Abstract
Artificial selection and experimental evolution document natural selection under controlled conditions. Collectively, these techniques are continuing to provide fresh and important insights into the genetic basis of evolutionary change, and are now being employed to investigate mating behaviour. Here, we focus on how selection techniques can reveal the genetic basis of post-mating adaptations to sexual selection and sexual conflict. Alteration of the operational sex ratio of adult Drosophila over just a few tens of generations can lead to altered ejaculate allocation patterns and the evolution of resistance in females to the costly effects of elevated mating rates. We provide new data to show how male responses to the presence of rivals can evolve. For several traits, the way in which males responded to rivals was opposite in lines selected for male-biased, as opposed to female-biased, adult sex ratio. This shows that the manipulation of the relative intensity of intra- and inter-sexual selection can lead to replicable and repeatable effects on mating systems, and reveals the potential for significant contemporary evolutionary change. Such studies, with important safeguards, have potential utility for understanding sexual selection and sexual conflict across many taxa. We discuss how artificial selection studies combined with genomics will continue to deepen our knowledge of the evolutionary principles first laid down by Darwin 150 years ago.
Keywords: selection, sperm competition, ejaculate allocation, selection experiment, reproductive isolation
1. Introduction
(a). Insights from selection experiments into evolutionary process
Darwin was acutely aware that artificial selection and breeding experiments could give powerful insight into evolutionary process (Darwin 1868) and he derived from these sources much of the evidence to support the main themes of The Origin of Species by Means of Natural Selection (Darwin 1859). Since Darwin's time, the study of natural selection under controlled conditions by using artificial selection and experimental evolution has provided evidence for mechanisms of inheritance, mutation accumulation and quantitative genetics, especially in the fruitfly and the mouse (Falconer 1992; Falconer & Mackay 1996; Mackay 2001; Barton & Keightley 2002). There has also been an extremely fruitful tradition of experimental evolution research using microbes (Mortlock 1984; Dykhuizen 1990), which is now also providing novel insights into mechanisms underlying social evolution (West et al. 2007; Buckling et al. 2009).
Among the many classic artificial selection experiments conducted in Drosophila melanogaster are those that have targeted bristle numbers, flight ability, ethanol tolerance, body size and life history (Mackay et al. 2009). These experiments show that sustained and replicated responses to selection are possible (e.g. Yoo 1980). They also reveal the contribution of standing genetic variation, new mutations and effective population size to selection responses (e.g. Weber 1990; Barton & Keightley 2002; Snook et al. 2009). The use of artificial selection or experimental evolution (i.e. the creation of a specific set of conditions to which a suite of traits may evolve) has also been an important tool in microbial genetics. Classic studies have illuminated the spread of new mutations (e.g. Atwood et al. 1951), biochemical adaptation (Mortlock 1984), the evolution of resistance to phage (e.g. Lenski 1988) and the effects of interspecific competition for limited resources (e.g. Gottschal et al. 1979). The value of these approaches looks set to increase in the future as their significance is realized across traditional disciplines. For example, recent reviews have emphasized the lack of knowledge of mechanisms of genetic adaptation to pervasive forces such as rising global temperatures (Gienapp et al. 2008).
(b). Insights from selection experiments into post-copulatory sexual selection and sexual conflict
There has been relatively less attention until recently on the application of artificial selection techniques to the study of behavioural ecology, sexual selection and sexual conflict. One exception is Manning's work on mating speed in D. melanogaster (Manning 1961, 1963). It was realized from the 1980s onwards that artificial selection and experimental evolution techniques could be used to investigate sexual selection and sexual conflict. Rice and colleagues pioneered the approach of exploring the consequences of the evolutionary manipulation of mating systems to alter the intensity of sexual selection and sexual conflict (e.g. Rice 1992, 1996; Holland & Rice 1999). Sexual conflict arises because of the different evolutionary interests of males and females (Parker 1979), and evolutionary approaches can reveal sexual conflict that occurs because of the expression of the same or different genes in males versus females (intra- versus interlocus sexual conflict, respectively, e.g. Rice 1992, 1996). For example, when males were allowed to evolve against a static female phenotype, alleles favouring an increase in fitness through the male lineage spread more easily because of the lack of counter selection in females (Rice 1996).
Selection experiments that have imposed different mating systems (e.g. monogamy versus polyandry) can reveal the effects on overall male and female fitness of the removal of sexual selection and conflict (Holland & Rice 1999; Hosken et al. 2001, 2009; Martin & Hosken 2003; Crudgington et al. 2005, 2009; Tilszer et al. 2006; Bacigalupe et al. 2007; Fricke & Arnqvist 2007; LaMunyon et al. 2007; Simmons & Garcia-Gonzalez 2008; Gay et al. 2009; Maklakov et al. 2009). For example, female D. melanogaster had longer lifespans following single matings to males from lines selected for 47 generations of monogamy, in comparison with females mated to polyandrous males (Holland & Rice 1999). These findings suggest that monogamous males evolve to be less harmful to females and that monogamous females can be more susceptible to mating costs. It is not yet clear whether costs that can arise from selection owing to sexual conflict can be offset via indirect benefits in subsequent generations (Kirkpatrick & Barton 1997; Cameron et al. 2003; Head et al. 2005; Priest et al. 2008) and, if possible, how often this occurs.
An attraction of applying regimes of monogamy and polyandry is that it can be done across many different taxa. For example, monogamous and polyandrous mating regimes have been applied in the fruitflies D. melanogaster and Drosophila pseudoobscura (Holland & Rice 1999; Crudgington et al. 2005, 2009; Bacigalupe et al. 2007), the seed beetle Callosobruchus maculatus (Fricke & Arnqvist 2007; Gay et al. 2009; Maklakov et al. 2009), the bulb mite Rhizoglyphus robini (Tilszer et al. 2006), the dung flies Sepsis synipsea and Scathophaga stercoraria (Hosken et al. 2001, 2009; Martin & Hosken 2003), the dung beetle Onthophagus taurus (Simmons & Garcia-Gonzalez 2008) and the round worm Caenorhabditis elegans (LaMunyon et al. 2007). Experiments which manipulate the degree of polyandry have also been performed (e.g. Wigby & Chapman 2004; Crudgington et al. 2005; Bacigalupe et al. 2007; Linklater et al. 2007; Reuter et al. 2008; Hosken et al. 2009). Together, these studies support the idea that experimental evolutionary manipulations of sexual selection and sexual conflict can lead to significant divergence in both pre- and post-mating traits.
The types of studies described above have also been useful in testing the important prediction that elevated sexual conflict can lead to antagonistic coevolution (e.g. Parker 1979; Holland & Rice 1998), which in turn can promote reproductive isolation leading to speciation (e.g. Parker & Partridge 1998; Rice 1998; Gavrilets 2000; Gavrilets et al. 2001). A distinctive prediction of sexual conflict is that reproductive isolation can evolve more quickly under large population sizes (Gavrilets 2000), in contrast to classical predictions (Lande 1981). There is not yet a consistent pattern of results, with some studies supporting a role for conflict in reproductive isolation (Martin & Hosken 2003; Hosken et al. 2009) and others not (Wigby & Chapman 2006; Bacigalupe et al. 2007; Gay et al. 2009). This may mean that conflict has a role to play in reproductive isolation only under some conditions, that such experiments have not yet run over a sufficiently long period of evolutionary time or that not all relevant traits have been studied (see below for discussion of further pitfalls).
There is also considerable interest in using selection techniques to study rates of adaptation. Theory states that sexual selection can speed up the rate of adaptation via the partitioning of genetic variation, with beneficial alleles being more effectively channelled through fewer breeding individuals because of mate choice (e.g. Lorch et al. 2003). Sexual selection can therefore reinforce natural selection and accelerate the rate of adaptation. This is an important prediction, but to date there are contradictory empirical results. Tests of population fitness in lines of D. melanogaster maintained with and without sexual selection revealed no, or negative, effects of sexual selection on the rate of adaptation (Holland 2002; Rundle et al. 2006). However, a study on adaptation to host shifts in seed beetles (C. maculatus) under polygamy and monogamy found that sexual selection increased the speed of adaptation, measured as fitness in the new environment (Fricke & Arnqvist 2007). In addition to the predicted beneficial effects of sexual selection, negative effects on population fitness can also arise owing to sexual conflict (Arnqvist & Rowe 2005). This may explain some of the discrepancies, as the extent of sexual conflict may counterbalance the beneficial effects of sexual selection.
An additional useful technique is to combine simultaneous evolutionary manipulations of mating systems and life histories. For example, Maklakov et al. (2009) combined selection for monogamy and polyandry with selection for early and late age reproduction in C. maculatus. The results showed that adaptation to life history had the most important effects on fitness, with the presence or absence of sexual selection having relatively little influence.
2. Artificial selection and experimental evolution in drosophila melanogaster
The breadth of organisms and traits targeted by experimental evolution and artificial selection is evident from the research summarized above. We expand one example below, focusing on our recent studies of the consequences of manipulating the intensity of sexual selection and conflict in D. melanogaster. We then also describe a new investigation of the evolution of male responses to rivals. To manipulate sexual selection and sexual conflict, we created experimental evolution lines in which the adult sex ratio was male-biased (MB, one female : three males), equal sex (ES) or female-biased (FB, three females : one male). Previously, females from these lines were tested for evidence of female resistance to the costly effects of elevated matings with males (Wigby & Chapman 2004). Given that the number of matings per female was significantly higher in the MB lines, we predicted that MB females would be selected to evolve resistance to mating costs. The survival of females from the MB lines in the presence of wild-type males was significantly higher than for females from the ES and then FB lines (Wigby & Chapman 2004). There were no intrinsic differences in female survival in the absence of males, which argues that females had evolved a specific resistance mechanism to counter the costly effects of elevated matings (Wigby & Chapman 2004).
The males from the MB and FB lines showed ejaculate allocation patterns consistent with their evolutionary history of sexual selection. Given that the risk of usurpation by other males is very high under MB conditions, males are predicted to allocate more ejaculate to their earlier mates, rather than to be prudent, and they therefore run the risk of ejaculate exhaustion. To test this idea, males from the MB and FB lines were mated to five wild-type virgin females in succession. MB males did indeed lose fertility significantly faster than did males from the FB lines (Linklater et al. 2007). The loss of fertility over successive mates was associated more strongly with a reduction in the size of the male accessory glands than with the testes.
(a). Evolutionary manipulations of male responses to rivals
The way in which males can tailor matings and ejaculate transfer in response to rivals was first demonstrated in insects (Gage & Baker 1991) and has subsequently been studied across a range of vertebrate and invertebrate taxa (reviewed by Wedell et al. 2002; see also Neff et al. 2003; Siva-Jothy & Stutt 2003; Pound & Gage 2004; Friberg 2006; Carazo et al. 2007). There is good evidence that males that experience, or perceive, high levels of sperm competition can ejaculate more sperm (Parker et al. 1996, 1997; Wedell et al. 2002; Engqvist & Reinhold 2005), and can transfer more seminal fluid proteins (Wigby et al. 2009).
Male D. melanogaster respond to the presence of rival males both before and during mating. However, it is primarily exposure to rivals prior to mating that evokes adaptive male responses resulting in significantly higher competitive reproductive success under conditions where sperm competition is strong (Bretman et al. 2009a). The data are consistent with the idea that it is the length of exposure to rivals that is most important, rather than the number of males or male density, although the exact mechanisms are not yet known (Bretman et al. 2009b). What is clear is that the plastic responses exhibited by males are tightly calibrated to their socio-sexual environment and result in significantly increased competitive reproductive success.
(b). Empirical study of male responses to rivals
It is not yet known whether the magnitude, presence or sign of the plastic responses of males to their rivals can themselves evolve. These questions were investigated in new empirical work that we report here. We tested whether responses to rivals had evolved in males from the MB and FB lines described above (see the electronic supplementary material for detailed methods). We predicted that changes in the relative balance of intra- versus intersexual selection, and specifically the evolutionary history of elevated male–male competition in the MB lines, would lead to stronger selection on male responses to rivals in MB when compared with FB lines. We also tested the idea that these manipulations would select for differences in female responses to matings. We examined how females from the lines responded to single matings with males that did and did not transfer an ejaculate component (sex peptide, SP) that modulates egg production, female receptivity and that mediates sexual conflict (Wigby & Chapman 2005; Fricke et al. 2009).
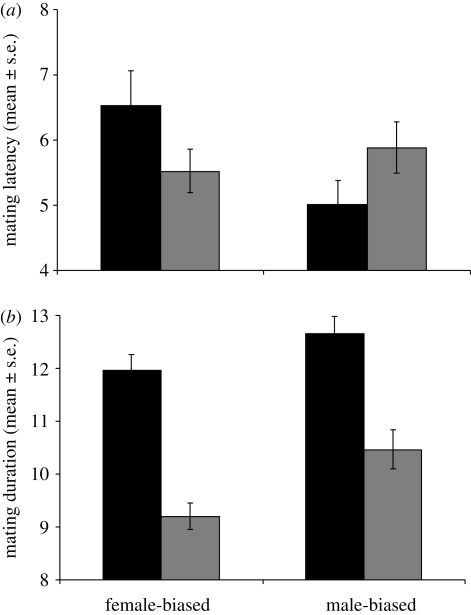
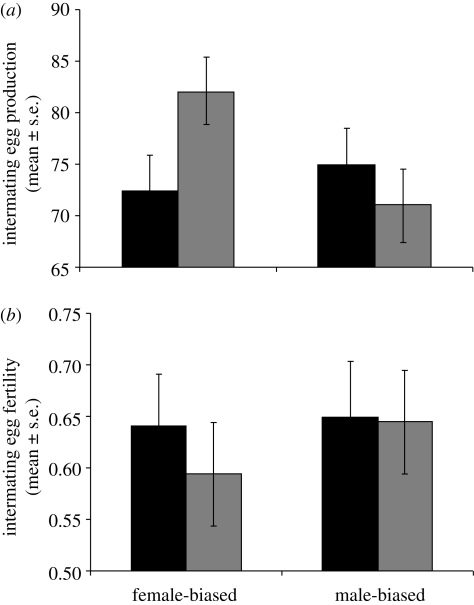
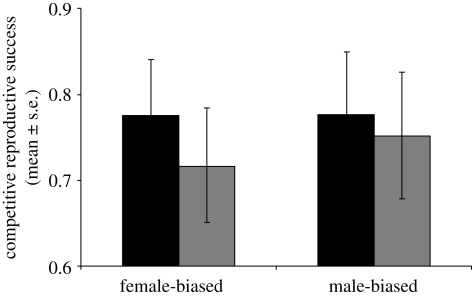
Our evolutionary manipulation of adult sex ratio produced opposing responses in mating latency in MB versus FB males (table 1 and figure 1a). MB males showed significantly decreased latency until mating and FB males significantly increased latency following exposure to rival males. Hence MB, but not FB, males retained the pattern found in wild-type males (Bretman et al. 2009a). Males from both lines showed significantly increased mating duration following exposure to rival males (table 1 and figure 1b). Hence, males from both regimes retained significant plasticity in mating duration, though there was a non-significant overall tendency for MB males to mate for longer. Egg production also showed a significant interaction (table 1). Females laid more eggs following matings to MB males that had been exposed to rivals and fewer eggs following matings to similarly exposed FB males (figure 2a). Hence, again MB males exhibited the wild-type pattern, whereas FB males showed the opposite. Further contrasts were evident in egg fertility, which was significantly lower in females mated to FB males without rivals, but not significantly different in mates of either group of MB males (table 1 and figure 2b). MB males fathered more, but not significantly more, progeny than FB males in a competitive context (p = 0.08; table 1 and figure 3), which may be linked to their longer mating duration (Bretman et al. 2009a). Both MB and FB males exposed to rivals prior to mating had significantly higher reproductive success, showing that plastic male responses to rivals were maintained in both regimes. Finally, the tests on females from the MB and FB lines showed, as expected based on the known phenotype of SP, significant differences in receptivity and intermating interval following receipt of SP. However, there were no consistent differences owing to selection regime (electronic supplementary material, table S1), and therefore no evidence that FB and MB females differed in receptivity and fecundity following single matings, or that they responded in a qualitatively different way to receipt of SP.
Table 1.
The effect of female-biased (FB) and male-biased (MB) selection regime and the presence or absence of rivals on variation in the latency to mate, mating duration, intermating egg production, intermating egg fertility and competitive reproductive success. Shown are the results of generalized linear models (for details, see electronic supplementary material).
| source | latency to mate | mating duration | intermating egg production | intermating egg fertility | competitive reproductive success |
|---|---|---|---|---|---|
| FB/MB selection regime | F1,4 = 1.615, p = 0.273 | F1,4 = 2.418, p = 0.195 | F1,4 = 1.866, p = 0.244 |
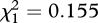 , p = 0.693 , p = 0.693 |
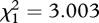 , p = 0.083 , p = 0.083 |
| ± rivals | F1,355 = 0.062, p = 0.804 | F1,356 = 62.569, p < 0.001 | F1,355 = 0.964, p = 0.327 |
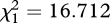 , p < 0.001 , p < 0.001 |
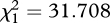 , p < 0.001 , p < 0.001 |
| FB/MB regime × rivals interaction | F1,355 = 5.538, p = 0.019 | F1,355 = 1.663, p = 0.198 | F1,355 = 3.826, p = 0.051 |
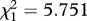 , p = 0.016 , p = 0.016 |
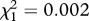 , p = 0.965 , p = 0.965 |
Figure 1.
(a) Mating latency (mean minutes ± s.e.) for males from the male-biased (MB) and female-biased (FB) selection lines either exposed or not exposed to rivals for 5 days prior to mating. (b) Mating duration (mean minutes ± s.e.) for males from the MB and FB selection lines either exposed or not exposed to rivals for 5 days prior to mating. Black bars,+rivals; grey bars,−rivals.
Figure 2.
(a) Female fecundity in the 24 h intermating interval (mean number of eggs ± s.e.) for females mated to males from the MB and FB selection lines either exposed or not exposed to rivals for 5 days prior to mating. (b) Female fertility in the 24 h intermating interval (proportion of eggs fertilized ± s.e.) for females mated to males from the MB and FB selection lines either exposed or not exposed to rivals for 5 days prior to mating. Black bars,+rivals; grey bars,−rivals.
Figure 3.
Competitive reproductive success (proportion of offspring sired ± s.e.) for males from the MB and FB selection lines either exposed or not exposed to rivals for 5 days prior to mating. Black bars,+rivals; grey bars,−rivals.
Overall, the study shows that experimental evolution under MB and FB regimes can lead to consistent and significant changes in the way that males respond to rivals, with males reared under MB conditions exhibiting responses similar to those of wild-type males. Differences were seen in both pre-mating and post-mating traits and there were significant interactions, where males from the FB and MB regimes (or the females to which they were mated) showed opposing responses. Consistent with these findings is the idea that the relaxation of male–male competition, and the potentially increased opportunity for female choice in the FB lines, led to the evolution of altered responses to rivals in FB males. An alternative explanation is the strong selection arising from the high frequency of male mating in the FB regimes to avoid sperm depletion, leading to constraints in how FB males responded to competitors.
Sexual selection can speed up the rate of adaptation because it can: (i) reinforce natural selection; (ii) speed up the fixation of advantageous alleles; and (iii) speed up the purging of deleterious alleles (Whitlock 2000; Lorch et al. 2003). However, these models are based on the assumption that both male–male competition and female choice are simultaneously elevated, or present/absent. The scenario employed in the creation of the MB and FB lines alters the balance of intra- to intersexual selection, and predictions for the speed of adaptation under these conditions have not yet been explored. Few significant effects owing to selection regime were found in our study, which is consistent with the idea that changes in the intensity of male–male competition relative to female choice (rather than elevated sexual selection per se) selected for altered responses to rivals.
Males exposed to rivals mate for longer and can transfer more seminal fluid proteins during those longer matings (Wigby et al. 2009). An artificial selection experiment on male accessory gland size suggested that the seminal fluid complement of a male can itself evolve (Wigby et al. 2009). Differences in the complement of male ejaculates could select for differences in how females respond to a single mating. However, there was no evidence that females from the FB, ES or MB lines differed in how they responded to single matings that either did or did not transfer the SP ejaculate component (electronic supplementary material). Collectively, these data show how relatively simple evolutionary manipulations to the way that adults interact can provide significant insight into the mechanisms underlying both pre- and post-mating sexual selection.
3. Discussion
(a). New avenues for artificial selection and experimental evolution in the study of sexual selection and sexual conflict
The potential value of the selection experiment in investigating evolutionary responses to factors such as adult sex ratio and the relative balance of intra- versus intersexual selection is significant, as illustrated above. There is now an exciting possibility of combining such selection studies with genomic profiling to identify genes that underpin these important fitness traits (Toma et al. 2002). To date there are relatively few such tests. One exception is a study on mating speed in D. melanogaster (Mackay et al. 2005) in which replicated artificial selection for fast and slow mating speed was performed. Transcriptional profiling of the responses to selection in fast and slow mating speed lines revealed a large fraction of genes (approx. 20% of the genome) with potentially altered expression. As with other studies of mating-related genes (e.g. Lawniczak & Begun 2004; McGraw et al. 2004), changes were subtle and generally less than twofold. A useful feature of this kind of microarray study is that it can test for biases in the chromosomal locations of the differentially expressed genes. This is of interest in the context of sexual conflict because there are predicted ‘hot spots’ for genes involved in mediating sexual antagonism (Gibson et al. 2001). The Mackay study found that the X chromosome harboured fewer differentially expressed genes than expected (Mackay et al. 2005). X-linked genes are filtered more often by their passage through the selective environment of females rather than males, and the X chromosome contains an excess of genes that are female-biased in their expression (Parisi et al. 2003; Ranz et al. 2003; Sturgill et al. 2007). Hence, the X chromosome potentially harbours many more ‘female benefit’ genes than expected.
(b). Avoiding the pitfalls
We have so far emphasized the utility of selection experiments for studying mechanisms of adaptation in response to sexual selection and sexual conflict. However, it is also necessary to consider the potential pitfalls (Harshman & Hoffman 2000; Fuller et al. 2005). For example, there has been, to date, perhaps a rather uncritical tendency to view inconsistencies between different studies testing for the evidence of reproductive isolation driven by sexual conflict to be the result of the focal-selective forces under study, rather than because of inadvertent selection or other causes. Other potentially confounding factors that can contribute to differences include differential genetic drift, inbreeding, feeding regimes, differential strengths of selection, gene × environment interactions, constancy of selective environment and differential accumulation of age-specific mutations. All these potential pitfalls have been discussed previously (Harshman & Hoffman 2000; Fuller et al. 2005); however, they have not yet been fully incorporated into recent investigations of sexual conflict using selection techniques. To give an example, it has been assumed that elevated sexual conflict sometimes does and sometimes does not lead to measurable differences in reproductive isolation (Hosken et al. 2009). However, there are other possibilities, for example (i) sexual conflict may not actually be manipulated in all studies; (ii) inadvertent selection may play a role; (iii) there may be differential inbreeding or genetic drift owing to large differences in effective population sizes; (iv) the use of constant environments and abundant food may obscure differences; (v) there may be differential gene × environment interactions between regimes. So far, the only factor that has been considered in detail is the effective population size, Ne (Wigby & Chapman 2004; Rice & Holland 2005; Snook et al. 2009). A recent review suggests that Ne is not likely to have confounded the differences between studies (Snook et al. 2009), though it clearly can have a significant effect (Reuter et al. 2008). The use of replicate populations to guard against the mistaken attribution of a response to selection when it is in fact drift has proved to be very useful.
In the study reported here, we saw significant, consistent responses in the different replicate lines in male responses to rivals, suggesting that those responses may have a common genetic underpinning. However, responses in females from the same lines to the effects of a single mating were more variable, perhaps indicating divergent responses. Differential genetic drift is unlikely to be an explanation (Snook et al. 2009). Inbreeding effects can be problematic, but can in general be countered by crossing between different replicate lines (Hosken & Ward 2001; Hosken et al. 2001). However, this procedure can then obscure coevolved differences within lines or indeed any distinct evolutionary trajectories that may have occurred. Whenever lines are tested in a different environment to that for which they are adapted, there is also the potential for differential gene × environment interactions to be expressed. In different environments, fitness may also be favoured by different combinations of alleles, potentially limiting the general utility of the results. An over-abundance of food might also represent a problem if it reduces the likelihood of the expression of differences between regimes. Regimes that employ discrete generations over extended periods may be more likely to accumulate age-specific mutations than are populations that can be maintained under overlapping generations (Harshman & Hoffman 2000), but the latter methodology is not always possible within the context of the selection regimes that are applied.
In short, it is necessary to consider all these factors in concluding whether divergence between different lines is because of the selective regime applied and more caution in this respect is required. The next generation of experiments should seek to equalize Ne as far as possible, increase the numbers of replicates (to provide rigorous tests of the possibility of different evolutionary trajectories, while ruling out drift) and provide fluctuating selection pressures through variable food supply, humidity and/or temperature. Studies in which the results from selection experiments converge with the outcomes from phenotypic manipulations and comparative studies will also be informative. When these principles are rigorously adopted, we will gain increasingly valuable insights into mechanisms of adaptation and evolutionary contingency.
4. Conclusion
Unique and powerful inferences have been gained since the time of Darwin by using artificial selection and experimental evolution techniques. Recently, the utility of these approaches has been realized in the context of studying the evolution and mechanistic basis of selection arising from sexual selection and sexual conflict. We highlighted with new empirical data the potential utility of selection approaches for testing how the responses of males to their rivals evolve. We found that alterations of the balance between male–male competition and female choice altered in a predictable and repeatable manner the way that males responded to mating rivals. Finally, we discussed the potential pitfalls and the necessary and important safeguards required in order to investigate important questions such as whether sexual conflict can lead to independent evolutionary trajectories ultimately resulting in reproductive isolation.
Acknowledgements
Thanks to Mike Bonsall and Brian Charlesworth for the invitation to participate in this discussion meeting; to the two referees and to Brian Charlesworth for their constructive comments; to the Royal Society and the Genetics Society for organizing the meeting; and to the Royal Society, BBSRC and NERC for funding.
Footnotes
One contribution of 18 to a Discussion Meeting Issue ‘Genetics and the causes of evolution: 150 years of progress since Darwin’.
References
- Arnqvist G., Rowe L.2005Sexual conflict. Princeton, NJ: Princeton University Press [Google Scholar]
- Atwood K. C., Schneider L. K., Ryan F. J.1951Selective mechanisms in bacteria. Cold Spring Harbor Symp. Quant. Biol. 16, 345–355 [DOI] [PubMed] [Google Scholar]
- Bacigalupe L. D., Crudgington H. S., Hunter F., Moore A. J., Snook R. R.2007Sexual conflict does not drive reproductive isolation in experimental populations of Drosophila pseudoobscura. J. Evol. Biol. 20, 1763–1771 (doi:10.1111/j.1420-9101.2007.01389.x) [DOI] [PubMed] [Google Scholar]
- Barton N. H., Keightley P. D.2002Understanding quantitative genetic variation. Nat. Rev. Genet. 3, 11–21 (doi:10.1038/nrg700) [DOI] [PubMed] [Google Scholar]
- Bretman A., Fricke C., Chapman T.2009aPlastic responses of male D. melanogaster to the level of sperm competition increase male reproductive fitness. Proc. R. Soc. B 276, 1705–1711 (doi:10.1098/rspb.2008.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretman A., Fricke C., Hetherington P., Stone R., Chapman T.2009bVariation in exposure to rivals and plastic responses to sperm competition in Drosophila melanogaster. Behav. Ecol. 21, 317–321 [Google Scholar]
- Buckling A., Maclean R. C., Brockhurst M. A., Colegrave N.2009The Beagle in a bottle. Nature 457, 824–829 (doi:10.1038/nature07892) [DOI] [PubMed] [Google Scholar]
- Cameron E., Day T., Rowe L.2003Sexual conflict and indirect benefits. J. Evol. Biol. 16, 1055–1060 [DOI] [PubMed] [Google Scholar]
- Carazo P., Font E., Alfthan B.2007Chemosensory assessment of sperm competition levels and the evolution of internal spermatophore guarding. Proc. R. Soc. B 274, 261–267 (doi:10.1098/rspb.2006.3714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crudgington H. S., Beckerman A. P., Brüstle L., Green K., Snook R. R.2005Experimental removal and elevation of sexual selection: does sexual selection generate manipulative males and resistant females? Am. Nat. 165, S72–S87 (doi:10.1086/429353) [DOI] [PubMed] [Google Scholar]
- Crudgington H. S., Fellows S., Badcock N. S., Snook R. R.2009Experimental manipulation of sexual selection promotes greater male mating capacity but does not alter sperm investment. Evolution 63, 926–938 (doi:10.1111/j.1558-5646.2008.00601.x) [DOI] [PubMed] [Google Scholar]
- Darwin C.1859On the origin of species by means of natural selection. London, UK: John Murray [Google Scholar]
- Darwin C.1868The variation of animals and plants under domestication. London, UK: John Murray [Google Scholar]
- Dykhuizen D. E.1990Experimental studies of natural selection in bacteria. Ann. Rev. Ecol. Syst. 21, 373–398 (doi:10.1146/annurev.es.21.110190.002105) [Google Scholar]
- Engqvist L., Reinhold K.2005Pitfalls in experiments testing predictions from sperm competition theory. J. Evol. Biol. 18, 116–123 (doi:10.1111/j.1420-9101.2004.00792.x) [DOI] [PubMed] [Google Scholar]
- Falconer D. S.1992Early selection experiments. Ann. Rev. Genet. 26, 1–14 (doi:10.1146/annurev.ge.26.120192.000245) [DOI] [PubMed] [Google Scholar]
- Falconer D. S., Mackay T. F. C.1996Introduction to quantitative genetics, 4th edn.Harlow, UK: Longman [Google Scholar]
- Friberg U.2006Male perception of female mating status: its effect on copulation duration, sperm defence and female fitness. Anim. Behav. 72, 1259–1268 (doi:10.1016/j.anbehav.2006.03.021) [Google Scholar]
- Fricke C., Arnqvist G.2007Rapid adaptation to a novel host in a seed beetle (Callosobruchus maculatus): the role of sexual selection. Evolution 61, 440–454 (doi:10.1111/j.1558-5646.2007.00038.x) [DOI] [PubMed] [Google Scholar]
- Fricke C., Bretman A., Chapman T.2009Female nutritional status determines the magnitude and sign of responses to a male ejaculate signal in Drosophila melanogaster. J. Evol. Biol. 23, 157–165 (doi:10.1111/j.1420–9101.2009.01882.x) [DOI] [PubMed] [Google Scholar]
- Fuller R. C., Baer C. F., Travis J.2005How and when selection experiments might actually be useful. Int. Comp. Biol. 45, 391–404 (doi:10.1093/icb/45.3.391) [DOI] [PubMed] [Google Scholar]
- Gage M. J. G., Baker R. R.1991Ejaculate size varies with sociosexual situation in an insect. Ecol. Entomol. 16, 331–337 (doi:10.1111/j.1365-2311.1991.tb00224.x) [Google Scholar]
- Gavrilets S.2000Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403, 886–889 (doi:10.1038/35002564) [DOI] [PubMed] [Google Scholar]
- Gavrilets S., Arnqvist G., Friberg U.2001The evolution of female mate choice by sexual conflict. Proc. R. Soc. Lond. B 268, 531–539 (doi:10.1098/rspb.2000.1382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L., Eady P. E., Vasudev R., Hosken D. J., Tregenza T.2009Does reproductive isolation evolve faster in larger populations via sexually antagonistic coevolution? Biol. Lett. 5, 693–696 (doi:10.1098/rsbl.2009.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. R., Chippindale A. K., Rice W. R.2001The X chromosome is a hot spot for sexually antagonistic fitness variation. Proc. R. Soc. Lond. B 269, 499–505 (doi:10.1098/rspb.2001.1863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gienapp P., Teplitsky C., Alho J. S., Mills J. A., Merilä J.2008Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178 (doi:10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- Gottschal J. C., De Vries S., Kuenen J. G.1979Competition between the facultatively chemolithotrophic Thiobacillus A2, an obligately chemolithotrophic Thiobacillus and a heterotrophic Spirillum for inorganic and organic substrates. Arch. Microbiol. 121, 241–249 (doi:10.1007/BF00425062) [Google Scholar]
- Harshman L., Hoffman A. A.2000Laboratory selection experiments using Drosophila: what do they really tell us? Trends Ecol. Evol. 15, 32–36 (doi:10.1016/S0169-5347(99)01756-5) [DOI] [PubMed] [Google Scholar]
- Head M. L., Hunt J., Jennions M. D., Brooks R.2005The indirect benefits of mating with attractive males outweigh the direct costs. PLoS Biol. 3, e33 (doi:10.1371/journal.pbio.0030033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland B.2002Sexual selection fails to promote adaptation to a new environment. Evolution 56, 721–730 [DOI] [PubMed] [Google Scholar]
- Holland B., Rice W. R.1998Chase-away sexual selection: antagonistic seduction versus resistance. Evolution 52, 1–7 (doi:10.2307/2410914) [DOI] [PubMed] [Google Scholar]
- Holland B., Rice W. R.1999Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA 96, 5083–5088 (doi:10.1073/pnas.96.9.5083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken D., Garner T., Ward P. I.2001Sexual conflict selects for male and female reproductive characters. Curr. Biol. 11, 489–493 (doi:10.1016/S0960-9822(01)00146-4) [DOI] [PubMed] [Google Scholar]
- Hosken D. J., Martin O. Y., Wigby S., Chapman T., Hodgson D. J.2009Sexual conflict and reproductive isolation in flies. Biol. Lett. 5, 697–699 (doi:10.1098/rsbl.2009.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M., Barton N. H.1997The strength of indirect selection on female mating preferences. Proc. Natl Acad. Sci. USA 94, 1282–1286 (doi:10.1073/pnas.94.4.1282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMunyon C. W., Bouban O., Cutter A. D.2007Postcopulatory sexual selection reduces genetic diversity in experimental populations of Caenorhabditis elegans. J. Heredity 98, 67–72 (doi:10.1093/jhered/esl052) [DOI] [PubMed] [Google Scholar]
- Lande R.1981Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725 (doi:10.1073/pnas.78.6.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawniczak M. K., Begun D. J.2004A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome 47, 900–910 (doi:10.1139/g04-050) [DOI] [PubMed] [Google Scholar]
- Lenski R. E.1988Experimental studies of pleiotropy and epistasis in Escherichia coli. II. Compensation for maladaptive effects associated with resistance to virus T4. Evolution 42, 433–440 (doi:10.2307/2409029) [DOI] [PubMed] [Google Scholar]
- Linklater J. R., Wertheim B., Wigby S., Chapman T.2007Ejaculate depletion patterns evolve in response to experimental manipulation of sex ratio in Drosophila melanogaster. Evolution 61, 2027–2034 (doi:10.1111/j.1558-5646.2007.00157.x) [DOI] [PubMed] [Google Scholar]
- Lorch P. D., Proulx S., Rowe L., Day T.2003Condition-dependent sexual selection can accelerate adaptation. Evol. Ecol. Res. 5, 867–881 [Google Scholar]
- Mackay T. F. C.2001Quantitative trait loci in Drosophila. Nat. Rev. Genet. 2, 11–20 (doi:10.1038/35047544) [DOI] [PubMed] [Google Scholar]
- Mackay T. F. C., Heinsohn S. L., Lyman R. F., Moehring A. J., Morgan T. J., Rollmann S. M.2005Genetics and genomics of Drosophila mating behavior. Proc. Natl Acad. Sci. USA 102, 6622–6629 (doi:10.1073/pnas.0501986102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay T. F. C., Stone E. A., Ayroles J. F.2009The genetics of quantitative traits: challenges and prospects. Nat. Rev. Genet. 10, 565–577 (doi:10.1038/nrg2612) [DOI] [PubMed] [Google Scholar]
- Maklakov A. A., Bonduriansky R., Brooks R. C.2009Sex differences, sexual selection, and ageing: an experimental evolution approach. Evolution 63, 2491–2503 (doi:10.1111/j.1558-5646.2009.00750.x) [DOI] [PubMed] [Google Scholar]
- Manning A.1961The effects of artificial selection for mating speed in Drosophila melanogaster. Anim. Behav. 9, 82–92 (doi:10.1016/0003-3472(61)90054-9) [Google Scholar]
- Manning A.1963Selection for mating speed in Drosophila melanogaster based on the behaviour of one sex. Anim. Behav. 11, 116–120 (doi:10.1016/0003-3472(63)90019-8) [Google Scholar]
- Martin O. Y., Hosken D. J.2003The evolution of reproductive isolation through sexual conflict. Nature 423, 979–982 (doi:10.1038/nature01752) [DOI] [PubMed] [Google Scholar]
- McGraw L. A., Gibson G., Clark A. G., Wolfner M. F.2004Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr. Biol. 14, 1509–1514 (doi:10.1016/j.cub.2004.08.028) [DOI] [PubMed] [Google Scholar]
- Mortlock R. P.1984Microorganisms as model systems for studying evolution. New York, NY: Plenum Press [Google Scholar]
- Neff B. D., Fu P., Gross M. R.2003Sperm investment and alternative mating tactics in bluegill sunfish (Lepomis macrochirus). Behav. Ecol. 14, 634–641 (doi:10.1093/beheco/arg032) [Google Scholar]
- Parisi M., Nuttall R., Naiman D., Bouffard G., Malley J., Andrews J., Eastman S., Oliver B.2003Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science 299, 697–700 (doi:10.1126/science.1079190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. A.1979Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum M. S., Blum N. A.), pp. 123–166 New York, NY: Academic Press [Google Scholar]
- Parker G. A., Partridge L.1998Sexual conflict and speciation. Phil. Trans. R. Soc. Lond. B 353, 261–274 (doi:10.1098/rstb.1998.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. A., Ball M. A., Stockley P., Gage M. J. G.1996Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. Lond. B 263, 1291–1297 (doi:10.1098/rspb.1996.0189) [Google Scholar]
- Parker G. A., Ball M. A., Stockley P., Gage M. J. G.1997Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. Lond. B 264, 1793–1802 (doi:10.1098/rspb.1990.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound N., Gage M. J. G.2004Prudent sperm allocation in Norway rats, Rattus norvegicus: a mammalian model of adaptive ejaculate adjustment. Anim. Behav. 68, 819–823 (doi:10.1016/j.anbehav.2004.02.004) [Google Scholar]
- Priest N. K., Galloway L. F., Roach D. A.2008Mating frequency and inclusive fitness in Drosophila melanogaster. Am. Nat. 171, 10–21 (doi:10.1086/523944) [DOI] [PubMed] [Google Scholar]
- Ranz J. M., Castillo-Davis C. I., Meiklejohn C. D., Hartl D. L.2003Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science 300, 1742–1745 (doi:10.1126/science.1085881) [DOI] [PubMed] [Google Scholar]
- Reuter M., Linklater J. R., Lehmann L., Fowler K., Chapman T., Hurst G. D. D.2008Adaptation to experimental alterations of the operational sex ratio in populations of Drosophila melanogaster. Evolution 62, 401–412 (doi:10.1111/j.1558-5646.2007.00300.x) [DOI] [PubMed] [Google Scholar]
- Rice W. R.1992Sexually antagonistic genes—experimental evidence. Science 256, 1436–1439 (doi:10.1126/science.1604317) [DOI] [PubMed] [Google Scholar]
- Rice W. R.1996Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381, 232–234 (doi:10.1038/381232a0) [DOI] [PubMed] [Google Scholar]
- Rice W. R.1998Intergenomic conflict, interlocus antagonistic coevolution and the evolution of reproductive isolation. In Endless forms species and speciation (eds Howard D. J., Berlocher S. H.), pp. 261–270 Oxford, UK: Oxford University Press [Google Scholar]
- Rice W. R., Holland B.2005Experimentally enforced monogamy: inadvertent selection, inbreeding, or evidence for sexually antagonistic coevolution? Evolution 59, 682–685 [PubMed] [Google Scholar]
- Rundle H. D., Chenoweth S. F., Blows M. W.2006The roles of natural and sexual selection during adaptation to a novel environment. Evolution 60, 2218–2225 [PubMed] [Google Scholar]
- Simmons L. W., Garcia-Gonzalez F.2008Evolutionary reduction in testes size and competitive fertilization success in response to the experimental removal of sexual selection in dung beetles. Evolution 62, 2580–2591 (doi:10.1111/j.1558-5646.2008.00479.x) [DOI] [PubMed] [Google Scholar]
- Siva-Jothy M. T., Stutt A. D.2003A matter of taste: direct detection of female mating status in the bedbug. Proc. R. Soc. Lond. B 270, 649–652 (doi:10.1098/rspb.2002.2260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook R. R., Brüstle L., Slate J.2009A test and review of the role of effective population size on experimental sexual selection patterns. Evolution 63, 1923–1933 (doi:10.1111/j.1558-5646.2009.00682.x) [DOI] [PubMed] [Google Scholar]
- Sturgill D., Zhang Y., Parisi M., Oliver B.2007Demasculinization of X chromosomes in the Drosophila genus. Nature 450, 238–241 (doi:10.1038/nature06330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilszer M., Antoszczyk K., Salek N., Zajac E., Radwan J.2006Evolution under relaxed sexual conflict in the bulb mite Rhizoglyphus robini. Evolution 60, 1868–1873 [DOI] [PubMed] [Google Scholar]
- Toma D. P., White K. P., Hirsch J., Greenspan R. J.2002Identification of genes involved in Drosophila melanogaster geotaxis, a complex behavioral trait. Nat. Genet. 31, 349–353 [DOI] [PubMed] [Google Scholar]
- Weber K. E.1990Increased selection response in larger populations.1. Selection for wing-tip height in Drosophila melanogaster at 3 population sizes. Genetics 125, 579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedell N., Gage M. J. G., Parker G. A.2002Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 (doi:10.1016/S0169-5347(02)02533-8) [Google Scholar]
- West S. A., Diggle S. P., Buckling A., Gardner A., Griffin A. S.2007The social lives of microbes. Ann. Rev. Ecol. Evol. Syst. 37, 53–77 [Google Scholar]
- Whitlock M. C.2000Fixation of new alleles and the extinction of small populations: drift load, beneficial alleles, and sexual selection. Evolution 54, 1855–1861 [DOI] [PubMed] [Google Scholar]
- Wigby S., Chapman T.2004Female resistance to male harm evolves in response to manipulation of sexual conflict. Evolution 58, 1028–1037 [DOI] [PubMed] [Google Scholar]
- Wigby S., Chapman T.2005Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15, 316–321 (doi:10.1016/j.cub.2005.01.051) [DOI] [PubMed] [Google Scholar]
- Wigby S., Chapman T.2006No evidence that experimental manipulation of sexual conflict drives premating reproductive isolation in Drosophila melanogaster. J. Evol. Biol. 19, 1033–1039 (doi:10.1111/j.1420-9101.2006.01107.x) [DOI] [PubMed] [Google Scholar]
- Wigby S., Sirot L. K., Linklater J. R., Buehner N., Calboli F. C. F., Bretman A., Wolfner M. F., Chapman T.2009Seminal fluid protein allocation and male reproductive success. Curr. Biol. 19, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo B. H.1980Long-term selection for a quantitative character in large replicate populations of Drosophila melanogaster. I. Response to selection. Genet. Res. 35, 1–17 (doi:10.1017/S0016672300013896) [DOI] [PubMed] [Google Scholar]