Abstract
The involvement of reactive oxygen species (ROS) in the pathophysiology of Sjögren’s syndrome (SS), an autoimmune disorder, and irradiation-induced impairments in salivary secretion has been reported. Meanwhile, the strong antioxidant astaxanthin (Ast) has been suggested to have therapeutic effects on various diseases. In the present study, we examined the ROS scavenging capacity of Ast using a human salivary gland epithelial cell line (HSY) and investigated the effects of Ast on salivary secretion in a mouse model of irradiation-induced salivary gland dysfunction. Furthermore, we performed a clinical study of Ast in six SS patients and six normal individuals, quantifying the volume of saliva secretion and the level of oxidative stress markers in the saliva. Ast partially suppressed hydrogen peroxide-induced ROS in HSY cells. The mouse model demonstrated that the pre-administration of Ast resulted in the suppression of irradiation-induced hyposalivation. Furthermore, the administration of Ast appeared to increase salivary output in both the SS and normal groups. The level of oxidative stress marker, hexanoyl-lysine, in the saliva was reduced after Ast intake. These results suggest that Ast might act as an ROS scavenger, providing benefits to SS patients with impaired salivary secretion.
Keywords: astaxanthin, sjögren’s syndrome, salivary secretion, reactive oxygen species
Introduction
Sjögren’s syndrome (SS) is an autoimmune disorder characterized by lymphocyte infiltration and the destruction of the lacrimal and salivary glands, accompanied by the systemic production of autoantibodies to the ribonucleoprotein particles SS-A/Ro and SS-B/La. The disease is not fatal, but the dysfunction of the exocrine glands causes the hyposecretion of tears and saliva, resulting in dry eyes and dry mouth. These are common problems among the elderly, and living with SS can be a devastating experience for the sufferer, decreasing the quality of life dramatically. The causes of SS have not yet been clarified; however, the involvement of environmental factors and oxidative stress from reactive oxygen species (ROS) [1] as well as genetic factors, immune disorders, and viral infections has been suggested in the onset and pathology of SS [2]. In particular, ROS such as superoxide and hydrogen peroxide are known to induce apoptosis [3] and cause oxidative damage to membrane proteins and lipids, leading to impaired functioning [4]. Similar mechanisms have also been implicated in the pathology of several diseases [5, 6]. Numerous reports have discussed possible mechanisms whereby various pathophysiological phenomena associated with autoimmune diseases develop through these processes [7, 8]. For example, increased levels of oxidative stress markers have been found in the synovial fluid [9, 10], serum [11–13], and urine of patients with rheumatoid arthritis [14] and type I diabetes mellitus [15, 16].
Antioxidants are expected to serve as potentially therapeutic agents for oxidative stress-related diseases. Astaxanthin (Ast) is a reddish orange compound that belongs to the carotenoid family, which is widely found in fishes such as sea bream and salmon as well as crustaceans such as crab and shrimp. An important function of carotenoids such as Ast is to intercept the chlorophyll triplet state, in order to prevent the formation of singlet oxygen, or to quench the singlet oxygen molecule directly [17, 18]. In recent years, the strong antioxidant action of Ast has been implicated in preventive mechanisms for many disorders [19, 20].
In this study, we examined the therapeutic effects of Ast on impairments in salivary secretion in a mouse model of irradiation-induced salivary hypofunction and SS patients with xerostomia. As part of a clinical study, a gumdrop containing Ast was administered to twelve subjects: six SS patients with xerostomia and six normal individuals. To investigate the capacity of Ast to scavenge intracellularly generated ROS, we used a human salivary gland epithelial cell line (HSY) and evaluated the amount of ROS generated by H2O2 using flow cytometry.
Materials and Methods
Experimental animals
Six-month-old male C57BL6J mice were purchased from Japan Clea Inc. (Tokyo, Japan) and maintained under specific pathogen-free conditions until use. All the experimental procedures were approved by the animal welfare committee of Tsurumi University (Kanagawa, Japan).
Irradiation
Each mouse was anesthetized using an intraperitoneal injection of 60 mg/kg sodium pentobarbital. A single acute exposure to a 10-MV X rays (MEVATRON74 DX40; Toshiba Medical Systems, Tokyo, Japan) at a dose rate of 3 Gy/min and a distance of 1,000 mm was performed. The effective radiation dose to the salivary gland was set using the percentage depth dose and was over 95% of the maximum dose delivered.
Administration of Ast
The Ast was provided by Fuji Chemical Industry Co., Ltd (Toyama, Japan). To examine the preventive and therapeutic effects of Ast on impaired salivary secretion mediated by ROS, the mice were freely fed food containing 2, 10 or 50 mg of Ast. In the model examining preventive effects, free feeding was performed beginning two weeks before irradiation and was continued until four weeks after irradiation in the model examining therapeutic effects, free feeding was performed for 4 weeks after irradiation.
Measurement of salivary secretion
The volume of saliva secreted by the Ast-treated mice was measured. The amount of saliva secretion was measured 1 week prior to irradiation and was used to classify the animals into administration groups with similar secretory capabilities. The mice were weighed and then anesthetized using an intraperitoneal injection of a mixture of xylazine (24 mg/kg) and ketamine (36 mg/kg). To stimulate salivation, pilocarpine (0.1 mg/kg) was injected intraperitoneally. The saliva secreted into the oral cavity during each 1-min period following the injection of pilocarpine was carefully collected using capillaries (Ringcaps; Hirschmann Laborgeräte GmbH & Co. KG, Eberstadt, Germany). The amount of total saliva secreted in 15 min was divided by the weight of the mouse.
Human salivary gland epithelial cell cultures
HSY cells derived from an adenocarcinoma of the parotid gland were used. HSY cells were cultured in growth medium containing high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (D6429; Sigma, St. Louis, MO) plus 10% fetal bovine serum (Sigma, St. Louis, MO) and 1% penicillin or streptomycin (Invitrogen, Carlsbad, CA) and maintained in a 100-mm culture dish in a humidified atmosphere containing 5% CO2 at 37°C. Cells were allowed to grow to 80% confluence before being passaged in trypsin-EDTA solution. The cells were then reseeded in a 100-mm culture dish and grown for 24 h before use in the experiments.
Measurement of ROS using fluorescence-activated cell sorting
We assessed ROS generation using the fluorescent probe CM-H2DCFDA (5 mM), a dye that is sensitive to a broad spectrum of ROS including H2O2 and •OH [21]. CM-H2DCFDA is a cell permeant indicator for ROS that is non-fluorescent until the removal of the acetate groups by oxidation within the cell. Cells were grown at a density of 1 × 106 cells per well in a 12-well plate and were maintained for 24 h in growth medium, then incubated for 1 h with Ast (5 mM) or N-acetyl-cysteine (NAC) (5 mM) and exposed for 15 min to CM-H2DCFDA. The HSY cells were then exposed to 1.0 mM H2O2 for 30 min to analyze the intracellular ROS level. Analyses were performed using a FACSCaliber flow cytometer and Cell Quest software (Becton Dickinson, Franklin Lakes, NJ).
Evaluation of Ast effects on SS patients and normal individuals
Tablets containing 2 mg of Ast extracted from Haematococcus pluvialis were used (provided by Fuji Chemical Industry Co., Ltd. Kamiichi-machi, Japan). Six SS patients with xerostomia who had a salivary output of less than 2 g, as determined using the Saxon test (SS group), and six normal individuals (normal group) were examined (Table 1). All twelve participants provided informed consent. Each participant took six test tablets per day (12 mg/day). Tests were performed before and 2 weeks after the oral intake of the Ast tablets. Participants were instructed not to eat food containing Ast during the examination period. A piece of sterilized gauze was weighed before and after being chewed by a participant for 2 min. The difference between the two measurements (dry weight before chewing and wet weight after chewing) was regarded as the salivary output. Pre-treated saliva samples were centrifuged at 10,000 rpm for 30 min and then passed through an ultrafiltration membrane (pore size, 0.22 µm). The filtered samples were subjected to an enzyme-linked immunosorbent assay for the measurement of the oxidative stress marker 8-hydroxy-2'-deoxyguanosine (8-OHdG) and the lipid peroxidation marker hexanoyl-lysine (HEL) using an anti-8-OHdG monoclonal antibody (N45.1; Institute for the Control of Aging, Shizuoka, Japan) and an anti-HEL monoclonal antibody (Institute for the Control of Aging, Shizuoka, Japan), respectively. The primary antibodies bound to the markers were probed with an HRP-conjugated anti-mouse IgG antibody (Zymed Laboratories, South San Francisco, CA) and assessed by measuring the color development at 490 nm.
Table 1.
Characteristic of the subjects in this study
| SS | Normal | |
|---|---|---|
| (n = 6) | (n = 6) | |
| age (years) | 64.7 ± 6.4 | 28.5 ± 4.0 |
| saliva flow rate (g/2 min) | 1.02 ± 0.4 | 6.2 ± 2.2 |
Values represent mean ± SEM.
SS: Sjögren syndrome
Statistical analysis
All the results were expressed as the mean ± SE. The Wilcoxon’s rank-sum test was performed to compare the results before and after the intake of the Ast tablets. Data were analyzed for statistical significance, and the significance level was set at a p value<0.05.
Ethics
Informed consent was obtained from all the patients, and the Ethical Committee of Tsurumi University approved this study.
Results
Comparison of Ast effects on irradiation-induced impaired salivary secretion
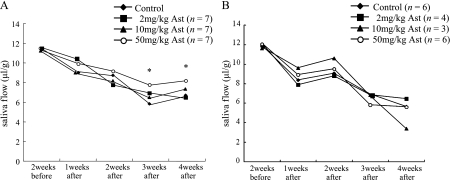
The present study showed that salivary flow had decreased at 1 week after irradiation in the control group, which was fed a conventional chow (Fig. 1). This result is consistent with that of a previous report. The administration of a high dose of Ast (50 mg/day) before irradiation resulted in the suppression of the salivary impairment observed in the control group at 3 and 4 weeks after irradiation (Fig. 1A). However, when Ast was administered after irradiation, the salivary secretion did not recover (Fig. 1B). These findings indicate that Ast has a preventive, but not a therapeutic, effect on irradiation-induced salivary dysfunction.
Fig. 1.
Effect of Ast on salivary secretion. A) Model examining preventive effects. Ast at a dose of 2 mg/kg, 10 mg/kg or 50 mg/kg was administered intravenously 2 weeks prior to irradiation and once a day beginning on the day after irradiation and continuing for 4 weeks. B) Model examining therapeutic effects. Ast at a dose 2 mg/kg, 10 mg/kg or 50 mg/kg was administered intravenously after irradiation and once a day beginning on the day after irradiation and continuing for 4 weeks. Saliva flow was expressed as the total output of saliva during the first 15 min after pilocarpine stimulation, normalized to the body weight. The asterisks indicate a significant decrease (*p<0.05), compared with the respective control.
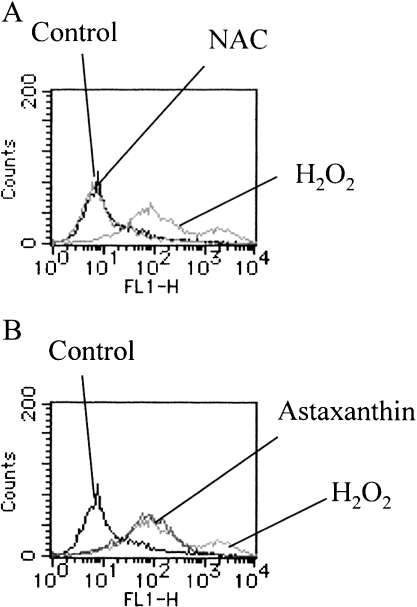
Confirmation of antioxidant activities of Ast and NAC using fluorescence-activated cell sorting flow cytometry
The intracellular generation of ROS at 20 min after the addition of H2O2 was measured using CM-H2DCFDA as a fluorescent indicator of ROS, such as H2O2 and hydroxyl radicals. The mean fluorescence intensity (MFI) of H2O2-stimulated HSY cells was elevated (MFI = 485.04). Pre-treatment with Ast partially suppressed the H2O2-induced MFI elevation (MFI = 117.51). Although the lower-phase intensity was not changed, the higher-phase intensity was almost completely inhibited (Fig. 2B). This result suggests the possibility that Ast scavenges some species of ROS induced by H2O2. On the other hand, pre-treatment with NAC, scavenges wide variety of ROS including H2O2 and •OH via glutathione generation [22], reverted the H2O2-induced MFI elevation to the unstimulated levels (MFI = 18.11) (Fig. 2A).
Fig. 2.
Flow cytometry analysis of ROS. HSY cells were incubated for 1 h with NAC (5 mM) (A) or Ast (5 mM) (B) and then exposed for 15 min to CM-H2DCFDA. ROS generation was assessed after exposure to 1.0 mM H2O2 for 30 min.
Effects of Ast on saliva secretion and oxidative stress markers in SS patients and normal individuals
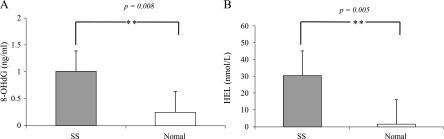
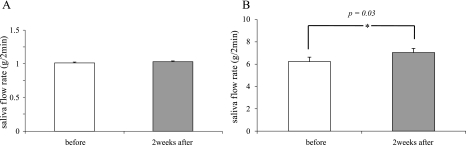
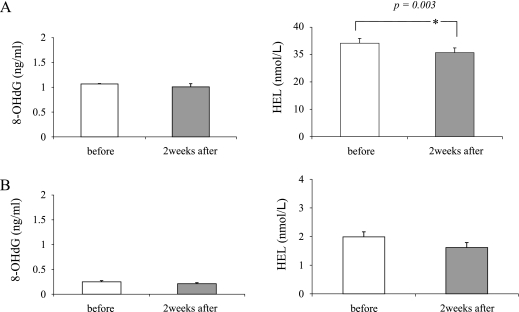
The involvement of oxidative stress in salivary gland dysfunction has been reported [1]. When the SS group was compared with the normal group, the levels of both 8-OHdG and HEL were significantly higher in the SS group than in the normal group (p = 0.008 and p = 0.005, respectively) (Fig. 3). This result was consistent with those of previous studies [1]. To evaluate the effects of Ast on salivary secretion and ROS production in SS patients, we measured the amount of salivary secretion and the level of 8-OHdG and HEL in saliva before and after examination. Although the increased amount of salivary secretion after the intake of Ast for 2 weeks was faint in the SS group (1.02 g/2 min to 1.04 g/2 min, p = 0.69) (Fig. 4A), a significant increase was observed in the normal group (6.23 g/2 min to 7.02 g/2 min, p = 0.03) (Fig. 4B). The level of the oxidative stress marker 8-OHdG did not change in either group (Fig. 5, left panels of A and B). However, the alternative oxidative stress marker HEL was significantly decreased in SS group (34.0 M to 30.58 M, p = 0.03) (Fig. 5, right panel of A). The decreased HEL level in the normal group was faint (Fig. 5, right panel of B). This suggests that the elevated stress marker levels shown in this study were not caused by hyposalivation. These results suggest that Ast administration has a beneficial effect on salivation in not only patients with SS, but also in normal individuals by inducing the scavenging of ROS.
Fig. 3.
8-OHdG (p = 0.008) and HEL (p = 0.005) levels of saliva in both groups. These levels were significantly higher in the SS group than in the normal group. The asterisks indicate significant increases (**p<0.005). Values are the mean ± SEM. A) SS group, B) normal group.
Fig. 4.
Measurement of salivary output upon stimulation using the Saxon test. In the normal group treated with Ast, the saliva flow rate after 2 weeks of treatment was significantly higher than that at baseline. The asterisks indicate a significant increase (*p<0.05). Values are the mean ± SEM. A) SS group, B) normal group.
Fig. 5.
Effect of Ast on 8-OHdG and HEL levels in saliva in both groups. In the SS group treated with Ast, the HEL level decreased significantly after treatment, compared with the baseline value. The asterisks indicate a significant decrease (*p<0.05). Values are the mean ± SEM. A) SS group, B) normal group.
Discussion
The possible causes of hyposalivation include organic disorders of glandular tissues, as exemplified by the autoimmune disease SS, as well as the adverse effects of drugs, pollution, and aging. High levels of oxidative stress markers such as 8-OHdG and HEL in the saliva of SS patients [1] and the involvement of ROS in the mechanism of irradiation-induced hyposalivation [23] have been reported. These findings suggest that the oxidative stress induced by various environmental factors causes salivary gland dysfunction [24]. Ast, a member of the carotenoid family, has been reported to have an extremely high level of antioxidant activity and to act as a scavenger of oxygen radicals [25]. For this reason, the clinical studies of Ast have been accumulated [26–28]. Satoh et al. showed the safety of new Ast-product and the efficiency of Ast on age-related decline in cognitive and psychomotor functions. Therefore, we examined the effects of Ast on the ROS scavenging capacity and the possible therapeutic effect of Ast on salivary gland dysfunction. We found that the levels of the oxidative stress markers 8-OHdG and HEL were significantly higher in the SS group than in the normal group (Fig. 3) and that the level of HEL in the SS group was significantly reduced by the intake of Ast (Fig. 5A). These results suggest that oxidative stress may be a cause of salivary gland dysfunction in SS. Although the Ast-induced inhibition of HEL in the SS group was higher than that in the normal group (Fig. 5), the effect of Ast on salivation was faint in the SS group but significant in the normal group (Fig. 4). These inconsistencies might have arisen for several reasons. First, the level of ROS production in the SS group might be too high to be compensated for by Ast under the study conditions, including the dose and/or term of treatment. Second, the species of ROS affecting salivary function might be generated as a byproduct of normal cellular respiration, associated primarily with mitochondrial electron transport [29]. In the SS group, in addition to the above mechanism, ROS might be generated following cell lysis and the oxidative burst produced by the immune response [30, 31]. Therefore, the increase in salivation in the SS group was not as strong as expected.
Takeda et al. [23] reported that NG-monomethyl-L-arginine (L-NMMA), a nitric oxide synthase inhibitor, alleviated hyposalivation in irradiated mice and inferred that nitrogen monoxide was involved in the impairment of salivary secretion. Based on these findings and the fact that Ast inhibits nitrogen monoxide synthesis [32], the alleviation of hyposalivation by Ast in an irradiation-induced hyposalivation mouse model shown in the present study (Fig. 1) might be due to a defense mechanism involving the inhibition of nitrogen monoxide synthesis. However, Ast did not show a therapeutic effect on irradiation-induced hyposalivation. Thus, the scavenging of ROS generated at the time of irradiation may be crucial. In the patients with SS, the reduction in HEL might have resulted from the Ast-induced scavenging of newly generated ROS, which accounts for a small portion of the total HEL in the saliva of patients with SS. Therefore, treatment with Ast might be useful in patients with salivary dysfunction associated with SS to prevent the condition from worsening.
The expression of various inflammatory cytokines including IL-1, IL-2, IFNγ and TGFβ, cell adhesion molecules, and chemokines is detected in salivary gland of SS patients [33–35]. In normal human mesangial cells (NHMCs), Ast suppresses high glucose induced ROS production. Accompanied with ROS production, Ast suppresses the activation of nuclear transcription factors such as nulclear factor kappa B (NFκB) and activator protein (AP-1), and the expression of transforming growth factor-beta 1 (TGFβ1), and monocyte chemoattractant protein-1 (MCP-1) [36]. Thus, Ast may be effective in the cytokine production following tissue destruction in the salivary gland of SS patients resulting in reduced salivary dysfunction.
Impairments in salivary secretion affect the quality of life and also cause various functional impairments, including dental caries, periodontal diseases, upper gastrointestinal disorders, aspiration pneumonia, dysphagia and infectious diseases. Therefore, the development of a medicine for impaired salivary secretion is of great significance, and Ast is a potent candidate for such a remedy. We plan to perform further clinical evaluations using a larger number of cases and to clarify the details of the mechanism of impaired salivary secretion in the future.
Acknowledgments
This work was partially supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports Science and Technology of Japan. The authors gratefully thank Judith Nishino for helpful discussions during the preparation of this manuscript.
Abbreviations
- ROS
reactive oxygen species
- SS
Sjögren’s syndrome
- Ast
astaxanthin
- HEL
hexanoyl-lysine
- HSY
human salivary gland epithelial cell line
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
References
- 1.Ryo K., Yamada H., Nakagawa Y., Tai Y., Obara K., Inoue H., Mishima K., Saito I. Possible involvement of oxidative stress in salivary gland of patients with Sjogren’s syndrome. Pathobiology. 2006;73:252–260. doi: 10.1159/000098211. [DOI] [PubMed] [Google Scholar]
- 2.Fox R.I. Sjögren’s syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 3.Maheshwari A., Misro M.M., Aggarwal A., Sharma R.K., Nandan D. Pathways involved in testicular germ cell apoptosis induced by H2O2 in vitro. FEBS J. 2009;276:870–881. doi: 10.1111/j.1742-4658.2008.06831.x. [DOI] [PubMed] [Google Scholar]
- 4.Dukan S., Farewell A., Ballesteros M., Taddei F., Radman M., Nyström T. Protein oxidation in response to increased transcriptional or translational errors. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5746–5749. doi: 10.1073/pnas.100422497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houstis N., Rosen E.D., Lander E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 6.Babbar N., Casero R.A. Jr. Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006;66:11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]
- 7.Saito I., Shimuta M., Terauchi K., Tsubota K., Yodoi J., Miyasaka N. Increased expression of human thioredoxin/adult T cell leukemia-derived factor in Sjögren’s syndrome. Arthritis Rheum. 1996;39:773–782. doi: 10.1002/art.1780390509. [DOI] [PubMed] [Google Scholar]
- 8.Bashir S., Harris G., Denman M.A., Blake D.R., Winyard P.G. Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann. Rheum. Dis. 1993;52:659–666. doi: 10.1136/ard.52.9.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyata T., Ishiguro N., Yasuda Y., Ito T., Nangaku M., Iwata H., Kurokawa K. Increased pentosidine, an advanced glycation end product, in plasma and synovial fluid from patients with rheumatoid arthritis and its relation with inflammatory markers. Biochem. Biophys. Res. Commun. 1998;244:45–49. doi: 10.1006/bbrc.1998.8203. [DOI] [PubMed] [Google Scholar]
- 10.Mantle D., Falkous G., Walker D. Quantification of protease activities in synovial fluid from rheumatoid and osteoarthritis cases: comparison with antioxidant and free radical damage markers. Clin. Chim. Acta. 1999;284:45–58. doi: 10.1016/s0009-8981(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 11.Ozkan Y., Yardým-Akaydýn S., Sepici A., Keskin E., Sepici V., Simsek B. Oxidative status in rheumatoid arthritis. Clin. Rheumatol. 2007;26:64–68. doi: 10.1007/s10067-006-0244-z. [DOI] [PubMed] [Google Scholar]
- 12.Baskol G., Demir H., Baskol M., Kilic E., Ates F., Karakukcu C., Ustdal M. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem. Funct. 2006;24:307–311. doi: 10.1002/cbf.1257. [DOI] [PubMed] [Google Scholar]
- 13.Jikimoto T., Nishikubo Y., Koshiba M., Kanagawa S., Morinobu S., Morinobu A., Saura R., Mizuno K., Kondo S., Toyokuni S., Nakamura H., Yodoi J., Kumagai S. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Mol. Immunol. 2002;38:765–772. doi: 10.1016/s0161-5890(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 14.Rall L.C., Roubenoff R., Meydani S.N., Han S.N., Meydani M. Urinary 8-hydroxy-2'-deoxyguanosine (8-OHdG) as a marker of oxidative stress in rheumatoid arthritis and aging: effect of progressive resistance training. J. Nutr. Biochem. 2000;11:581–584. doi: 10.1016/s0955-2863(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 15.Reznick A.Z., Shehadeh N., Shafir Y., Nagler R.M. Free radicals related effects and antioxidants in saliva and serum of adolescents with Type 1 diabetes mellitus. Arch. Oral Biol. 2006;51:640–648. doi: 10.1016/j.archoralbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Hata I., Kaji M., Hirano S., Shigematsu Y., Tsukahara H., Mayumi M. Urinary oxidative stress markers in young patients with type 1 diabetes. Pediatr. Int. 2006;48:58–61. doi: 10.1111/j.1442-200X.2006.02156.x. [DOI] [PubMed] [Google Scholar]
- 17.Tatsuzawa H., Maruyama T., Misawa N., Fujimori K., Nakano M. Quenching of singlet oxygen by carotenoids produced in escherichia coli—attenuation of singlet oxygen-mediated bacterial killing by carotenoids. FEBS Lett. 2000;484:280–284. doi: 10.1016/s0014-5793(00)02149-9. [DOI] [PubMed] [Google Scholar]
- 18.Cantrell A., McGarvey D.J., Truscott T.G., Rancan F., Böhm F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003;412:47–54. doi: 10.1016/s0003-9861(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder W.A., Johnson E.A. Singlet oxygen and peroxyl radicals regulate carotenoid biosynthesis in Phaffia rhodozyma. J. Biol. Chem. 1995;270:18374–18379. doi: 10.1074/jbc.270.31.18374. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Dor A., Steiner M., Gheber L., Danilenko M., Dubi N., Linnewiel K., Zick A., Sharoni Y., Levy J. Carotenoids activate the antioxidant response element transcription system. Mol. Cancer Ther. 2005;4:177–186. [PubMed] [Google Scholar]
- 21.Wedgwood S., Black S.M. Induction of apoptosis in fetal pulmonary arterial smooth muscle cells by a combined superoxide dismutase/catalase mimetic. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L305–312. doi: 10.1152/ajplung.00382.2002. [DOI] [PubMed] [Google Scholar]
- 22.Aruoma O.I., Halliwell B., Hoey B.M., Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 23.Takeda I., Kizu Y., Yoshitaka O., Saito I., Yamane G.Y. Possible role of nitric oxide in radiation-induced salivary gland dysfunction. Radiat. Res. 2003;159:465–470. doi: 10.1667/0033-7587(2003)159[0465:pronoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Vitolo J.M., Cotrim A.P., Sowers A.L., Russo A., Wellner R.B., Pillemer S.R., Mitchell J.B., Baum B.J. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin. Cancer Res. 2004;10:1807–1812. doi: 10.1158/1078-0432.ccr-03-0194. [DOI] [PubMed] [Google Scholar]
- 25.Kim J.H., Choi W., Lee J.H., Jeon S.J., Choi Y.H., Kim B.W., Chang H.I., Nam S.W. Astaxanthin inhibits H2O2-mediated apoptotic cell death in mouse neural progenitor cells via modulation of P38 and MEK signaling pathways. J. Microbiol. Biotechnol. 2009;19:1355–1363. doi: 10.4014/jmb.0906.06003. [DOI] [PubMed] [Google Scholar]
- 26.Satoh A., Tsuji S., Okada Y., Murakami N., Urami M., Nakagawa K., Ishikura M., Katagiri M., Koga Y., Shirasawa T. Preliminary clinical evaluation of toxicity and efficacy of a new astaxanthin-rich haematococcus pluvialis extract. J. Clin. Biochem. Nutr. 2009;44:280–284. doi: 10.3164/jcbn.08-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussein G., Sankawa U., Goto H., Matsumoto K., Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006;69:443–449. doi: 10.1021/np050354+. [DOI] [PubMed] [Google Scholar]
- 28.Guerin M., Huntley M.E., Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 29.Richter C., Ferrier J. Continuously active sodium channels in osteoblastic ROS 17/2.8 cells. Bone Miner. 1991;15:57–71. doi: 10.1016/0169-6009(91)90110-l. [DOI] [PubMed] [Google Scholar]
- 30.Bellavite P. The superoxide-forming enzymatic system of phagocytes. Free Radic. Biol. Med. 1988;4:225–261. doi: 10.1016/0891-5849(88)90044-5. [DOI] [PubMed] [Google Scholar]
- 31.Giulian D., Vaca K., Corpuz M. Brain glia release factors with opposing actions upon neuronal survival. J. Neurosci. 1993;13:29–37. doi: 10.1523/JNEUROSCI.13-01-00029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S.J., Bai S.K., Lee K.S., Namkoong S., Na H.J., Ha K.S., Han J.A., Yim S.V., Chang K., Kwon Y.G., Lee S.K., Kim Y.M. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol. Cells. 2003;16:97–105. [PubMed] [Google Scholar]
- 33.Fox R.I., Kang H.I., Ando D., Abrams J., Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjögren’s syndrome. J. Immunol. 1994;152:5532–5539. [PubMed] [Google Scholar]
- 34.Boumba D., Skopouli F.N., Moutsopoulos H.M. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren’s syndrome. Br. J. Rheumatol. 1995;34:326–333. doi: 10.1093/rheumatology/34.4.326. [DOI] [PubMed] [Google Scholar]
- 35.Cuello C., Palladinetti P., Tedla N. Di, Girolamo. N., Lloyd A.R., McCluskey P.J., Wakefield D. Chemokine expression and leucocyte infiltration in Sjögren’s syndrome. Br. J. Rheumatol. 1998;37:779–783. doi: 10.1093/rheumatology/37.7.779. [DOI] [PubMed] [Google Scholar]
- 36.Manabe E., Handa O., Naito Y., Mizushima K., Akagiri S., Adachi S., Takagi T., Kokura S., Maoka T., Yoshikawa T. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J. Cell Biochem. 2008;103:1925–1937. doi: 10.1002/jcb.21583. [DOI] [PubMed] [Google Scholar]