Abstract
Ustiloxins A–F are antimitotic heterodetic cyclopeptides containing a 13–membered cyclic core structure with a synthetically challenging chiral tertiary alkyl–aryl ether linkage. The first total synthesis of ustiloxin D was achieved in 31 linear steps using an SNAr reaction. An nOe study of this synthetic product showed that ustiloxin D existed as a single atropisomer. Subsequently, highly concise and convergent syntheses of ustiloxins D and F were developed by utilizing a newly discovered ethynyl aziridine ring–opening reaction in a longest linear sequence of 15 steps. The approach was further optimized to achieve a better macrolactamization strategy. Ustiloxins D, F and eight analogues (14–MeO–ustiloxin D, four analogues with different amino acid residues at the C–6 position, and three (9R, 10S)–epi–ustiloxin analogues) were prepared via the second generation route. Evaluation of these compounds as inhibitors of tubulin polymerization demonstrated that variation at the C–6 position is tolerated to a certain extent. In contrast, the S configuration of the C–9 methylamino group and a free phenolic hydroxyl group are essential for inhibition of tubulin polymerization.
Antimitotic agents
Antimitotic agents have long been of interest to scientists and physicians, even before their precise mechanism of action could be articulated. Most of these compounds interfere with microtubule assembly and functions thus resulting in mitotic arrest of eukaryotic cells. There are a number of natural and synthetic compounds that interfere with tubulin function to inhibit the formation of microtubules.1 Such antimitotic agents exhibit a broad range of biological activities and can be used for medicinal and agrochemical purposes, acting as anticancer, antifungal, or anthelmintic agents. Moreover, tubulin binding compounds are valuable for understanding basic mechanisms involved in the dynamics of the microtubule network.2 Mitotic arrest is of importance in cancer chemotherapy, since tubulin is an established chemotherapeutic target. In addition to the widely used vinca alkaloids and taxoids, many other antimitotic agents are currently in clinical or preclinical development.3 Newer potential targets that may cause mitotic arrest are in development as well. Although cancer chemotherapy is a very important goal being extensively investigated, the mechanism of action of the naturally occurring peptides that inhibit tubulin polymerization remains a challenging problem. Attempts to establish a relationship among the tubulin binding peptides have only had limited success.4-8 As a result, the mechanism of action of peptidic antimitotic agents is not completely elucidated and deserves further investigation.
Ustiloxins
The first peptide shown to interact with tubulin was phomopsin A (Error! Reference source not found.).9-13 Since then, a number of peptides and depsipeptides were found to disrupt cellular microtubules. These compounds universally inhibit the assembly of the α,β–tubulin dimer into its polymer and, in the cases investigated, strongly suppress microtubule dynamics at low concentrations. Among these compounds are ustiloxins A–F (1–5) (Error! Reference source not found.), which were isolated from the water extracts of false smut balls, caused by the parasitic fungus Ustilaginoidea virens, found on the panicles of the rice plant.14-16
Ustiloxins are structurally similar to phomopsin A, and their structures were elucidated by amino acid analyses, X–ray analysis of ustiloxin A16 and other spectroscopic data. Ustiloxins cause mycotoxicosis and inhibit the polymerization of brain tubulin at micromolar concentrations but have no growth inhibitory effect on bacteria or fungi.17 Interestingly, the ustiloxins and phomopsin A are relatively weak cytotoxins compared with other antimitotic agents, which are lethal to cells at sub–nanomolar concentrations.5
The structures of the ustiloxin congeners contain a 13–membered heterodetic peptide macrocycle that includes a rare chiral tertiary alkyl–aryl ether linkage. The ustiloxin congeners (1–5) are composed of permutations of two separate sites: the side chain attached to the macrocyclic aromatic ring para to the ether linkage (see R1 in Error! Reference source not found.) and the presence of either alanine or valine in the macrocycle (see R2 in Error! Reference source not found.). Both ustiloxins A (1) and B (2) have a sulfinyl norvaline based amino acid attached to the aromatic ring at the R1 position. Ustiloxins A and B differ only in the side chain of a single macrocyclic amino acid. Ustiloxin C (3) contains a hydroxyethanesulfinyl group at the R1 position and the alanine variation of the macrocycle. Ustiloxins D (4) and F (5) have no sulfinyl appendage on the aromatic ring; they are differentiated from each other only by the presence of valine or alanine in the macrocycle. A major difference between the phomopsins12 and the ustiloxins16 is that, although both have a hydroxyl substituent at C–10, they are of opposite configurations.
It is important to determine the mechanism of action of the naturally occurring peptides that inhibit tubulin polymerization so that they may be more efficiently applied in cancer chemotherapy. In the literature, descriptions of potential binding sites of antimitotic peptides and depsipeptides to tubulin are somewhat contradictory.6,18-20 The ustiloxins appeared to provide fertile ground for studying antimitotic peptide natural products and their inhibitory effects on tubulin polymerization. Synthesis of ustiloxins could provide access to a variety of analogues of the natural product for SAR studies, which would elucidate the interaction between tubulin and the ustiloxin ligands. Ultimately, these analogues would provide information that could lead to a better understanding of the underlying mechanism of microtubule assembly inhibition, as well as the dynamics and impact of modulating these processes on cancer chemotherapy. Given our longstanding interest in the synthesis of cyclodepsipeptides, we present two generations of total syntheses of ustiloxins D and F and a full account of recent progress in the total synthesis of several analogues.
Synthetic strategy and retrosynthetic analysis
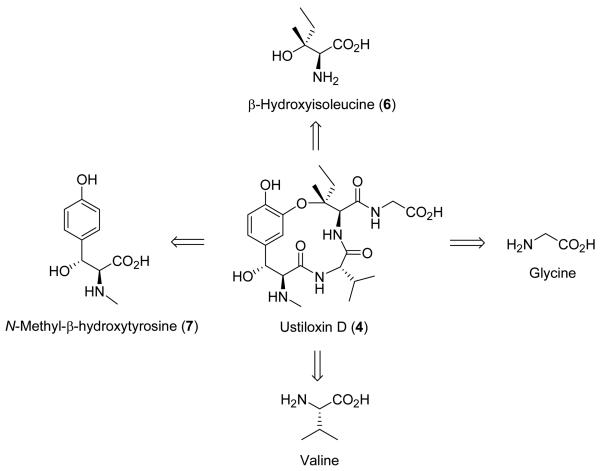
Ustiloxin D was chosen as the lead compound because it was one of the simplest congeners of the ustiloxin family and it possessed high biological activity. The total synthesis of ustiloxin D provided a scaffold for the syntheses of other congeners and analogues. From a synthetic perspective, ustiloxin D is comprised of four amino acid residues (Figure 2). Two of them are naturally occurring glycine and valine. The other two are nonproteinogenic amino acid residues, namely β–hydroxyisoleucine (6) and N–methyl–β–hydroxytyrosine derivative 7. A chiral tertiary alkyl–aryl ether linkage exists between compounds 6 and 7. The main challenges of the total synthesis are the construction of 6 and 7 by short and stereoselective approaches and the formation of a chiral tertiary alkyl–aryl ether between these two amino acid residues without affecting other functional groups and the existing stereochemistry. The latter specification was especially challenging, given the very limited methods that were available for the transformation and the highly functionalized structure of the ustiloxins.
Figure 2.
Amino acid components of ustiloxin D.
The challenges
An alkyl–aryl ether may be retrosynthetically disconnected in two ways: one providing an alkyl alcohol and an aromatic compound with a leaving group. The C–O bond may be formed using a nucleophilic aromatic substitution (SNAr) reaction or a transition metal–catalyzed coupling reaction between the same reagents. The other disconnection would provide a phenol derivative and an aliphatic compound with a leaving group. In this approach, the most common methods are nucleophilic substitution of an alkyl halide by a phenol or a Mitsunobu reaction between a phenol derivative and an alkyl alcohol. Although these methods are available, the formation of a tertiary alkyl–aryl ether remains a challenging transformation. Stereoselective formation of this functionality is even more difficult. In the first disconnection, the alcohol substrate needs to be a tertiary alcohol. Tertiary alkoxides are more often employed as non–nucleophilic bases, but rarely used as nucleophiles because of steric bulkiness and low nucleophilicity. The second disconnection is not a good alternative since tertiary alkyl species with a leaving group tend to form tertiary alkyl cations readily, which would destroy the stereochemistry of the tertiary carbon center. The competing elimination reaction would inhibit the effectiveness of the nucleophilic substitution reaction. As a result, very few reactions have been reported to form tertiary alkyl–aryl ethers stereoselectively. To the best of our knowledge, only four types of reactions have been reported to construct such an ether motif, namely, SNAr reactions,21-23 Pd–catalyzed intermolecular cross–coupling of aryl halides with alcohols,24-26 Mitsunobu–type reactions27-29 and Pd–catalyzed asymmetric allylic O–alkylation (AAA) reactions.30-33
Since the project began in our laboratory, extensive investigations were carried out to identify a method to form the challenging tertiary alkyl–aryl ether bond. It was envisioned that a mild reaction tolerant of a broad range of functionalities would lead to an expedient total synthesis. The Mitsunobu reaction was the first choice. We had successfully synthesized a number of cyclopeptide alkaloids bearing alkyl–aryl ether linkages using the Mitsunobu approach.34,35 Model studies used phenol derivative 8, which was synthesized in 6 steps from tyrosine. Among all the Mitsunobu reaction conditions tested, the best result was obtained by treatment of 8 with methyl–2–hydroxyisobutyrate (9) with PPh3 and DEAD in THF, which provided the desired ether 10 in 35% yield (Scheme 1a).
Scheme 1.
Ether formation study using the Mitsunobu reaction.
However, when using a more complex tertiary alcohol 12, the desired ether 13 was not produced under Mitsunobu conditions (PPh3, DEAD). A newly developed Mitsunobu reagent, cyanomethylenetrimethylphosphorane (CMMP),36 was also used but failed to give the desired ether (Scheme 1b). When propargyl tertiary alcohol 15 was treated with isovanillin (14) under a Mitsunobu protocol, tertiary alkyl-aryl ether 16 was obtained in a very low yield (Scheme 1c). Although the propargylic alcohol 15 is a more reactive Mitsunobu substrate, no further improvement to this reaction was achieved. Our unsuccessful model studies indicated that the scope of substrates in the Mitsunobu reaction for the tertiary ether formation was limited.37,38 As a result, we investigated the SNAr reaction as an alternative approach towards stereoselective alkyl–aryl synthesis.
The advantage of the SNAr reaction for generation of alkyl–aryl ethers is retention of configuration of the chiral alcohol substrates, as no new stereogenic centers are formed in the transformation. For many years, our group has focused on building the ether linkage in cyclopeptide alkaloids using SNAr reactions. These efforts resulted in the total syntheses of sanjoinine A39,40 and sanjoinine G1.41 We used 4–fluorobenzonitrile as an effective reagent for the arylation of alcohols,42 and several SNAr reactions on activated aryl halides have been reported.23,43,44 Wandless and co–workers developed an efficient method for the preparation of hindered alkyl–aryl ethers using potassium bis(trimethylsilyl)amide (KHMDS) to generate the alkoxide.23 The enantiopure tertiary alcohols were prepared prior to this challenging bond formation, and no selectivity issues were noted during the reaction. However, functional group compatibility remained an issue as the reaction uses strong basic conditions. Nonetheless, Zhu and co–workers applied the intramolecular SNAr reaction to synthesize a cyclic depsipeptide using tetrabutylammonium fluoride (TBAF) as the base.44
The first total synthesis of ustiloxin D
Based on the reported success of SNAr reactions, this approach was used in our first generation total synthesis of ustiloxin D. The key aspects of our synthetic strategy were the installation of the ether linkage at an early stage to circumvent potential problems at the later stages and the stereospecific generation of the β–hydroxytyrosine moiety in a single step. The first total synthesis of ustiloxin D began with the preparation of the protected amino alcohol 19 (Scheme 2). This alcohol was synthesized from D–serine in five steps according to a literature procedure.45 Treatment of D–serine with di–tert–butyl dicarbonate and NaHCO3 protected the amino group as its Boc carbamate, and the carboxylic acid was then coupled with N,O–dimethylhydroxylamine to afford Weinreb amide 17 using 1–ethyl–(3–dimethylaminopropyl) carbodiimide hydrochloride (EDCI·HCl) as the coupling reagent. The amino and hydroxyl groups of 17 were protected as the N–tert–butoxycarbonyl–N,O–isopropylidene derivative. Treatment with methyllithium converted the Weinreb amide to methyl ketone 18. Addition of ethylmagnesium bromide to the ketone proceeded with very good diastereoselectivity, giving rise to the anti–Felkin diastereomer of tertiary alcohol 19 as the major product. The absolute configuration of the newly generated stereocenter was confirmed by X–ray crystallography.
Scheme 2.
Synthesis of chiral tertiary alkyl–aryl ether 24 via SNAr reaction.
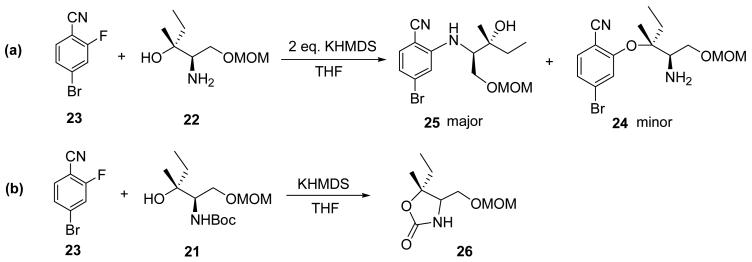
Treatment of 19 with p–toluenesulfonic acid in methanol gave diol 20 and the primary hydroxyl group was selectively protected as its MOM ether 21. Subsequent removal of the Boc group with trifluoroacetic acid (TFA) afforded amino alcohol 22. Treatment of 22 with aryl fluoride 23 and 1.1 equiv of KHMDS in THF23 successfully constructed the ether linkage in 24 in 78% yield. A number of problems were encountered during this SNAr reaction. Two of these are shown in Scheme 3: (a) when 22 and 23 were treated with 2.0 equiv of KHMDS, two products were observed with the undesired aryl amine 25 as the major product due to a faster reaction between the in situ generated amide and aryl fluoride 23; (b) when the amino group was protected as its N–carbamate 21, an undesired oxazolidinone 26 was produced via intramolecular cyclization. The SNAr reaction is highly sensitive to the functional groups on the aromatic ring as well. When the cyano group was replaced by a nitro group, no reaction occurred under the same reaction conditions.38
Scheme 3.
Problems observed during the SNAr reaction optimization
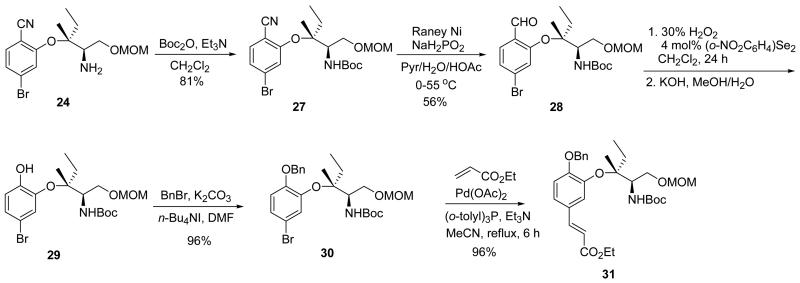
With ether 24 in hand, the amino group was protected as its Boc carbamate 27 (Scheme 4). Later we found that the three–step sequence from compound 21 to 27 could be performed without purification of crude intermediates to provide better overall yield (78% for 3 steps). Raney nickel reduction with NaH2PO2 in aqueous buffer solution converted nitrile 27 to aldehyde 28. Treatment of 28 with 30% H2O2 and catalytic di–(o–nitrophenyl)diselenide via Dakin oxidation afforded the corresponding formate, and subsequent hydrolysis with KOH in methanol gave phenol 29, which was protected as a benzyl ether 30. Treatment of 30 with ethyl acrylate, Pd(OAc)2 and (o–tolyl)3P under Heck conditions afforded cinnamate 31 in 96% yield.
Scheme 4.
Synthesis of intermediate 31 for use in the Sharpless aminohydroxylation.
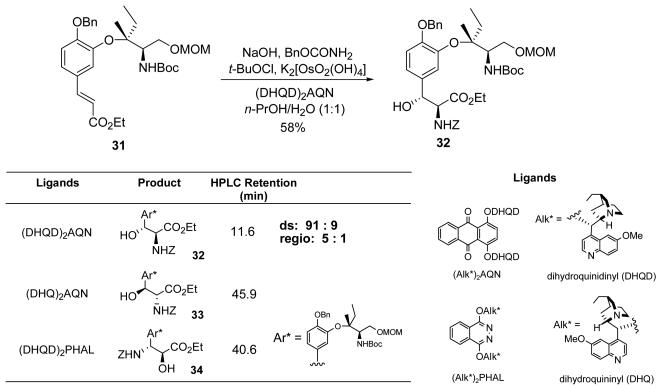
With cinnamate 31 in hand, the regio–reversed Sharpless asymmetric aminohydroxylation (AA) protocol46 was successfully applied to install two stereocenters in a single step, giving the desired (2S,3R)–β–hydroxy–α–amino ester 32 in 58% yield (Figure 3). To determine the regioselectivity and diastereoselectivity of this reaction, the diastereomer 33 and the regioisomer 34 of compound 32 were synthesized using different chiral ligands. With (DHQD)2AQN or (DHQ)2AQN as the ligand, isomer 32 and the diastereomeric (2R,3S)–β–hydroxy–α–amino ester 33 were the major products, respectively. When (DHQD)2PHAL was used as the ligand, the regioisomeric (2S,3R)–α–hydroxy–β–amino ester 34 was the major product. Using isomers 33 and 34 as internal references, regioselectivity (5:1) and diastereoselectivity (91:9) were determined. The desired compound 32 was separated from other isomers by flash column chromatography on aluminum oxide.
Figure 3.
Synthesis of β–hydroxy–α–amino ester via regio–reversed Sharpless AA reaction.
With the amino ester 32 in hand, the Boc group was removed by TFA to afford an amine, which was then coupled with N–Boc–L–valine using the DEPBT reagent to give 35 (Scheme 5). Treatment of 35 with TBSOTf protected the secondary hydroxyl group as its silyl ether, as well as transformed the tert–butyl carbamate to the corresponding tert–butyldimethylsilyl carbamate. Basic hydrolysis with LiOH in tert–BuOH–H2O afforded amino acid 36, which was then cyclized via macrolactamization to afford macrocycle 37 in 78% yield using EDCI–HOBt in a mixed solvent DMF–CH2Cl2 (1:5) as the optimized conditions for macrocyclization. The MOM group was removed using B–bromocatecholborane at −78 °C to afford alcohol 38 in 88% yield (Scheme 5).
Scheme 5.
Synthesis of protected macrocycle 40.
In order to differentiate the nitrogen of the tyrosine α–amino group from the two backbone nitrogens in the macrocycle and to achieve methylation later in the synthesis, conversion of the N–carbamate to N–sulfonamide was necessary to decrease the pKa of the nitrogen. The benzyl carbamate was selectively removed by catalytic hydrogenolysis in the presence of the benzyl ether, using 2,2′–dipyridyl as a catalyst poison (Scheme 5). The intermediate amine was subsequently protected as the 2–nitrobenzenesulfonamide 39 in 85% yield for two steps. Sequential oxidation of the primary hydroxyl group in 39 with the Dess–Martin reagent and then with NaClO2 afforded a carboxylic acid, which was coupled with glycine benzyl ester to give 40. Selective deprotonation of sulfonamide 40 was achieved with the base MTBD (1,3,4,6,7,8–hexahydro–1–methyl–2H–pyrimido[1,2–a]–pyrimidine),47 and subsequent methylation with methyl 4–nitrobenzenesulfonate afforded 41 (Scheme 6). Treatment of compound 41 with n–Bu4NF–HOAc deprotected the silyl ether to give alcohol 42. Removal of the 2–nitrobenzenesulfonyl group with β–mercaptoethanol and DBU (1,8–diazabicyclo[5.4.0]undec–7–ene) gave secondary amine 43.48,49 The final step involved hydrogenolysis to remove both the benzyl ester and the benzyl ether. Direct hydrogenolysis with 10% Pd/C, as well as transfer hydrogenolysis with 10% Pd/C and 1,4–hexadiene, removed the benzyl ester but not the benzyl ether. A more active catalyst, palladium black, was needed to remove the protecting groups and afford ustiloxin D (4) in 85% yield. The 1H NMR, 13C NMR, HRMS and optical rotation of synthetic ustiloxin D were identical to those of the natural product.
Scheme 6.
Completion of the synthesis of ustiloxin D.
Atropisomerism
Like many other macrocyclic natural products, such as vancomycins, ustiloxin D may potentially exist as two atropisomers. To clarify the atropisomerism issue, a 2D 1H–1H NOESY experiment was carried out. Ustiloxin D exhibited diagnostic nOe crosspeaks between protons on C–16 and C–10, C–12 and C–9, and C–16 and C–21 (Figure 4). No nOe crosspeaks were observed between protons on C–12 and C–10, C–16 and C–9, or C–12 and C–21. This observation suggested that ustiloxin D was present as a single atropisomer instead of as a mixture of two atropisomers. The three–dimensional structure in Figure 4 was generated based on the information from nOe signals.
Figure 4.
Assignment of atrop–stereochemistry of ustiloxin D by 1H–1H nOe study.
The first total synthesis of ustiloxin D (4) was accomplished from readily available D–serine and aryl fluoride starting materials. The longest linear sequence of the synthetic route was 31 steps, and the average yield was 82% for each step.50 We next initiated efforts to improve the efficiency of the synthesis. The strategy was based on an intramolecular SNAr reaction at a late stage to make the synthesis more convergent. Although the linear precursor was synthesized very efficiently, many attempts using different SNAr reaction conditions failed to afford the desired macrocycle.51
It was clear that a new method to construct the chiral tertiary alkyl–aryl ether linkage was necessary for a more efficient and convergent synthesis. The new reaction should ideally be performed after β–hydroxyisoleucine 6 and N–methyl–β–hydroxytyrosine derivative 7 were built. The conditions for ether formation had to be mild to tolerate all the functionalities of these substrates and not affect any existing chiral centers. Furthermore, as the main difference between the macrocyclic core structures of ustiloxins and phomopsins12,16 is the stereochemistry of the C–10 hydroxyl group of the β–hydroxytyrosine moiety, adoption of a single method to access all the diastereomers of N–methyl–β–hydroxytyrosine was preferred as it would be easily modifiable for the synthesis of phomopsin natural products.
Investigation of AAA reaction
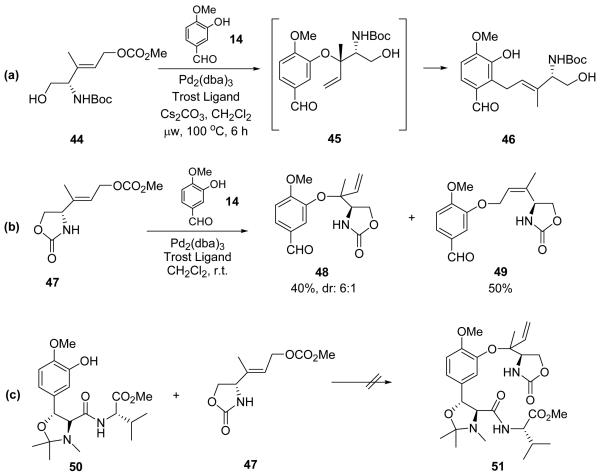
The Pd–catalyzed AAA reaction has been widely used to construct chiral alkyl–aryl ethers in many natural product syntheses.31,32 However, applications of the AAA reaction to form a tertiary alkyl–aryl ether stereoselectively are rare because of steric effects.52 Nonetheless, the mild reaction conditions and broad functional group tolerance would be beneficial for the late–stage synthesis of highly functionalized natural products such as ustiloxins with a proper allylic methyl carbonate. To investigate this approach, allylic carbonate 44, bearing a nitrogen–containing chiral carbon center and an adjacent hydroxyl group, was subjected to standard AAA reaction conditions with isovanillin (14, Scheme 7a).
Scheme 7.
Studies of the AAA reaction.
No reaction was observed at room temperature but a small amount of product 46 was isolated when the reaction was heated to 100 °C in a microwave reactor. The initial Pd–catalyzed allylic O–alkylation presumably formed the desired tertiary alkyl–aryl ether 45, but it was subsequently converted to the isolated product via a Claisen rearrangement because of the high temperature. To increase the reactivity of the allylic carbonate and allow for lower reaction temperatures, allylic carbonate 47 was synthesized38 and reacted with isovanillin to provide products 48 and 49 in a 4:5 ratio. Product 48 was shown by 1H NMR to be a diastereomeric mixture in a 6:1 ratio. Although introduction of this cyclic constraint improved the reactivity with isovanillin, carbonate 47 failed to participate in the AAA reaction with dipeptide 50. Attempts to favor the AAA pathway by either adding a base to increase the concentration of phenoxide nucleophile or using the naphthoyl–based ligand did not provide any desired product (51). A low conversion was observed in toluene and THF, but the major product was the undesired regioisomer. Using an iridium phosphoramidite catalyst53 also did not yield any product.
Based on these results, we concluded that the more substituted allylic carbon center was too sterically hindered or that the nucleophile was too bulky. At that time, Wandless and co–workers reported a total synthesis of ustiloxin D utilizing an AAA reaction.54,55 However, a 2:1 ratio of diastereoselectivity was obtained with a relatively simple allylic carbonate, and this inseparable mixture was carried through all subsequent manipulations to the end of the total synthesis.54,55 Based on these results and our model studies, we decided to develop a new stereoselective method to construct tertiary alkyl–aryl ethers.
Discovery of an aziridine ring–opening reaction by phenol derivatives
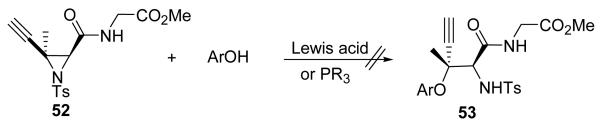
We next investigated methods used for the formation of tertiary alkyl–aryl ethers with the aim to extend these to a stereoselective method. The aziridine ring–opening reaction by phenol derivatives was very attractive for this purpose.56-60 Having learned from the results of our investigations of the Mitsunobu reaction that the propargylic carbon center was more reactive toward nucleophilic attack than a normal tertiary carbon center, ethynyl aziridine 52 was used for the study of tertiary alkyl–aryl ether formation by aziridine ring–opening. Investigation of a trisubstituted aziridine ring–opening by phenol derivatives with Lewis acids51 or phosphines57 did not provide 53 in acceptable yield (Figure 5). Our results suggested that aziridine ring–opening at the tertiary carbon center was very difficult because of steric effects. The size and nucleophilicity of the phenol were also significant factors.
Figure 5.
Attempted Lewis acid promoted ring–opening of aziridine by phenol derivatives.
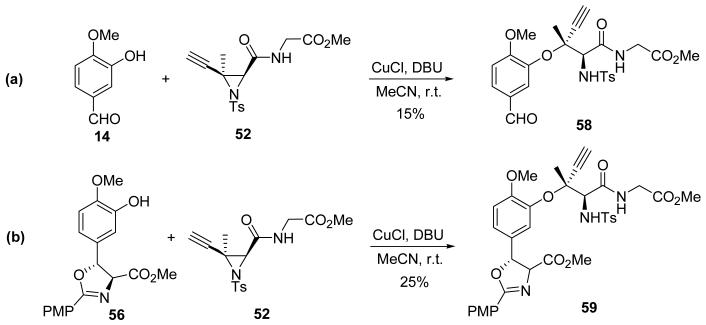
The formation of aryl 1,1–dimethylpropargyl ethers mediated by copper catalysts appeared to be worthy of investigation.61,62 The reaction conditions were mild, and the yields were excellent. Although the method had not been used to form stereogenic centers, we felt that a stereochemical induction from a vicinal stereocenter might provide useful levels of selectivity. The chiral amino acid carbon center next to the C–2 center could potentially induce diastereoselectivity. Subjecting propargylic carbonate 54 and isovanillin (14) to the conditions of the copper–catalyzed aryl 1,1–dimethylpropargyl etherification afforded the desired tertiary alkyl–aryl ether 55 in 37% yield (Figure 6a). The more highly functionalized β–hydroxytyrosine derivative 56 also exhibited similar reactivity to isovanillin, albeit in poor yield.
Figure 6.
Diastereoselective copper–catalyzed tertiary alkyl–aryl ether formation.
Though these results were very promising, reaction optimization and determination of the stereochemistry of the coupled product were problematic. The reactive propargylic carbonate 54 participated in various nonproductive reaction pathways, and a less reactive leaving group was desired. Aziridine–2–carboxylate 52, prepared in the previous study of the Lewis acid aziridine ring–opening, had shown low reactivity where the strained N–C bond behaved as a leaving group. Therefore, we investigated the reaction between isovanillin (14) and 52 and found that it afforded a tertiary alkyl–aryl ether 58 as a single product in modest yield. An advantage of this aziridine ring–opening reaction was that the product stereochemistry was easily controlled since it was stereoselective and yielded a single diastereomer. Should the C–2 configuration prove to be opposite of what we proposed, the desired product could be obtained from a diastereomer of aziridine 52. The reaction between 52 and β–hydroxytyrosine derivative 56 afforded the tertiary alkyl–aryl ether 59 as well (Figure 7).
Figure 7.
Ethynyl aziridine ring–opening reactions.
A number of conditions, including different copper species, bases, solvents, and temperatures were screened to optimize the reaction shown in Figure 7a. Toluene was the solvent of choice. During the screening of different copper species, CuOAc was found to provide the best reaction when combined with DBU as the base. Using these optimized conditions, the desired tertiary alkyl–aryl ether product 58 was isolated in 60% yield after 24 h at room temperature. Moreover, X–ray structure analysis showed that the stereochemical configuration of the product matched that of ustiloxin D.63
Second generation total synthesis of ustiloxins D and F
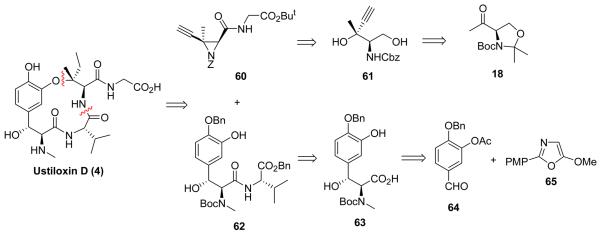
Given the mild reaction conditions and broad functional group tolerance, the new regio– and stereoselective ethynyl aziridine ring–opening reaction provided an ideal solution for a convergent ustiloxin total synthesis. Two key intermediates (60 and 62) were necessary for the construction of tertiary alkyl–aryl ether via ethynyl aziridine ring–opening reaction (Figure 8).
Figure 8.
Convergent approach to ustiloxin D.
First convergent approach toward ustiloxin
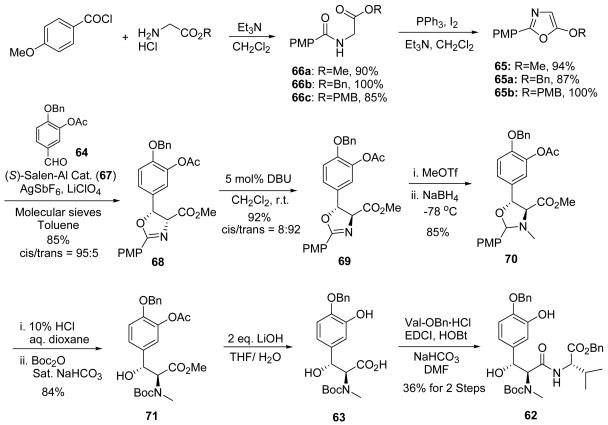
The synthesis of fragment 62 started from 5–methoxy–2–(4–methoxyphenyl)–oxazole) 65 (Scheme 8). The oxazole 65 was originally obtained by dehydration of (4–methoxybenzoylamino)acetic acid methyl ester using P2O5.64 However, the reaction was low yielding and not easy to handle, especially under humid conditions. Furthermore, replacing the 5–methoxy group with other alkoxy groups, such as a benzyloxy group, failed to give the desired oxazoles. The cyclodehydration conditions developed by Wipf65 provided the first successful synthesis of oxazoles 65a–b. The condensation of p–anisoyl chloride with glycine derivatives smoothly afforded 66a–c in excellent yield. Treatment of 66a–c with triphenylphosphine, iodine and triethylamine presumably formed a carbene that underwent a formal cyclodehydration to yield the oxazoles 65 and 65a–b.
Scheme 8.
Synthesis of β–hydroxytyrosine fragment 62.
A salen–aluminum complex (67) catalyzed the aldol–type reaction between oxazole 65 and orthogonally protected 3,4–dihydroxybenzaldehyde 64 afforded the desired oxazoline–2–carboxylate 68.66 Electron–rich aldehydes undergo slower turnover, so catalyst loading was increased to 10% to insure full conversion of aldehyde 64 to 68. Other investigators reported nearly identical results in this aldol–type reaction.54 The diimine ligand was easily recovered by flash column chromatography, and the catalyst could be regenerated without loss of activity and stereoselectivity. The enantiopure cis–oxazoline 68 was obtained in 85% yield after a single recrystallization. However, when oxazole 65a was subjected to the aluminum–salen catalyzed aldol–type reaction, only a small amount of the cis–product was formed even with high catalyst loadings. No cis–product was observed when oxazole 65b was used in the aldol–type reaction. It appeared that the bulky aluminum–salen catalyst was sensitive to the increased size of the benzyl or para–methoxybenzyl substituents, and it could not effectively promote the aldol–type reactions of oxazoles 65a and 65b. The electronic properties of the aromatic ring are also critical. No reaction occurred when the acetyl group was replaced by a more electron–donating silyl ether.
DBU–mediated isomerization of 68 afforded the thermodynamically more stable trans–oxazoline 69,66 with the correct absolute stereochemistry for the construction of ustiloxins. At this stage, it was decided to install the requisite N–methyl group by formation of an oxazolinium ion with methyl triflate, followed by in situ reduction.67 This protocol would replace the four–step sequence for selective N–methylation employed in our first total synthesis.
During the in situ reduction of the oxazolinium ion, we encountered the same over–reduction problem that Wandless and co–workers had observed.54 While applying the commonly used conditions of NaBH4 in a 1:4 mixture of methanol and THF at 0 °C to the methoxy phenol analogue of 69, the desired N,O–acetal was obtained without any difficulty. However, when 69 was subjected to the same protocol, the C–O bond was cleaved to afford 72 as the major product (Table 1). The relative stereochemical configuration of the adjacent two carbon centers was confirmed by an X–ray crystallographic analysis of 72.
Table 1.
Investigation of iminium ion reduction.
 | ||||
|---|---|---|---|---|
| Reductant | Solvents | Temp. | Producta | Yieldb |
| NaBH4 | MeOH/THF (1:4) | 0° C | 72 | 64% |
| L–Selectride | CH2Cl2 | 0° C | 73 | 67% |
| NaBH4 | H2O/THF (1:4) | 0° C | 73 | 63% |
| NaBH4 | EtOH | −78° C | 70 | 85% |
major product;
yield of major product
L–Selectride or NaBH4 in a mixture of water and THF as the reductants provided compound 73 as the major product. Wandless and co–workers developed a biphasic system which successfully prevented over–reduction from occurring,54 but we found that using ethanol as the solvent and lowering the reaction temperature to −78 °C provided the desired oxazolidine 70 in higher yield. Since diastereomers were formed upon reduction, the para–methoxybenzylidene 70 was cleaved by 10% aqueous HCl, and the resulting secondary amine was protected as Boc–carbamate 71 in one pot. Simultaneous hydrolysis of the acetate and methyl ester of compound 71 was next performed using lithium hydroxide, followed by coupling with valine benzyl ester to afford dipeptide 62 in a sequence of eight steps (Scheme 8).
The ethynyl aziridines were synthesized utilizing the methyl ketone intermediate 18 of the first total synthesis of ustiloxin D. The synthesis of ketone 18 was improved68 via a four–step sequence without column chromatography with an overall 60% yield. Grignard addition of ethynylmagnesium bromide to 18 afforded tertiary alcohol 15 in 80% yield with an 11:1 diastereomeric ratio. The amino alcohol was liberated by acidic full deprotection of intermediate 15, followed by protection of the primary amino group as its Cbz carbamate (61) or nosyl sulfonamide (74) in one pot. A one–step TEMPO catalyzed oxidation protocol directly oxidized the primary hydroxyl group of diols 61 and 74 to carboxylic acids 75a–b, which were coupled with glycine esters to provide the dipeptides without affecting the tertiary hydroxyl group. The Mitsunobu reaction converted the amino alcohols 76–78 to the ethynyl aziridines 60, 79, and 80 (Scheme 9).63
Scheme 9.
Trisubstituted ethynyl aziridine synthesis.
The aziridine ring–opening reaction between 60 and 62 successfully generated the desired tertiary alkyl–aryl ether 81. A Pd–catalyzed transfer hydrogenolysis simultaneously removed the Cbz carbamate, benzyl ester and benzyl ether and reduced the ethynyl group to an ethyl group to provide the linear precursor 82 (Scheme 10).
Scheme 10.
First attempt to ustiloxin D via aziridine ring–opening.
To our disappointment, macrolactamization of 82 was unsuccessful with various coupling reagents, bases and solvents. Wandless and co–workers also experienced difficulties in macrolactamization with a linear ustiloxin precursor. Although only macrolactamization and simultaneous deprotection of the tert–butyl ester and the Boc carbamate were needed to finish the total synthesis of ustiloxin D in 14 steps, no further progress was achieved.
Total synthesis of ustiloxins D and F, and three C–6 analogues
Based on these results, it was decided that the macrolactamization between the N–terminus of β–hydroxyisoleucine and the C–terminus of valine was not viable. Another choice was to attempt macrocyclization between the N–terminus of valine and the C–terminus of β–hydroxytyrosine, as both previous total syntheses of ustiloxin D closed the macrocycle at this position.
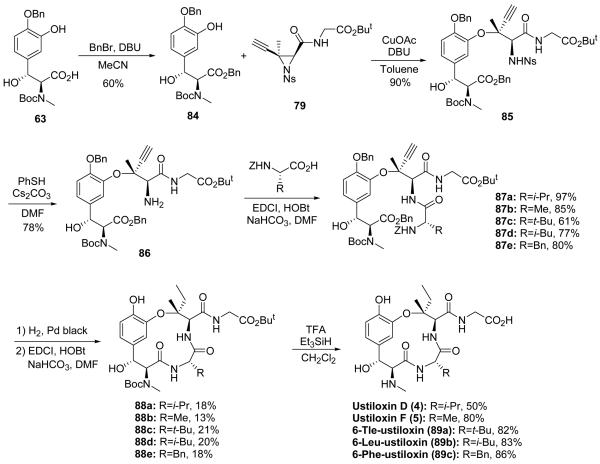
To improve the protecting group strategy, acid 63 was selectively protected as its benzyl ester 84 (Scheme 11). The ethynyl aziridine ring opening reaction of 84 with 79 successfully provided the desired chiral tertiary alkyl–aryl ether 85 in 90% yield. The o–nosyl group was removed to afford the primary amine 86 in 78% yield.69 The amine was coupled with N–Cbz–valine and N–Cbz–alanine using EDCI and HOBt to install all the required amino acid components for ustiloxin D and F respectively. Palladium black catalyzed hydrogenolysis of 87a–b simultaneously removed the benzyl carbamate, the benzyl ester, and the benzyl ether, and reduced the ethynyl group to the requisite ethyl group to afford the linear precursors, which underwent macrolactamization to provide macrocycles 88a–b in moderate yield in two steps. Cleavage of the Boc carbamate and tert–butyl ester by TFA in the presence of triethylsilane70 completed the total syntheses of ustiloxin D (4)71 and ustiloxin F (5).51 The longest linear sequence of this second generation approach was 15 steps. Since the amino acid residue at the C–6 position is one of the two variable positions in the ustiloxin family, it was valuable to investigate the structural requirements at this position. As the new approach introduces the C–6 amino acid residue at the late stage, three analogues (6–Tle–ustiloxin, 6–Leu–ustiloxin, and 6–Phe–ustiloxin; 89a–c) were synthesized using the same strategy.
Scheme 11.
Synthesis of ustiloxins D and F and C–6 analogues.
An improved total synthesis strategy
Although the new total syntheses of ustiloxins D and F were convergent, concise, and versatile, the approach suffered from a low yielding macrocyclization step. To facilitate the synthesis of other analogues, the macrolactamization needed to be improved. The yields of macrocyclization were consistently in the range of 10–20%, and coupling reagents seemed to have little effect on the yield. However, in a nonpolar solvent, such as methylene chloride, no product was observed, whereas in a polar solvent, such as DMF, macrolactamization occurred with various coupling reagents. This observation suggested that the solution conformation of the linear precursor, likely due to intramolecular H–bonding, could be preventing macrocyclization. A comparison of the linear precursors of the new route with those of the previous two routes50,54,55 showed a major difference between the precursors: the phenolic hydroxyl group ortho to the tertiary alkyl–aryl ether linkage was not protected in the new linear precursor, while the other two were both protected as their benzyl ethers in the macrocyclization reaction. A study of the unusual effects remote substituents may have on macrocyclization reactions was reported by Boger and Yohannes.72 The formation of 17–membered cyclic tripeptides bearing a diaryl ether was thoroughly examined using a full range of substrates. The study showed that remote substituents have substantial, conformationally derived effects on the rate of macrocyclization reactions. Interestingly, in contrast with our results, substrates bearing a free phenol were found to undergo macrocyclization more productively.73
The observation led us to hypothesize that intramolecular H–bonding between the free phenolic hydroxyl group and other oxygen or nitrogen atoms in the linear precursor was the problem for the macrocyclization reaction. Computational results supported the hypothesis, as some of the lowest energy conformations of the linear precursor calculated by MacroModel® indicated hydrogen bonding between the phenolic hydroxyl group and other oxygen and nitrogen atoms causing the distance between the N–terminus and C–terminus to be too large for amide bond formation.
Model study: Synthesis of 14–MeO–ustiloxin
To achieve a better macrocyclization reaction, a linear precursor with a protected phenolic hydroxyl group was needed. At that time, aryl methyl ether 90 was available. This intermediate was not only ideal for the model study, but also could provided an analogue to test the importance of the free phenolic functionality for biological activity.
It was realized that if the model study went as proposed and the benzyl ether was present for the macrocyclization, this approach would require avoiding hydrogenolysis in the linear precursor formation as in the total synthesis route. The protecting strategy was changed accordingly to employ the acid labile para–methoxybenzyl (PMB) ester and Boc carbamate to protect the termini of the linear precursor so that the linear precursor would be generated by treatment with acid without affecting the phenolic benzyl ether. After macrolactamization, protecting group removal by hydrogenolytic cleavage would provide the natural product analogue.
The ethynyl aziridine ring–opening reaction of 80 with β–hydroxytyrosine derivative 90 successfully formed the tertiary alkyl–aryl ether 91. Deprotection of the o–nosyl group and coupling of the resulting free amine 92 and N–Boc–valine provided compound 93. Treatment of 93 with TFA liberated the carboxylic acid and free amino group and deprotected the TBS ether simultaneously. The linear precursor was cyclized to give 94 in 38% yield for the two steps, which was twice the yield that was obtained when the linear precursor had a free phenolic hydroxyl group. This result confirmed the hypothesis that the free phenol made macrocyclization more difficult. The final hydrogenolysis step deprotected the Cbz carbamate and benzyl ester and reduced the ethynyl group to the ethyl group to provide the 14–MeO–ustiloxin D analogue 95 (Scheme 12).51
Scheme 12.
Synthesis of 14–MeO–ustiloxin D.
Final optimization of the new total synthesis route
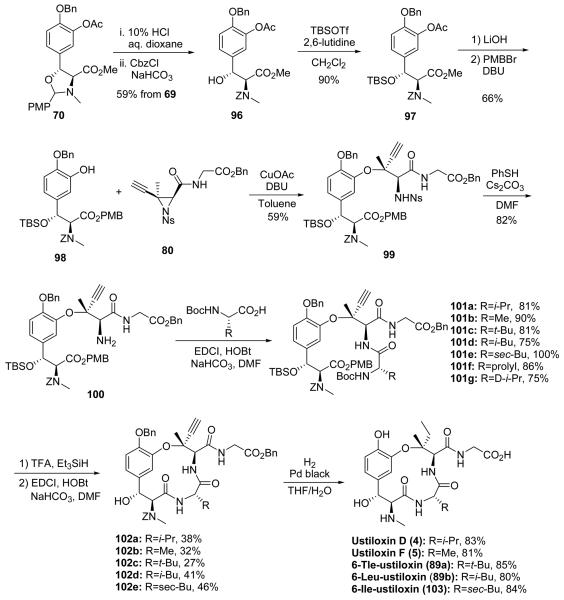
With the results from the previous model study, a slight modification of the second–generation synthesis was pursued. In this new plan, β–hydroxytyrosine 98 was reacted with the ethynyl aziridine 80 used in the model study. (Scheme 13). The synthesis of β–hydroxytyrosine derivative 98 started from trans–oxazolidine 70. Acidic deprotection of the N,O–acetal, followed by Cbz carbamate formation of the free amino group, afforded product 96 that, unlike the Boc carbamate 71, easily formed an oxazolidinone through an intramolecular cyclization under basic conditions. The C–9 benzylic hydroxyl was masked as its TBS ether 97. Following hydrolysis of acetate and methyl ester, the free acid was selectively protected as the PMB ester to give compound 98.
Scheme 13.
Improved synthesis of ustiloxins.
The ethynyl aziridine ring–opening reaction between the β–hydroxytyrosine derivative 98 and aziridine 80 successfully constructed the desired tertiary alkyl–aryl ether 99. The free amino group of 100, liberated from the thiol assisted nosyl sulfonamide cleavage, was coupled with various Boc protected amino acids. The resulting products 101a–g were then subjected to TFA with triethylsilane as a cation scavenger. These acidic conditions simultaneously cleaved the Boc carbamate, the PMB ester and the TBS ether to afford the linear precursors. Macrolactamization successfully provided the macrocycle 102a–e. The 2–step yields (102a–d) were significantly better than those obtained in Scheme 11. Unfortunately, proline and D–valine based linear precursors 101f–g did not undergo successful macrolactamization to provide ustiloxin analogues. The (6R)–ustiloxin D would have provided data on the role of configuration at C–6 for biological activity. Simultaneous reduction of the ethynyl group to the ethyl group and deprotection of Cbz carbamate, benzyl ester and benzyl ether by hydrogenolysis afforded ustiloxin D (4), ustiloxin F (5), 6–Tle–ustiloxin (89a), 6–Leu–ustiloxin (89b), and 6–Ile–ustiloxin (103). A mixture of THF–H2O instead of ethanol was the solvent of choice for the hydrogenolysis, because it was observed that the carboxylic acid of the glycine side chain was easily esterified under acidic conditions, during both the first total synthesis of ustiloxin D and the synthesis of ustiloxin F.
Syntheses of (9R, 10S)–epi–ustiloxin analogues
Another goal of the research program was to prepare (9R, 10S)–epi–ustiloxin analogues to evaluate their ability to inhibit tubulin polymerization. Comparison of the ustiloxin and phomopsin12,16 core structures shows that the configuration of the C–10 hydroxyl group is not critical for tubulin binding, because the two natural products have opposite configurations at C–10. This observation suggested that the C–10 hydroxyl group may not play a role in the interaction of these peptides with tubulin. Since it is known that tubulin polymerization activity is sensitive to dimethylation of the β–hydroxytyrosine nitrogen,74 we felt that investigation of the stereochemical configuration of the 9–amino group was important. As we had developed a robust route to all diastereomers of the β–hydroxytyrosine moiety using Evans' aldol type chemistry, we chose to prepare (9R, 10S)–epi–ustiloxin analogues, and their synthesis is shown in Scheme 14.
Scheme 14.
Synthesis of three (9R, 10S)–epi–ustiloxin analogues.
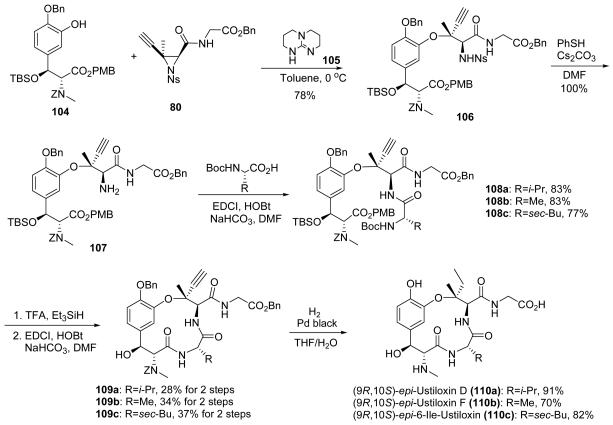
β–Hydroxytyrosine 104 was successfully prepared by the same approach used for its enantiomer (98) using R–salen–aluminum catalyst. At this stage, our investigations showed that the use of TBD (105) as a base gave better results than DBU and CuOAc combined and also minimized side reactions.75 Indeed, the ethynyl aziridine ring–opening reaction of 104 and 80 in the presence of TBD gave superior results and afforded the chiral tertiary alkyl–aryl ether 106 in 78% yield. An additional advantage was that a 1:1 ratio of 104 and 80 was enabled by the use of TBD versus a 1.5:1 ratio when DBU was employed.76 This result greatly improved the material throughput for the synthesis of analogues, and this efficient joining of the complex β–hydroxytyrosine and aziridine components highlights the convergence of our synthetic route. The N–nosyl group was cleaved by treatment with benzenethiol to provide the free amine 107 in quantitative yield. The amine was used as a branching point to incorporate three amino acid residues, specifically valine, alanine, and isoleucine. The amino acids valine and alanine were chosen because these residues are found in ustiloxin D (4) and F (5), respectively. In previous investigations, an active 6–Ile–ustiloxin analogue had been prepared, so we chose to introduce isoleucine to provide a comparable (9R, 10S)–epi–6–Ile–ustiloxin analogue.
The coupling of amine 107 with valine, alanine, or isoleucine provided the desired protected linear precursors 108a–c, respectively, in excellent yields. Using the improved macrolactamization and same end game strategy, (9R, 10S)–epi–ustiloxin analogues 110a–c were prepared efficiently. These three (9R, 10S)–epi–ustiloxin analogues are the first analogues synthesized to probe the structural basis for the interaction of the β–hydroxytyrosine region of the ustiloxin family with tubulin.
Bioactivity
Disturbance of the dynamic equilibrium of microtubules has critical consequences for cell survival. Since disruption of the mitotic pathway represents a challenging and significant target for oncology, the investigation of ligands that interact with tubulin is essential to better understand their mechanisms of action and biological activity. The course of our investigations led to the preparation of suitable analogues to initiate structure activity relationship studies in the ustiloxin–phomopsin class of antimitotic peptides. The analogues were designed to probe the importance of different structural regions of these peptides for biological activity. Thus far, modifications at the valine residue (C–6), the β–hydroxytyrosine residue (C–9 and C–10), and the phenolic hydroxyl have been investigated.
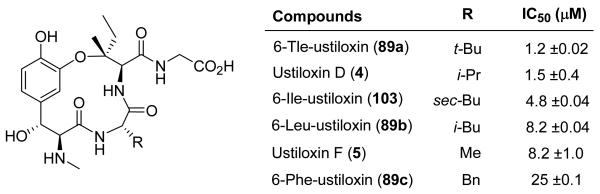
The biological property we examined was the effect of these compounds on the polymerization of purified tubulin. Tubulin showed a tolerance for structural variation at the C–6 macrocyclic position of the ustiloxins (Figure 9). The most active analogues had the highest degree of branching at the C–6 position, with the synthetic 6–Tle–ustiloxin (89a) and ustiloxin D (4) displaying comparable activities (IC50 = 1.2 and 1.5 μM, respectively). When an isoleucine residue was incorporated at C–6 (6–Ile–ustiloxin 103), a slight decrease in activity was observed. The isoleucine residue possesses the same degree of branching as the valine residue in 4, but is extended by an additional methylene unit, which may cause less effective binding of 103 relative to 4. When the branching element at the C–6 position was moved further from the macrocycle or removed, as in 6–Leu–ustiloxin (89b) or ustiloxin F (5), respectively, inhibition of tubulin polymerization was further decreased (IC50 = 8.2 μM). An aromatic residue, such as phenylalanine (6–Phe–ustiloxin 89c), resulted in a still less active compound (IC50 = 25 μM). These results suggest that a relatively compact, but highly branched aliphatic residue at the C–6 position is optimal for inhibition of tubulin polymerization.
Figure 9.
Evaluation of C–6 substitution on inhibition of tubulin polymerization
Biological evaluation of 14–MeO–ustiloxin D (95) showed no inhibition of tubulin polymerization (IC50 > 40 μM), demonstrating that the phenol moiety of the ustiloxin family is essential for binding to the α,β–tubulin dimer. Iwasaki also reported loss of activity upon methylation of the phenol in a semisynthetic ustiloxin analogue.74 Similarly, (9R, 10S)–epi–ustiloxin D (110a), (9R, 10S)–epi–ustiloxin F (110b), and (9R, 10S)–epi–6–Ile ustiloxin (110c) yielded IC50 values > 40 μM (Figure 10). Since the phomopsin peptides13 have an S configuration at C–10, these results imply that the naturally occurring 9S–methylamino group is critical for binding to the α,β–tubulin heterodimer. Structural changes that alter the macrocyclic conformation, such as stereochemical modifications, likely place the 9R–methylamino group at a distance too great to make contact with the tubulin protein and results in the observed loss of activity. Future studies to investigate other regions of the ustiloxin/phomopsin structure should reveal other critical points for binding to tubulin and advance our understanding of the interaction of tubulin with these heterodetic peptides.
Figure 10.
Inactivity of 14–MeO–ustiloxin D and (9R, 10S)–epi–ustiloxin analogues.
Conclusion
Two total syntheses of ustiloxin natural products were achieved. The first total synthesis of ustiloxin D featured application of an SNAr reaction to construct the tertiary alkyl–aryl ether and regio–reversed Sharpless aminohydroxylation to install the two chiral centers of the β–hydroxytyrosine moiety in one step. The synthesis provided a scaffold for further development. The second generation synthesis took advantage of a newly developed trisubstituted aziridine ring–opening by phenol derivatives and resulted in a concise and convergent synthesis. Ustiloxin D, ustiloxin F, and eight ustiloxin analogues were prepared using this highly versatile strategy. Studies on the inhibition of tubulin polymerization revealed that limited variation at the C–6 position is tolerated and that the free phenolic hydroxyl group and the S configuration of the C–9 methylamino group are essential for bioactivity. Investigation of other analogues of the ustiloxin family is ongoing.
Supplementary Material
Figure 1.
Structures of ustiloxins and phomopsin A.
Acknowledgment
The authors thank NIH (CA–40081), NSF grant 0515443, Wyeth Research and the University of Pennsylvania Research Foundation for financial support.
Footnotes
Supporting Information Available: Experimental detail and characterization for all new compounds in the synthesis of ustiloxin D, ustiloxin F, and eight analogues.
References
- 1.Downing KH. Annu. Rev. Cell. Dev. Biol. 2000;16:89. doi: 10.1146/annurev.cellbio.16.1.89. [DOI] [PubMed] [Google Scholar]
- 2.Walczak CE. Curr. Opin. Cell Biol. 2000;12:52. doi: 10.1016/s0955-0674(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 3.Jordan A, Hadfield JA, Lawrence NJ. Med. Res. Rev. 1998;18:250. doi: 10.1002/(sici)1098-1128(199807)18:4<259::aid-med3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Hamel E. Biopolymers. 2002;66:142. doi: 10.1002/bip.10255. [DOI] [PubMed] [Google Scholar]
- 5.Hamel E, Covell DG. Curr. Med. Chem.: Anti-Cancer Agents. 2002;2:19. doi: 10.2174/1568011023354263. [DOI] [PubMed] [Google Scholar]
- 6.Mitra A, Sept D. Biochemistry. 2004;43:13955. doi: 10.1021/bi0487387. [DOI] [PubMed] [Google Scholar]
- 7.Poncet J, Busquet M, Roux F, Pierre A, Atassi G, Jouin P. J. Med. Chem. 1998;41:1524. doi: 10.1021/jm970800t. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt J, Bernd M, Kutscher B, Kessler H. Biorg. Med. Chem. Lett. 1998;8:385. doi: 10.1016/s0960-894x(98)00034-1. [DOI] [PubMed] [Google Scholar]
- 9.Culvenor CCJ, Beck AB, Clarke M, Cockrum PA, Edgar JA, Frahn JL, Jago MV, Lanigan GW, Payne AL, Peterson JE, Petterson DS, Smith LW, White RR. Aust. J. Biol. Sci. 1977;30:269. doi: 10.1071/bi9770269. [DOI] [PubMed] [Google Scholar]
- 10.Culvenor CCJ, Cockrum PA, Edgar JA, Frahn JL, Gorst-Allman CP, Jones AJ, Marasas WFO, Murray KE, Smith LW, Steyn PS, Vleggaar R, Wessels PL. Chem. Commun. 1983:1259. [Google Scholar]
- 11.Culvenor CCJ, Edgar JA, Mackay MF, Gorst-Allman CP, Marasas WOF, Steyn PS, Vleggar R, Wessels PL. Tetrahedron. 1989;45:2351. [Google Scholar]
- 12.Mackay MF, Van Donkelaar AV, Culvenor CCJ. J. Chem. Soc. Chem. Commun. 1986:1219. [Google Scholar]
- 13.Tonsing EM, Steyn PS, Osborn M, Weber K. Eur. J. Cell. Biol. 1984;35:156. [Google Scholar]
- 14.Koiso Y, Li Y, Iwasaki S, Hanaoka K, Kobayashi T, Sonoda R, Fujita Y, Yaegashi H, Sato Z. J. Antibiot. 1994;47:765. doi: 10.7164/antibiotics.47.765. [DOI] [PubMed] [Google Scholar]
- 15.Koiso Y, Morisaki N, Yamashita Y, Mitsui Y, Shirai R, Hashimoto Y, Iwasaki S. J. Antibiot. 1998;51:418. doi: 10.7164/antibiotics.51.418. [DOI] [PubMed] [Google Scholar]
- 16.Koiso Y, Natori M, Iwasaki S, Sato S, Sonoda R, Fujita Y, Yaegashi H, Sato Z. Tetrahedron Lett. 1992;33:4157. [Google Scholar]
- 17.Iwasaki S. Med. Res. Rev. 1993;13:183. doi: 10.1002/med.2610130205. [DOI] [PubMed] [Google Scholar]
- 18.Bai R, Cowell DG, Taylor GF, Kepler JA, Copeland TD, Nguyen NY, Pettit GR, Hamel E. J. Biol. Chem. 2004;279:30731. doi: 10.1074/jbc.M402110200. [DOI] [PubMed] [Google Scholar]
- 19.Barbier P, Gregoire C, Sarrazin M, Peyrot V. Biochemistry. 2001;40:13510. doi: 10.1021/bi010926z. [DOI] [PubMed] [Google Scholar]
- 20.Ravi M, Zask A, Rush III TS. Biochemistry. 2005;44:15871. doi: 10.1021/bi051268b. [DOI] [PubMed] [Google Scholar]
- 21.Coutts IGC, Southcott MR. J. Chem. Soc. Perkin Trans. 1. 1990:767. [Google Scholar]
- 22.Masada H, Oishi Y. Chem. Lett. 1978:57. [Google Scholar]
- 23.Woiwode TF, Rose C, Wandless TJ. J. Org. Chem. 1998;63:9594. [Google Scholar]
- 24.Mann G, Hartwig JF. J. Am. Chem. Soc. 1996;118:13109. [Google Scholar]
- 25.Mann G, Hartwig JF. J. Org. Chem. 1997;62:5413. [Google Scholar]
- 26.Palucki M, Wolfe JP, Buchwald SL. J. Am. Chem. Soc. 1997;119:3395. [Google Scholar]
- 27.Hughes DL. Org. React. 1992;42:355. [Google Scholar]
- 28.Hughes DL. Org. Prep. Proced. Int. 1996;28:127. [Google Scholar]
- 29.Mitsunobu O. Synthesis. 1981:1. [Google Scholar]
- 30.Trost BM. Chem. Pharm. Bull. 2002;50:1. doi: 10.1248/cpb.50.1. [DOI] [PubMed] [Google Scholar]
- 31.Trost BM. J. Org. Chem. 2004;69:5813. doi: 10.1021/jo0491004. [DOI] [PubMed] [Google Scholar]
- 32.Trost BM, Crawley ML. Chem. Rev. 2003;103:2921. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]
- 33.Trost BM, Tang W. J. Am. Chem. Soc. 2002;124:14542. doi: 10.1021/ja0283394. [DOI] [PubMed] [Google Scholar]
- 34.Heffner RJ, Jiang JJ, Joullié MM. J. Am. Chem. Soc. 1992;114:10181. [Google Scholar]
- 35.Heffner RJ, Joullié MM. Tetrahedron Lett. 1989:7021. [Google Scholar]
- 36.Tsunoda T, Nagino C, Oguri M, Itô S. Tetrahedron Lett. 1996:2459. [Google Scholar]
- 37.Cao B. University of Pennsylvania; Philadelphia, PA: 2002. Ph. D. Thesis. [Google Scholar]
- 38.Li P. University of Pennsylvania; Philadelphia, PA: 2005. Ph. D. Thesis. [Google Scholar]
- 39.East SP, Joullié MM. Tetrahedron Lett. 1998;39:7211. [Google Scholar]
- 40.Xiao D, East SP, Joullié MM. Tetrahedron Lett. 1998;39:9631. [Google Scholar]
- 41.East SP, Shao F, Williams L, Joullié MM. Tetrahedron. 1998;54:13371. [Google Scholar]
- 42.Williams L, Zhang Z, Shao F, Carroll PJ, Joullié MM. Tetrahedron. 1996;52:11673. [Google Scholar]
- 43.Laïb T, Zhu J. Synlett. 2000;9:1363. [Google Scholar]
- 44.Laïb T, Zhu J. Tetrahedron Lett. 1999:83. [Google Scholar]
- 45.Ageno G, Banfi L, Cascio G, Guanti G, Manghisi E, Riva R, Rocca V. Tetrahedron. 1995;51:8121. [Google Scholar]
- 46.Park H, Cao B, Joullié MM. J. Org. Chem. 2001;66:7223. doi: 10.1021/jo010482c. [DOI] [PubMed] [Google Scholar]
- 47.Miller SC, Scanlan TS. J. Am. Chem. Soc. 1997;119:2301. [Google Scholar]
- 48.Fukuyama T, Jow C-K, Cheung M. Tetrahedron Lett. 1995;36:6373. [Google Scholar]
- 49.Kan T, Fukuyama T. Chem. Commun. 2004:353. doi: 10.1039/b311203a. [DOI] [PubMed] [Google Scholar]
- 50.Cao B, Park H, Joullie MM. J. Am. Chem. Soc. 2002;124:520. doi: 10.1021/ja017277z. [DOI] [PubMed] [Google Scholar]
- 51.Li P, Evans CD, Forbeck EM, Park H, Bai R, Hamel E, Joullié MM. Bioorg. Med. Chem. Lett. 2006;16:4804. doi: 10.1016/j.bmcl.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 52.Trost BM, Toste FD. J. Am. Chem. Soc. 1998;120:9074. [Google Scholar]
- 53.Lopez F, Ohmura T, Hartwig JF. J. Am. Chem. Soc. 2003;125:3426. doi: 10.1021/ja029790y. [DOI] [PubMed] [Google Scholar]
- 54.Sawayama AM, Tanaka H, Wandless TJ. J. Org. Chem. 2004;69:8810. doi: 10.1021/jo048854f. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka H, Sawayama AM, Wandless TJ. J. Am. Chem. Soc. 2003;125:6864. doi: 10.1021/ja035429f. [DOI] [PubMed] [Google Scholar]
- 56.Fan R-H, Hou X-L. J. Org. Chem. 2003;68:726. doi: 10.1021/jo025983s. [DOI] [PubMed] [Google Scholar]
- 57.Hou X-L, Fan R-H, Dai L-X. J. Org. Chem. 2002;67:5295. doi: 10.1021/jo016230t. [DOI] [PubMed] [Google Scholar]
- 58.Palais L, Chemla F, Ferreira F. Synlett. 2006:1039. [Google Scholar]
- 59.Pineschi M, Bertolini F, Haak RM, Crotti P, Macchia F. Chem. Commun. 2005:1426. doi: 10.1039/b416517a. [DOI] [PubMed] [Google Scholar]
- 60.Prasad BAB, Sanghi R, Singh VK. Tetrahedron. 2002;58:7355. [Google Scholar]
- 61.Bell D, Davies MR, Geen GR, Mann IS. Synthesis. 1995:707. [Google Scholar]
- 62.Godfrey JD, Jr., Mueller RH, Sedergran TC, Soundararajan N, Colandrea VJ. Tetrahedron Lett. 1994;35:6405. [Google Scholar]
- 63.Li P, Forbeck EM, Evans CD, Joullié MM. Org. Lett. 2006;8:5105. doi: 10.1021/ol062010w. [DOI] [PubMed] [Google Scholar]
- 64.Karrer P, Miyamichi E, Storm HC, Widmer R. Helv. Chim. Acta. 1925;8:205. [Google Scholar]
- 65.Wipf P, Miller CP. J. Org. Chem. 1993;58:3604. [Google Scholar]
- 66.Evans DA, Janey JM, Magomedov N, Tedrow JS. Angew. Chem., Int. Ed. Engl. 2001;40:1884. [PubMed] [Google Scholar]
- 67.Nordin IC. J. Heterocycl. Chem. 1966;3:531. [Google Scholar]
- 68.Campbell AD, Raynham TM, Taylor RJK. Synthesis. 1998:1707. [Google Scholar]
- 69.Nihei K, Kato MJ, Yamane T, Palma MS, Konno K. Synlett. 2001:1167. [Google Scholar]
- 70.Mehta A, Jaouhari R, Benson TJ, Douglas KT. Tetrahedron Lett. 1992;33:5441. [Google Scholar]
- 71.Li P, Evans CD, Joullié MM. Org. Lett. 2005;7:5325. doi: 10.1021/ol052287g. [DOI] [PubMed] [Google Scholar]
- 72.Boger LD, Yohannes D. Tetrahedron Lett. 1989;30:5061. [Google Scholar]
- 73.Boger LD, Yohannes D. J. Org. Chem. 1990;55:6000. [Google Scholar]
- 74.Morisaki N, Mitsui Y, Yamashita Y, Koiso Y, Shirai R, Hashimoto Y, Iwasaki S. J. Antibiot. 1998;51:423. doi: 10.7164/antibiotics.51.423. [DOI] [PubMed] [Google Scholar]
- 75.Forbeck EM, Evans CD, Gilleran JA, Li P, Joullié MM. J. Am. Chem. Soc. 2007;129:14463. doi: 10.1021/ja0758077. [DOI] [PubMed] [Google Scholar]
- 76.Evans CD. University of Pennsylvania; Philadelphia, PA: 2007. Ph. D. Thesis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.