 magnification. One of the resulting triple-infected A. albopictus lines was named HouR (derived from the Hou recipient strain and the Drosophila simulans Riverside donor strain).
Rearing and selection of microinjected lines.
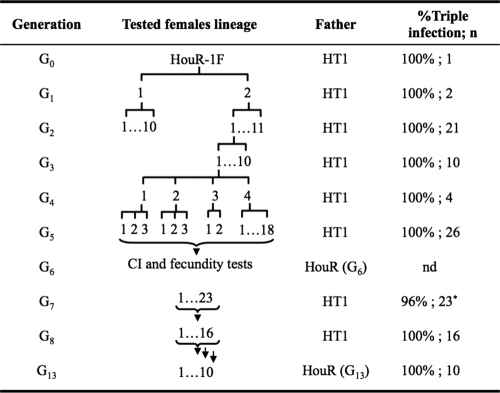
Females of the parent generation (G0) were isolated as virgins and were mated with HT1 males (Fig. 1). After oviposition, G0 females and males were assayed for infection by using PCR. Daughters (G1) from triple-infected G0 females were isolated as virgins and were outcrossed with HT1 males. All ovipositing females were tested for Wolbachia infection by PCR. Subsequently, PCR-guided selection was performed for 5 generations (i.e., G1 to G5). At G6, individuals were combined and used for CI crosses. In the G7 and G8 generations, the progeny from positive mothers were pooled, and selection continued. After G8, the HouR strain was closed (i.e., not outcrossed with HT1 males, but crossed with HouR males), and PCR was used to monitor the frequency of infection periodically through generations.
FIG. 1.
Genealogy and transinfection rates following the generation of the A. albopictus HouR strain. n, number of females tested. The asterisk indicates that for one G7 female, only the wAlbA and wAlbB infections were detected by the PCR assay (i.e., wRi infection was not detected).
Infection status testing via PCR amplification.
DNA was extracted from dissected adult ovaries by the STE method (16) or from whole adult mosquitoes. For the latter procedure, an individual adult mosquito was placed with a 2-mm-diameter autoclaved glass bead and 100 μl of squash buffer (10 mM Tris, 1 mM EDTA, 50 mM NaCl [pH 8.2]) in a 1.5-ml Eppendorf tube. Homogenization using a Mini-BeadBeater (Glen Mills Inc., Clifton, NJ) was followed by incubation at 100°C for 5 min. Samples were then held on ice for 2 min before centrifugation at maximum speed (14,000 rpm) for 5 min using an Eppendorf centrifuge, model 5415C (Brinkmann Instruments, Westbury, NY). The supernatant was transferred to a fresh Eppendorf tube for PCR analysis or storage at −20°C.
Adults putatively infected with Wolbachia were screened by type-specific PCR amplification (Table 1). PCR amplification was performed in a PTC-200 thermal cycler (MJ Research Inc., Waltham, MA) in a final volume of 20 μl containing 0.25 mM deoxynucleoside triphosphate (dNTP) mixture, 0.5 μM each primer, 1.5 mM MgCl2, 1 U of Taq DNA polymerase (New England Biolabs Inc., Ipswich, MA), and 1 μl DNA template. The PCR conditions were as follows: 4 min at 94°C; 35 cycles of 1 min of denaturation at 94°C, 1 min of annealing (specific annealing temperatures and primer sequences are listed in Table 1), and 1 min of extension at 72°C; and an additional 10-min extension at 72°C.
TABLE 1.
Diagnostic primers for determining the Wolbachia type
| Wolbachia strain/phage | Gene | Primer | Primer sequence (5′ to 3′) | Hybridization temp (°C) | Expected amplicon size (bp) | Reference |
|---|---|---|---|---|---|---|
| wAlbA | wsp | 328F | CCA GCA GAT ACT ATT GCG | 55 | 379 | 32 |
| 691R | AAA AAT TAA ACG CTA CTC CA | |||||
| wAlbB | wsp | 183F | AAG GAA CCG AAG TTC ATG | 55 | 501 | 32 |
| 691R | AAA AAT TAA ACG CTA CTC CA | |||||
| wRi | wsp | 169F | ATT GAA TAT AAA AAG GCC ACA GAC A | 52 | 523 | 32 |
| 691R | AAA AAT TAA ACG CTA CTC CA | |||||
| WO | orf7 | WOF | CCC ACA TGA GCC AAT GAC GTC TG | 57 | 353 | 14 |
| WOR | CGT TCG CTC TGC AAG TAA CTC CAT TAA AAC | |||||
| 12S rRNAa | 12s A1 | AAA CTA GGA TTA GAT ACC CTA TTA T | 55 | 400 | 16 | |
| 12s B1 | AAG AGC GAC GGG CGA TGT GT |
Control for template quality.
Cytoplasmic incompatibility and fitness assays.
The following 4 crosses (female × male) were set up with the triple-infected HouR line at G6: Hou × HouR, HouR × Hou, HouR × HouR, and Hou × Hou. For each cross type, 10 virgin females and males (<48 h posteclosion) were mated. Five to six replicates were made for the four crosses. Mosquitoes were blood fed once a week, and oviposition papers were collected weekly. Three oviposition papers were collected and allowed to embryonate for 5 days prior to hatching. Egg hatch rates were measured 3 days after the oviposition papers were submerged in deoxygenated water. The fecundity per female was estimated from the total number of eggs laid within 4 days by each group of females, divided by 10. For each replicate of each cross type, the hatch rate and fecundity were obtained by averaging the data from the three egg papers collected.
Statistics.
The normality of the data was examined by a Kolmogorov-Smirnov normality test using StatView (SAS Institute, Cary, NC) software. Significant differences among mean egg hatch rates and among fecundity levels were tested by analysis of variance (ANOVA), followed by statistical comparison using a Scheffé test.
RESULTS
A triple Wolbachia infected strain of A. albopictus was generated by microinjecting embryos of the naturally double infected Hou strain with cytoplasm from wRi-infected D. simulans (Riverside) embryos (Table 2). Triple-infected adults were obtained in each of two microinjection experiments. As in prior experiments (28), the survival of injected embryos (∼ 5%) was the primary limitation. The rate of survival of larvae to the adult stage was relatively high, resulting in a total of seven females and seven males.
TABLE 2.
Survival and infection levels of A. albopictus Hou strain mosquitoes injected as embryos with Wolbachia wRi from D. simulans
| Expt | G0 survival |
G0 infection status |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of larvae/eggs (hatch rate [%]) | No. of pupae/larvae (pupation rate [%]) | No. of adults/pupae (eclosion rate [%]) | No. of females/total (sex ratio [%]) | Female |
Male |
|||
| No. of samples tested | % Triple infecteda | No. of samples tested | % Triple infecteda | |||||
| 1 | 8/170 (4.7) | 3/8 (37.5) | 3/3 (100.0) | 1/3 (33.3) | 1 | 100 | 2 | 0 |
| 2 | 14/241 (5.8) | 14/14 (100.0) | 11/14 (78.6) | 6/11 (54.5) | 6 | 16.7 | 5 | 0 |
Coinfected with wAlbA, wAlbB, and wRi.
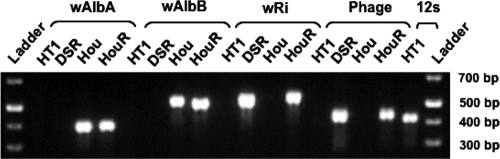
As illustrated in Fig. 2, DNA from both Hou (wild type; double infected) and triple-infected individuals was amplified by PCR assays with the 328F/691R and 183F/691R primer sets, indicating the presence of Wolbachia wAlbA and wAlbB. In contrast, PCR with the 169F/691R or WOF/WOR primer set yielded amplicons only for the triple-infected and Drosophila simulans Riverside individuals, indicating successful transfer of Wolbachia wRi into the Hou strain.
FIG. 2.
PCR assays of the triple-infected A. albopictus HouR line using primers specific for the wAlbA, wAlbB, and wRi strains and bacteriophage WO. 12S rRNA primers were used to check the DNA template quality of the negative control (HT1). DSR, Drosophila simulans Riverside.
Of the 14 surviving G0 adults, 2 (14.3%) triple-infected adult females were obtained (Table 2). The G0 females were outcrossed with HT1 males, and the progeny were tested via PCR assay. In the two G1 lines derived from one of the G0 females, the triple infection was detected in all 21 G2 individuals tested (Fig. 1). One line (named “HouR”) derived from the second infected female (G1) was selected for subsequent characterization.
Crosses between all four combinations of the Hou and HouR strains revealed a typical unidirectional CI pattern (Table 3). Relative to the compatible crosses, the egg hatch rate was reduced by ∼80% in crosses of HouR males with wild-type Hou females. In contrast, wild-type Hou males were compatible with HouR females, resulting in a mean hatch rate of 78%, similar to the egg hatch rate resulting from crosses between individuals with similar infection types. ANOVA indicated significant differences in egg hatch rates between the incompatible cross (Hou × HouR) and the three compatible crosses (F, 79.97; P, <0.0001). The hatch rates of the three compatible crosses (HouR × Hou, HouR × HouR, and Hou × Hou) were not significantly different.
TABLE 3.
Crossing results for the triple Wolbachia infected A. albopictus HouR line (G6)
| Expected CI type | Crossa | Infection types |
Hatch rate (%)b | No. of eggs scored | |
|---|---|---|---|---|---|
| Female | Male | ||||
| Unidirectional CI | Hou × HouR | wAlbA, wAlbB | wAlbA, wAlbB, wRi | 16.0 ± 9.3 A | 5,153 |
| HouR × Hou | wAlbA, wAlbB, wRi | wAlbA, wAlbB | 78.1 ± 12.1 B | 5,752 | |
| Compatible | HouR × HouR | wAlbA, wAlbB, wRi | wAlbA, wAlbB, wRi | 75.9 ± 3.2 B | 4,106 |
| Hou × Hou | wAlbA, wAlbB | wAlbA, wAlbB | 80.2 ± 8.2 B | 7,736 | |
Female × male.
Expressed as the mean for 5 to 6 replicates/cross type ± standard deviation. Different capital letters following the data indicate significant differences (P < 0.001).
No obvious impact of the triple infection on female fecundity was observed. Specifically, HouR and Hou females produced similar numbers of eggs when mated with Hou males (38.4 ± 11.3 and 51.6 ± 9.4 eggs/female, respectively).
Transmission efficiencies were monitored from G1 to G8 (Fig. 1). HouR females were randomly selected at each generation for PCR assays to determine the infection status. Triple infection was detected in 101/102 (99.0%) females tested. PCR assays failed to detect the wRi infection in only one female (Fig. 1, G7). At G13, the maternal inheritance rate for triple infection was tested by PCR assays of 10 females and 10 males from five separate lines (i.e., 2 females and 2 males from each of five lines were assayed), and an overall 95% transmission rate of triple infection was observed: 100% for females and 90% for males. PCR failed to detect the wAlbA infection in one of 10 males tested (i.e., it detected wAlbB and wRi infections only).
DISCUSSION
The results demonstrate that A. albopictus can stably support wRi from D. simulans as a superinfection, that the triple infection is unidirectionally incompatible with the wild-type infection, and that the triple infection is maternally transmitted at high rates. The results presented here are similar to prior observations of the wRi single infection of A. albopictus, which also displayed stability and high levels of maternal inheritance (28). The results are consistent with the hypothesis that wRi causes CI when introduced into naturally double infected populations. In a prior examination of the wRi single infection of A. albopictus, the HTR strain (singly infected with wRi) failed to rescue the Wolbachia-induced sperm modification caused by either wAlbA, wAlbB, or their combination (28). Furthermore, infection with wAlbA or wAlbB, or superinfection with wAlbA and wAlbB, failed to rescue sperm modification in crosses with wRi males. Thus, wRi displays bidirectional cytoplasmic incompatibility with the wAlbA and wAlbB infection types. Here we have observed that wRi continues to induce CI when coinfecting the HouR strain along with the wAlbA and wAlbB infection types. The CI level resulting in the Hou × HouR crosses (hatch rate, ∼16%) is similar to the CI level observed with the HTR strain (hatch rate, ∼14%) (28). Thus, coinfection with the three Wolbachia types did not have a measurable effect on the CI level induced by wRi infection, relative to that with wRi alone.
Of the 122 HouR mosquitoes assayed by PCR, only 1 male failed to demonstrate a triple Wolbachia infection. wAlbB and wRi were detected in this male (i.e., the natural wAlbA infection was not detected). This could represent an artifact of the PCR assay (i.e., false negative for the wAlbA infection). Alternatively, loss of the wAlbA infection could reflect the previously reported lower density of wAlbA versus wAlbB infections (7). The infrequent loss of wAlbA in males is not expected to impact Wolbachia infection dynamics substantially in A. albopictus, since males are a dead end for the maternally inherited infections.
Insects that are naturally infected with three Wolbachia types have been reported (26), and triple Wolbachia infected insects have been artificially generated by microinjecting a third strain into a double-infected Drosophila line (18). Mouton et al. (15) studied the regulation of Wolbachia strains in triple-, double-, and single-infected Leptopilina heterotoma (Thomson) (Hymenoptera: Cynipoidea: Eucoilidae) wasps and found that the total Wolbachia counts (total number of Wolbachia cells/wasp) are not at the same level for the different infection lines. Notably, the density (cell number per milligram [fresh weight]) of each Wolbachia strain remained unchanged in different infection lines; thus, the infection levels in the wasp strains were independent of co-occurring Wolbachia infections. Likewise, the artificial introduction of a third Wolbachia strain into double-infected D. simulans resulted in an increase of the total Wolbachia density in the host (18). Therefore, the HouR strain provides an additional system for studying Wolbachia regulation.
The Wolbachia wRi strain is known to be infected by an active bacteriophage named WO (10, 14). In contrast, no phage has been described in association with Wolbachia wAlbA or wAlbB. During microinjection, phage WO was transferred together with Wolbachia wRi, and it can be detected within the triple-infected HouR mosquito strain (Fig. 2). This provides an opportunity for the study of the interaction of phage WO with the wAlbA and/or wAlbB infection. Specifically, the interaction among the phage, Wolbachia, and the insect host (14, 19) could be studied in HouR sublines that contain only wAlbA and/or wAlbB (e.g., generated from HouR treated with moderate antibiotic levels [3]).
The artificial strain resulting from this study displays stable triple Wolbachia infection and high maternal inheritance rates in A. albopictus with no observed fecundity effect. These features are consistent with the traits desired for an efficient population replacement strategy (23). Furthermore, the reduced hatch rate observed in crosses between naturally infected females and HouR males indicates a CI phenotype, suggesting that the HouR strain is potentially useful for field application.
Acknowledgments
We thank Zhiyong Xi for assistance with Wolbachia transinfection techniques, Lok-Sze Ng for help with injection and screening, and James W. Mains and Corey L. Brelsfoard for assistance with mosquito blood feeding.
This research was supported by a grant from the National Institutes of Health (AI-67434).
Footnotes
Published ahead of print on 2 July 2010.
Publication 10-08-015 of the University of Kentucky Agricultural Experiment Station.
REFERENCES
- 1.Beaty, B. J. 2000. Genetic manipulation of vectors: a potential novel approach for control of vector-borne diseases. Proc. Natl. Acad. Sci. U. S. A. 97:10295-10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourtzis, K., and T. A. Miller (ed.). 2003. Insect symbiosis. CRC Press, Boca Raton, FL.
- 3.Dedeine, F., F. Vavre, F. Fleury, B. Loppin, M. E. Hochberg, and M. Bouletreau. 2001. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl. Acad. Sci. U. S. A. 98:6247-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobson, S. L. 2003. Reversing Wolbachia-based population replacement. Trends Parasitol. 19:128-133. [DOI] [PubMed] [Google Scholar]
- 5.Dobson, S. L., E. J. Marsland, and W. Rattanadechakul. 2001. Wolbachia-induced cytoplasmic incompatibility in single- and superinfected Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 38:382-387. [DOI] [PubMed] [Google Scholar]
- 6.Dobson, S. L., and W. Rattanadechakul. 2001. A novel technique for removing Wolbachia infections from Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 38:844-849. [DOI] [PubMed] [Google Scholar]
- 7.Dutton, T. J., and S. P. Sinkins. 2004. Strain-specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing condition. Insect Mol. Biol. 13:317-322. [DOI] [PubMed] [Google Scholar]
- 8.Enserink, M. 2001. Two new steps towards a ‘better mosquito.’ Science 293:2370-2371. [DOI] [PubMed] [Google Scholar]
- 9.Eritja, R., R. Escosa, J. Lucientes, E. Marquès, D. Roiz, and S. Ruiz. 2005. Worldwide invasion of vector mosquitoes: present European distribution and challenges for Spain. Biol. Invas. 7:87-97. [Google Scholar]
- 10.Gavotte, L., F. Vavre, H. Henri, M. Ravallec, R. Stouthamer, and M. Bouletreau. 2004. Diversity, distribution and specificity of WO phage infection in Wolbachia of four insect species. Insect Mol. Biol. 13:147-153. [DOI] [PubMed] [Google Scholar]
- 11.Gratz, N. G. 2004. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18:215-227. [DOI] [PubMed] [Google Scholar]
- 12.James, A. A. 2005. Gene drive systems in mosquitoes: rules of the road. Trends Parasitol. 21:64-67. [DOI] [PubMed] [Google Scholar]
- 13.Kitrayapong, P., V. Baimai, and S. L. O'Neill. 2002. Field prevalence of Wolbachia in the mosquito vector Aedes albopictus. Am. J. Trop. Med. Hyg. 66:108-111. [DOI] [PubMed] [Google Scholar]
- 14.Masui, S., S. Kamoda, T. Sasaki, and H. Ishikawa. 2000. Distribution and evolution of bacteriophage WO in Wolbachia, the endosymbiont causing sexual alterations in arthropods. J. Mol. Evol. 51:491-497. [DOI] [PubMed] [Google Scholar]
- 15.Mouton, L., H. Henri, M. Bouletreau, and F. Vavre. 2003. Strain-specific regulation of intracellular Wolbachia density in multiply infected insects. Mol. Ecol. 12:3459-3465. [DOI] [PubMed] [Google Scholar]
- 16.O'Neill, S. L., R. Giordano, A. M. Colbert, T. L. Karr, and H. M. Robertson. 1992. 16S RNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. U. S. A. 89:2699-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiter, P., D. Fontenille, and C. Paupy. 2006. Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem? Lancet Infect. Dis. 6(8):463-464. [DOI] [PubMed] [Google Scholar]
- 18.Rousset, F., H. R. Braig, and S. L. O'Neill. 1999. A stable triple Wolbachia infection in Drosophila with nearly additive incompatibility effects. Heredity 82:620-627. [DOI] [PubMed] [Google Scholar]
- 19.Sanogo, Y. O., and S. L. Dobson. 2006. WO bacteriophage transcription in Wolbachia-infected Culex pipiens. Insect Biochem. Mol. Biol. 36:80-85. [DOI] [PubMed] [Google Scholar]
- 20.Scoles, G. A., and S. Kambhampati. 1995. A polymerase chain reaction-based method for the detection of canine heartworm (Filarioidea: Onchocercidae) in mosquitoes (Diptera: Culicidae) and vertebrate hosts. J. Med. Entomol. 32:864-869. [DOI] [PubMed] [Google Scholar]
- 21.Simon, F., H. Savini, and P. Parola. 2008. Chikungunya: a paradigm of emergence and globalization of vector-borne diseases. Med. Clin. North Am. 92:1323-1343. [DOI] [PubMed] [Google Scholar]
- 22.Sinkins, S. P., and F. Gould. 2006. Gene drive systems for insect disease vectors. Nat. Rev. Genet. 7:427-435. [DOI] [PubMed] [Google Scholar]
- 23.Sinkins, S. P. 2004. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem. Mol. Biol. 34:723-729. [DOI] [PubMed] [Google Scholar]
- 24.Sinkins, S. P., H. R. Braig, and S. L. O'Neill. 1995. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc. R. Soc. Lond. B Biol. Sci. 261:325-330. [DOI] [PubMed] [Google Scholar]
- 25.Stouthamer, R., J. A. Breeuwer, and G. D. Hurst. 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53:71-102. [DOI] [PubMed] [Google Scholar]
- 26.Vavre, F., F. Fleury, D. Lepetit, P. Fouillet, and M. Bouletreau. 1999. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol. Biol. Evol. 16:1711-1723. [DOI] [PubMed] [Google Scholar]
- 27.Werren, J. H. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42:587-609. [DOI] [PubMed] [Google Scholar]
- 28.Xi, Z., C. C. H. Khoo, and S. L. Dobson. 2006. Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc. R. Soc. Lond. B Biol. Sci. 273:1317-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xi, Z., J. L. Dean, C. Khoo, and S. L. Dobson. 2005. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem. Mol. Biol. 35:903-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xi, Z., and S. L. Dobson. 2005. Characterization of Wolbachia transfection efficiency by using microinjection of embryonic cytoplasm and embryo homogenate. Appl. Environ. Microbiol. 71:3199-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zabalou, S., M. Riegler, M. Theodorakopoulou, C. Stauffer, C. Savakis, and K. Bourtzis. 2004. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. U. S. A. 101:15042-15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou, W., F. Rousset, and S. O'Neill. 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B Biol. Sci. 265:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]