Abstract
In many angiosperm species, populations are reproductively subdivided into distinct sexual morphs including females, males and hermaphrodites. Sexual polymorphism is maintained by frequency-dependent selection, leading to predictable sex ratios at equilibrium. Charles Darwin devoted much of his book ‘The Different Forms of Flowers on Plants of the Same Species’ (1877) to investigating plant sexual polymorphisms and laid the foundation for many problems addressed today by integrating theory with empirical studies of the demography and genetics of populations. Here, we summarize our recent work on the ecological and genetic mechanisms influencing variation in sex ratios and their implications for evolutionary transitions among sexual systems. We present the results of a survey of sex ratios from 126 species from 47 angiosperm families and then address two general problems using examples from diverse angiosperm taxa: (i) the mechanisms governing biased sex ratios in dioecious species; (ii) the origins and maintenance of populations composed of females, males and hermaphrodites. Several themes are emphasized, including the importance of non-equilibrium conditions, the role of life history and demography in affecting sex ratios, the value of theory for modelling the dynamics of sex ratio variation, and the utility of genetic markers for investigating evolutionary processes in sexually polymorphic plant populations.
Keywords: dioecy, frequency-dependent selection, non-equilibrium conditions, sex ratios
1. Introduction
Flowering plants possess diverse reproductive systems, including species that are polymorphic for sexual traits. In these species, populations are composed of distinct mating groups, or sexual morphs, which can be distinguished by their reproductive organs. This variation ranges from the coexistence within populations of various combinations of hermaphroditic and unisexual individuals (females and males), to discrete differences in floral design between hermaphroditic morphs. The maintenance of sexual polymorphism results from negative frequency-dependent selection, whereby the reproductive success of an individual depends on the frequency of sexual morphs with which it can mate (Clarke et al. 1988). Disassortative mating between sexual morphs generally leads to equal ratios at equilibrium (Fisher 1930), although unequal ratios can also occur at equilibrium when morphs differ in their ability to mate with each other (Barrett et al. 2004), or when non-equilibrium conditions prevail (Eckert & Barrett 1995). Understanding the origin and maintenance of sexual polymorphisms has been an enduring problem that has attracted considerable interest since the birth of evolutionary biology.
Charles Darwin's book ‘The Different Forms of Flowers on Plants of the Same Species’ (1877) was largely concerned with the evolution and function of polymorphic sexual systems. Why some species have evolved separate female and male plants (dioecy) and others were hermaphrodite fascinated Darwin. He was also interested in the occurrence of females, males and hermaphrodites within populations (subdioecy) and interpreted this as evidence that dioecy could evolve gradually from hermaphroditism (Darwin 1877, p. 281). ‘Forms of Flowers’ identifies the beginning of evolutionary studies on polymorphic sexual systems in plants and the work provides a conceptual framework for understanding floral adaptations that promote cross-fertilization, and the mechanisms responsible for transitions between sexual systems.
Darwin's investigations were conducted without knowledge of the genetic basis of inheritance, although it should be noted that the progeny ratios he found in his studies of distylous Primula were consistent with the ratios that Gregor Mendel obtained to establish the principles of inheritance (Charlesworth & Charlesworth 2009). However, once the science of genetics was established at the beginning of the twentieth century, plant sexual polymorphisms contributed substantially to our understanding of several fundamental concepts including inheritance, linkage, recombination, epistasis, supergenes and polymorphic equilibria through the work of W. Bateson, R. A. Fisher, J. B. S. Haldane, S. Wright, C. Correns and A. Ernst. Their investigations made a significant contribution to the growth of ecological and evolutionary genetics and laid the foundations for theoretical work on the evolution and maintenance of sexual systems using population genetic approaches (Charlesworth & Charlesworth 1979). Today, the integration of theory with empirical studies using genetic markers, and investigations of the ecology of populations, has enabled workers to investigate many problems first raised by Darwin in ‘Forms of Flowers’.
Here, we address several topics concerned with the ecological genetics of polymorphic sexual systems using examples from diverse angiosperm taxa. We begin by presenting data on sex ratios in a large sample of dioecious species and consider ecological and genetic factors that cause biased sex ratios. We then investigate the coexistence of three sexual morphs within populations and consider the origins and maintenance of subdioecy. A recurrent theme in our treatment is the need to consider the roles of life history and demography in affecting the maintenance and frequency of sexual morphs in populations.
2. Sex ratio variation in dioecious plant populations
Dioecy is relatively infrequent in angiosperms (approx. 6% of species) but has evolved repeatedly from hermaphroditism with at least 100 independent origins (Charlesworth 2002). Although much effort has focused on understanding the selective mechanisms responsible for this transition (reviewed in Geber et al. 1999), less attention has been devoted to investigating sex ratio evolution in plants. Darwin first discussed the role of natural selection in sex ratio evolution in ‘The Descent of Man and Selection in Relation to Sex’ (Darwin 1871, p. 316), but withdrew this from the second edition published in 1874 (see Edwards 1998). In discussions of plant sex ratios, Darwin tended to use group selection arguments and considered maternal fitness the sole target of fertility selection (Darwin 1877, p. 260, 282, 304). Since then a large body of theoretical and empirical work has been developed to explain sex ratio variation and evolution, particularly in animals (reviewed in Edwards 2000; Hardy 2002; West 2009).
If the costs of producing males and females are equal, offspring sex ratios should generally be close to unity after the period of parental investment. Although theory predicts that a 1 : 1 sex ratio should be maintained by negative frequency-dependent selection, there is evidence from surveys of plant populations of frequent departures from equality (reviewed in Delph 1999). To investigate this problem further we surveyed the literature for studies of sex ratios in dioecious species to determine how often bias occurs, and to what extent it is more likely to be female or male biased. Here, we discuss the main patterns observed; a more detailed analysis of the correlates of sex ratio variation will be presented elsewhere.
(a). General patterns
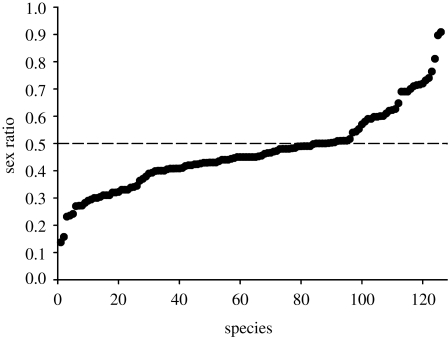
Population sex ratios of 126 dioecious species (representing 70 genera and 47 families) show nearly continuous variation from strong male to strong female bias (range 0.14–0.91; figure 1). Only 33 per cent of species in our survey (n = 42) exhibited close to even sex ratios, based on arbitrarily classifying male bias as mean sex ratios less than 0.4 and female bias as ratios greater than 0.6. This indicates that biased sex ratios are common, with male bias (46% of species, n = 58) being over twice as frequent as female bias (21% of species, n = 26). However, several caveats apply when interpreting estimates of sex ratio. First, in virtually all species included in our survey, sex ratios were obtained from reproductive individuals because of the inability to identify the gender of non-reproductive plants. Second, many dioecious species are clonal and in these cases sex ratios were based on samples of flowering shoots (ramets) rather than genets. These issues highlight the inherent limitations of studying sex ratios in long-lived and clonal perennial species, and emphasize the need for sex-specific genetic markers to enable an assessment of sex ratios throughout the life cycle.
Figure 1.
Variation in sex ratios in 126 dioecious species from 70 genera and 47 families of flowering plants. Sex ratio was calculated as females/(females + males), so that low values indicate male bias and high values female bias. Dashed line indicates 1 : 1 sex ratio. A weighted average was calculated for species with more than one sex ratio sample (mean number of populations sampled per species 5.4, range 1–67).
(b). Male-biased ratios
Differential expenditure on reproduction between males and females is probably the primary mechanism responsible for the common occurrence of male-biased sex ratios. At least three factors related to the costs of reproduction can contribute to male bias: (i) earlier onset of male flowering, (ii) more frequent flowering in males, and (iii) greater gender-specific mortality in females. Numerous cases are reported in the literature linking male-biased sex ratios to sex-differential reproductive costs (reviewed in Delph 1999). These effects are likely to become amplified in longer lived species due to repeated episodes of reproduction.
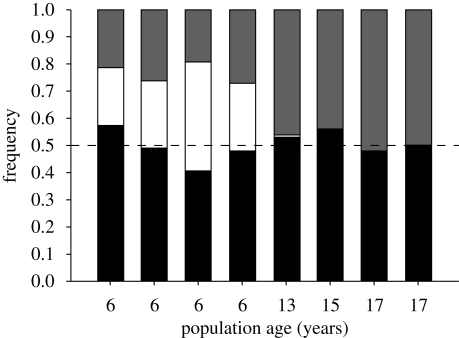
An example of the first of these mechanisms is evident in the dioecious fire-adapted shrub Leucadendron gandogeri (Proteaceae), native to fynbos vegetation of the Cape region of South Africa. Populations establish synchronously after fire, from seed released from serotinous cones in the canopy, and are therefore even-aged. Samples of female, male and non-reproductive plants exhibit male-biased sex ratios in four younger populations, but close to even sex ratios in older populations in which all individuals were reproductively mature (figure 2). This pattern is most probably explained by the longer time required for females to reach reproductive maturity because of costs associated with serotinous cone production (see Harris & Pannell 2010), and assumes that non-flowering individuals in the younger populations examined are largely female. Populations of most dioecious species are composed of mixed age cohorts and it is therefore more difficult to assess the influence of age on sex ratio dynamics.
Figure 2.
Relation between sex ratio and population age in eight dioecious populations of Leucadendron gandogeri sampled near Hermanus, Western Cape, South Africa. Sampling was conducted in September–October 2003 using belt transects (50 × 2 m) in the four younger populations, and quadrats (20, 5 × 5 m or 2, 100 × 5 m depending on plant density) in the four older populations. All plants in each sample were recorded as female (grey bar), male (black bar) or non-reproductive (vegetative; white bar). Age was estimated based on annual shoot growth, since each internode represents a single year of growth.
(c). Female-biased sex ratios
Several mechanisms have been proposed to account for female bias including: (i) differential performance of male- versus female-determining microgametophytes, a phenomenon known as certation (Correns 1922); (ii) X-linked meiotic drive and selfish gene elements (Taylor & Ingvarsson 2003); (iii) gender-specific differences in mortality (Lloyd 1974); and (iv) local mate competition (de Jong et al. 2002). Unfortunately, definitive experimental evidence is generally lacking for most of these mechanisms because of the difficulty in establishing at what stages bias occurs during the life cycle.
It has been suggested that, for species with sex chromosomes where males are the heterogametic sex, degeneration of the non-recombining Y-chromosome may contribute to female bias through differences in viability of the sexes and/or the performance of the sex-chromosomal genotypes of microgametophytes during pollination and fertilization (Smith 1963). Although the specific genetic mechanism(s) responsible has not been established, recent studies on wind-pollinated Rumex nivalis (Polygonaceae), a species with heteromorphic sex chromosomes (females XX and males XY1Y2), indicate that both certation and gender-based mortality cause the female-biased sex ratios that characterize populations (Stehlik & Barrett 2005, 2006; Stehlik et al. 2007, 2008). By using sex-specific markers, and sampling across different life cycle stages, these workers demonstrated that female bias is first expressed in pollen, and then subsequently increases in intensity from seeds to vegetative plants to flowering. They also demonstrated that demographic aspects of the local mating environment influence progeny sex ratios (Stehlik et al. 2008). Females closer to males captured more pollen and produced more female-biased sex ratios than more isolated females. Experimental studies involving the manipulation of pollination intensity also demonstrated a relation between pollen load size and sex ratio bias (Stehlik & Barrett 2006). These findings are consistent with Correns' certation hypothesis, which posits that larger pollen loads should intensify gametophytic competition favouring selective fertilization by female-determining pollen tubes.
Differential mortality may also interact with life history to contribute to sex ratio variation in dioecious species. For example, if growth and viability differences between the sexes occur, we might expect that sex ratios become increasingly biased as plants age, with significantly more bias in perennials compared with annuals. To investigate this possibility we examined sex ratio variation in the annual Rumex hastatulus. We found that, although sex ratios were mostly female biased (mean flowering sex ratio = 0.62, range 0.50–0.71, n = 46 populations; M. Pickup, C. de Waal & S. C. H. Barrett, unpublished data, 2009), the degree of bias was less than in the longer lived R. nivalis (mean flowering sex ratio = 0.82, range 0.69–0.95, n = 18; Stehlik & Barrett 2005). Future studies using sex-specific markers are needed to identify the proximate genetic mechanisms causing female bias, and to evaluate whether the poor performance of male gametophytes and sporophytes is a consequence of the accumulation of deleterious mutations on portions of the non-recombining Y-chromosomes.
3. Coexistence of three sexes
Darwin (1877) identified several species in which populations were composed of females, males and hermaphrodites. Sexual diversity of this type is now reported from numerous species, raising the question of how it originates and whether it represents a stable sexual system, or is simply a transient condition associated with the evolution of dioecy, as Darwin surmised (Lloyd 1976; Charlesworth & Charlesworth 1978; Ehlers & Bataillon 2007). Theoretical investigations of the evolutionary maintenance of subdioecy indicate that coexistence of three sexes can occur as a stable polymorphism, although under restrictive conditions (Maurice & Fleming 1995; Ehlers & Bataillon 2007), or simply cannot be maintained (Wolf & Takebayashi 2004). Several hypotheses may account for the discrepancy between these theoretical expectations and empirical observations. First, resource-dependent gender plasticity may lessen the trade-off between female and male fitness and help maintain subdioecy (Ehlers & Bataillon 2007). Second, subdioecy may persist at the metapopulation level as a result of selection for hermaphrodites during colonization, and unisexuals in established colonies due to inbreeding avoidance and optimal resource allocation (Pannell 2006). Here, we investigate this problem further by focusing instead on non-equilibrium conditions in perennial populations. Specifically, we consider the coexistence of three sexual morphs over ecological time scales and explore how aspects of life history and the inheritance of sex determination influence sex ratio dynamics.
(a). Ecological maintenance of subdioecy in clonal populations
Many dioecious and subdioecious species are long-lived perennials, propagating through both sexual and clonal reproduction. Sex ratio evolution is therefore often extremely protracted and historical contingencies associated with the sex genotypes of founders that initiate populations have the potential to influence pathways to equilibrium. Here, we explore the occurrence of subdioecy as a non-equilibrium state that can be maintained for long periods of time before the transition to dioecy or hermaphroditism. We used an explicit genetic model to examine founder pools, modes of reproduction and levels of adult survival, on the trajectories of sexual morphs and time to equilibrium in subdioecious populations.
Our model was motivated by empirical data from clonal Sagittaria latifolia (Alismataceae), reviewed in the next section. This species is composed of dioecious and monoecious populations and populations containing all three sexual morphs can arise through hybridization between the sexual systems. Our goal was to investigate how long coexistence of three morphs could be maintained given these conditions. Sex determination in our model was governed by simple Mendelian segregation of alleles at two linked loci, with males heterozygous at both the male and female sterility loci (Charlesworth & Guttman 1999); this is the mode of sex determination in S. latifolia (Dorken & Barrett 2004a). We used a set of recursion equations to track the frequencies of the three sexual morphs over time for populations reproducing both sexually and clonally (see appendix A for details).
Under a scenario of unisexual invasion of hermaphrodite populations, mode of reproduction and rates of survival strongly affected the persistence of subdioecy and the time required for populations to reach equilibrium. Even when conditions strongly favoured the spread of unisexuals, increasing the rate of survival from 20 to 80 per cent increased the time to equilibrium from 50 to approximately 400 time units. However, simultaneously increasing levels of clonal reproduction and survival had the most pronounced effect, resulting in subdioecious populations persisting for up to 800 time units as a non-equilibrium state. With levels of clonal propagation reaching 80 per cent and less favourable conditions for the spread of unisexuals (e.g. γo = 0.48, γp = 0.48, δ = 0.6; see appendix), the time to equilibrium increased to well over 2000 time units. These results demonstrate that aspects of life history can prolong the persistence of populations containing three sexual morphs, resulting in functional subdioecy.
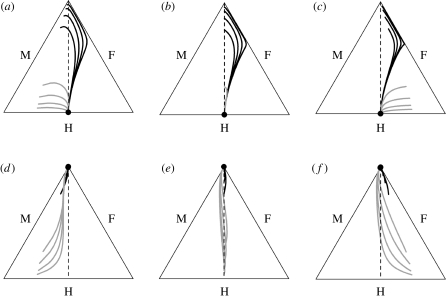
For the invasion of unisexuals into hermaphrodite populations, male-biased sex ratios were evident in non-equilibrium populations (figure 3a–c). This occurs because our model presumes that a single double-recessive genotype (SufSm/SufSm: see appendix for details) determines the female phenotype, and hybridization creates no new opportunities for females to arise. In contrast, following hybridization, two hermaphrodite and two male genotypes can be produced. As long as one male is present among the invaders, this pattern will occur regardless of the number or direction of sex bias among founding individuals. For the alternative scenario involving the spread of hermaphrodites into unisexual populations, the genetic system had little effect on the direction or magnitude of deviation from 1 : 1 ratios (figure 3d–f). Rather, male or female bias occurred only because of skewed frequencies among the founding individuals of dioecious populations. The morph frequency trajectories followed different paths (figure 3), depending on the direction of the invasion and whether conditions favoured the spread of hermaphrodites or unisexuals. Our results demonstrate that in subdioecious populations the genetics of sex determination and composition of the founder pool result in characteristic sexual morph frequencies, providing testable predictions regarding their origins.
Figure 3.
De Finetti diagrams depicting sex ratio dynamics of males (M), females (F) and hermaphrodites (H) in simulated plant populations. The simulations consider populations of unisexuals invaded by hermaphrodites (grey lines) and hermaphrodites invaded by unisexuals (black lines). The distance of the line from a side (M, F, H) represents the frequency of a morph in the population. Lines in each plot connect the frequencies from each successive generation following invasion of recipient unisexual or hermaphrodite populations of different sizes (n = 200, 100, 50, 25) by eight individuals of the other sexual system. Initial frequencies of the recipient and invading individuals were either female biased (a,d), had equal sex ratios (b,e), or were male biased (c,f) under conditions favouring unisexuals (a–c) or those favouring hermaphrodites (d–f). Dashed line indicates equal frequencies of males and females. The filled circle represents the equilibrium frequency of sex phenotypes. All models run for 4000 time units under high adult survival and high clonal reproduction (see appendix for details).
(b). Using molecular markers to investigate the origins of subdioecy
Subdioecy is most often considered a legacy of the gradual evolution of dioecy via the gynodioecy pathway. This hypothesis envisions that populations stall along the pathway to dioecy and as a result comprise unisexuals and a low frequency of ‘inconstant males’ with hermaphrodite sex expression (Lloyd 1976; Charlesworth & Charlesworth 1978). However, an alternative hypothesis for the origin of subdioecy that has not been considered involves hybridization between dioecious and hermaphrodite populations. In many gender dimorphic groups it is not uncommon to find sexually dimorphic and monomorphic populations (e.g. Ecballium elaterium; Costich 1995) and, if these occur in sympatry (e.g. Wurmbea dioica; Case & Barrett 2001), this could provide opportunities for gene flow between the sexual systems. Here, we briefly demonstrate that genetic markers can distinguish between alternative hypotheses for the origin of subdioecy.
We have investigated geographical patterns of sex ratio variation in S. latifolia in eastern N. America. Populations over much of the range are commonly either monoecious or dioecious (Dorken et al. 2002). However, at the northern range limit of dioecious populations in Ontario and Quebec many populations are subdioecious, containing significant frequencies of hermaphrodite plants coexisting with females and males (n = 25 populations, mean hermaphrodite frequency = 0.31, range 0.11–0.54; mean female frequency = 0.29, range 0.02–0.53; mean male frequency = 0.40, range 0.02–0.68; S. Yakimowski & S. C. H. Barrett, unpublished data, 2009). Because both monoecious and dioecious populations occur throughout this region, and crossing studies indicate no barriers to inter-fertility (Dorken & Barrett 2004a), hybridization could account for the origin of subdioecious populations. However, an alternative hypothesis is that subdioecious populations of S. latifolia have arisen directly from dioecious populations through an increase in the frequency of ‘inconstant’ males. These different pathways to subdioecy should be distinguishable, since previous studies (Dorken et al. 2002; Dorken & Barrett 2004b) have demonstrated that populations belonging to the two sexual systems are genetically differentiated at marker loci.
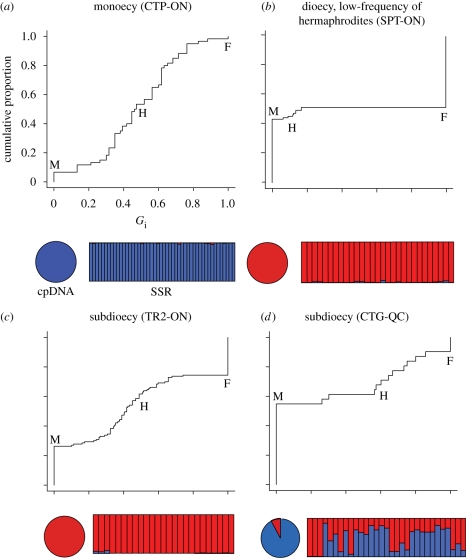
We used cpDNA (Dorken & Barrett 2004b) and microsatellite (SSR) markers (Yakimowski et al. 2009) to investigate patterns of molecular variation in 10 monoecious, 12 dioecious and 14 subdioecious populations (S. B. Yakimowski & S. C. H. Barrett, unpublished data, 2009). Here, we present the results from four representative populations to illustrate the contrasting patterns obtained. Details of the complete survey will be published elsewhere. Monoecious (CTP-ON) and dioecious (SPT-ON) populations were clearly distinguishable by their cpDNA haplotypes and by variation at 11 SSR loci (figure 4a,b). These patterns were typical of the sample as a whole, with monoecious and dioecious populations fixed for alternative cpDNA haplotypes and falling into two distinct (SSR) clusters using Structure (Pritchard et al. 2000). Given this genetic differentiation, we then inferred the ancestry of subdioecious populations using the reference set of monoecious and dioecious populations and assigning individuals to either group.
Figure 4.
Sexual diversity and variation at molecular markers in four populations of Sagittaria latifolia: (a) monoecy (CTP-ON); (b) dioecy, low-frequency of hermaphrodites (SPT-ON); (c) subdioecy (TR2-ON); (d) subdioecy (CTG-QC). For each population the sex phenotype distribution (Gi) is represented by a plot of the cumulative frequency distribution of standardized phenotypic femaleness (Gi = 0 for males (M), Gi = 1 for females (F)), based on a sample of 50–100 flowering ramets per population. The vertical line extending up from Gi = 0 is the frequency of males, the vertical line extending down from Gi = 1 is the frequency of females, and hermaphroditic (H) individuals connect these two extremes. Below each gender plot is a pie diagram showing the proportion of individuals in populations exhibiting cpDNA haplotypes D (blue) and G (red) from Dorken & Barrett (2004b). To the right is a bar-plot of proportional Structure assignments (Pritchard et al. 2000) of individuals to two clusters (k = 2), where each vertical bar represents one individual from the population. We used an admixture model, which allows a proportion of 11 SSR loci in each individual to be assigned to each of k groups, in this case k = 2. An individual for which 95 per cent or more of its genome is assigned to one group is considered ‘pure’, while individuals that are assigned to more than one group for more than 5 per cent of their genome are considered admixed (hybrids).
The sample of 14 subdioecious populations could be divided unambiguously into two groups of which TR2-ON and CTG-QC are representatives (figure 4c,d, respectively). Population TR2-ON has the molecular signature of a dioecious population and probably originated simply by an increase in the frequency of hermaphrodites. The selective mechanisms favouring sex inconstancy in male plants are not known but may be associated with the benefits of hermaphroditism at northern range limits. This could occur because of: (i) the benefits of hermaphroditism for colony establishment through reproductive assurance, and (ii) the reduced cost of limited female function in comparison to females in marginal environments. Of relevance to these hypotheses are the observations that monoecious populations extend much further north than dioecious populations, and female frequencies are substantially reduced in subdioecious populations at the northern range limit.
In striking contrast to TR2-ON, subdioecious population CTG-QC displayed clear evidence of genetic admixture between monoecious and dioecious groups for both cpDNA and SSR markers. Significantly, no hermaphrodite individual exhibited a pure monoecious or dioecious genotype. Overall these results indicate that subdioecy in S. latifolia can arise by two distinct pathways, although hybridization is the most common mechanism among our sample of populations. Given the results of our theoretical models, it seems probable that many subdioecious populations of S. latifolia have been maintained for a considerable period in a non-equilibrium state because of prolific clonal propagation and limited sexual recruitment. Similar situations have been reported in the clonal, tristylous aquatics Pontederia cordata (Morgan & Barrett 1988) and Decodon verticillatus (Eckert & Barrett 1995), which commonly display biased morph ratios.
4. Conclusions
Polymorphic sexual systems offer several advantages over sexual monomorphism for studies in ecological genetics. These include: (i) the morphs can be identified under field conditions; (ii) the form of selection maintaining the polymorphisms—frequency-dependent selection—is well understood; (iii) the genetic basis of polymorphism is relatively simple, and (iv) theoretical models are straightforward to develop and can be used to explore conditions influencing equilibrium states. Fisher (1930) predicted that in dioecious populations at equilibrium there should be equal sex ratios. However, since then surveys of numerous plant species have often revealed biased sex ratios. These departures reflect the fact that ecological, life history and demographic factors weaken the influence of frequency-dependent selection in many species. This can arise when progress to equilibrium is delayed through clonal propagation so that a non-equilibrium state is the norm. The frequent occurrence of deviations from equilibrium predictions highlights the general importance of distinguishing between ecological and evolutionary time scales when considering how deterministic versus stochastic forces affect polymorphisms in plant populations.
Acknowledgements
We thank Michael Bonsall and Brian Charlesworth for the opportunity to participate in the Discussion Meeting, and Discovery Grants from the Natural Sciences and Engineering Research Council of Canada and the Canada Research Chair's Program that funded this work.
Appendix A
We developed a genetic model to track the frequencies of the three sexual morphs over time. We used the model of two linked loci and notation of Charlesworth & Guttman (1999), which is the mode of sex determination in S. latifolia. In this model S determines male fertility and Su determines female fertility. In monoecious populations, hermaphrodites are of genotype SufSM/SufSM. Males from dioecious populations are heterozygous with genotype SuFSM/SufSm, where SuF is a dominant female suppressor of female fertility and Sm is a recessive male sterility factor. Females from dioecious populations have a single double-recessive genotype SufSm/SufSm. Following hybridization between monoecious and dioecious individuals, two additional genotypes are possible: a hybrid male SuFSM/SufSM, and a hybrid hermaphrodite SufSM/SufSm.
Let g1, g2, g3, g4 and g5 be the number of individuals in a population at time t that are hermaphrodite, female, male, hybrid male and hybrid hermaphrodite, respectively. We let gi′ be the total number of individuals of ith genotype at time t + 1, which is the sum of all components of asexual and sexual reproduction and year-to-year adult survival:
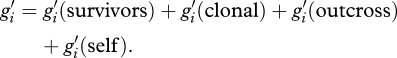 |
A1 |
We let S be the proportion of adult individuals surviving from time t to time t + 1, and π be the number of clones produced by ith genotype. Hence, our model is age structured but with constant survival probability and clonal reproduction at each time interval. With these assumptions we derive recursion equations to calculate the number of survivors and clones in the next time interval:
| A2 |
and
| A3 |
For sexual reproduction, females produce λo ovules, whereas hermaphrodites are expected to make fewer ovules than females, due to the benefits of sexual specialization and optimal resource allocation; we thus set their ovule production to λoγo (0 < γo < 1). Similarly, hermaphrodites are expected to make less pollen than males so we set the relative pollen production of males to 1 and hermaphrodites to γp (0 < γp < 1).
Sexual reproduction occurs by random mating, with the frequencies of each genotype at time t + 1 depending on female and male gametic frequencies at time t. The total amount of pollen in the pollen pool P at time t is:
| A4 |
Unisexual genotypes are obligate outcrossers; however, females (g2) can arise through the selfing of hybrid hermaphrodites (g5). Hermaphrodite ovules are fertilized by either outcrossing or selfing. We assume that, for an hermaphrodite, a proportion (gi–1)γp/P of their ovules is pollinated via outcrossing. The remainder, γp/P, is the fraction of ovules pollinated via selfing through intra-ramet selfing, which we assume to be inversely proportional to the size of the pollen pool. When genotypes reproduce clonally (π > 0), mating between ramets of the same genotype can occur in hermaphrodites, which is equivalent to selfing. The frequency of such inter-ramet mating is dependent on the relative amount of asexual versus sexual reproduction in the population, given by
| A5 |
The frequency of inter-ramet selfing is proportional to the number of clones produced per hermaphrodite, and hence to c. The total proportion of self-fertilization of an hermaphrodite is thus increased to γp/P + (gi–1)cγp/P, where the second term represents the chance of fertilization of an individual by different ramets belonging to the same clone as itself. Lastly, we assume that self-pollinated progeny are less viable than outcrossed progeny with a proportion, δ, of selfed seeds being inviable (inbreeding depression).
With these assumptions, we can obtain recursion equations for each component of sexual reproduction:
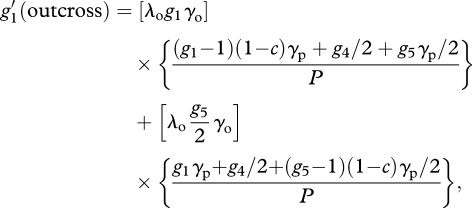 |
A6 |
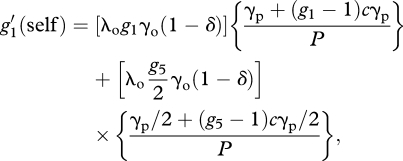 |
A7 |
 |
A8 |
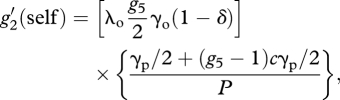 |
A9 |
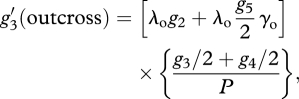 |
A10 |
 |
A11 |
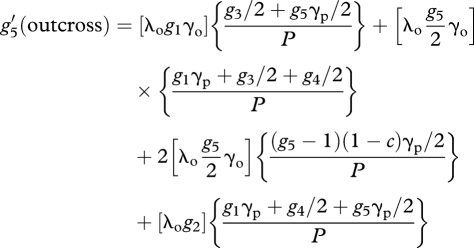 |
A12 |
and
 |
A13 |
The frequencies of each genotype were tracked under the set of conditions for each model, assuming a carrying capacity of K = 10 000, ovule production λo = 30, and iterated for 4000 time units. The model was iterated under conditions favouring unisexuals: γo = 0.4, γp = 0.4, δ = 0.8; and conditions favouring hermaphrodites: γo = 1.0, γp = 1.0, δ = 0.2, unless otherwise stated. Models were also run for low and high levels of both adult survival (S = 0.2, S = 0.8, respectively) and clonal reproduction (π = 0.2, π = 0.6, respectively).
Footnotes
One contribution of 18 to a Discussion Meeting Issue ‘Genetics and the causes of evolution: 150 years of progress since Darwin’.
References
- Barrett S. C. H., Harder L. D., Cole W. W.2004Correlated evolution of floral morphology and mating-type frequencies in a sexually polymorphic plant. Evolution 58, 964–975 (doi:10.1554/03-417) [DOI] [PubMed] [Google Scholar]
- Case A. L., Barrett S. C. H.2001Ecological differentiation of combined and separate sexes of Wurmbea dioica (Colchicaceae) in sympatry. Ecology 82, 2601–2616 (doi:10.1890/0012-9658(2001)082[2601:EDOCAS]2.0.CO;2) [Google Scholar]
- Charlesworth D.2002Plant sex determination and sex chromosomes. Heredity 88, 94–101 (doi:10.1038/sj.hdy.6800016) [DOI] [PubMed] [Google Scholar]
- Charlesworth B., Charlesworth D.1978A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997 (doi:10.1086/283342) [Google Scholar]
- Charlesworth D., Charlesworth B.1979The evolutionary genetics of sexual systems in flowering plants. Proc. R. Soc. Lond. B 205, 513–530 (doi:10.1098/rspb.1979.0082) [DOI] [PubMed] [Google Scholar]
- Charlesworth B., Charlesworth D.2009Darwin and genetics. Genetics 183, 757–766 (doi:10.1534/genetics.109.109991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D., Guttman D. S.1999The evolution of dioecy and plant sex chromosome systems. In Sex determination in plants (ed. Ainsworth C. C.). Oxford, UK: BIOSIS Scientific Publishers Ltd [Google Scholar]
- Clarke B. C., Partridge L., Robertson A.1988Frequency-dependent selection. New York, NY: Cambridge University Press [Google Scholar]
- Correns C.1922Sex determination and numerical proportion of genders in common sorrel (Rumex acetosa) (translated from German). Biol. Zentralblatt 42, 465–480 [Google Scholar]
- Costich D. E.1995Gender specialization across a climatic gradient—experimental comparison of monoecious and dioecious Ecballium. Ecology 76, 1036–1050 (doi:10.2307/1940914) [Google Scholar]
- Darwin C. R.1871The descent of man and selection in relation to sex. London, UK: John Murray [Google Scholar]
- Darwin C. R.1877The different forms of flowers on plants of the same species. London, UK: John Murray [Google Scholar]
- Delph L. F.1999Sexual dimorphism in life history. In Gender and sexual dimorphism in flowering plants (eds Geber M. A., Dawson T. E., Delph L. F.), pp. 149–174 Berlin, Germany: Springer [Google Scholar]
- Dorken M. E., Barrett S. C. H.2004aSex determination and the evolution of dioecy from monoecy in Sagittaria latifolia (Alismataceae). Proc. R. Soc. Lond. B 271, 213–219 (doi:10.1098/rspb.2003.2580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorken M. E., Barrett S. C. H.2004bChloroplast haplotype variation among monoecious and dioecious populations of Sagittaria latifolia (Alismataceae) in eastern North America. Mol. Ecol. 13, 2699–2707 (doi:10.1111/j.1365-294X.2004.02246.x) [DOI] [PubMed] [Google Scholar]
- Dorken M. E., Friedman J., Barrett S. C. H.2002The evolution and maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae). Evolution 56, 31–41 (doi:10.1554/0014-3820(2002)056[0031:TEAMOM]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Eckert C. G., Barrett S. C. H.1995Style morph ratios in tristylous Decodon verticillatus (Lythraceae): selection vs. historical contingency. Ecology 76, 1051–1066 (doi:10.2307/1940915) [Google Scholar]
- Edwards A. W. F.1998Natural selection and the sex ratio: Fisher's sources. Am. Nat. 151, 564–569 (doi:10.1086/286141) [DOI] [PubMed] [Google Scholar]
- Edwards A. W. F.2000Carl Düsing (1884) on the regulation of the sex-ratio. Theor. Popul. Biol. 58, 255–257 (doi:10.1006/tpbi.2000.1482) [DOI] [PubMed] [Google Scholar]
- Ehlers B. K., Bataillon T.2007‘Inconstant males’ and the maintenance of labile sex expression in subdioecious plants. New Phytol. 174, 194–211 (doi:10.1111/j.1469-8137.2007.01975.x) [DOI] [PubMed] [Google Scholar]
- Fisher R. A.1930The genetical theory of natural selection. London: Oxford University Press [Google Scholar]
- Geber M. A., Dawson T. E., Delph L. F.1999Gender and sexual dimorphism in flowering plants. Berlin, Germany: Springer [Google Scholar]
- Hardy I. C. W.2002Sex ratios: concepts and research methods. Cambridge, UK: Cambridge University Press [Google Scholar]
- Harris M. S., Pannell J. R.2010Canopy seed storage is associated with sexual dimorphism in the woody dioecious genus Leucadendron. J. Ecol. 98, 509–515 (doi:10.1111/j.1365-2745.2009.01623.x) [Google Scholar]
- de Jong T. J., de Van Batenburg F. H. D., Van Dijk J.2002Seed sex ratio in dioecious plants depends on relative dispersal of pollen and seeds: an example using a chessboard simulation model. J. Evol. Biol. 15, 373–379 (doi:10.1046/j.1420-9101.2002.00398.x) [Google Scholar]
- Lloyd D. G.1974Female-predominant sex ratios in angiosperms. Heredity 32, 35–44 (doi:10.1038/hdy.1974.3) [Google Scholar]
- Lloyd D. G.1976Transmission of genes via pollen and ovules in gynodioecious angiosperms. Theor. Popul. Biol. 9, 299–316 (doi:10.1016/0040-5809(76)90050-2) [DOI] [PubMed] [Google Scholar]
- Maurice S., Fleming T. H.1995The effect of pollen limitation on plant reproductive systems and the maintenance of sexual polymorphisms. Oikos 74, 55–60 (doi:10.2307/3545674) [Google Scholar]
- Morgan M. T., Barrett S. C. H.1988Historical factors and anisoplethic population structure in tristylous Pontederia cordata L.: a re-assessment. Evolution 42, 496–504 (doi:10.2307/2409034) [DOI] [PubMed] [Google Scholar]
- Pannell J. R.2006Effects of colonization and metapopulation dynamics on the evolution of plant sexual systems. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 223–238 Oxford, UK: Oxford University Press [Google Scholar]
- Pritchard J. K., Stephens M., Donnelly P.2000Inference of population structure using multilocus genotype data. Genetics 155, 945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. W.1963The mechanism of sex determination in Rumex hastatulus. Genetics 48, 1265–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik I., Barrett S. C. H.2005Mechanisms governing sex-ratio variation in dioecious Rumex nivalis. Evolution 59, 814–825 [PubMed] [Google Scholar]
- Stehlik I., Barrett S. C. H.2006Pollination intensity influences sex ratios in dioecious Rumex nivalis, a wind-pollinated plant. Evolution 60, 1207–1214 (doi:10.1554/06-026.1) [PubMed] [Google Scholar]
- Stehlik I., Kron P., Barrett S. C. H., Husband B. C.2007Sexing pollen reveals female bias in a dioecious plant. New Phytol. 175, 185–194 (doi:10.1111/j.1469-8137.2007.02093.x) [DOI] [PubMed] [Google Scholar]
- Stehlik I., Friedman J., Barrett S. C. H.2008Environmental influence on primary sex ratio in a dioecious plant. Proc. Natl Acad. Sci. USA 105, 10 847–10 852 (doi:10.1073/pnas.0801964105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. R., Ingvarsson P. K.2003Common features of segregation distortion in plants and animals. Genetica 117, 27–35 (doi:10.1023/A:1022308414864) [DOI] [PubMed] [Google Scholar]
- West S. A.2009Sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- Wolf D. E., Takebayashi N.2004Pollen limitation and the evolution of androdioecy from dioecy. Am. Nat. 163, 122–137 (doi:10.1086/380493) [DOI] [PubMed] [Google Scholar]
- Yakimowski S. B., Rymer P. D., Stone H., Barrett S. C. H., Dorken M. E.2009Isolation and characterization of 11 microsatellite markers from Sagittaria latifolia (Alismataceae). Mol. Ecol. Res. 9, 579–581 (doi:10.1111/j.1755-0998.2008.02400.x) [DOI] [PubMed] [Google Scholar]