Abstract
In working environments, especially in confined spaces like greenhouses, elevated concentrations of airborne microorganisms may become a problem for workers' health. Additionally, the use of microbial pest control agents (MPCAs) may increase exposure to microorganisms. The aim of this study was to investigate tomato growers' exposure to naturally occurring bioaerosol components [dust, bacteria, fungi, actinomycetes, (1→3)-β-d-glucans, and endotoxin] and MPCAs applied by drip irrigation. Airborne dust was collected with filter samplers and analyzed for microorganisms by plate counts and total counts using a microscope. Analysis of (1→3)-β-d-glucan and endotoxin content was performed by kinetic, chromatic Limulus amoebocyte lysate tests. The fungal strain (Trichoderma harzianum) from the biocontrol product Supresivit was identified by PCR analysis. Measurements were performed on the day of drip irrigation and 1 week, 1 month, and 3 months after the irrigation. T. harzianum from Supresivit could be detected only on the day of treatment. Streptomyces griseoviridis, an applied MPCA, was not detected in the air during this investigation. We found that bioaerosol exposure increases during the growth season and that exposure to fungi, bacteria, and endotoxin can reach levels during the harvest period that may cause respiratory symptoms in growers. The collected data indicate that MPCAs applied by drip irrigation do not become airborne later in the season.
In greenhouses, the growth conditions for plants are optimized to increase crop yield. However, the confined environment can increase growers' exposure to bioaerosols (fungal spores, bacteria, pollen, etc.) and agents for plant protection (25). It is well documented that workers in agriculture are exposed to high concentrations of airborne microorganisms due to the handling of organic materials (31, 52), but less is known about greenhouse workers' exposure.
Inhalation of microorganisms can cause respiratory symptoms and lung function impairment (2, 4, 10, 11, 29, 56). The components (1→3)-β-d-glucan (β-glucan) and endotoxin are found to be associated with respiratory symptoms as well (8, 40, 41). Endotoxin is found predominantly on the outer surface of Gram-negative bacteria, and β-glucans are a part of the cell wall in fungi, as well as in some plants. There are strong indications that inhalation of propagules from the actinomycete Streptomyces may evoke an immunological response leading to respiratory diseases (16). Occupational asthma and rhinitis have been reported for greenhouse workers sensitized to workplace flowers or molds (42, 43, 45, 46). Another health concern is that some fungal species, e.g., the thermophilic Aspergillus fumigatus, can infect immunosuppressed people (30).
Microbial pest control agents (MPCAs) are species of microorganisms that can suppress plant pests and pathogens (5). Application of biocontrol products that contain MPCAs may increase growers' exposure to the microbial agent (9, 19, 26, 27). The biocontrol product Supresivit contains the ascomycete Trichoderma harzianum (Rifai 1969) which belongs to the family Hypocreaceae. The genus Trichoderma is beneficial in vegetable production due to its ability to outcompete plant-pathogenic microorganisms. It is also a parasite on other fungi and can induce systemic and localized resistance to pathogens in plants (21). T. harzianum is applied to foliage, seeds, or soil but can also be applied to plant wounds as a paste. Another fungal species used in biocontrol products is, e.g., Beauveria bassiana, which is applied by soil treatment or spraying. The biocontrol product Mycostop contains the actinomycete Streptomyces griseoviridis strain K61. Actinomycetes are Gram-positive bacteria that reproduce through spore release. S. griseoviridis can suppress a range of seed- and soilborne fungal pathogens through the release of antibiotics (28).
The aim of this study was to investigate growers' exposure to MPCAs and other bioaerosol components during the growth season of greenhouse tomatoes. We monitored the exposure to dust, microorganisms, endotoxin, and β-glucans from the time of application of MPCAs by drip irrigation in late winter to tomato harvest in late spring.
MATERIALS AND METHODS
Greenhouse environment.
The investigation was conducted in a tomato greenhouse (10,000 m2) at a producer of organic vegetables. In a previous study, the producer was labeled T-3 (19). Investigations in the greenhouse were planned after agreement by the vegetable producer. Tomato plants had been supplied by a plant nursery and transplanted into plastic-covered loam soil in the greenhouse. Plants were treated with T. harzianum prior to arrival at the greenhouse. On the 1st sampling day (20 February 2008), the biocontrol products Supresivit and Mycostop were applied to the plant roots by drip irrigation. The active organisms of the biocontrol products were termed T. harzianum Su and S. griseoviridis My. MPCAs were prepared as follows: one bag of Supresivit (500 g, 1.4 × 1010 spores g−1; Fytovita Ltd., Prague, Czech Republic) and eight bags of Mycostop (25 g, >108 CFU g−1; Verdera, Espoo, Finland) were mixed with 10 liters of water and poured into the watering tank connected to the irrigation system. The mixing was performed by one of the growers in a separate room connected to the greenhouse. Inside the greenhouse, air sampling was performed for 50 min prior to initiation of drip irrigation and for 6 h after the initiation. Windows were closed the entire working day.
Measurements of bioaerosols were performed again 1 week (26 February 2008), 1 month (28 March 2008), and 3 months (20 May 2008) after the 1st sampling day. On the 2nd and 3rd sampling days, the windows were opened slightly at times. On the 4th sampling day, the windows were fully open the entire working day. On the first three sampling days, four or five growers were nurturing young plants in the greenhouse. On the last sampling day, nine growers were working in the greenhouse, primarily with harvesting. The sampling days and number of samples are given in Table 1.
TABLE 1.
Air samples collected in the tomato greenhouse
| Sampling day and details | Main taska | No. of samples collected |
|
|---|---|---|---|
| Personal | Stationary | ||
| 1st, 20 February 2008 | Nurturing | ||
| Prior to MPCA application | 4 | ||
| During handling of MPCAs | 1 | 1 | |
| Post-MPCA application | 4 | 3 | |
| 2nd, 26 February 2008 | Nurturing | 4 | |
| 3rd, 18 March 2008 | Nurturing | 5 | |
| 4th, 20 May 2008 | Harvesting | 9 | 3 |
Main task of growers on the day of measurement.
Air sampling.
To sample inhalable dust, personal samplers (GSP; CIS inhalable sampler [BGI, Waltham, MA]) were attached to the growers' clothing. Airflow was adjusted to 3.5 liters min−1. With the exception of the first sampling day, during which the samplings were split into the time “prior to” and the time “after” the application of MPCAs, all samplings were done throughout the working day (approximately 6 h total of sampling). The personal samplers were turned off twice a day due to scheduled breaks.
Stationary air sampling of “total dust” was performed simultaneously with personal air sampling on the 1st and 4th sampling day. Total dust was sampled using 25-mm closed-face cassettes (Millipore, Bedford, MA). Airflow was adjusted to 1.9 liters min−1, corresponding to an inlet velocity of 1.25 m s−1. The samplers were placed between plant rows 1.5 m above ground. Because the growers moved about, the distance varied between stationary samplers and the work activities. For outdoor reference measurements, stationary samplers were placed upwind from the greenhouse. Reference measurements were performed on the 1st and 4th sampling days (once on each day).
Personal and stationary samplers were mounted with a polycarbonate filter (pore size, 1 μm; Osmonics Inc., Minnetonka, MN) for culturable counts, total counts, and quantification of β-glucans, or a Teflon filter (pore size, 1 μm; Millipore, Bedford, MA) for gravimetric analysis of dust and quantification of endotoxin. Each grower and each stationary sampling station carried a polycarbonate filter and a Teflon filter.
Gravimetric and endotoxin analysis.
Dust mass was quantified by gravimetric analysis of Teflon filters. Prior to being weighed, filters were acclimatized for 12 to 24 h at constant air temperature and humidity. For personal samplers, the limit of detection was 0.028 mg m−3, and for stationary samplers, it was 0.022 mg m−3. After gravimetric analysis, dust was extracted by orbital shaking in 6 ml pyrogen-free water with 0.05% Tween 20 (300 rpm) at room temperature for 60 min and centrifugation (1,000 × g) for 15 min. Prior to endotoxin analysis, the supernatant was stored at −80°C. Analysis was performed in duplicate with the kinetic, chromatic Limulus amoebocyte lysate test (Kinetic-QCL endotoxin kit; Biowhittaker, Walkersville, MD) with β-glucan blocker as described by the manufacturer. The detection limit was 0.05 endotoxin units (EU) ml−1.
Analysis of β-glucans.
Extracts from polycarbonate filters were analyzed in duplicate for β-glucans using the kinetic, chromatic Fungitic G test (Seikaga Co., Tokyo, Japan). The triple-helix structure of the β-glucans was made water soluble by adding 0.3 M NaOH (14) and incubating the β-glucans for 10 min. The detection level (DL) was 4 ng ml−1.
Quantification of culturable microorganisms.
The day after sampling, dust was extracted and resuspended in 10 ml (personal samples) or in 6 ml (stationary samples) of sterile solution (0.05% Tween 80, 0.85% NaCl) by orbital shaking for 15 min (500 rpm) at room temperature. Part of the extract was kept at −80°C for β-glucan and PCR analyses. Tenfold dilution series were prepared, and 100-μl aliquots were plated on agar plates.
Measurements of mesophilic fungi and A. fumigatus were obtained on DG-18 plates (DG-18 agar; Oxoid, Basingstoke, England) with chloramphenicol (100 mg liter−1; Oxoid, Hampshire, United Kingdom) at 25°C and 45°C, respectively. The numbers of CFU of Trichoderma sp. were quantified on THSM (T. harzianum selective medium) after incubation at 22°C for 5 to 10 days as described by Williams et al. (55). This medium contains a range of antibiotics with both antibacterial and antifungal activities, increasing the specificity toward Trichoderma spp. Colonies with different morphological appearances grew on the THSM plates. Hence, specific PCR amplification of DNA was used for final identification. Measurements of Beauveria spp. were obtained on selective media under conditions described previously (39).
Measurements of mesophilic bacteria and thermophilic actinomycetes were obtained by plate counts on nutrient agar (Oxoid, Basingstoke, England) with cycloheximide (50 mg liter−1; Applichem, Darmstadt, Germany) incubated at 25°C and 55°C, respectively. Quantifications of airborne bacteria have been reported previously from this greenhouse (19).
Mesophilic actinomycetes were quantified on 10% nutrient agar with cycloheximide (50 mg liter−1) supplemented with 15 g agar (Fisher Scientific, Leicestershire, United Kingdom) and incubated at 25°C. S. griseoviridis was cultivated under the same conditions as mesophilic actinomycetes. All agar plates, with the exception of THSM plates (see above), were incubated for 7 days before CFU were counted.
Visual identification of S. griseoviridis My was performed by comparing colonies of mesophilic actinomycetes from samples with culturable S. griseoviridis obtained from Mycostop. The detection levels for culturable microorganisms sampled for 6 h were 80 CFU m−3 for personal samplers and 88 CFU m−3 for stationary samplers.
Total counts of bacteria and fungi.
Total counts of airborne bacteria and fungi were performed after staining the nucleic acids in the cells with 20 ppm acridine orange (Difco, Sparks, MD) in acetate buffer for 30 s with subsequent filtration through a polycarbonate filter (0.4 μm; Nuclepore, Cambridge, MA). Bacteria and fungi were counted at a magnification of ×1,250 using epifluorescence microscopy (Orthoplan; Leitz, Wetzlar, Germany). The microorganisms were distinguished from each other by morphology and size; particles with sizes of 0.8 to 1.8 μm were defined as bacterial cells, and particles with sizes of 1.8 to 10 μm were defined as fungal spores. Only personal samples were investigated by microscopy.
Identification of Trichoderma harzianum Su by PCR analysis.
DNA from fungal isolates and air samples was extracted by a freeze-thaw-boil technique, in which −80°C samples were thawed to room temperature and then heated at 100°C for 10 min. After centrifugation at 15,000 × g for 10 min, the supernatant was recovered and used for PCR. However, for comparison of extraction efficiencies, DNA was extracted from a few samples using the FastDNA Spin kit for soil (Q-Biogene, Carlsbad, CA). The MoBio PowerClean DNA cleanup kit (Mo Bio Laboratories, Inc., Carlsbad, CA) was used for cleaning DNA extracted by the freeze-thaw-boil technique where poor results were obtained. Extracted DNA was stored at −80°C.
Fungal isolates were identified as T. harzianum by PCR analysis of extracted DNA by using the primers described by Hagn et al. (17) The PCR conditions were as follows. A PCR tube contained 25 μl of PCR mix [13.25 μl DNase-free water, 2.5 μl of MgCl2 (25 mM), 2.5 μl 10× PCR buffer with 200 mM (NH4)2SO4 (Fermentas, St. Leon-Rot, Germany), 2.5 μl deoxynucleoside triphosphate (0.25 mM) (Fermentas, St. Leon-Rot, Germany), 1 μl each of primers (100 pM) uTf and uTr (17) (Eurofins MWG Operon, Ebersberg, Germany), 1.25 μl Taq DNA polymerase (1U μl−1; Fermentas, St. Leon-Rot, Germany)]. For the template, 1 μl DNA extract was added. PCR conditions were optimized at 3 min at 95°C; 30 cycles of 30 s at 94°C, 30 s at 58°C, 30 s at 72°C; 10 min at 72°C; and final cooling at 4°C.
In order to further identify the T. harzianum Su strain present, specific PCR primers were developed. Initially, the Trichoderma-specific fragment amplified with primers uTf/uTr was sequenced and compared to published sequences using BLAST software (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The analysis showed that this sequence was not sufficiently unique to construct robust primers. Using the random amplified polymorphic DNA (RAPD) method (20) with the random primer OPA03 (RAPD 10mer kit; Eurofins MWG Operon, Ebersberg, Germany), a fragment unique to T. harzianum Su of approximately 650 bp was identified, cloned using the TOPO TA cloning kit (no. K4500-01; Invitrogen A/S, Taastrup, Denmark), and sequenced by Eurofins MWG Operon (Ebersberg, Germany). Based on the sequence and by use of the primer3 software (http://sourceforge.net/projects/primer3/files/), specific primers were identified as the forward primer SuOPA03+F (5′-AGTCAGCCACGAACATAGAAGG-3′) and reverse primer SuOPA03+R (5′-AGTCAGCCACCTCAGCAATATG-3′). The master mix and PCR conditions were as described for uTf/uTr except that the annealing temperature was 69°C. The primer set was found to be specific to T. harzianum Su when tested against 12 Trichoderma strains of which five were T. harzianum, as well as 15 other closely related fungal species (36). This primer set was used to further identify T. harzianum isolates to the level of T. harzianum Su.
T. harzianum and T. harzianum Su were also searched for directly in DNA extracted from air samples by using the above-mentioned primer sets and by nested PCR. In brief, by nested PCR, a large fragment of DNA was initially amplified using the general Trichoderma primer Tri18S-F and the reverse primer uTr (17). Each PCR tube contained the same PCR mix as described above, except that primer Tri18S-F (Eurofins MWG Operon, Ebersberg, Germany) replaced uTf and the annealing temperature was 65°C. The PCR product was purified using the Qiaquick PCR purification kit (Qiagen, Copenhagen, Denmark) prior to the second PCR analysis with the primers uTf/uTr targeting T. harzianum. PCR products were determined by gel electrophoresis.
Treatment of data.
Measurements based on personal samplings were termed “exposures,” and measurements based on stationary samplings were termed “concentrations.” Both were calculated as time-weighted averages. The median value and the multiplicative standard deviation (s*) (32) were calculated for each bioaerosol component on each day and are presented together with the range of values for the bioaerosol component. Exposure and concentration data for dust, endotoxin, β-glucans, and microorganisms were logarithmically transformed for statistical analysis to approximate normality. For statistical analysis, values below the detection level were replaced by half of the value of the calculated detection level. Statistical analysis (Proc GLM) was performed using SAS software.
RESULTS
Exposure to biocontrol products during the growth season.
The exposure levels to bioaerosols other than MPCAs during preparation of biocontrol products are given in Table 2. PCR analysis confirmed that T. harzianum Su was detectable during the preparation of MPCAs (Table 3). After the initiation of irrigation, two of the four growers were also found to be exposed to T. harzianum Su (Table 3). Culturable T. harzianum Su was also detected by a stationary air sampler in the greenhouse (65 CFU m−3). The presence of T. harzianum Su could be confirmed neither in uncultured samples collected during preparation of MPCAs nor in the personal or stationary samplers where spores of T. harzianum Su had been detected on THSM. It was also not possible to detect DNA from T. harzianum Su in any other samples. No other Trichoderma strains were detected in the greenhouse. Culturable S. griseoviridis My was detected neither in the air during preparation of MPCAs nor at any other time during this study (data not shown).
TABLE 2.
Measurements of bioaerosol components other than Trichoderma sp. during the preparation of biocontrol products and inside the greenhouse during working hours
| Parameter | Value(s) determined ona: |
|||
|---|---|---|---|---|
| 1st sampling day (20 February 2008) |
4th sampling day (20 May 2008), harvesting tomatoes, stationary samplersd (n = 3) | |||
| Preparing MPCAs, personal samplerb (n = 1) | Preparing MPCAs, stationary samplerc (n = 1) | Nurturing plants, stationary samplersd (n = 3) | ||
| Dust (mg m−3) | 0.71 | 0.072 | 0.031 (0.018-0.047) (0.63) | 0.098 (0.079-0.13) (1.25) |
| Fungi | ||||
| β-glucans (ng m−3) | 44 | <DL | ND | ND |
| Total fungi (spores m−3) | 1.4 × 105 | ND | ND | ND |
| Culturable, mesophilic fungi (CFU m−3) | 1.2 × 103 | 7.9 × 103 | 2.0 × 103 (830-3.2 × 103) (1.99) | 3.7 × 104 (4.2 × 103-4.3 × 104)(3.68) |
| Beauveria sp. (CFU m−3) | <DL | <DL | <DL | <DL |
| Bacteria | ||||
| Endotoxin (EU m−3) | 31 | 1.7 | 2.7 (1.3-3.5) (1.67) | ND |
| Total bacteria (cell m−3) | 4.9 × 104 | ND | ND | ND |
| Culturable, mesophilic bacteria (CFU m−3) | 9.3 × 103 | 1.2 × 103 | 250 (<DL-310) (2.92) | 110 (<DL-150) (1.89) |
| Actinomycetes | ||||
| Culturable, mesophilic actinomycetes (CFU m−3) | 190 | 74 | 46 (<DL-170) (2.22) | <DL |
| Culturable, thermophilic actinomycetes (CFU m−3) | 1.3 × 103 | 900 | <DL (<DL-84) (1.54) | <DL |
Values for stationary samplers during nurturing of plants and harvesting are medians, with ranges shown in the first set of parentheses and the multiplicative standard deviation (s*) in the second. ND, not determined.
<DL, below the detection level of 650 CFU m−3 for 44 min of personal sampling.
<DL, below the detection level of 357 CFU m−3 for 44 min of stationary sampling.
<DL, below the detection level of 88 CFU m−3 for 6 h of stationary sampling.
TABLE 3.
Exposure to T. harzianum Su from application of Supresivit to harvest of tomatoes
| Time of sampling and grower's activity | Type of samplera | No. of positive samples/total no. of samples | No. of CFU m−3, median (range) (s*) |
|---|---|---|---|
| 1st sampling day (20 February, 2008) | |||
| Nurturing plants in the greenhouse (prior to application of Supresivit) | P | 0/4 | <DLc |
| Preparing Supresivitb in separate room | P | 1/1 | 1.0 × 105 |
| Preparing Supresivitb in separate room | S | 1/1 | 3.7 × 103 |
| Nurturing plants in the greenhouse (after application of Supresivit) | Pb | 2/4 | 51 (<DLd-290) (4.74) |
| 2nd sampling day (26 February 2008); nurturing plants in the greenhouse | P | 0/5 | <DLe |
| 3rd sampling day (18 March 2008); nurturing plants in the greenhouse | P | 0/5 | <DLe |
| 4th sampling day (20 May 2008); harvesting tomatoes in the greenhouse | P | 0/9 | <DLe |
P, personal; S, stationary.
Preparing Supresivit involved mixing Supresivit with water.
DL = 171 CFU m−3 for 50 min of personal sampling.
DL = 29 CFU m−3 for 5 h of personal sampling.
DL = 24 CFU m−3 for 6 h of personal sampling.
Exposure to other bioaerosol components during the growth season.
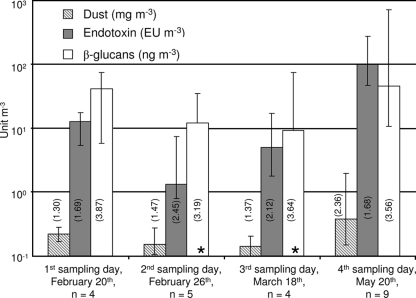
Growers' exposure levels to dust were not significantly different on the first three sampling days nor did the exposures on the 1st (0.22 mg m−3) and 4th (0.38 mg m−3) sampling day differ significantly (P < 0.05) (Fig. 1). The highest dust exposure (1.9 mg m−3) was observed for a grower picking tomatoes.
FIG. 1.
Exposure to dust, endotoxin, and β-glucans (personal samples). Note that units differ for each parameter. Columns show median exposures, bars show ranges, and multiplicative standard deviations are shown in parentheses. Asterisks indicate that the lowest value of exposure was below the detection level (4 ng m−3).
Endotoxin exposures did not differ significantly on the first three sampling days; however, an increase from 13 EU m−3 to 100 EU m−3 was observed between the 1st and the 4th sampling day (P < 0.05). The highest endotoxin exposure (270 EU m−3) was detected for a grower harvesting tomatoes.
β-Glucan exposures did not show any significant differences during the investigated period (P = 0.21), and exposures were approximately 43 ng m−3 (Fig. 1). The highest exposure (710 ng m−3) was detected for a grower picking tomatoes.
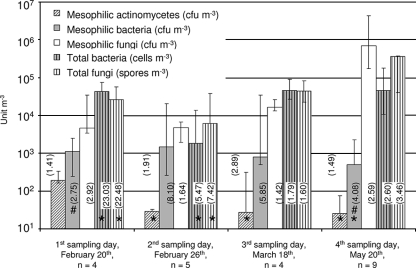
Culturable, mesophilic actinomycetes constituted the smallest fraction of the airborne biota (Fig. 2). The highest exposure, with a median value of 189 CFU m−3, was seen on the 1st sampling day, followed by a significant decrease to a median value of approximately 27 CFU m−3 the following three sampling days (P < 0.05). The highest exposure was 333 CFU m−3 (Fig. 2). Exposures to culturable, thermophilic actinomycetes were measured only on the 1st and 4th sampling days and were not significantly different from each other (P = 0.76). The median exposure was 130 CFU m−3, and exposures ranged from below the detection level to 519 CFU m−3 (data not shown).
FIG. 2.
Exposure to actinomycetes, bacteria, and fungi (personal samples). Columns show median exposures, bars show ranges, and multiplicative standard deviations are shown in parentheses. Asterisks indicate that the lowest exposure was below the detection level (mesophilic actinomycetes and mesophilic bacteria, 40 CFU m−3; total bacteria and total fungi, 500 CFU m−3). #, data from Hansen et al. (19).
Counts by microscopy and plate counts revealed that fungi were the dominant microorganism in the air (Fig. 2). Penicillium and Cladosporium spp. were observed to be the most prevalent fungal species. Similar to the results for dust, the exposures to fungi were not significantly different on the first three sampling days. The median exposures to culturable fungi were 5 × 103 CFU m−3 on the first two sampling days. On the 4th sampling day, the median exposure had increased to 6.9 × 105 CFU m−3 (P < 0.05). The median exposure to A. fumigatus was below the detection level on the 1st as well as the 2nd sampling day (data not shown). However, exposure reached as high as 88 CFU m−3 on the mentioned sampling days. Beauveria spp. were not detected by personal samplers (data not shown). Analysis of samples by microscopy showed that the median exposures to total fungi ranged from 2.6 × 104 spores m−3 to 3.7 × 105 spores m−3.
The exposures to culturable bacteria on the sampling days did not differ from each other (P = 0.21) and had an average value of 960 CFU m−3. However, exposure was observed to reach 3.4 × 104 CFU m−3 for a grower nurturing plants on the 3rd sampling day (Fig. 2). Cycloheximide-resistant fungi were prevalent on the agar plates for bacterial counts. The total count of bacteria showed that exposure to bacteria on the 2nd sampling day was less than the exposures on the 3rd and the 4th sampling day (P < 0.05). Besides this difference, bacterial exposures did not differ on the sampling days (Fig. 2).
Bioaerosol components measured by stationary samplers inside and outside the greenhouse.
Stationary samples were collected only on the 1st and 4th sampling days (Table 2). The concentrations of dust and fungal spores increased (P < 0.05) between the two sampling days. The concentrations of bacteria were at the same level on the investigated days. The concentrations of airborne mesophilic and thermophilic actinomycetes decreased from a detectable level on the 1st sampling day to below the detection level on the 4th sampling day.
The dust concentration measured by stationary samplers inside the greenhouse was significant lower than the exposure measured by personal samplers (P < 0.05). This difference was not observed for culturable fungi and bacteria (P = 0.08 and 0.50, respectively).
In outdoor reference samples (data not shown), the concentrations of culturable bacteria and mesophilic actinomycetes were below the detection level on the 1st and 4th sampling days. For culturable fungi, the concentration was below the detection limit on the 1st sampling day and 3.4 × 103 CFU m−3on the 4th sampling day.
DISCUSSION
The grower preparing MPCAs was exposed to a measurable level of T. harzianum Su, while the exposure to S. griseoviridis My was below the detection level. This difference is expected to be due to the packaging and formulation of the products. Also, the concentration of MPCA in the products and the amount of product used have likely influenced the exposure level. On the day of application of the biocontrol products, four growers worked in the greenhouse, but only in two growers' samplers did we detect T. harzianum Su. The exposure may have occurred in the greenhouse or in the room where the biocontrol products had been prepared. In the latter case, it would have been after the MPCAs had been prepared for drip irrigation. T. harzianum Su was also isolated from a stationary sampler in the greenhouse, showing that MPCAs can become airborne and be transported about on a day of treatment by irrigation. However, the exposure levels must be considered to be low and are less than the no-effect level of 3.5 × 105 spores m−3 determined in a short-term human inhalation study (38). T. harzianum Su was not detected for the rest of the investigation period. S. griseoviridis My was likewise not detected from February to May.
The results suggest that microorganisms applied by drip irrigation generally do not become airborne in high concentrations after application. Covering the soil with plastic has probably helped to limit the MPCAs in the air.
The application method and the ability of MPCAs to become airborne are important parameters for determining the risk of exposure during work. Previous studies have shown growers to be exposed to Bacillus thuringiensis applied by spraying (19, 26, 27). The three biocontrol organisms included in this study (T. harzianum, Beauveria sp., and S. griseoviridis) were not observed to be a natural part of the airborne microbiota in the greenhouse. This indicates that tomato growers are not exposed to these organisms, unless biocontrol products are used. A review study has shown that Trichoderma spp. can be detected frequently in agricultural settings but only in low concentrations (<15 CFU m−3) (35).
As plants matured, the growers' exposure to bioaerosols increased. However, on the first three sampling days, the exposure levels to dust, endotoxin, β-glucans, fungi, and bacteria were stable. A decrease could be observed on the 2nd and 3rd sampling days, but this was significant only for dust. The decrease was likely due to the opening of windows in the ceiling. The highest exposure to dust and microbial particles was measured in late May even though the windows were open throughout the working day. This is likely due to an increase in the microbiota together with accumulation of dead microorganisms, which counterbalances the effect of the open windows.
Exposure to dust during harvest was lower than the Danish Occupational Exposure Limit (OEL) of 3 mg m−3 (1) by a factor of 9. The exposure was similar to or lower than exposures in other vegetable-producing greenhouses (37, 51). Madsen et al. reported dust exposure to be 0.46 mg m−3 and 2.3 mg m−3 during harvest and clearing of tomato plants in greenhouses (n = 2), respectively (37). However, in cucumber greenhouses (n = 2), exposures ranged from 0.86 to 12 mg m−3 during harvest (37). In flower greenhouses, exposures have been quantified to be lower than those in tomato and cucumber greenhouses (37, 43, 45). The variations observed between producers of different crops may be due to differences in plant sizes and leaf morphologies. Mature tomato plants are tall, and the release of settled dust from above is likely to increase the growers' exposure. As reported for cucumber greenhouses (37), the concentration of dust measured by stationary samplers was significantly lower than the exposure measured by personal samplers. This indicates that the working activities between the rows of plants do increase the level of airborne particles.
Exposure to endotoxin increased by a factor of 8 from late winter to late spring. The endotoxin exposure during tomato harvest (range = 0.79 to 270 EU m−3) was at the same level as that previously reported for tomato production (37, 51). Even though the highest exposures to endotoxin in cucumber production (37, 51) were found to reach twice the maximum exposure found in this study, the median exposures in cucumber greenhouses are similar to the median exposures in the tomato greenhouse.
In flower greenhouses, endotoxin exposures are most often lower (1.5- to 18-fold) than the exposure reported here (33, 43, 45, 51). Higher exposures, however, have been detected in chicory and flower bulb nurseries (51). There is no internationally accepted threshold limit value (TLV) for endotoxin. Suggested TLVs or calculated “no-effect values” for inhalable or “total” endotoxin exposure are between 30 and 800 EU m−3 (37). In this study, the exposure to endotoxin will be related to a calculated “no-effect level” of 150 EU m−3 for Dutch animal feed workers (49, 50). On the first 3 days, the exposure to endotoxin did not exceed 150 EU m−3 (n = 13). During harvest, 22% of growers were exposed to endotoxin levels exceeding 150 EU m−3, with no exposures exceeding 800 EU m−3 (n = 9).
Airborne β-glucans have not previously been measured in greenhouses. In this study, we report the β-glucan exposure during harvest to range between 11 and 710 ng m−3. During nurturing of plants in late winter, the exposure ranged between 6 and 74 ng m−3. Measurements in other environments have shown various concentrations of β-glucans (12, 47, 48). For waste collectors, the exposure to β-glucans is similar or less than what we observed for tomato growers (15, 23, 53). β-Glucans have been shown to have inflammatory properties (7, 24). However, it has not been possible to reproduce these effects consistently in human inhalation studies, and no conclusions on the association between β-glucan exposure and effects can be drawn at this point (7).
Exposure to fungi is higher in the tomato greenhouse than in flower greenhouses (43, 45). For the stationary samplers, the fungal concentration was found to be 10-fold higher inside the tomato greenhouse than outside. In a Venlo-type tomato greenhouse, stationary samplings showed fungal concentrations inside and outside the greenhouse to be the same (44). Penicillium and Cladosporium spp. were the most frequent fungal species in this study, corresponding with findings from other greenhouses (6, 43-45). A study group has suggested that fungal concentrations above 104 CFU m−3 in total or above 500 CFU m−3 for a specific group of potential pathogenic species should be considered a threat to workers' health (22). For Penicillium spp., the lowest observed level of effect has been reported to be 104 spores m−3 (38). Based on a large review study, Eduard (10) suggests that an exposure level of 105 spores m−3 would be an appropriate baseline for an OEL for spores from various fungi, although this is probably too high if spores from mycotoxin-producing or pathogenic species are prevalent (10). On the first three sampling days, 2 of 13 growers were exposed to more than 104 CFU m−3 of fungi. However, during harvest, all growers were exposed to levels above 105 CFU m−3.
Enya et al. have shown that the populations of bacteria on tomato plants increase as the plants mature (13). However, exposures to bacteria did not differ significantly from late winter to late spring in this study. Bacteria are sensitive to unfavorable conditions (3, 54) and might therefore not accumulate. Bacterial cells may also have been suppressed by cycloheximide-resistant fungi growing on the plates used for bacterial CFU counts. In another investigation, tomato growers were exposed to 310 CFU m−3 and 100 CFU m−3 during harvest activities and 5.3 × 104 CFU m−3 and 1.4 × 104 CFU m−3 during clearing of old plants in the same greenhouses (19). Higher exposures to bacteria have been observed in flower greenhouses (43, 45). An internationally accepted OEL for bacteria has not been determined. However, threshold limit values (TLV) have been suggested for a mixed bacterial population (104 CFU m−3) and for Gram-negative bacteria (103 CFU m−3, due to the production of endotoxin) (22). In this study 10% of the growers were exposed to bacterial levels above 104 CFU m−3 and 38% of the growers were exposed to bacterial levels above 103 CFU m−3 (n = 22). The prevalence of Gram-negative bacteria was not quantified here.
In the tomato greenhouse, the highest exposures to mesophilic actinomycetes were detected early in the year. However, the exposure was well below the Russian maximum allowable concentration of 103 to 104 CFU m−3 for specific species of actinomycetes (10). Growers' exposure to mesophilic actinomycetes, as well as thermophilic actinomycetes, is low compared to the exposures reported from other occupational settings where organic matter is handled (18, 34).
Vegetable production is a seasonal occupation where the workforce is adjusted according to the labor need. In this study, growers' exposure to bioaerosols increased during the growth season and exposures to fungi, bacteria, and endotoxin reached levels that may cause health symptoms during the high-labor-demand harvest period. It is possible that even higher exposures can be observed later in the growth season. The data show that growers in tomato greenhouses are not exposed to high concentrations of bioaerosols all year. This makes it more complicated to investigate the health effects of working in tomato greenhouses. Short-term exposure to elevated concentrations of bioaerosols is, however, also a health risk and should be avoided (11). In this study, personal samplers were a valuable tool to investigate growers' exposure to airborne MPCAs. The collected data indicate that MPCAs applied by drip irrigation do not become airborne later in the season.
Acknowledgments
We thank Tina Olsen, Signe Nielsen, Anne-Grethe Holm-Jensen, and Tina Thrane for technical assistance and Kira Tendal for assistance with copyediting.
This research was partly funded by the Danish Environmental Protection Agency. No other funding from outside sources was received.
Footnotes
Published ahead of print on 9 July 2010.
REFERENCES
- 1.Anonymous. 2007. At-vejledning, stoffer og materialer C.0.1, Grænseværdier for stoffer og materialer, August 2007, p. 51. (DWEA—Guide, C.0.1 Limit values for substances and materials.) Danish Working Environment Authority (Arbejdstilsynet), Copenhagen, Denmark. http://www.at.dk/REGLER/At-vejledninger-mv/Stoffer-og-materialer/At-vejledninger-om-stoffer-og-materialer/C0-Generelt-og-diverse/PDF-C01-Graensevaerdi-for-stoffer-og-mat.aspx?sc_lang-da.
- 2.Atkinson, R. W., D. P. Strachan, H. R. Anderson, S. Hajat, and J. Emberlin. 2006. Temporal associations between daily counts of fungal spores and asthma exacerbations. Occup. Environ. Med. 63:580-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bale, M. J., P. M. Bennett, J. E. Beringer, and M. Hinton. 1993. The survival of bacteria exposed to desiccation on surfaces associated with farm buildings. J. Appl. Bacteriol. 75:519-528. [DOI] [PubMed] [Google Scholar]
- 4.Bush, R. K., J. M. Portnoy, A. Saxon, A. I. Terr, and R. A. Wood. 2006. The medical effects of mold exposure. J. Allergy Clin. Immunol. 117:326-333. [DOI] [PubMed] [Google Scholar]
- 5.Copping, L. G., and J. J. Menn. 2000. Biopesticides: a review of their action, applications and efficacy. Pest Manag. Sci. 56:651-676. [Google Scholar]
- 6.Davies, P. D. O., R. Jacobs, J. Mullins, and B. H. Davies. 1988. Occupational asthma in tomato growers following an outbreak of the fungus Verticillium albo-atrum in the crop. J. Soc. Occup. Med. 38:13-17. [DOI] [PubMed] [Google Scholar]
- 7.Douwes, J. 2005. (1→3)-β-d-glucans and respiratory health: a review of the scientific evidence. Indoor Air 15:160-169. [DOI] [PubMed] [Google Scholar]
- 8.Douwes, J., P. Thorne, N. Pearce, and D. Heederik. 2003. Bioaerosol health effects and exposure assessment: progress and prospects. Ann. Occup. Hyg. 47:187-200. [DOI] [PubMed] [Google Scholar]
- 9.Eaton, K. K., T. J. Hennessy, D. J. Snodin, and D. W. McNulty. 1986. Verticillium lecanii. Allergological and toxicological studies on work exposed personnel. Ann. Occup. Hyg. 30:209-217. [DOI] [PubMed] [Google Scholar]
- 10.Eduard, W. 2009. Fungal spores: a critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit. Rev. Toxicol. 39:799-864. [DOI] [PubMed] [Google Scholar]
- 11.Eduard, W., J. Douwes, R. Mehl, D. Heederik, and E. Melbostad. 2001. Short term exposure to airborne microbial agents during farm work: exposure-response relations with eye and respiratory symptoms. Occup. Environ. Med. 58:113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elfman, L., M. Riihimaki, J. Pringle, and R. Walinder. 2009. Influence of horse stable environment on human airways. J. Occup. Med. Toxicol. 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enya, J., H. Shinohara, S. Yoshida, T. T. H. Negishi, K. Suyama, and S. Tsushima. 2007. Culturable leaf-associated bacteria on tomato plants and their potential as biological control agents. Microb. Ecol. 53:524-536. [DOI] [PubMed] [Google Scholar]
- 14.Fogelmark, B., J. Thorn, and R. Rylander. 2001. Inhalation of (1→3)-beta-d-glucan causes airway eosinophilia. Mediators Inflamm. 10:13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gladding, T., J. Thorn, and D. Stott. 2003. Organic dust exposure and work-related effects among recycling workers. Am. J. Ind. Med. 43:584-591. [DOI] [PubMed] [Google Scholar]
- 16.Górny, R. L., G. Mainelis, S. A. Grinshpun, K. Willeke, J. Dutkiewicz, and T. Reponen. 2003. Release of Streptomyces albus propagules from contaminated surfaces. Environ. Res. 91:45-53. [DOI] [PubMed] [Google Scholar]
- 17.Hagn, A., S. Wallisch, V. Radl, J. C. Munch, and M. Schloter. 2007. A new cultivation independent approach to detect and monitor common Trichoderma species in soils. J. Microbiol. Methods 69:86-92. [DOI] [PubMed] [Google Scholar]
- 18.Halstensen, A. S., K. C. Nordby, I. M. Wouters, and W. Eduard. 2007. Determinants of microbial exposure in grain farming. Ann. Occup. Hyg. 51:581-592. [DOI] [PubMed] [Google Scholar]
- 19.Hansen, V. M., J. Eilenberg, and A. M. Madsen. 2010. Occupational exposure to airborne Bacillus thuringiensis kurstaki HD1 and other bacteria in greenhouses and vegetable fields. Biocontr. Sci. Technol. 20:605-619. [Google Scholar]
- 20.Hansen, B. M., and A. Winding. 1997. Detection of Pseudomonas putida B in rhizophere by RAPD using extracted DNA. Lett. Appl. Microbiol. 24:249-252. [Google Scholar]
- 21.Harman, G. E., C. R. Howell, A. Viterbo, I. Chet, and M. Lorito. 2004. Trichoderma species—opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2:43-56. [DOI] [PubMed] [Google Scholar]
- 22.Heida, H., F. Bartman, and S. C. van der Zee. 1995. Occupational exposure and indoor air quality monitoring in a composting facility. Am. Ind. Hyg. Assoc. J. 56:39-43. [DOI] [PubMed] [Google Scholar]
- 23.Heldal, K. K., A. S. Halstensen, J. Thorn, P. Djupesland, I. Wouters, W. Eduard, and T. S. Halstensen. 2003. Upper airway inflammation in waste handlers exposed to bioaerosols. Occup. Environ. Med. 60:444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holck, P., M. Sletmoen, B. T. Stokke, H. Permin, and S. Norn. 2007. Potentiation of histamine release by microfungal (1→3)- and (1→6)-beta-d-glucans. Basic Clin. Pharmacol. Toxicol. 101:455-458. [DOI] [PubMed] [Google Scholar]
- 25.Illing, H. P. 1997. Is working in greenhouses healthy? Evidence concerning the toxic risks that might affect greenhouse workers. Occup. Med. (Lond.) 47:281-293. [DOI] [PubMed] [Google Scholar]
- 26.Jensen, G. B., P. Larsen, B. L. Jacobsen, B. Madsen, L. Smidt, and L. Andrup. 2002. Bacillus thuringiensis in fecal samples from greenhouse workers after exposure to B. thuringiensis-based pesticides. Appl. Environ. Microbiol. 68:4900-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen, G. B., P. Larsen, B. L. Jacobsen, B. Madsen, A. Wilcks, L. Smidt, and L. Andrup. 2002. Isolation and characterization of Bacillus cereus-like bacteria from faecal samples from greenhouse workers who are using Bacillus thuringiensis-based insecticides. Int. Arch. Occup. Environ. Health 75:191-196. [DOI] [PubMed] [Google Scholar]
- 28.Jespersen, A. B. K., and M. A. De Waard. 1993. Natural products in plant protection. Neth. J. Plant Pathol. 99:109-117. [Google Scholar]
- 29.Lacey, J., and J. Dutkiewicz. 1994. Bioaerosols and occupational lung disease. J. Aerosol Sci. 25:1371-1404. [Google Scholar]
- 30.Latgé, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, S. A., A. Adhikari, S. A. Grinshpun, R. Mckay, R. Shukla, and T. Reponen. 2006. Personal exposure to airborne dust and microorganisms in agricultural environments. J. Occup. Environ. Hyg. 3:118-130. [DOI] [PubMed] [Google Scholar]
- 32.Limpert, E., A. Stahel, and M. Abbt. 2001. Log-normal distributions across the sciences: keys and clues. Bioscience 51:341-352. [Google Scholar]
- 33.Madsen, A. M. 2006. Airborne endotoxin in different background environments and seasons. Ann. Agric. Environ. Med. 13:81-86. [PubMed] [Google Scholar]
- 34.Madsen, A. M. 2006. Exposure to airborne microbial components in autumn and spring during work at Danish biofuel plants. Ann. Occup. Hyg. 50:821-831. [DOI] [PubMed] [Google Scholar]
- 35.Madsen, A. M., V. M. Hansen, N. V. Meyling, and J. Eilenberg. 2007. Human exposure to airborne fungi from genera used as biocontrol agents in plant production. Ann. Agric. Environ. Med. 14:5-24. [PubMed] [Google Scholar]
- 36.Madsen, A. M., V. M. Hansen, N. V. Meyling, N. B. Hendriksen, A. Winding, K. Tendal, and J. Eilenberg. Human eksponering for mikrobiologiske bekæmpelsesmidler, deres naturligt forekommende slægtninge og andre mikroorganismer, in press. Pesticide research no. 131. Danish Environmental Protection Agency, Copenhagen, Denmark.
- 37.Madsen, A. M., V. M. Hansen, S. H. Nielsen, and T. T. Olesen. 2009. Exposure to dust and endotoxin of employees in cucumber and tomato nurseries. Ann. Occup. Hyg. 53:129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer, H. W., K. A. Jensen, K. F. Nielsen, J. Kildesø, S. Norn, H. Permin, L. K. Poulsen, H. J. Malling, S. Gravesen, and F. Gyntelberg. 2005. Double blind placebo controlled exposure to molds: exposure system and clinical results. Indoor Air 15:73-80. [DOI] [PubMed] [Google Scholar]
- 39.Meyling, N. V., and J. Eilenberg. 2006. Isolation and characterisation of Beauveria bassiana isolates from phylloplanes of hedgerow vegetation. Mycol. Res. 110:188-195. [DOI] [PubMed] [Google Scholar]
- 40.Michel, O., A. M. Nagy, M. Schroeven, J. Duchateau, J. Neve, P. Fondu, and R. Sergysels. 1997. Dose-response relationship to inhaled endotoxin in normal subjects. Am. J. Respir. Crit. Care Med. 156:1157-1164. [DOI] [PubMed] [Google Scholar]
- 41.Milton, D. K., D. Wypij, D. Kriebel, M. D. Walters, S. K. Hammond, and J. S. Evans. 1996. Endotoxin exposure-response in a fiberglass manufacturing facility. Am. J. Ind. Med. 29:3-13. [DOI] [PubMed] [Google Scholar]
- 42.Monsó, E. 2004. Occupational asthma in greenhouse workers. Curr. Opin. Pulm. Med. 10:147-150. [DOI] [PubMed] [Google Scholar]
- 43.Monsó, E., R. Magarolas, I. Badorrey, K. Radon, D. Nowak, and J. Morera. 2002. Occupational asthma in greenhouse flower and ornamental plant growers. Am. J. Respir. Crit. Care Med. 165:954-960. [DOI] [PubMed] [Google Scholar]
- 44.Okushima, L., M. Ishii, S. Sase, and M. Saito. 2004. An evalution of floating dust particles and molds in commercial greenhouses. Acta Hort. (ISHS) 639:359-366. [Google Scholar]
- 45.Radon, K., B. Danuser, M. Iversen, E. Monso, C. Weber, J. Hartung, K. J. Donham, U. Palmgren, and D. Nowak. 2002. Air contaminants in different European farming environments. Ann. Agric. Environ. Med. 9:41-48. [PubMed] [Google Scholar]
- 46.Riu, E., E. Monso, A. Marin, R. Magarolas, K. Radon, J. Morera, F. Andreo, and D. Nowak. 2008. Occupational risk factors for rhinitis in greenhouse flower and ornamental plant growers. Am. J. Rhinol. 22:361-364. [DOI] [PubMed] [Google Scholar]
- 47.Rylander, R., M. Norrhall, U. Engdahl, A. Tunsater, and P. G. Holt. 1998. Airways inflammation, atopy, and (1→3)-beta-d-glucan exposures in two schools. Am. J. Respir. Crit. Care Med. 158:1685-1687. [DOI] [PubMed] [Google Scholar]
- 48.Rylander, R., J. Thorn, and R. Attefors. 1999. Airways inflammation among workers in a paper industry. Eur. Respir. J. 13:1151-1157. [DOI] [PubMed] [Google Scholar]
- 49.Smid, T. 1993. Exposure to organic dust and respiratory disorders: an epidemiological study in the animal feed industry. CIP gegevens Koinklijke Bibliotheek, Den Haag, Netherlands.
- 50.Smid, T., D. Heederik, R. Houba, and P. H. Quanjer. 1992. Dust- and endotoxin-related respiratory effects in the animal feed industry. Am. Rev. Respir. Dis. 146:1474-1479. [DOI] [PubMed] [Google Scholar]
- 51.Spaan, S., I. M. Wouters, I. Oosting, G. Doekes, and D. Heederik. 2006. Exposure to inhalable dust and endotoxins in agricultural industries. J. Environ. Monit. 8:63-72. [DOI] [PubMed] [Google Scholar]
- 52.Swan, J. R. M., and B. Crook. 1998. Airborne microorganisms associated with grain handling. Ann. Agric. Environ. Med. 5:7-15. [PubMed] [Google Scholar]
- 53.Thorn, J., L. Beijer, and R. Rylander. 1998. Airways inflammation and glucan exposure among household waste collectors. Am. J. Ind. Med. 33:463-470. [DOI] [PubMed] [Google Scholar]
- 54.Tong, Y. Y., and B. Lighthart. 1998. Effect of simulated solar radiation on mixed outdoor atmospheric bacterial populations. FEMS Microbiol. Ecol. 26:311-316. [Google Scholar]
- 55.Williams, J., J. M. Clarkson, P. R. Mills, and R. M. Cooper. 2003. A selective medium for quantitative reisolation of Trichoderma harzianum from Agaricus bisporus compost. Appl. Environ. Microbiol. 69:4190-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhiping, W., P. Malmberg, B. M. Larsson, K. Larsson, L. Larsson, and A. Saraf. 1996. Exposure to bacteria in swine-house dust and acute inflammatory reactions in humans. Am. J. Respir. Crit. Care Med. 154:1261-1266. [DOI] [PubMed] [Google Scholar]