Abstract
Astaxanthin (ATX), a naturally occurring carotenoid pigment, is a powerful biological antioxidant. In the present study, we investigated whether ATX pharmacologically offers neuroprotection against oxidative stress by cerebral ischemia. We found that the neuroprotective efficacy of ATX at the dose of 30 mg/kg (n = 8) was 59.5% compared with the control group (n = 3). In order to make clear the mechanism of ATX neuroprotection, the up-regulation inducible nitric oxide synthase (iNOS) and heat shock proteins (HSPs) together with the oxygen glucose deprivation (OGD) in SH-SY5Y cells were also investigated. The induction of various factors involved in oxidative stress processes such as iNOS was suppressed by the treatment of ATX at 25 and 50 µM after OGD-induced oxidative stress. In addition, Western blots showed that ATX elevated of heme oxygenase-1 (HO-1; Hsp32) and Hsp70 protein levels in in vitro. These results suggest that the neuroprotective effects of ATX were related to anti-oxidant activities in global ischemia.
Keywords: astaxanthin, global ischemia, oxidative stress, neuroprotection, nitric oxide synthase, heat shock protein
Introduction
Ischemic stroke occurs when the blood supply to the brain is obstructed. It is a common cause of death and disability in westernized society. Involvement of oxidative stress in neuronal loss after stroke is well established. Ischemia-induced neuronal loss is initiated by the release of excitatory neurotransmitters, which leads to membrane depolarization, increase in intracellular calcium concentration, and production of nitric oxide (NO) and reactive oxygen species (ROS), that is, superoxide anion radical, hydrogen peroxide and highly cytotoxic byproduct hydroxyl radical. Several components of ROS have been found to be generated after ischemic reperfusion injury and play an important role in the neuronal loss after cerebral ischemia [1]. However, many antioxidants (tempol, LY231617, LY178002 and U-101033E) are reported to reduce ROS mediated reactions and rescue the neurons from reperfusion induced neuronal loss in animal models of cerebral ischemia [2–5].
Astaxanthin (3,3'-dihydroxy-β,β-carotene-4,4'-dione; ATX; Fig. 1) is widely distributed in nature, and is the principal pigment in crustacean shell, salmonoids, and many other organisms. Animals cannot synthesize carotenoids de novo, thus ultimately they must obtain these pigments from the plants and algae that support their food chains [6]. ATX has important metabolic functions in animals, including conversion to vitamin A [7], enhancement of immune response [8] and protection against diseases such as cancer by scavenging oxygen radicals [9]. Ohgami et al. [10] reported that ATX inhibits NO production as well as lipopolysaccharide (LPS)-induced inflammation in in vivo and in vitro. The antioxidant activity of ATX has been reported to be approximately 10 times stronger than that of other carotenoids tested, including zeaxanthin, lutein, canthaxanthin, and β-carotene, and 550 to 1000 times greater than that of α-tocopherol (V-E) [11, 12]. The role of ATX, as well as that of many other antioxidant carotenoids (e.g. lycopene, β-carotene), in reducing atherogenesis and lowering the risk of some coronary heart diseases has been previously studied [13]. In another study, ATX showed neuroprotective effects in the ischemia-induced impairment of spatial memory in mice [14]. However there is no previous reported research about the neuroprotective action of ATX against global cerebral ischemia.
Fig. 1.
Chemical structure of astaxanthin.
In this study, we investigated the neuroprotective effect of ATX in a four-vessel occlusion (4-VO) induced global cerebral ischemia model in rats. In addition, we also studied possible neuroprotection by measuring the amount of NO and by comparing the activities of enzymatic scavengers such as inducible nitric oxide synthase (iNOS) and induction of heat shock proteins (HSPs) in an attempt to elucidate possible mechanisms underlying ATX protection against oxygen glucose deprivation (OGD)-induced death in SH-SY5Y neuroblastoma cell line.
Materials and Methods
Materials
Dulbecco’s modified Eagle medium (DMEM), penicillin/streptomycin solution, and trypsin-EDTA solution were obtained from Gibco RBL (Grand Island, NY). Fetal calf serum was purchased from Hyclone Laboratories (Logan, UT). Poly-D-lysine, Hank’s Balance Salt Solution, 3-(4,5-dimethylthioazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT), and all the other chemicals used in this study were purchased from Sigma Chemical Co. (St. Louis, MO).
Cell culture
SH-SY5Y human neuroblastoma cells were purchased from ATCC (Manassas, VA). Cells were cultured in DMEM/Nutrient Mixture Ham’s F-12 (1:1) media, supplemented with 10% fetal bovine serum, penicillin (100 IU/ml), streptomycin (100 µg/ml), and L-glutamine (2 mM); the cell culture medium was replaced every 2 days. The cultures were maintained at 37°C in 95% air–5% CO2 in a humidified incubator. The cells were grown on culture dishes pre-coated with poly-D-lysine (50 mg/ml in sterile water) overnight.
4-Vessel occlusion for in vivo ischemia
Male Wistar rats (SLC, Tokyo, Japan), weighing 190 to 220 g (6 to 7 weeks of age) were purchased from Dae-Han BioLab (Chungcheongbuk-do, South Korea). The animal procedures were performed in accordance with National Institutes of Health animal care guidelines. Transient forebrain ischemia was induced by 4-VO according to the method of Pulsinelli et al. [15]. In brief, after the animals were positioned in stereotaxic ear bars (Kopf; Tujunga, CA) with the head tilted down at ≈30° to the horizontal, incision of 1 cm in length was made behind the occipital bone directly overlying the first two cervical vertebrae. The paraspinal muscles were separated from the midline, and the right and left alar foramina of the first cervical vertebrae were exposed with the use of an operating microscope. A 0.5-mm diameter electrocautery needle was inserted through each alar foramen and both vertebral arteries electrocauterized and permanently occluded. Next, both common carotid arteries were isolated via a ventral, midline cervical incision. An atraumatic arterial clasp was loosely placed around each common carotid artery without interrupting carotid blood flow and the incision was closed with a single suture. On the following day, 10 min of 4-VO ischemia was induced by tightening the clasp around the common carotid arteries. Carotid clasps were then removed following 10 min to restore carotid blood flow. To minimize variability among animals, following criteria were strictly applied for the 10 min ischemic period and 20 ± 5 min post-ischemic coma: loss of righting reflex and bilateral pupil dilation. Body temperature was monitored and maintained at 37 ± 0.5°C with a rectal thermistor coupled to a heating blanket (Homeothermic Blanket Control Unit, Harvard apparatus; Edenbridge, UK). Sham-operated animals that underwent surgery were used for non-ischemic control.
OGD for in vitro ischemia
Cultures were placed in an anaerobic chamber (Forma Scientific) that contained a gas mixture of 5% CO2, 10% H2 and 85% N2 (<0.2% O2). Culture medium was replaced by thorough exchange with deoxygenated, glucose-free balanced salt solution containing the following (mmol/L): 116 NaCl, 5.4 KCl, 0.8 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 1.8 CaCl2, 0.01 glycine and 0.6 L-arginine; plates were then placed in a 37°C humidified incubator within the chamber for 90 min. Cultures were subsequently removed from the incubator, the exposure medium was exchanged with oxygenated MEM, and cultures were returned to a 37°C 5% CO2-containing normoxic (21% O2) incubator. The medium was supplemented with L-arginine to prevent this amino acid substrate of NOS from being limiting.
Histology
At 7 days after reperfusion, the animals were anesthetized and perfused with heparinized physiologic saline, and their brains fixed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were quickly removed from the skull and post-fixed by immersion, cryoprotected in 30% sucrose. Fixed brains were serially sectioned parallel to the coronal plane on a sliding microtome (HM440E, Zeiss, Germany) with the thickness set at 30 µm and the sections were stained with 0.5% cresyl violet. The number of intact neurons in a 1 mm length of the middle portion of the hippocampal CA1 subfield in the total three coronal sections per animal was counted by three blinded observers using a microscope at ×400 magnification. Only whole neurons with visible nucleus were counted. The histogram was plotted on the basis of the mean ± standard deviation values. Data from each group was analyzed by use of Student’s t test.
Administration of ATX
For the assessment of neuroprotective effects, ATX (purchased from Sigma, St. Louis, MO) was dissolved in pure ethanol. Animals that had been subjected to 10 min ischemia as described below were randomly divided into two groups. Either a solution of ATX (30 mg/kg) or ethanol was intra-peritoneally injected into the two groups of animals at 0 and 90 min of cerebral reperfusion.
Cell viability assay
SH-SY5Y cells were seeded at a density of 3 × 104 cells per well on collagen coated 96-well plates and maintained at 37°C in a 5% CO2 atmosphere. ATX was dissolved and diluted with 0.1% DMSO (10, 25, 50 and 100 µM). Final concentrations of organic solvents were always less than 0.1%, and they have no effects on cell viability. SH-SY5Y cells were pretreated with diluted ATX for 90 min before the exposure to OGD. After treatment, 50 µl of MTT (2 mg/ml stock solution, Sigma) was added and the plates were incubated for an additional 4 h. 150 µl of DMSO was added to dissolve the formazan and 20 µl of Sorenson’s glycine buffer was added to obtain the highest absorbance at 570 nm. After 20 min of gentle shaking, each plate was read at 570 nm using a microplate reader. The IC50 values were determined by plotting the drug concentration versus the survival ratio of the treated cells. All the experiments were performed in triplicate, and graphic results are shown as mean ± SEM corresponding to 6 separate experiments.
Measurement of NO
Nitrite, measured by Griess reaction, was taken as a measure of NO production. Briefly, 100 µl of culture supernatant (24 h) was reacted with an equal volume of Griess reagent (1 part 0.1% naphthylethylene-diamine, 1 part 1% sulfanilamide in 5% H3PO4) in 96-well tissue culture plates for 10 min at room temperature in the dark. The absorbance at 540 nm was determined using a microplate reader (spectraMAX 340, Molecular Devices, Sunnyvale, CA).
Immunobloting
Total protein extraction and western blot analysis was performed as described in our earlier studies [16]. For this study, the protein transferred membranes were incubated with rabbit anti-mouse iNOS (1:2000, Calbiochem, San Diego, CA), Hsp70 (1 µg/ml, Calbiochem), β-actin (1:5000, Calbiochem), PARP-1 (1:1000, Santacruz) and Heme oxygenase-1 (Hsp32; HO-1) (1 mg/ml, Stressgene). The proteins were detected by a chemiluminescence detection system according to the manufacturer’s instructions (ECL, Amersham, Berkshire, UK). The band intensity was quantified with a densitometric scanner (PDI, Huntington Station, NY).
Statistical analysis
All data were presented as mean ± SEM. Statistical comparison between different treatments was done by Student’s t test. Differences with p value<0.05 were considered statistically significant.
Results
Neuroprotective effect of ATX on global cerebral ischemia
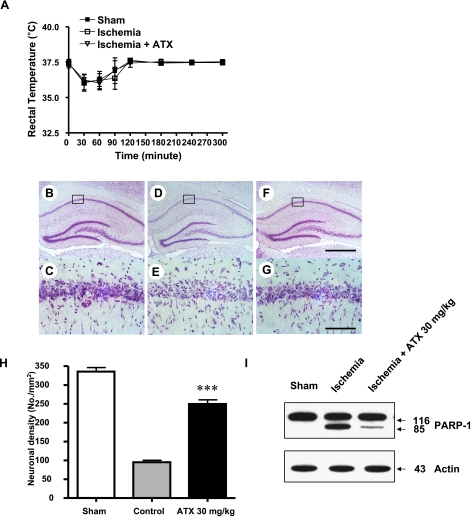
The body temperature of animals exposed to ischemia was monitored for 5 h after ischemia-reperfusion. There was no significant difference in body temperature between saline treated group and ATX treated groups at any time point recorded indicating that neuroprotective effects of ATX were not due to decrease in body temperature (Fig. 2A). The neuroprotective effect of ATX on CA1 hippocampal neurons was evaluated by measuring the neuronal cell density in the CA1 hippocampal region at 7 days after 10 min ischemia. The brain tissue sections of the dorsal hippocampus were stained with cresyl violet to display the selective delayed neuronal loss in the CA1 hippocampal region. In the sham operated group, the most of the CA1 pyramidal neurons in the CA1 region showed an unchanged staining pattern (Fig. 2-B: a, d). In the saline treated group (control group), the CA1 region was weakly stained, and the neuronal cell damage limitedly occurred in the CA1 region. Pyramidal neurons underwent a coagulative cellular change and were damaged with characteristic apparent gliosis (Fig. 2-B: b and e). The ATX treated group (at a dose 30 mg/kg, i.p.) was significantly reduced due to the number of coagulated and damaged pyramidal neurons in the CA1 region (Fig. 2-B: c and f). Apoptosis was induced in the control group shown by Fig. 2-B: b and e. Apoptotic cells were induced due to some external or internal stimuli-cells shrank, losing their intrinsic shapes established according to their differentiation. Additionally, as the shrinkage broke the junctions with surrounding cells, the interaction was interrupted between apoptotic cells and the cells adjacent to them. The characteristic pyramidal morphology of the apoptotic cells changed. In the saline treated group, neuronal cells were found to undergo apoptosis (Fig. 2-B: e). Also, in the ATX treated group apoptotic cells appeared in the detached cells from CA1 region. In contrast, the attached neuronal cells in the CA1 region showed morphology similar to the pyramidal normal cells (Fig. 2-B: f). ATX (at a dose 30 mg/kg) was intraperitoneally administered at 30 min after ischemia. The control group was orally administered with a saline solution (administration volume, 1.0 ml/kg). Viable neuronal cells were measured to be 338.3 ± 19.5 neurons/mm2 in the sham-operated group and 103.6 ± 11.3 neurons/mm2 in the control group. In the ATX treated group, viable neuronal cells at a dose of 30 mg/kg were measured to be 247.6 ± 10.7 neurons/mm2 (Fig. 2-C). The neuroprotective percent at a dose of 30 mg/kg is 59.5%. (***p<0.001). Based on our findings showing that ischemia causes apoptotic death of nuoronal cells, we assessed whether ATX inhibits apoptotic cell death. As poly (ADP-ribose) polymerase (PARP-1) cleavage is a hallmark of apoptosis. PARP-1 is a protein involved in a number of cellular processes involving mainly DNA repair and programmed cell death. PARP-1 is also known for its role in transcription through remodelling of chromatin by PARylating histones and relaxing chromatin structure, thus allowing transcription complex to access genes. As shown in Fig. 2-I, ATX treatment of ischemia rats at this dose (20 mg/kg) inhibited PARP-1 cleavage compared with ischemia rats group.
Fig. 2.
Neuroprotective effect of ATX on global cerebral ischemia. (A) Rectal temperatures were measured in rats for 5 h after global ischemia-reperfusion. Animals were administered with ATX of 30 mg/kg (n = 7) at 0 and 300 min following 10-min ischemia, respectively. Number in parenthesis represents the number of animals. B–H. Representative photomicrographs of Cresyl violet-stained hippocampal regions of sham-operated animals (B, C) or animals that had been subjected to 10 min ischemia followed by treatment with either saline (D, E) or 30 mg/kg of ATX (F, G). Boxed regions in B, D and F are shown in C, E and G, respectively. The 10 min ischemia caused selective and delayed neuronal cell loss in the hippocampal CA1 region (D, E). In contrast, ATX treatment conferred neuroprotection by markedly reducing the number of damaged pyramidal cells in the CA1 subfield (F, G). Scale bar is 100 µm. (H) Assessment of neuroprotective effects of ATX. Either ethanol or ATX (10, 20 and 30 mg/kg) was intraperitoneally administered into animals following 10 min ischemia. Seven days later, neuronal cell density in CA1 region was measured by Nissl staining. Asterisks indicate statistically significant differences from saline-treated ischemic group (p<0.05). Sham, sham-operated animals (n = 6); Saline, ethanol-treated animals following ischemia (n = 5); ATX, ATX-treated animals following ischemia (n = 5 for 10 mg/kg, 20 mg/kg and 30 mg/kg). (I) Lysates containing equal amounts of protein (20 µg) were separated by SDS-PAGE and immunoblotted. Actin was used to confirm the equal amount of proteins loaded in each lane.
Inhibition of NO and iNOS by ATX
To further understand the neuroprotective mechanisms of ATX, we next investigated whether ATX protects SH-SY5Y neuronal cells from oxidative injury. In the cells treated with ATX (at a concentration of 10, 25 and 50 µM) for 30 min before being exposed OGD, the viability was measured to be 73.81%, 92.95% and 110.76%, respectively (Fig. 3-A, *p<0.05, **p<0.01, ***p<0.001). Next, the cells were treated with OGD alone or with various concentrations of ATX for 24 h. NO production was assayed by measuring the levels of a stable NO metabolite, nitrites in the conditioned medium. At the concentrations (10–50 µM) used in this study, none of the OGD or ATX treatments caused toxicity to cells as judged by the MTT assays (Fig. 3-A). For determining the concentration-dependent relationship, increasing concentrations of drug are used in a semi-log scale. We are found that ATX acts from 5 to 100 µM both the highest ineffective and the lowest effective concentrations, respectively (Fig. 3-B). ATX treatment of SH-SY5Y cells after OGD stress time-dependently decreased nitrite accumulation. ATX at 50 µM concentration effectively inhibited NO production by 70% by 24 h of culturing time in OGD-stimulated SH-SY5Y cells (Fig. 3-C). To determine whether the inhibitory ability of ATX on NO production was due to a decrease in the cytosolic iNOS protein level, SH-SY5Y cells were treated with OGD and ATX for 24 h, and the levels of iNOS protein were detected by Western blotting. As shown in Fig. 3-D, treatment with ATX led to a significant decrease in iNOS protein levels. ATX (25 and 50 µM) inhibited OGD-induced iNOS protein production by 20–60%.
Fig. 3.
Effect of ATX against oxidative stress in OGD-induced SH-SY5Y cells. (A, B) Cells were subjected to OGD then various concentrations of ATX (10, 25 and 50 µM) were added for 24 h. ATX was not toxic to SH-SY5Y cells without OGD as compared with SH-SY5Y cells with OGD. (C) Cells were OGD-induced then various concentrations of ATX (10, 25 and 50 µM) were added for 24 h. Nitrite content was measured using the Griess reaction. Values indicate nitrite production from cells exposed to culture supernatants of OGD treated alone and cells exposed to OGD plus ATX. (D) Cell lysates were prepared and subjected to Western blotting from control or cell OGD-induced, alone or in combination with increasing concentrations (25 and 50 µM) of ATX for 24 h. Depicted is a Western blot of iNOS protein expression (upper panel) and the statistical analysis of the changes of iNOS protein (lower panel). The β-actin level was considered as an internal control. Values represent the mean ± SEM of five separate experiments on SH-SY5Y cells. Means data points were analyzed by one-way ANOVA, followed by Dunnett’s posttest to compare all data with OGD-group. (*p<0.05, **p<0.01, ***p<0.001).
ATX increases HSPs protein levels in the absence of oxidative stress
We found that in the absence of OGD, ATX robustly stimulated HO-1 (Hsp32) levels in a dose-dependent manner (Fig. 4-A). HO-1 (Hsp32) is an anti-inflammatory enzyme, shown to be important in the resolution of acute inflammatory responses. Also, HO-1 (Hsp32) is known to be induced by a variety of stress-stimuli, such as heavy metals, UV radiation and heat shock. To assess whether HO-1 (Hsp32) induction might contribute to the anti-oxidant effects of ATX in SH-SY5Y cells, we tested to see if ATX could induce HO-1 (Hsp32) production in SH-SY5Y cells. The increases in HO-1 (Hsp32) levels reached a maximum at 25 µM of ATX concentration. However, OGD alone did not stimulate HO-1 (Hsp32) induction in SH-SY5Y cells nor did it affect the ability of ATX to stimulate HO-1 (Hsp32) (Data not shown). We also examined the ability of other HSP to induce HO-1 (Hsp32) production in SH-SY5Y cells. In addition to stimulating HO-1 (Hsp32), ATX also induced an increase in another protective enzyme, Hsp70. As shown in Fig. 4-B, the levels of Hsp70 increased in SH-SY5Y cells treated with ATX (10 µM). The induction of HO-1 (Hsp32) and the hsp70 could possibly represent upregulation of antioxidant defense mechanisms in a cellular response to oxidative stress. These data suggest that the stimulation of HO-1 (Hsp32) and Hsp70 by ATX is a consequence of oxidative stress as is decreased production of NO by inhibition of iNOS. However, our data do not rule out the possibility that other forms of oxidative stress not examined in this study might be induced by ATX.
Fig. 4.
Modulation of OGD-induced Hsp70 and HO-1 (Hsp32) expression by ATX. Cell lysates were prepared and subjected to Western blotting from control or cell OGD-induced, alone or in combination with increasing concentrations (10, 25, 50 and 100 µM) of ATX for 24 h. Depicted is data of (A) Hsp70 or (B) Hsp32 protein expression (upper panel) and the statistical analysis of the changes of (A) Hsp70 or (B) Hsp32 protein (lower panel). The β-actin level was considered as an internal control. Data represent the mean ± SEM of three independent experiments.
Discussion
Recognition of the involvement of oxidative stress in neurodegenerative diseases has generated substantial interest in investigating the ability of naturally occurring antioxidant phytochemicals to ameliorate neuronal damage associated with neuro-degeneration and aging [17]. Previous studies from our laboratory have demonstrated the ability of falcarindial and baicalein, a antioxidants isolated from plants, to ameliorate neuronal damage resulting from cerebral ischemia [18, 19]. In the present study, we demonstrated that administration of ATX showed neuroprotective effects in a 4-VO-induced global cerebral ischemia model in rats. ATX also significantly inhibited OGD-stimulated NO production and iNOS protein expression in SH-SY5Y cell lines.
In the animal study, ATX (30 mg/kg body wt) was injected into Wistar rats within 30 min after ischemia. Treatment with ATX after cerebral ischemia significantly reduced the CA1 neuronal cell death up to 78% as compared to the control group. Death of CA1 hippocampal neurons following cerebral ischemia caused a variety of neurological dysfunctions. Although the exact mechanism of neurological damage caused by cerebral ischemia has yet to be elucidated, the involvement of excitotoxicity, activation of voltage-gated calcium channels, inflammatory cytokines, and oxidative stress was reported [20]. One of the possible ways to prevent free radical mediated cellular injuries is to augment the oxidative defense capacity through intake of antioxidants. ATX has a quenching capability against damage from NO in vitro 80 times stronger than α-tocopherol and twice as strong as β-carotene [21]. Hence, we cannot exclude the possibility that the neuroprotection of ATX against ischemia-induced neuronal damage may partially depend on its anti-inflammatory and anti-oxidation activity. Although our study used lower doses than those reported by Hussein et al. [14], comparable protective effects were obtained. Since most of these studies examined cell death at one time point after ischemia/reperfusion, it is not possible to conclude whether the protective effects of ATX are not simply delaying the cell death process. Recently, a report indicated that ATX significantly protected mitochondria be a ROS scavenging in hyperglycemia-induced oxidative damage [22]. In addition, ATX has been beneficial effects for cognitive and psychomotor function in preliminary clinical [23]. Consequently, more studies are needed to examine the effects of ATX at prolonged times after ischemia.
NO production is enhanced at all stages of cerebral ischemia and this increase is accompanied by up regulation of both NOS activity and NOS gene expression [24]. While NO has been reported to be toxic to cells through several different mechanisms [25], we here demonstrate that NO triggered by iNOS induction may contribute to an enhancement of OGD-induced neuronal death. The iNOS can be induced in neuronal cells after OGD insult in vitro. Furthermore, this inducement occurs in a short period of time (2–3 h after ischemia), suggesting that NO can play an important pathogenic role in cell damage that occurs in early stages of cerebral ischemia [26].
Our studies showed that ATX in a dose dependent manner can decrease the delivery of NO and the activity of iNOS in OGD-induced SH-SY5Y cells. Moreover, we have found that ATX can protect rat cerebral cortical and hippocampus slices from injury induced by H2O2 (data not shown), which is consistent with the protective effect of ATX, which may be related to its antioxidant properties. Despite the incomplete understanding of molecular mechanisms involved with the proposed neuroprotective action of ATX, we suggest that its inhibition of the NO/iNOS system plays a significant role in reducing OGD-induced neuron cells damage. The detailed mechanism by which ATX inhibits the NO/iNOS system remains a subject for further investigation. We investigated the effect of ATX on OGD-induced NO production and iNOS protein expression in the SH-SY5Y cells. Treatment of ATX inhibited iNOS protein expression and NO accumulation after OGD stimulation. This implies that ATX, which inhibits NO production through inhibiting iNOS enzyme activity, has beneficial therapeutic effects in the treatment of inflammation associated neurodegenerative disease. Moreover the compound, even at the concentration of 50 µM, did not change cell viability. The data are in agreement with previous reports showing that 10 to 50 µM ATX do not cause cytotoxicity in RAW246.7 cell lines [10]. This implies that the inhibition of OGD-induced NO production by ATX is not the result of its cytotoxicity on SH-SY5Y cells.
HSPs are found in all organisms, and are induced in all organs of all animals, including the brain, by heat, heavy metals, ischemia and other types of stress [27]. Transient whole-body hyperthermia or mild cerebral ischemia protect against subsequent cerebral ischemic injury [28, 29]. In the nervous system, HSPs are induced in a variety of pathological conditions, including cerebral ischemia, neurodegenerative disorders, epilepsy, and trauma. A number of in vitro studies show that both heat shock and HSP overproduction protect CNS cells against both necrosis and apoptosis. Although prolonged exposure to conditions of extreme stress is harmful and can lead to cell death, induction of HSP synthesis can result in stress tolerance and cytoprotection against stress-induced molecular damage. There is now strong evidence that overproduction of HSPs leads to protection in several different models of nervous system injury. Previous studies have shown that Hsp70 over-expression protects cells from death induced by various insults that cause either necrosis or apoptosis, including hypoxia and ischemia/reperfusion, by inhibiting multiple cell death pathways [30]. Two found that treatment of the cells with low concentrations of ATX resulted in high resistance to OGD-induced cell death, and ATX induced HO-1 (Hsp32) and Hsp70 expression at low concentration in SH-SY5Y cell lines. In the previous study, however, the presence of NOS inhibitors suppressed both nitrite accumulation and HO-1 mRNA expression. Modulation of HO-1 mRNA expression by iNOS-derived NO following stimulation with OGD has also been reported in different brain regions, particularly in the hippocampus and substantia nigra in the in vivo rat model [31]. In previously reported, in the in hemorrhage/resuscitation-induced injury reduced cellular caspase-3 activity, and to preserve cellular ATP levels thorough HSP-70 overexpression and then subsequently to inhibit iNOS expression [32]. In addition, overexpression of HSP 70 mRNA and of HSP 70 and HO-1 proteins and inhibition of iNOS mRNA induction in endotoxin-induced hypertension rats with arsenite treatment [33]. The effects of ATX relation to NOS activity and HSPs induction need to be further investigated.
In conclusion, our results demonstrate that ATX protects SH-SY5Y cells in an in vitro model of ischemia/reperfusion through inhibition of NO production and HSPs modulation. Also, the administration of ATX during the early stage of cerebral ischemia/reperfusion protects against neuronal cell death in the hippocampal CA1 area of animal model. Additional in vitro and in vivo studies are necessary to determine whether ATX can be used as a preventive agent against acute neurodegenerative conditions, or to reduce the progression of chronic and age-associated neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease.
Acknowledgements
This work was supported by the Korean Research Foundation Grant Fund by the Korean Government (MOEHRD, Basic Research Fund, KRF-214-2006-1-E00014). Also, this study was conducted in part by research funds from Gwangju University, South Korea in 2010.
References
- 1.Oliver C.N., Starke-Reed P.E., Stadtman E.R., Liu G.J., Carney J.M., Floyd R.A. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc. Natl. Acad. Sci. 1990;87:5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemens J.A., Ho P.P., Panetta J.A. LY178002 reduces rat brain damage after transient global forebrain ischemia. Stroke. 1991;22:1048–1052. doi: 10.1161/01.str.22.8.1048. [DOI] [PubMed] [Google Scholar]
- 3.Soehle M., Heimann A., Kempski O. Postischemic application of lipid peroxidation inhibitor U-101033E reduces neuronal damage after global cerebral ischemia in rats. Stroke. 1998;29:1240–1246. doi: 10.1161/01.str.29.6.1240. [DOI] [PubMed] [Google Scholar]
- 4.Fuson K.S., Mark R.J., Panetta J.A., May P.C. Characterization of LY231617 protection against hydrogen peroxide toxicity. J. Neurochem. 1999;72:1154–1160. doi: 10.1046/j.1471-4159.1999.0721154.x. [DOI] [PubMed] [Google Scholar]
- 5.Cuzzocrea S., McDonald M.C., Mazzon E., Siriwardena D., Costantino G., Fulia F., Cucinotta G., Gitto E., Cordaro S., Barberi I., De Sarro A., Caputi A.P., Thiemermann C. Effects of tempol, a membrane-permeable radical scavenger, in a gerbil model of brain injury. Brain. Res. 2000;875:96–106. doi: 10.1016/s0006-8993(00)02582-8. [DOI] [PubMed] [Google Scholar]
- 6.Britton G.S., Liaaen-Jensen, Pfander. H. In: Carotenoids today and challenges for the future. Britton G., Liaaen-Jensen S., Pfander H., editors. 1995. in Carotenoids vol. 1A: Isolation and Analysis, [Google Scholar]
- 7.Bendich A., Olson J.A. Biological actions of carotenoids. FASEB J. 1989;3:1927–1932. [PubMed] [Google Scholar]
- 8.Jyonouchi H., Zhang-Shanbhag L., Tomita Y., Yokoyama H. Nucleotide-free diet impairs T-helper cell functions in antibody production in response to T-dependent antigens in normal C57B1/6 mice. J. Nutr. 1994;124:475–484. doi: 10.1093/jn/124.4.475. [DOI] [PubMed] [Google Scholar]
- 9.Jyonouchi H., Sun S., Iijima K., Gross M.D. Antitumor activity of astaxanthin and its mode of action. Nutr. Cancer. 2000;36:59–65. doi: 10.1207/S15327914NC3601_9. [DOI] [PubMed] [Google Scholar]
- 10.Ohgami K., Shiratori K., Kotake S., Nishida T., Mizuki N., Yazawa K., Ohno S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 2003;44:2694–2701. doi: 10.1167/iovs.02-0822. [DOI] [PubMed] [Google Scholar]
- 11.Krinsky N.I. Antioxidant functions of carotenoids. Free. Radic. Biol. Med. 1989;7:617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 12.Naguib Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000;48:1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- 13.Giugliano D. Dietary antioxidants for cardiovascular prevention. Nutr. Metab. Cardiovasc. Dis. 2000;10:38–44. [PubMed] [Google Scholar]
- 14.Hussein G., Nakamura M., Zhao Q., Iguchi T., Goto H., Sankawa U., Watanabe H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol. Pharm. Bull. 2005;28:47–52. doi: 10.1248/bpb.28.47. [DOI] [PubMed] [Google Scholar]
- 15.Pulsinelli W.A., Brierley J.B. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 16.Suk K., Kim S.Y., Leem K., Kim Y.O., Park S.Y., Hur J., Baek J., Lee K.J., Zheng H.Z., Kim H. Neuroprotection by methanol extract of Uncaria rhynchophylla against global cerebral ischemia in rats. Life Sci. 2002;70:2467–2480. doi: 10.1016/s0024-3205(02)01534-5. [DOI] [PubMed] [Google Scholar]
- 17.Youdim K.A., Joseph J.A. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radic. Biol. Med. 2001;30:583–594. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim J.M., Lee P., Son D., Kim H., Kim S.Y. Falcarindiol inhibits nitric oxide-mediated neuronal death in lipopolysaccharide-treated organotypic hippocampal cultures. Neuroreport. 2003;14:1941–1944. doi: 10.1097/00001756-200310270-00012. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.O., Leem K., Park J., Lee P., Ahn D.K., Lee B.C., Park H.K., Suk K., Kim S.Y., Kim H. Cytoprotective effect of Scutellaria baicalensis in CA1 hippocampal neurons of rats after global cerebral ischemia. J. Ethnopharmacol. 2001;77:183–188. doi: 10.1016/s0378-8741(01)00283-5. [DOI] [PubMed] [Google Scholar]
- 20.Pulsinelli W.A., Jacewicz M., Levy D.E., Petito C.K., Plum F. Ischemic brain injury and the therapeutic window. Ann. N.Y. Acad. Sci. 1997;835:187–193. doi: 10.1111/j.1749-6632.1997.tb48629.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Willén R., Wadström T. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob. Agents Chemother. 2000;44:2452–2457. doi: 10.1128/aac.44.9.2452-2457.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manabe E., Handa O., Naito Y., Mizushima K., Akagiri S., Adachi S., Takagi T., Kokura S., Maoka T., Yoshikawa T. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J. Cell. Biochem. 2008;103:1925–1937. doi: 10.1002/jcb.21583. [DOI] [PubMed] [Google Scholar]
- 23.Satoh A., Tsuji S., Okada Y., Murakami N., Urami M., Nakagawa K., Ishikura M., Katagiri M., Koga Y., Shirasawa T. Preliminary Clinical Evaluation of Toxicity and Efficacy of A New Astaxanthin-rich Haematococcus pluvialis Extract. J. Clin. Biochem. Nutr. 2009;44:280–284. doi: 10.3164/jcbn.08-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z., Huang P.L., Panahian N., Dalkara T., Fishman M.C., Moskowitz M.A. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 26.De Alba J., Cárdenas A., Moro M.A., Leza J.C., Lorenzo P., Boscá L., Lizasoain I. Down regulation of neuronal nitric oxide synthase by nitric oxide after oxygen-glucose deprivation in rat forebrain slices. J. Neurochem. 1999;72:248–254. doi: 10.1046/j.1471-4159.1999.0720248.x. [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulos M.C., Giffardm R.G., Bell B.A. An introduction to the changes in gene expression that occur after cerebral ischaemia. Br. J. Neurosurg. 2000;14:305–312. doi: 10.1080/026886900417261. [DOI] [PubMed] [Google Scholar]
- 28.Chopp M., Chen H., Ho K.L., Dereski M.O., Brown E., Hetzel F.W., Welch K.M. Transient hyperthermia protects against subsequent forebrain ischemic cell damage in the rat. Neurology. 1989;39:1396–1398. doi: 10.1212/wnl.39.10.1396. [DOI] [PubMed] [Google Scholar]
- 29.Chen H., Chopp M., Welch K.M. Effect of mild hyperthermia on the ischemic infarct volume after middle cerebral artery occlusion in the rat. Neurology. 1991;41:1133–1135. doi: 10.1212/wnl.41.7.1133. [DOI] [PubMed] [Google Scholar]
- 30.Giffard R.G., Yenari M.A. Many mechanisms for hsp70 protection from cerebral ischemia. J. Neurosurg. Anesthesiol. 2004;16:53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Scapagnini G., D’Agata V., Calabrese V., Pascale A., Colombrita C., Alkon D., Cavallaro S. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain. Res. 2002;954:51–59. doi: 10.1016/s0006-8993(02)03338-3. [DOI] [PubMed] [Google Scholar]
- 32.Kiang J.G. Inducible heat shock protein 70 kD and inducible nitric oxide synthase in hemorrhage/resuscitation-induced injury. Cell. Res. 2004;14:450–459. doi: 10.1038/sj.cr.7290247. [DOI] [PubMed] [Google Scholar]
- 33.Hauser G.J., Dayao E.K., Wasserloos K., Pitt B.R., Wong H.R. HSP induction inhibits iNOS mRNA expression and attenuates hypotension in endotoxin-challenged rats. Am. J. Physiol. 1996;271:2529–2535. doi: 10.1152/ajpheart.1996.271.6.H2529. [DOI] [PubMed] [Google Scholar]