Abstract
Escherichia coli biotin ligase can attach biotin molecules to a lysine residue of biotin acceptor peptide (BAP), and biotinylation of particular BAP-fused proteins in cells was carried out by coexpression of E. coli biotin ligase (in vivo biotinylation). This in vivo biotinylation technology has been applied for protein purification, analysis of protein localization, and protein-protein interaction in eukaryotic cells, while such studies have not been reported in bacterial cells. In this study, in vivo biotinylation of bacterial magnetic particles (BacMPs) synthesized by Magnetospirillum magneticum AMB-1 was attempted by heterologous expression of E. coli biotin ligase. To biotinylate BacMPs in vivo, BAP was fused to a BacMP surface protein, Mms13, and E. coli biotin ligase was simultaneously expressed in the truncated form lacking the DNA-binding domain. This truncation-based approach permitted the growth of AMB-1 transformants when biotin ligase was heterologously expressed. In vivo biotinylation of BAP on BacMPs was confirmed using an alkaline phosphatase-conjugated antibiotin antibody. The biotinylated BAP-displaying BacMPs were then exposed to streptavidin by simple mixing. The streptavidin-binding capacity of BacMPs biotinylated in vivo was 35-fold greater than that of BacMPs biotinylated in vitro, where BAP-displaying BacMPs purified from bacterial cells were biotinylated by being mixed with E. coli biotin ligase. This study describes not only a simple method to produce biotinylated nanomagnetic particles but also a possible expansion of in vivo biotinylation technology for bacterial investigation.
Biotin/streptavidin binding is the strongest noncovalent interaction known in nature (Kd [dissociation constant], ∼10−15 M) (10), and this tight binding is one of the most general tools for biological research and has been widely used for biomolecular detection (11, 12), immobilization (14, 19), and recovery (15). Therefore, it is of great significance to biotinylate biomolecules, in particular, proteins without functional inhibition. For this purpose, the method for site-selective biotinylation of proteins had been developed using biotin ligase. Biotin ligase catalyzes the posttranslational biotinylation of biotin enzymes, such as acetyl coenzyme A (acetyl-CoA) carboxylase, and introduces biotin into a specific lysine residue of a biotin carboxyl carrier protein (BCCP), a subunit of biotin enzymes (13). In early studies, BCCP (∼100 amino acid residues) had been fused with the proteins of interest for biotinylation by biotin ligase (7); however, there was a concern that fused BCCP might disrupt the function of target proteins. Recently, biotin acceptor peptides (BAPs) had replaced BCCP due to the advantage of small size. BAPs, with 15 to 23 amino acid residues, were screened from a peptide library as peptide tags biotinylated by Escherichia coli biotin ligase (4, 25). BAP-fused proteins can be biotinylated outside the cells by adding biotin and purified E. coli biotin ligase with Mg2+ and ATP (in vitro biotinylation). Furthermore, it is also possible to biotinylate BAP-fused proteins inside the cells with coexpression of E. coli biotin ligase (in vivo biotinylation) because BAP is specifically recognized only by E. coli biotin ligase. This in vivo biotinylation technology has been applied in eukaryotic cells to purify the proteins by using streptavidin-immobilized resin (8, 24, 28), because biotin/streptavidin interaction permits stringent washing to eliminate the nonspecific binding. Specific biotinylation can be applied also for protein localization analysis. Using fluorophore- or gold nanoparticle-labeled streptavidin, biotinylated proteins were clearly observed in a previous study (27). Recently, a novel technique to detect protein-protein interaction by fusing BAP and biotin ligase was developed by Ting's group. BAP and biotin ligase were fused to different two proteins, and then the interaction of these proteins was successfully evaluated via biotinylation of BAP (9). In vivo biotinylation technology using heterologously expressed E. coli biotin ligase should be equally useful for prokaryotes; however, such studies have not been reported for bacterial cells.
Magnetospirillum magneticum AMB-1, a magnetotactic bacterium, synthesizes intracellular nanosized bacterial magnetic particles (BacMPs) of 50 to 100 nm; these are surrounded by a lipid bilayer membrane, possess a single magnetic domain of magnetite, and exhibit strong ferrimagnetism (18). Furthermore, functional proteins have been displayed on BacMP surfaces through gene fusion techniques (21, 30, 31). BacMP membrane proteins, including Mms13, were used as anchor proteins; this approach permits functional proteins to be localized efficiently and oriented appropriately on BacMPs (31). We recently reported a novel method for the simple production of biotin-labeled magnetic particles through protein display techniques, where introduction of the biotin moiety onto BacMPs was carried out by the endogenous biotin ligase (17). For the biotinylation of BacMPs, we screened the gene encoding BCCP in the AMB-1 genome and displayed it on the surface of BacMPs using an anchor protein, Mms13. BCCP-displaying BacMPs were biotinylated by endogenous AMB-1 biotin ligase in the cells with high efficiency. This in vivo modification approach could be applied for construction of BacMP-quantum dot nanocomposites toward multicolor labeling of cancer cells, where BCCP and antibody carrier protein (protein G) were simultaneously displayed in tandem (16). However, the size of BCCP, with 149 amino acid residues and a mass of 15.6 kDa, makes it rather large for use as a labeling tag. Although it would be preferable to use a smaller peptide, BAP, for the tag to minimize effects on the flanking proteins for future applications, BAP was not recognized and biotinylated by endogenous AMB-1 biotin ligase (17).
In this study, in vivo biotinylation of BacMPs was attempted by heterologous expression of E. coli biotin ligase and Mms13-BAP fusion protein in AMB-1 cells. First, the method for effective expression of E. coli biotin ligase in bacterial cells was optimized. Then site-selective biotinylation of BAP on BacMPs was confirmed. Finally, the obvious advantage of in vivo biotinylation of BAP-displaying BacMPs compared with the in vitro biotinylation method was demonstrated.
MATERIALS AND METHODS
Materials.
E. coli biotin ligase was purchased from Avidity L.L.C. (Aurora, CO). Chicken anti-biotin ligase antibody was purchased from ProSci Incorporated (Poway, CA). Alkaline phosphatase (ALP)-labeled anti-chicken/turkey IgG was from Invitrogen (Carlsbad, CA). ALP-labeled antibiotin antibody was purchased from Rockland Immunochemicals, Inc. (Gilbertsville, PA). ALP-labeled streptavidin was purchased from Millipore (Billerica, MA). Tetramethyl rhodamine isocyanate (TRITC)-labeled streptavidin was purchased from Beckman Coulter (Fullerton, CA). (+)-Biotin, magnesium chloride hexahydrate, Lumi-Phos 530, and Tween 20 were purchased from Wako Pure Chemical Industries (Osaka, Japan). All other reagents were commercially available analytical reagents of laboratory grade. Deionized distilled water was used in all procedures.
Bacterial strains and culture conditions.
E. coli strain EPI300 (AR Brown Co., Ltd., Tokyo, Japan) was used as a host for gene cloning. Cells were cultured in LB medium containing 50 μg/ml ampicillin at 37°C. M. magneticum AMB-1 was microaerobically cultured in magnetic spirillum growth medium (MSGM) at 25°C as previously described (20). Microaerobic conditions were established by purging the cultures with argon gas. AMB-1 transformants were cultured under the same conditions in the presence of 5 μg/ml ampicillin.
Preparation of BacMPs.
M. magneticum AMB-1 cells were collected by centrifugation at 11,344 × g for 10 min at 4°C, resuspended in 40 ml 10 mM phosphate-buffered saline (PBS), pH 7.4, and disrupted by three passes through a French press cell (Ohtake Works Co. Ltd., Tokyo, Japan) at 1,500 kg/cm2. BacMPs were collected from the disrupted cell fraction using a columnar neodymium-boron (Nd-B) magnet and washed 10 times with 10 mM HEPES buffer, pH 7.4. The concentration of BacMPs in suspension was determined by measuring the optical density of the solution at 660 nm (OD660) with a spectrophotometer (UV-2200; Shimadzu, Kyoto, Japan). A value of 1.0 corresponded to 172 μg (dry weight) BacMPs/ml.
Construction of expression vectors.
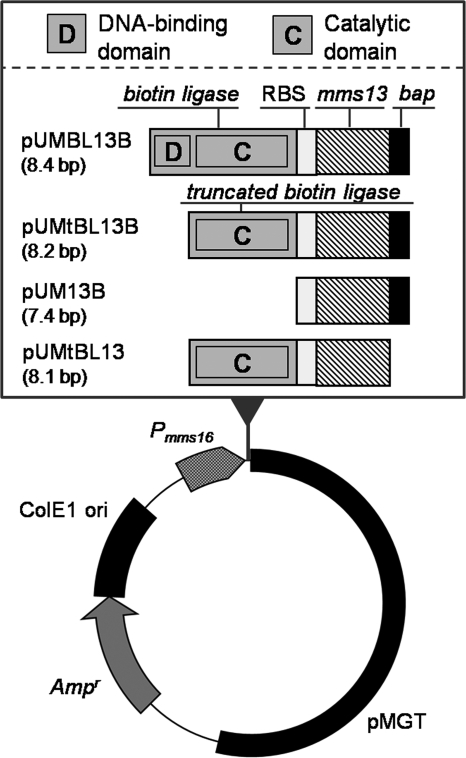
The plasmids pUMBL13B, pUM13B, pUMtBL13, and pUMtBL13B were derived from pUMP16 (Ampr; 7.0 kbp; Fig. 1) (32). For the construction of pUMBL13B and pUMtBL13B, the genes encoding intact biotin ligase and truncated biotin ligase were generated by PCR amplification using E. coli JM109 genomic DNA as a template and the primer sets XbaI-EcoRV-RBS-BL-R (5′-TTTTTCTAGAGATATCGTTATTCCTCCAACCCGGTTATTTTTCTGCAC-3′) and XbaI-BL-F (5′-AAAATCTAGAATGAAGGATAACACCGTGCC-3′) or XbaI-tBL-F (5′-AAAATCTAGAATGATCCAGTTACTTAATGCTAAAC-3′). Each PCR product was cloned into pCR4-BluntTOPO (Invitrogen, Carlsbad, CA). Plasmids were digested with XbaI, and the fragment containing the biotin ligase or truncated biotin ligase gene was cloned into SpeI-digested pUMP16. These plasmids were designated pUMBL and pUMtBL, respectively. The mms13-bap fusion gene was then amplified using pUMGP16M13 (31) as a template with the primer set Mms13-F (5′-ATGGTGAGCAAGGGCGC-3′) and BAP-Mms13-R (5′-TCACTCGTGCCATTCGATCTTCTGGGCCTCGAAGATGTCGTTCAGGCCGGCCAGTTCGTCCC-3′). The PCR product was cloned into EcoRV-digested pUMBL and pUMtBL. For the construction of pUMtBL13, the mms13 gene was amplified using pUMGP16M13 as a template and the primer set Mms13-F and EcoRV-Mms13-R (5′-TCAGATATCGGCCAGTTCGTCCC-3′). The PCR product was cloned into EcoRV-digested pUMtBL. For the construction of pUM13B, the mms13-bap fusion gene was amplified using pUMtBL13B as a template with the primer set XbaI-Mms13-F (5′-CCCCTCTAGAATGCCCTTTCACCTTGCC-3′) and XbaI-BAP-R (5′-GGGGTCTAGATCACTCGTGCCATTCGAT-3′). The PCR product was digested with XbaI and cloned into SpeI-digested pUMP16. Plasmids were transformed into wild-type M. magneticum AMB-1 by electroporation (23).
FIG. 1.
Plasmids used in this study encoding Mms13-BAP and E. coli biotin ligase are shown. pUMBL13B expresses Mms13-BAP and intact E. coli biotin ligase, including both the DNA-binding domain and the catalytic domain. pUMtBL13B expresses Mms13-BAP and truncated E. coli biotin ligase, which lacks the DNA-binding domain. pUM13B expresses Mms13-BAP fusion protein. pUMtBL13 expresses Mms13 and truncated biotin ligase. Simultaneous expression of two proteins was controlled by a promoter for the mms16 gene (Pmms16), where both genes were aligned in tandem across the ribosome-binding site (RBS) found in Pmms16.
SDS-PAGE and Western blotting.
For detection of truncated E. coli biotin ligase expressed in M. magneticum AMB-1, 5 × 108 AMB-1 cells were collected by centrifugation, suspended in 25 μl 1% (wt/vol) SDS in aqueous solution, and boiled for 30 min. Following addition of SDS sample buffer to a final concentration of 62.5 mM Tris-HCl, pH 6.8, 5% 2-mercaptoethanol, 2% SDS, 5% sucrose, and 0.002% bromophenol blue, proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) through a 12.5% (wt/vol) gel and transferred to a polyvinylidene difluoride membrane. Biotin ligase was detected using chicken anti-E. coli biotin ligase antibody (1/20,000 dilution from stock in PBS containing 0.05% Tween 20 [PBST]) and ALP-labeled rabbit anti-chicken IgG (1/2,000 dilution from stock in PBST). BCIP/NBT-Blue (Sigma, St. Louis, MO) was used as the ALP substrate for visualization.
For detection of the biotin moiety on the Mms13-BAP fusion protein, aliquots of suspension containing 100 μg BacMPs were dispensed to microtubes and the BacMPs were magnetically collected. After removal of supernatant, BacMPs were suspended in 25 μl 1% (wt/vol) SDS in aqueous solution and boiled for 30 min. Magnetite was removed by centrifugation and magnetic separation. BacMP membrane proteins in the supernatant were mixed with SDS sample buffer, subjected to SDS-PAGE through a 20% (wt/vol) polyacrylamide gel, and transferred to a polyvinylidene difluoride membrane. Biotin was detected using ALP-labeled antibiotin antibody.
In vitro biotinylation reaction.
BacMPs (700 μg) were magnetically collected using a columnar Nd-B magnet and suspended in 1,400 μl PBS containing 15 μg/ml E. coli biotin ligase, 10 μM (+)-biotin, 5 mM magnesium chloride hexahydrate, and 100 mM ATP disodium salt followed by incubation for 60 min at room temperature with pulsed sonication every 10 min. BacMPs were magnetically separated from the reaction mixture by using a Nd-B magnet and washed once with 1,400 μl PBST with sonication.
Introduction of antibody or streptavidin to BacMPs.
BacMPs (50 μg) were magnetically collected and added to a solution of ALP-labeled antibiotin antibody (10 μg/ml) or ALP-labeled streptavidin (1/100 dilution from stock in PBST) in 50 μl PBST. Mixtures were incubated for 30 min at room temperature with pulsed sonication every 5 min. BacMPs were then magnetically separated, washed three times with 100 μl PBST, and resuspended in 50 μl PBS. Fifty microliters Lumi-Phos 530 was added as luminescence substrate. After 5 min of incubation, luminescence intensity was measured with a luminometer (Aloka, Tokyo, Japan).
BacMPs (100 μg) were magnetically collected and added to 200 μl TRITC-labeled streptavidin in PBST. The mixture was incubated for 60 min at room temperature with pulsed sonication every 5 min. BacMPs were magnetically separated and washed three times with 200 μl PBST followed by suspension of 50 μg BacMPs in 500 μl PBS and measurement of fluorescence intensity of the solution (λexcitation = 550 nm, λemission = 580 nm) using a spectrofluorometer (Horiba Itech, Tokyo, Japan). To estimate the amount of streptavidin bound to BacMPs, a calibration curve was constructed by measuring the fluorescent intensities of 500-μl aliquots of PBS containing 50 μg BacMPs and various concentrations of TRITC-labeled streptavidin.
RESULTS
Heterologous expression of E. coli biotin ligase and Mms13-BAP in M. magneticum AMB-1.
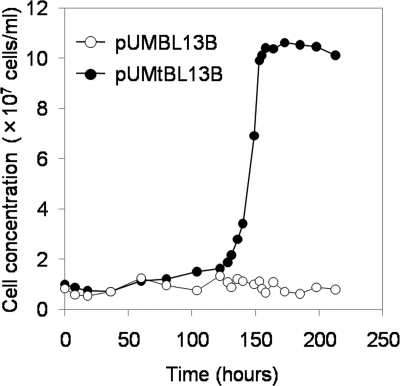
For in vivo biotinylation of BacMPs, E. coli biotin ligase was coexpressed in M. magneticum AMB-1 with BAP (GLNDIFEAQKIEWHE) fused to anchor protein Mms13, which is tightly bound to the BacMP surface. Two kinds of proteins were simultaneously expressed under the control of a strong promoter, Pmms16 (30), where both genes were aligned in tandem across the ribosome-binding site found in the same Pmms16 (Fig. 1). In the initial trial, an intact gene encoding the full length (321 amino acid residues) of the biotin ligase was cloned into expression vector pUMBL13B and introduced into wild-type AMB-1 by electroporation. After transformation, bacterial cells were cultured in MSGM containing ampicillin, and cell growth was monitored by microscopic observation. However, transformants harboring pUMBL13B were not obtained even after 3 weeks of culture, and it was speculated that full-length E. coli biotin ligase showed cytotoxicity when it was overexpressed in the bacterial cells. E. coli biotin ligase is a bifunctional protein and serves not only as a biotin-protein ligase but also as a biotin repressor, binding the biotin operator to repress transcription of the biotin biosynthetic operon (2, 3). E. coli biotin ligase has a DNA-binding domain (29). Generally, overexpression of proteins containing a DNA-binding domain tends to show the cytotoxicity for host cells (5, 6). Based on these observations, we hypothesized that the DNA-binding domain of E. coli biotin ligase is also cytotoxic in AMB-1 cells as a result of binding to chromosomal DNA and disturbing cellular metabolism. A truncated gene which encodes a truncated mutant of biotin ligase lacking the DNA-binding domain (Δ2K-63P, consisting of 259 amino acids) was cloned into the expression vector pUMtBL13B, and the growth of transformants harboring pUMBL13B and that of transformants harboring pUMtBL13B were compared. No growth of transformants expressing the intact form of biotin ligase was observed (Fig. 2, open circles), while transformants expressing the truncated biotin ligase lacking the DNA-binding domain showed steady growth (Fig. 2, closed circles). Truncation of the DNA-binding domain permitted effective transformation of AMB-1.
FIG. 2.
Growth curve of transformants harboring pUMBL13B or pUMtBL13B. Each expression vector (500 ng) was mixed with 1 × 108 competent cells of wild-type M. magneticum AMB-1 and introduced by electroporation. Transformed cells were diluted in 40 ml MSGM containing 5 μg/ml ampicillin.
Site-selective biotinylation of Mms13-BAP on BacMPs.
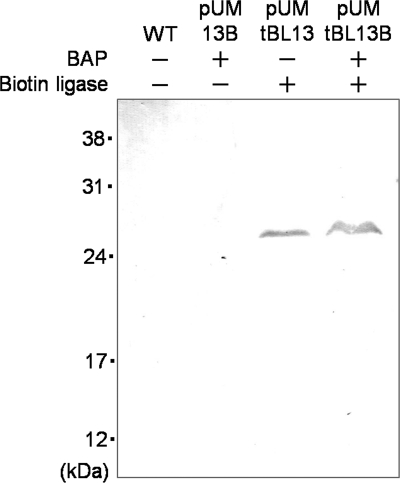
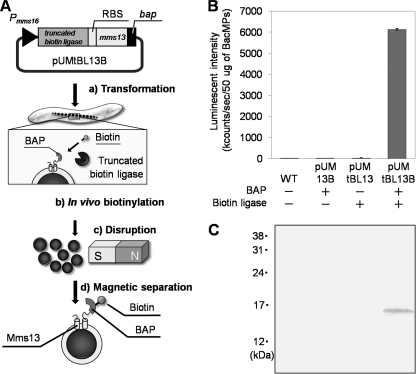
To evaluate the biotinylation of BacMPs by heterologously expressed E. coli biotin ligase, a further two kinds of plasmids, in which truncated biotin ligase or bap was deleted from pUMtBL13B, were constructed (Fig. 1, pUM13B and pUMtBL13). Three kinds of plasmids (pUM13B, pUMtBL13, and pUMtBL13B) were respectively introduced into wild-type AMB-1, and heterologous expression of truncated biotin ligase in AMB-1 was analyzed by Western blotting using anti-biotin ligase antibody. Truncated E. coli biotin ligase was observed in the fraction of whole-cell lysates from pUMtBL13 and pUMtBL13B at the predicted mass (29 kDa) (Fig. 3). In vivo attachment of biotin to BAP-displaying BacMPs (BAP-BacMPs) by truncated biotin ligase was evaluated (Fig. 4). Figure 4A shows the principle of in vivo biotinylation in magnetotactic bacteria and purification of BAP-BacMPs. After extraction of BacMPs from wild-type AMB-1 and from transformants harboring pUM13B lacking the truncated biotin ligase gene, pUMtBL13 lacking the bap gene, and pUMtBL13B, BacMPs were exposed to ALP-labeled antibiotin antibody followed by addition of luminescence substrate and measurement of luminescence intensity. High luminescence intensity was observed in BAP-BacMPs extracted from pUMtBL13B transformants (Fig. 4B). To confirm the specific biotinylation of the Mms13-BAP fusion proteins in pUMtBL13B transformants, the protein fraction of the BacMP membrane was analyzed by Western blotting with ALP-labeled antibiotin antibody and the specific band was observed at the predicted mass (Fig. 4C).
FIG. 3.
Expression analysis of the truncated form of E. coli biotin ligase in AMB-1 wild type (WT) and transformants harboring pUM13B, pUMtBL13, or pUMtBL13B. Cell debris was denatured and analyzed by SDS-PAGE and Western blotting using chicken anti-biotin ligase antibody and ALP-labeled anti-chicken IgG antibody.
FIG. 4.
In vivo biotinylation of BacMPs. (A) Schematic diagram of the preparation and in vivo biotinylation of BacMPs. Plasmid pUMtBL13B containing the truncated biotin ligase and mms13-bap gene was introduced into wild-type AMB-1 (step a). BacMPs were biotinylated by coexpressed truncated E. coli biotin ligase and Mms13-BAP (step b). The AMB-1 transformant harboring pUMtBL13B was disrupted to release biotinylated BAP-BacMPs (step c). Biotinylated BAP-BacMPs were magnetically separated and purified by stringent washing (step d). (B and C) The in vivo biotinylation of BAP-BacMPs was evaluated using ALP-labeled antibiotin antibody and luminescent substrate in a method based on enzyme-linked immunosorbent assay (B) and Western blotting (C). WT, wild type.
Immobilization of streptavidin on BAP-BacMPs biotinylated in vitro or in vivo.
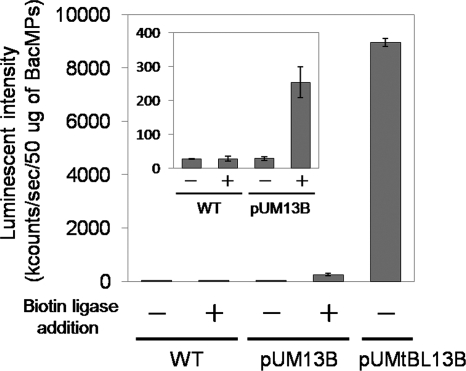
The interaction between biotin and streptavidin is enormously stable. Therefore, various functional materials can be immobilized on biotinylated BacMPs. Here, we compared the amounts of streptavidin immobilized on BAP-BacMPs biotinylated in vitro and in vivo. In vitro biotinylation was carried out using purified E. coli biotin ligase and BAP-BacMPs extracted from pUM13B transformants. Commercialized E. coli biotin ligase was added to extracted BAP-BacMPs by an optimized biotinylation method (17). ALP-labeled streptavidin was immobilized on BAP-BacMPs biotinylated in vivo and in vitro by simply mixing them. Then luminescence substrate was added and luminescence intensity was measured. In vitro biotinylation was confirmed by the observation of increased luminescence intensity compared to that of wild-type BacMPs (Fig. 5, inset); in vivo-biotinylated BAP-BacMPs showed 35-fold-greater luminescence than did in vitro-biotinylated particles (Fig. 5), indicating that the biotinylation reaction of BAP-BacMPs in the bacterial cells (in vivo biotinylation) was much more effective than that outside the cells (in vitro biotinylation). The number of streptavidins immobilized on BacMPs was quantitated using TRITC-labeled streptavidin. Approximately 700 ng of streptavidin was immobilized per 1 mg of in vivo-biotinylated BAP-BacMPs, and this amount corresponds to eight molecules of streptavidin for a single in vivo-biotinylated BAP-BacMP (molecular mass of streptavidin, 60 kDa; BacMP diameter, 75 nm; BacMP density, 5.2 g/cm3). Similar results were obtained in a previous study of in vivo biotinylation of BCCP-displaying BacMPs (17).
FIG. 5.
Immobilization of ALP-labeled streptavidin on BacMPs extracted from wild-type AMB-1 (WT) or transformants harboring pUM13B or pUMtBL13B was determined by luminescence intensity. In vitro biotinylation of BAP-BacMPs from pUM13B transformants was carried out by addition of 15 μg/ml E. coli biotin ligase, 10 μM (+)-biotin, 5 mM magnesium chloride hexahydrate, and 100 mM ATP disodium salt (biotin ligase addition).
DISCUSSION
Efficient biotinylation of BacMPs in magnetotactic bacteria was successfully demonstrated. For in vivo biotin labeling of BacMPs, E. coli biotin ligase and Mms13-BAP were overexpressed in AMB-1 cells. While in vivo biotinylation of BAP-fused proteins by heterologously expressed E. coli biotin ligase is used as a powerful tool to characterize the protein of interest in eukaryotic cells, this is the first report to apply this method to prokaryotes. Initially, full-length biotin ligase was expressed and was observed to be cytotoxic to the AMB-1 cells. Similar toxicity has been reported in E. coli when biotin ligase was overexpressed (5, 6, 22). Fusion of biotin ligase to glutathione S-transferase (GST) decreased the DNA-binding activity of biotin ligase in E. coli cells (22). We employed the truncation approach to overcome the cytotoxic effect of biotin ligase. Site-directed mutagenesis of the bifunctional biotin ligase had revealed that the C-terminal domain of E. coli biotin ligase is required for catalytic activity (6). Crystallographic analysis had also indicated that E. coli biotin ligase is composed of two domains; the N-terminal domain contains a helix-turn-helix motif for DNA binding and the C-terminal domain contains a biotin- or ATP-binding motif for biotin attachment. The N- and C-terminal domains are connected by a linker sequence (29). Based on these findings, the N-terminal domain of E. coli biotin ligase was truncated to generate biotin ligase lacking the DNA-binding activity for use in this study. Although it is still a matter for speculation that the DNA-binding domain of E. coli biotin ligase can bind to AMB-1 genomic DNA and disturb the metabolism of host cells, this truncation strategy clearly permitted growth of the transformants harboring expression vectors for E. coli biotin ligase.
Simultaneous expression of the genes encoding truncated biotin ligase and Mms13-BAP was carried out under the control of the promoter Pmms16 (30). In vivo biotinylation of Mms13-BAP on BacMPs was confirmed using ALP-labeled antibiotin antibody. In previous work, we had already demonstrated the in vitro biotin labeling of BAP-displaying BacMPs by E. coli biotin ligase in the presence of biotin, Mg2+, and ATP after purification of BAP-BacMPs from AMB-1 transformants harboring plasmid pUM13BAP, which encodes the fusion protein of Mms13 and 23 amino acid residues of BAP (17). However, the efficiency of in vitro biotinylation was quite low despite its being carried out under optimized conditions. In this study, we successfully demonstrated in vivo biotinylation of BAP-BacMPs with greater efficiency than in vitro biotinylation. This was likely due to the reaction environment for biotinylation of Mms13-BAP. In magnetotactic bacteria, synthesis of BacMPs is initiated with the invagination of the cytoplasmic membrane and with the vesicle formation which serves as the precursor of the BacMP membrane. Then, ferrous ions are accumulated in the vesicles and magnetite crystals are formed (1). In this procedure, various proteins, including Mms13, are translocated from the cytoplasmic membrane to the BacMP membrane at the invagination stage (26). For in vitro biotinylation, extracted BAP-BacMPs were mixed with purified E. coli biotin ligase, and interaction between Mms13-BAP and E. coli biotin ligase was limited on the surface of the BacMP membrane. In contrast, for in vivo biotinylation, Mms13-BAP could interact with E. coli biotin ligase not only on the surface of BacMPs but also in the cytoplasm or on the cytoplasmic membrane before the first stage of BacMP synthesis. Mms13-BAP is assumed to have a high mobility prior to translocation into the BacMP membrane in vivo, allowing for more efficient biotinylation relative to in vitro biotinylation. Actually specific biotinylation of Mms13-BAP in the cytoplasm and cytoplasmic membrane was detected by the Western blotting of each protein fraction (data not shown).
In conclusion, BacMPs were biotinylated in vivo by using the truncated form of E. coli biotin ligase and Mms13-BAP. Streptavidin was readily immobilized on biotinylated BacMPs. The conjugation system developed in this study provides a simple, low-cost method for producing biotin- and streptavidin-immobilized magnetic nanoparticles. This site-selective technique for modification of magnetic nanoparticles will be valuable in future development of functionalized magnetic conjugates. Furthermore, in vivo biotinylation of bacterial protein with coexpression of E. coli truncated biotin ligase can also be applied in basic studies, such as analysis of protein localization and protein-protein interaction in bacterial cells.
Acknowledgments
This work was funded by the Industrial Technology Research Grant Program in 2009 from the New Energy and Industrial Technology Development Organization of Japan. Y. Maeda thanks the education project Human Resource Development Program for Scientific Powerhouse through the Tokyo University of Agriculture and Technology for financial support.
Footnotes
Published ahead of print on 9 July 2010.
REFERENCES
- 1.Arakaki, A., H. Nakazawa, M. Nemoto, T. Mori, and T. Matsunaga. 2008. Formation of magnetite by bacteria and its application. J. R. Soc. Interface 5:977-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker, D. F., and A. M. Campbell. 1981. The birA gene of Escherichia coli encodes a biotin holoenzyme synthetase. J. Mol. Biol. 146:451-467. [DOI] [PubMed] [Google Scholar]
- 3.Barker, D. F., and A. M. Campbell. 1981. Genetic and biochemical characterization of the birA gene and its product: evidence for a direct role of biotin holoenzyme synthetase in repression of the biotin operon in Escherichia coli. J. Mol. Biol. 146:469-492. [DOI] [PubMed] [Google Scholar]
- 4.Beckett, D., E. Kovaleva, and P. J. Schatz. 1999. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 8:921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buoncristiani, M. R., and A. J. Otsuka. 1988. Overproduction and rapid purification of the biotin operon repressor from Escherichia coli. J. Biol. Chem. 263:1013-1016. [PubMed] [Google Scholar]
- 6.Chapman-Smith, A., T. D. Mulhern, F. Whelan, J. E. Cronan, Jr., and J. C. Wallace. 2001. The C-terminal domain of biotin protein ligase from E. coli is required for catalytic activity. Protein Sci. 10:2608-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cronan, J. E., Jr. 1990. Biotination of proteins in vivo. A post-translational modification to label, purify, and study proteins. J. Biol. Chem. 265:10327-10333. [PubMed] [Google Scholar]
- 8.de Boer, E., P. Rodriguez, E. Bonte, J. Krijgsveld, E. Katsantoni, A. Heck, F. Grosveld, and J. Strouboulis. 2003. Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 100:7480-7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Suarez, M., T. S. Chen, and A. Y. Ting. 2008. Protein-protein interaction detection in vitro and in cells by proximity biotinylation. J. Am. Chem. Soc. 130:9251-9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green, N. M. 1990. Avidin and streptavidin. Methods Enzymol. 184:51-67. [DOI] [PubMed] [Google Scholar]
- 11.Hatakeyama, K., T. Tanaka, M. Sawaguchi, A. Iwadate, Y. Mizutani, K. Sasaki, N. Tateishi, and T. Matsunaga. 2009. Microfluidic device using chemiluminescence and a DNA-arrayed thin film transistor photosensor for single nucleotide polymorphism genotyping of PCR amplicons from whole blood. Lab Chip 9:1052-1058. [DOI] [PubMed] [Google Scholar]
- 12.Howarth, M., K. Takao, Y. Hayashi, and A. Y. Ting. 2005. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl. Acad. Sci. U. S. A. 102:7583-7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosow, D. P., and M. D. Lane. 1962. Propionyl holocarboxylase formation: covalent bonding of biotin to apocarboxylase lysyl epsilon-amino groups. Biochem. Biophys. Res. Commun. 7:439-443. [DOI] [PubMed] [Google Scholar]
- 14.Larsson, C., M. Rodahl, and F. Höök. 2003. Characterization of DNA immobilization and subsequent hybridization on a 2D arrangement of streptavidin on a biotin-modified lipid bilayer supported on SiO2. Anal. Chem. 75:5080-5087. [DOI] [PubMed] [Google Scholar]
- 15.Liao, S., and N. C. Seeman. 2004. Translation of DNA signals into polymer assembly instructions. Science 306:2072-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda, Y., T. Yoshino, and T. Matsunaga. 2009. Novel nanocomposites consisting of in vivo-biotinylated bacterial magnetic particles and quantum dots for magnetic separation and fluorescent labeling of cancer cells. J. Mater. Chem. 19:6361-6366. [Google Scholar]
- 17.Maeda, Y., T. Yoshino, M. Takahashi, H. Ginya, J. Asahina, H. Tajima, and T. Matsunaga. 2008. Noncovalent immobilization of streptavidin on in vitro- and in vivo-biotinylated bacterial magnetic particles. Appl. Environ. Microbiol. 74:5139-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsunaga, T., and S. Kamiya. 1987. Use of magnetic particles isolated from magnetotactic bacteria for enzyme immobilization. Appl. Microbiol. Biotechnol. 26:328-332. [Google Scholar]
- 19.Matsunaga, T., Y. Maeda, T. Yoshino, H. Takeyama, M. Takahashi, H. Ginya, J. Aasahina, and H. Tajima. 2007. Fully automated immunoassay for detection of prostate-specific antigen using nano-magnetic beads and micro-polystyrene bead composites, ‘Beads on Beads’. Anal. Chim. Acta 597:331-339. [DOI] [PubMed] [Google Scholar]
- 20.Matsunaga, T., T. Sakaguchi, and F. Tadokoro. 1991. Magnetite formation by a magnetic bacterium capable of growing aerobically. Appl. Microbiol. Biotechnol. 35:651-655. [Google Scholar]
- 21.Nakamura, C., T. Kikuchi, J. G. Burgess, and T. Matsunaga. 1995. Iron-regulated expression and membrane localization of the magA protein in Magnetospirillum sp. strain AMB-1. J. Biochem. 118:23-27. [DOI] [PubMed] [Google Scholar]
- 22.O'Callaghan, C. A., M. F. Byford, J. R. Wyer, B. E. Willcox, B. K. Jakobsen, A. J. McMichael, and J. I. Bell. 1999. BirA enzyme: production and application in the study of membrane receptor-ligand interactions by site-specific biotinylation. Anal. Biochem. 266:9-15. [DOI] [PubMed] [Google Scholar]
- 23.Okamura, Y., H. Takeyama, T. Sekine, T. Sakaguchi, A. T. Wahyudi, R. Sato, S. Kamiya, and T. Matsunaga. 2003. Design and application of a new cryptic-plasmid-based shuttle vector for Magnetospirillum magneticum. Appl. Environ. Microbiol. 69:4274-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ooi, S. L., J. G. Henikoff, and S. Henikoff. 2010. A native chromatin purification system for epigenomic profiling in Caenorhabditis elegans. Nucleic Acids Res. 38:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schatz, P. J. 1993. Use of peptide libraries to map the substrate specificity of a peptide-modifying enzyme: a 13 residue consensus peptide specifies biotinylation in Escherichia coli. Nat. Biotechnol. 11:1138-1143. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka, M., Y. Okamura, A. Arakaki, T. Tanaka, H. Takeyama, and T. Matsunaga. 2006. Origin of magnetosome membrane: proteomic analysis of magnetosome membrane and comparison with cytoplasmic membrane. Proteomics 6:5234-5247. [DOI] [PubMed] [Google Scholar]
- 27.Viens, A., F. Harper, E. Pichard, M. Comisso, G. Pierron, and V. Ogryzko. 2008. Use of protein biotinylation in vivo for immunoelectron microscopic localization of a specific protein isoform. J. Histochem. Cytochem. 56:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, J., S. Rao, J. Chu, X. Shen, D. N. Levasseur, T. W. Theunissen, and S. H. Orkin. 2006. A protein interaction network for pluripotency of embryonic stem cells. Nature 444:364-368. [DOI] [PubMed] [Google Scholar]
- 29.Wilson, K. P., L. M. Shewchuk, R. G. Brennan, A. J. Otsuka, and B. W. Matthews. 1992. Escherichia coli biotin holoenzyme synthetase/bio repressor crystal structure delineates the biotin-and DNA-binding domains. Proc. Natl. Acad. Sci. U. S. A. 89:9257-9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshino, T., and T. Matsunaga. 2005. Development of efficient expression system for protein display on bacterial magnetic particles. Biochem. Biophys. Res. Commun. 338:1678-1681. [DOI] [PubMed] [Google Scholar]
- 31.Yoshino, T., and T. Matsunaga. 2006. Efficient and stable display of functional proteins on bacterial magnetic particles using Mms13 as a novel anchor molecule. Appl. Environ. Microbiol. 72:465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshino, T., M. Takahashi, H. Takeyama, Y. Okamura, F. Kato, and T. Matsunaga. 2004. Assembly of G protein-coupled receptors onto nano-sized bacterial magnetic particles using Mms16 as an anchor molecule. Appl. Environ. Microbiol. 70:2880-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]