Abstract
Antibiotic-resistant bacteria (ARB) have been surveyed widely in water bodies, but few studies have determined the diversity of ARB in sediment, which is the most taxon-abundant habitat in aquatic environments. We isolated 56 extended-spectrum β-lactamase (ESBL)-producing bacteria from a single sediment sample taken from an urban river in China. All strains were confirmed for ESBL-producing capability by both the clavulanic acid combination disc method and MIC determination. Of the isolated strains, 39 were classified as Enterobacteriaceae (consisting of the genera Escherichia, Klebsiella, Serratia, and Aeromonas) by 16S rRNA gene sequencing and biochemical analysis. The present study identifies, for the first time, ESBL-producing strains from the families Brucellaceae and Moraxellaceae. The blaCTX-M gene was the most dominant of the ESBL genes (45 strains), while the blaTEM gene was the second-most dominant (22 strains). A total of five types of blaCTX-M fragments were identified, with both known and novel sequences. A library of blaCTX-M cloned from the sediment DNA showed an even higher diversity of blaCTX-M sequences. The discovery of highly diverse ESBL-producing bacteria and ESBL genes, particularly blaCTX, in urban river sediment raises alarms for potential dissemination of ARB in communities through river environments.
Antibiotic-resistant bacteria (ARB) have been found widely in aquatic environments (1, 18, 24). ARB in rivers may originate from anthropogenic sources, such as hospital, municipal, and aquaculture effluents (3, 16, 23); in addition, they could occur naturally, since many acquired resistance mechanisms originated in producers of antibiotics, such as actinomycetes (12). Both anthropogenic and naturally occurring ARB in water environments may compromise human health, since people may be infected by ARB through drinking water, aquatic products, and direct contact with water bodies. Moreover, the ARB may transfer the antibiotic resistance genes to other pathogens through horizontal gene transfer (1).
Sediment has the highest microbial diversity in water environments. The species richness and abundance of the sediment community are comparable to those of soil and are orders of magnitude higher than those of the planktonic community in the upper water layer (10, 20). It is reasonable to deduce that a wide variety of ARB might exist in sediment environments, as they are taxon-rich habitats, particularly in sediments receiving wastewater. Nevertheless, despite extensive studies surveying ARB in water columns, the diversity of antibiotic resistance in sediment environments has seldom been investigated.
The present study therefore focused on an urban river sediment environment as a model, concentrating on the diversity of extended-spectrum β-lactamase (ESBL)-producing bacteria. ESBL-producing organisms have been emerging both in nosocomial and in community settings since the 1980s (4, 13, 14). In aquatic environments, ESBL-producing bacteria have been found in sewage and water samples (11, 13, 16), but their diversity in sediment habitats has never been analyzed before. In the present study, the diversity of ESBL-producing organisms was studied by both culture-dependent and culture-independent methods. A wide variety of ESBL-producing bacteria were isolated using a vast array of different nutrient media. The ESBL-producing abilities of the isolated strains were determined by both the clavulanic acid combination disc method and MIC determination. ESBL genes were identified and characterized by PCR and sequencing. A clone library of the blaCTX-M gene, which was the most abundant type of ESBL gene found in the isolated bacteria, was cloned directly from the sediment and sequenced. The data revealed a diverse community of ESBL-producing bacteria that exists in urban aquatic sediment.
MATERIALS AND METHODS
Sampling and isolation of ESBL-producing bacteria.
Sediment samples were taken from Jinxi River at a site around 1 km downstream of a large teaching hospital in Guangzhou, China. We collected the surface 0 to 5 cm of sediment using a stainless steel scoop at a wadeable site close to the river bank. A total of ∼250 g of four subsamples within 1 m was collected and pooled in a Ziploc plastic bag. The sediment was kept on ice during delivery, stored in a 4°C refrigerator, and used freshly within 12 h. We mixed the sediment well and used 10 g for isolating bacteria and 1 g for DNA extraction. The sediment was sieved, and 10 g was dissolved in 90 ml of 0.9% NaCl water and shaken for 30 min. After standing for 20 min, the supernatant was diluted serially, and 1 ml was spread on each agar plate supplemented with ampicillin (30 mg liter−1) or cefotaxime (60 mg liter−1) (Sigma). Numerous different types of selective media, including EMB, PIA, NA, SS, 10-fold-diluted nutrient agar (NA 10−1), AMB, TCBS, R2A, and EA (Difco), were used to grow as many different ESBL-producing bacteria as possible. Colonies grown on the above-mentioned plates that showed different morphologies were isolated and purified for further ESBL activity determination and species identification. To avoid repeatedly isolating the clonal strains from the same unique bacterium, on every plate, we picked only 1 or 2 colonies for those with similar colony morphologies and preferred colonies with very singular shapes.
Identification of ESBL production.
ESBL-producing activity was first screened with a phenotypic confirmatory test (clavulanic acid combination disc) as recommended by the CLSI (6). The MIC of strains with ESBL activity was further determined using the Phoenix 100 system (7). The MICs of the antibiotics tetracycline, trimethoprim-sulfamethoxazole, cefazolin, ceftazidime, cefotaxime, cefepime, aztreonam, ampicillin, piperacillin, amoxicillin/clavulanate, ampicillin/sulbactam, piperacillin-tazobactam, imipenem, meropenem, amikacin, gentamicin, chloramphenicol, ciprofloxacin, and levofloxacin were determined.
Identification of isolated bacteria.
The isolated ESBL-producing bacteria were identified using both 16S rRNA gene sequencing and Phoenix 100 phenotyping. The 16S rRNA gene was amplified using primer set 27f (5′-AGAGTTTGATYMTGGCTCAG-3′) and 1492r (5′-TACGGYTACCTTGTTACGACT-3′) and was sequenced using the 1492r primer. The 16S rRNA sequences were compared to the DNA sequences deposited in all available public databases by BLASTn (http://www.ncbi.nlm.nih.gov/BLAST/). For Phoenix 100 system typing, the Phoenix ID broth was inoculated with 0.5 to 0.6 McFarland standards. After the transfer of 25 μl of the ID broth suspension to the Phoenix AST broth, the suspension was poured into the ID side of the combo panel. Once inoculated, the panel was logged and loaded into the Phoenix 100, in which kinetic measurements of colorimetric and fluorimetric signals were collected for biochemical identification.
Determination of ESBL genes.
The following primers were used to amplify the ESBL genes: TEM (TEM1, 5′-ATAAAATTCTTGAAGACGAAA-3′; TEM2, 5′-GACAGTTACCAATGCTTAATCA-3′), SHV (SHV1, 5′-TTGAATTCCGCCGGGTTATTCTTATTTGTCGC-3′; SHV2, 5′-TTGGATCCTCTTTCCGATGCCGCCGCCAGTCA-3′), OXA (OXA1, 5′-GTCTTTCGAGTACGGCATTA-3′; OXA2, 5′-ATTTTCTTAGCGGCAACTTAC-3′), and IMP (IMP1, 5′-CTACCGCAGCAGAGTCTTTG-3′; IMP2 5′-AACCAGTTTTGCCTTACCAT-3′) (8) and CTX-M (CTX-F, 5′-SCVATGTGCAGYACCAGTAA-3′; CTX-R, 5′-GCTGCCGGTYTTATCVCC-3′) (developed in the present study). PCR conditions for the above primers were as follows: 94°C for 2 min, followed by 30 cycles consisting of 94°C for 15 s, 55°C for 30 s and 72°C for 45 s, with a final extension at 74°C for 10 min. The PCR cocktail was ExTaq (Takara).
To investigate further the diversity of the blaCTX-M gene in the studied environment, a clone library of blaCTX-M was constructed. Sediment DNA was extracted using the Ultraclean soil DNA kit (Mobio), and the blaCTX-M gene was amplified using the CTX-F and CTX-R primer set. The PCR product was linked into the pGEM-Teasy vector (Promega) and transformed into Escherichia coli DH5α. The colonies were screened and sequenced.
Nucleotide sequence accession numbers.
The sequence data reported in this paper have been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession numbers GQ983155 through GQ983300.
RESULTS AND DISCUSSION
Isolation and identification of ESBL-producing bacteria.
Studies surveying the environmental distribution of ARB have typically focused on certain groups of pathogens or opportunistic pathogens using limited types of culture media (8, 18, 23). Because the present study intended to explore the diversity of ESBL-producing bacteria in one habitat, we employed a variety of culture media to obtain as many different species of bacteria as possible. Meanwhile, either of two types of β-lactams, ampicillin or cefotaxime, was used to supplement the media during the initial screen step to reduce potential biases of antibiotics.
From a single sediment sample, we obtained nearly 250 β-lactam-resistant bacterial isolates; these were identified by their different colony morphologies after growth on each type of medium plate supplemented with β-lactam. We initially analyzed their ESBL-producing capabilities through the clavulanic acid combination disc method as recommended by the CLSI, and 76 strains were designated tentatively as potential ESBL-producing bacteria. These 76 strains were characterized further for MIC with the Phoenix 100 system, and 56 of them were designated ESBL-producing bacteria. Biochemical analysis of the 56 strains was performed simultaneously using the Phoenix 100 system, and 51 strains were characterized further through 16S rRNA sequencing.
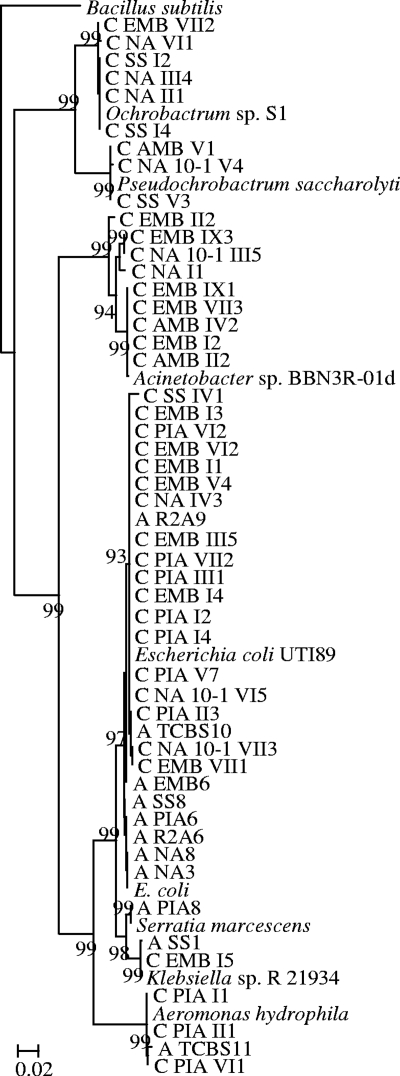
Enterobacteriaceae was the dominant order in our isolates; 39 out of 56 isolated strains belonged to this taxon. As one of the major threats among multidrug-resistant bacteria, ESBL-producing Enterobacteriaceae have been studied extensively in clinical settings (5, 15). This group has emerged within the community setting as an important cause of urinary tract infections since the late 1990s (5, 14). In natural environments, ESBL-producing Enterobacteriaceae have been found in water bodies in Portugal, Spain, Brazil, and India (11, 13, 16, 19). Nevertheless, the species diversity and ESBL genes of Enterobacteriaceae isolated from environmental habitats have been studied rarely. Our results show that these Enterobacteriaceae strains clustered with Escherichia, Klebsiella, Serratia, and Aeromonas (Fig. 1). The high level of abundance of ESBL-producing pathogens, like Escherichia, Klebsiella, and Serratia strains, suggested that the river was heavily contaminated by the upstream hospital effluent.
FIG. 1.
Phylogenetic tree of 16S rRNA of ESBL-producing isolates. The tree was rerooted with Bacillus subtilis (EU755383) as the root. The branch numbers refer to the percent confidence as estimated by a bootstrap analysis with 100 replications. Scale bar indicates percent divergence. (The first letter of strain names, C or A, indicates the antibiotics used for enrichment, with C for cefotaxime and A for ampicillin. The second word of strain names indicates the isolation medium.)
In addition to Enterobacteriaceae, the isolation of Ochrobactrum (six strains), Pseudochrobactrum (three strains), and Acinetobacter (nine strains) was unexpected because no strains from the families Brucellaceae or Moraxellaceae have been previously reported to produce ESBLs. The present results are robust: the Brucellaceae or Moraxellaceae strains were identified by both phylogenetic (16S rRNA sequence) and phenotypic (Phoenix 100 biochemical analysis) methods, and their ESBL-producing abilities were confirmed by both the clavulanic acid combination disc method (CLSI method) and MIC determination (Phoenix 100 system). In addition, ESBL genes (both blaTEM and blaCTX-M) were identified and sequenced from these strains (see Table S1 in the supplemental material). We propose to pay more attention to ESBL-producing Brucellaceae and Moraxellaceae strains in clinical settings.
Antibiotic resistance pattern of the isolated strains.
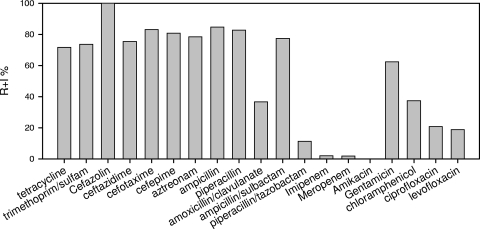
For the most part, ESBL-producing isolates were resistant to β-lactams, including penicillin, monobactam (aztreonam), and narrow-spectrum to “fourth-generation” cephalosporins (Fig. 2) (see Table S1 in the supplemental material). Of the three determined ESBL enzyme inhibitors, piperacillin-tazobactam was the most potent for inhibiting growth, while ampicillin-sulbactam was the least efficient. The carbapenems (imipenem and meropenem) were still active against our isolated ESBL-producing strains. For antibiotics other than β-lactams, only amikacin failed to impede the growth of all isolates tested, while chloramphenicol and fluoroquinolones (ciprofloxacin and levofloxacin) were effective against ∼80% of the isolates. Less than 50% of the ESBL-producing isolates were still sensitive to the remaining antibiotics, which included gentamicin, tetracycline, and trimethoprim-sulfamethoxazole. In general, the antibiotic resistance patterns of the ESBL-producing bacteria isolated from the sediment habitat were similar to those determined in clinical settings (22), and the Brucellaceae and Moraxellaceae strains showed grossly similar patterns with the Enterobacteriaceae strains (see Table S1 in the supplemental material).
FIG. 2.
Antibiotic resistance percentages for 56 isolated strains. y axis, percentage of resistant and intermediate (R+I) strains among all tested bacteria.
Antibiotic resistance genes in isolated strains.
Using PCR and sequence analysis, we determined five types of ESBL genes: blaCTX-M, blaTEM, blaSHV, blaOXA, and blaIMP. According to the PCR result (see Table S1 in the supplemental material), the most abundant ESBL gene was blaCTX-M (identified in 45 out of 56 strains from all three major orders), and the second-most prevalent gene was blaTEM (found in 22 strains). The blaOXA and blaIMP genes were identified only once, while the blaSHV gene was not found. In epidemiological studies, the blaCTX-M gene has been increasingly prevalent in global clinical settings (5), but it has seldom been identified in environmental isolates. Very recently, Reinthaler et al. reported that the blaCTX-M gene was dominant in ESBL-producing bacteria isolated from Austrian sewage sludge, with the blaTEM gene as the second-most prevalent type (17). The present result was in concordance with the above study, but our data further indicated that the CTX-M-type ESBL gene had already been prevalent in a river sediment environment.
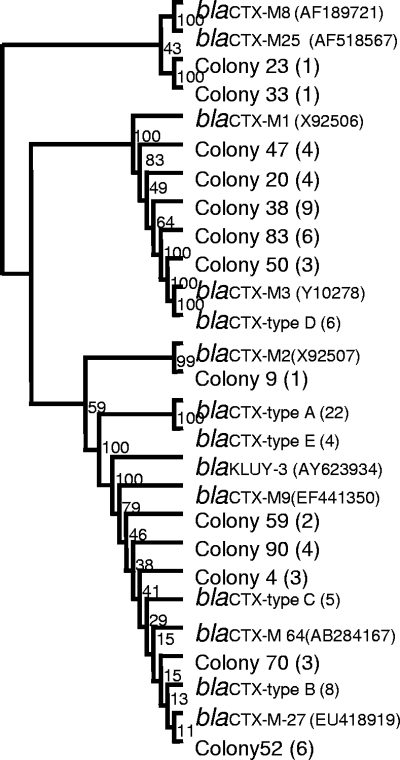
According to the sequencing results, there were five types of blaCTX-M genes (see Table S1 in the supplemental material). The type A gene was identified in 22 strains, which showed 96% nucleotide sequence identity with blaCTX-M27 (AB545827) by BLASTn; the type B gene was determined in 8 strains, and it shared 100% identity with blaCTX-M27 (AB545827); the type C gene existed in 5 strains, and it was 100% identical with blaCTX-M64 (AB284167); the type D gene was found in 6 strains, with 100% identity to blaCTX-M3 (GU125712); and the type E gene was determined in 4 strains, showing 95% identity to blaKluY-3 (AY623934). It was interesting to find both known and novel blaCTX-M genes coexisting in the same habitat, indicating a high diversity of blaCTX-M genes in the sediment. However, to fully prove the identification of a novel CTX-M gene, the full length of those sequenced genes needs to be cloned, and their functions should be tested in the future. In comparison, all blaTEM genes sequenced in the 22 strains were the same, showing 100% identity with blaTEM_139 (GQ149344).
Because a variety of both known and novel blaCTX-M sequences were found in the isolated bacteria, we used a clone library approach to study the diversity of the blaCTX-M gene in the sediment. A total of 60 colonies were sequenced and 47 of them were identified as CTX-M coding sequence using BLASTX analysis. These 47 colonies showed 13 different sequences, and they formed two major clades clustering with the blaCTX-M sequences determined from the isolated bacteria (Fig. 3). Such a high diversity of blaCTX-M genes was quite surprising. We acknowledge that mutations incurred during PCR amplification as well as sequencing errors could be potential sources of the novel sequences; however, since many of these sequences were determined in more than one colony, it was highly possible that novel blaCTX-M sequences existed in the sediment environment naturally.
FIG. 3.
Dendrogram tree of blaCTX-M gene sequences. blaCTX-M types A to E were identified from the cultured bacteria, and colonies were isolated from the library. The number in parentheses shows the number of times the sequence was determined in the isolated bacterium or library. The branch numbers refer to the percent confidence as estimated by a bootstrap analysis with 100 replications.
The blaCTX-M group is unique among ESBL genes. Since the first identification of CTX-M in 1989, the CTX-M pandemic has changed the prevalence of ESBLs, and it has rapidly become the dominant ESBL worldwide (4, 9, 22). Previous studies suggest that the CTX-Ms had several different environmental origins, in contrast to TEM and SHV, with single ancestors (2). CTX-M not only is associated with nosocomial outbreaks but is also mostly found in community-acquired infections (5). Nevertheless, the environmental source for community infection is still unclear (21). In our present study, the high degree of diversity of blaCTX-M genes in the sediment could have two potential sources: the upstream hospital effluent or naturally present strains. Our results indicate that novel blaCTX-M genes could be identified from the environment and that the river sediment could be a potential reservoir of novel ESBL genes that may pose a potential risk to public health.
In conclusion, the present study showed a rich reservoir of diverse ESBL-producing bacteria and ESBL genes, in particular, the blaCTX-M gene, in an urban river sediment environment. A number of novel ESBL-producing species, particularly the Brucellaceae and Moraxellaceae strains, and blaCTX-M genes were determined. It should be pointed out that this diversity is underevaluated: the isolation procedure employed could not culture all ESBL-producing species in the habitat, and the molecular methods used did not identify all ESBL genes due to the limitations of the PCR. Since people could be exposed to river water through aquatic products, drinking water, direct interaction, or aerosol inhalation, such a high variety of ESBL-producing bacteria in urban river sediment could pose a significant risk to the public health. The study of resistance mechanisms in environmental ARB may have indications for future clinical treatments. Moreover, we suggest paying more attention to the heavy contamination of urban river sediments by ARB, which can increase the risk of community infection.
Supplementary Material
Acknowledgments
We thank He Min-Chang, Zhu Pin-Ting, and Li Xiao-Xu for help in isolating ESBL-producing bacteria.
This work was supported financially by the Guangdong Natural Science Foundation (no. 7300224), the Ph.D. Programs Foundation of the Education Ministry of China (no. 20094433120017), the Key Discipline Construction Project under the 3rd Stage of the 211 Project, Guangdong Province (GW201019), and the Natural Science Foundation of China (no. 30971193).
Footnotes
Published ahead of print on 16 July 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Baquero, F., J. L. Martinez, and R. Canton. 2008. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 19:260-265. [DOI] [PubMed] [Google Scholar]
- 2.Barlow, M., R. A. Reik, S. D. Jacobs, M. Medina, M. P. Meyer, J. E. McGowan, Jr., and F. C. Tenover. 2008. High rate of mobilization for blaCTX-Ms. Emerg. Infect. Dis. 14:423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabello, F. C. 2006. Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ. Microbiol. 8:1137-1144. [DOI] [PubMed] [Google Scholar]
- 4.Canton, R., and T. M. Coque. 2006. The CTX-M beta-lactamase pandemic. Curr. Opin. Microbiol. 9:466-475. [DOI] [PubMed] [Google Scholar]
- 5.Canton, R., A. Novais, A. Valverde, E. Machado, L. Peixe, F. Baquero, and T. M. Coque. 2008. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 14:144-153. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing: informational supplement M100-S18. CLSI, Wayne, PA.
- 7.Donay, J. L., D. Mathieu, P. Fernandes, C. Pregermain, P. Bruel, A. Wargnier, I. Casin, F. X. Weill, P. H. Lagrange, and J. L. Herrmann. 2004. Evaluation of the Automated Phoenix System for potential routine use in the clinical microbiology laboratory. J. Clin. Microbiol. 42:1542-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, D., M. Yang, J. Hu, J. Zhang, R. Liu, X. Gu, Y. Zhang, and Z. Wang. 2009. Antibiotic-resistance profile in environmental bacteria isolated from penicillin production wastewater treatment plant and the receiving river. Environ. Microbiol. 11:1506-1517. [DOI] [PubMed] [Google Scholar]
- 9.Livermore, D. M., R. Canton, M. Gniadkowski, P. Nordmann, G. M. Rossolini, G. Arlet, J. Ayala, T. M. Coque, I. Kern-Zdanowicz, F. Luzzaro, L. Poirel, and N. Woodford. 2007. CTX-M: changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 59:165-174. [DOI] [PubMed] [Google Scholar]
- 10.Lozupone, C. A., and R. Knight. 2007. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. U. S. A. 104:11436-11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado, E., T. M. Coque, R. Canton, J. C. Sousa, D. Silva, M. Ramos, J. Rocha, H. Ferreira, and L. Peixe. 2009. Leakage into Portuguese aquatic environments of extended-spectrum-beta-lactamase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 63:616-618. [DOI] [PubMed] [Google Scholar]
- 12.Martinez, J. L. 2008. Antibiotics and antibiotic resistance genes in natural environments. Science 321:365-367. [DOI] [PubMed] [Google Scholar]
- 13.Mesa, R. J., V. Blanc, A. R. Blanch, P. Cortes, J. J. Gonzalez, S. Lavilla, E. Miro, M. Muniesa, M. Saco, M. T. Tortola, B. Mirelis, P. Coll, M. Llagostera, G. Prats, and F. Navarro. 2006. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J. Antimicrob. Chemother. 58:211-215. [DOI] [PubMed] [Google Scholar]
- 14.Perez, F., A. Endimiani, K. M. Hujer, and R. A. Bonomo. 2007. The continuing challenge of ESBLs. Curr. Opin. Pharmacol. 7:459-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitout, J. D. D., and K. B. Laupland. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 8:159-166. [DOI] [PubMed] [Google Scholar]
- 16.Prado, T., W. C. Pereira, D. M. Silva, L. M. Seki, A. P. Carvalho, and M. D. Asensi. 2008. Detection of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in effluents and sludge of a hospital sewage treatment plant. Lett. Appl. Microbiol. 46:136-141. [DOI] [PubMed] [Google Scholar]
- 17.Reinthaler, F. F., G. Feierl, H. Galler, D. Haas, E. Leitner, F. Mascher, A. Melkes, J. Posch, I. Winter, G. Zarfel, and E. Marth. 2010. ESBL-producing E. coli in Austrian sewage sludge. Water Res. 44:1981-1985. [DOI] [PubMed] [Google Scholar]
- 18.Sapkota, A. R., F. C. Curriero, K. E. Gibson, and K. J. Schwab. 2007. Antibiotic-resistant enterococci and fecal indicators in surface water and groundwater impacted by a concentrated swine feeding operation. Environ. Health Perspect. 115:1040-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma, A., P. Dour, and T. N. Singh. 2008. The prevalence of extended-spectrum beta-lactamase in environmental isolates of Enterobacter. Indian J. Pathol. Microbiol. 51:130-136. [DOI] [PubMed] [Google Scholar]
- 20.Shaw, A. K., A. L. Halpern, K. Beeson, B. Tran, J. C. Venter, and J. B. Martiny. 2008. It's all relative: ranking the diversity of aquatic bacterial communities. Environ. Microbiol. 10:2200-2210. [DOI] [PubMed] [Google Scholar]
- 21.Smet, A., A. Martel, D. Persoons, J. Dewulf, M. Heyndrickx, B. Catry, L. Herman, F. Haesebrouck, and P. Butaye. 2008. Diversity of extended-spectrum beta-lactamases and class C beta-lactamases among cloacal Escherichia coli isolates in Belgian broiler farms. Antimicrob. Agents Chemother. 52:1238-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki, S., N. Shibata, K. Yamane, J.-I. Wachino, K. Ito, and Y. Arakawa. 2009. Change in the prevalence of extended-spectrum-beta-lactamase-producing Escherichia coli in Japan by clonal spread. J. Antimicrob. Chemother. 63:72-79. [DOI] [PubMed] [Google Scholar]
- 23.Watkinson, A. J., G. B. Micalizzi, G. M. Graham, J. B. Bates, and S. D. Costanzo. 2007. Antibiotic-resistant Escherichia coli in wastewaters, surface waters, and oysters from an urban riverine system. Appl. Environ. Microbiol. 73:5667-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xi, C., Y. Zhang, C. F. Marrs, W. Ye, C. Simon, B. Foxman, and J. Nriagu. 2009. Prevalence of antibiotic resistance in drinking water treatment and distribution systems. Appl. Environ. Microbiol. 75:5714-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.