Abstract
Alleviated melanin formation in the skin through inhibition of tyrosine-tyrosinase reaction is one of the major targets of cosmetics for whitening ability. Since melanin has a pivotal role for photoprotection, there are pros and cons of inhibition of melanin formation. This study applying electron spin resonance (ESR)-spin trapping method revealed that •H and •OH are generated through tyrosine-tyrosinase reaction. When deuterium water was used instead of H2O, the signal of 5,5-dimethyl-1-pyrroline N-oxide (DMPO)-H (a spin adduct of DMPO and •H) greatly decreased, whilst DMPO-OH (a spin adduct of DMPO and •OH) did not. Thus, it is suggested that •H was derived from H2O, and •OH through oxidative catalytic process of tyrosine to dopaquinone. Our study suggests that tyrosinase inhibitors might contribute to alleviate the oxidative damage of the skin by inhibiting •OH generation via the enzyme reaction.
Keywords: tyrosine, tyrosinase, radical species
Introduction
Exposure of ultraviolet (UV) irradiation to the skin causes acute and chronic detrimental cutaneous effects, which may result in photocarcinogenesis [1–7]. Native human melanin includes eumelanin and pheomelanin that contains sulfur, and eumelanin has been found in almost every type of human skin [8, 9]. In the skin, melanin synthesized in melanocytes located in the basal layer and hair bulbs transfers to keratinocytes. Melanin in keratinocytes acts as a photoprotector through body coloration and scavenging reactive oxygen species [10–15]. In spite of photoprotective role of melanin, there are many cosmetics developed to prevent melanin formation in the skin because of aesthetic satisfaction by whitening ability. Of these, inhibitor of tyrosinase, which is a pivotal enzyme for melanin synthesis [16], has become major ingredient of cosmetics [17–21]. Tyrosinase is an enzyme which catalyzes the biological conversion of tyrosine to dopaquinone with dioxygen at the dinuclear copper active site under physiological conditions [22–24]. Other than oxidative catalysis of substrates by tyrosinase, a few studies on radical formation by tyrosinase have been reported. For instance, it was reported that tyrosinase-dependent activation of hydroxybenzenes formed reactive compounds including free radical [25], and some radicals were produced during the tyrosinase reaction and dopa-melanin formation [26]. However, in these few studies, radical species have not been determined. We have studied the tyrosine-tyrosinase reaction in terms of melanin formation and ROS scavenging ability of melanin [15]. In a battery of studies, we found radical formation through the enzyme reaction, and discuss the formation mechanism in this paper.
Materials and Methods
Test materials and reagents
Reagents were purchased from the following sources: L-tyrosine, phosphate buffer solution (PB, pH 6.5) and catalase (from bovine liver) from Wako Pure Chemicals (Osaka, Japan); tyrosinase (from mushroom), and superoxide dismutase (SOD from bovine erythrocytes) from Sigma-Aldrich Corp. (St. Louis, MO); 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) from Labotec (Tokyo, Japan); deuterium water (D2O) from Tokyo chemical Industry Co., Ltd (Tokyo, Japan). All other reagents used were of analytical grade.
Electron spin resonance (ESR)-spin trapping determinations of ROS generated by tyrosine-tyrosinase reaction
Tyrosine was dissolved in 1 M HCl to be 200 mM. Then 1 mM tyrosine solution was prepared by mixing 5 µl of 200 mM tyrosine solution with 5 µl of 1 M NaOH and 990 µl of 0.2 M PB. DMPO was dissolved in ultrapure water to be 4.5 M. The reaction mixture was prepared to contain different activity of tyrosinase, 20 µl of 4.5 M DMPO, 60 µl of 1 mM tyrosine and 0.2 M PB which was added to adjust a total volume of 200 µl. Immediately after mixing the mixture was transferred to an ESR spectrometry cell, and the ESR measurement was started after 45 s. The measurement conditions of ESR (JES-FA-100, JEOL, Tokyo, Japan) were as follows: field sweep, 330.80–340.80 mT; field modulation frequency, 100 kHz; filed modulation width, 0.07 mT; amplitude, 400; sweep time, 1 min; time constant, 0.1 s; microwave frequency, 9.430 GHz; microwave power, 4–5 mW.
Deuterium water (D2O) effect on radical species generated by tyrosine-tyrosinase reaction
Tyrosine was dissolved in 1 M HCl to be 200 mM. Then 1 mM tyrosine solution was prepared by mixing 5 µl of 200 mM tyrosine solution with 5 µl of 1 M NaOH and 990 µl of ultrapure water or D2O. Tyrosinase was dissolved in ultrapure water to be 100 U/µl. Immediately before the measurement tyrosinase was diluted to be 10 U/µl with ultrapure water or D2O. DMPO (8.9 M) was diluted to be 4.5 M with ultrapure water or D2O. The reaction mixture was prepared to contain 100 µl of D2O, 20 µl of 10 U/µl tyrosinase (90% D2O solution), 20 µl of 4.5 M DMPO (50% D2O solution), and 60 µl of 1 mM tyrosine (99% D2O solution). Immediately after mixing the mixture was transferred to an ESR spectrometry cell, and the ESR measurement was started after 60 s. As a control, the reaction mixture was prepared to contain 100 µl of H2O (ultrapure water), 20 µl of 10 U/µl tyrosinase dissolved in H2O, 20 µl of 4.5 M DMPO dissolved in H2O, and 60 µl of 1 mM tyrosine dissolved in H2O, and was similarly subjected to ESR measurement. The measurement conditions of ESR were the same as those described above.
Results and Discussion
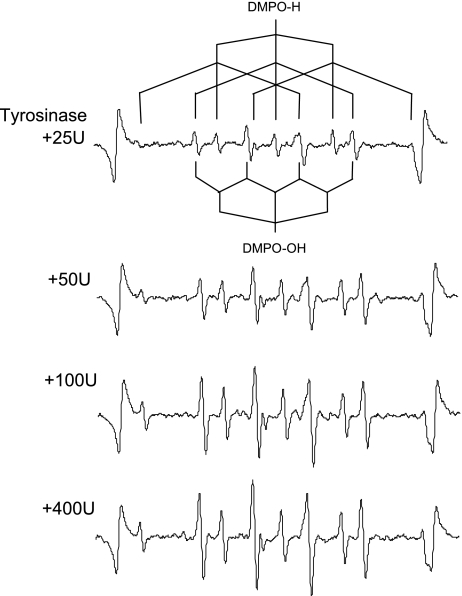
Representative ESR spectra obtained from tyrosine-tyrosinase reaction with different activity of tyrosinase are summarized in Fig. 1. Each spectrum consists of quartet segments (intensity ratio, 1:2:2:1) and triplet of triplet segments (intensity ratio, 1:1:2:1:2:1:2:1:1). The former segment was assigned to DMPO-OH, a spin adduct derived from •OH (hyperfine coupling constant, aN = 1.49; aH = 1.49 mT). The latter segment was assigned to DMPO-H, a spin adduct derived from •H (hyperfine coupling constant, aN = 1.63; aH = 2.25 mT) as reported in a previous study [27]. The ratio of DMPO-H and DMPO-OH generated in the tyrosine-tyrosinase reaction was approximately 1:2.
Fig. 1.
Representative ESR spectra of DMPO-H and DMPO-OH obtained from the tyrosine-tyrosinase reaction with different concentration of tyrosine.
To examine if O2•− and H2O2 are involved in a downstream of the generation process of •H and •OH, ESR signals of DMPO-H and DMPO-OH generated by the tyrosine-tyrosinase reaction were analyzed in the presence of SOD, a scavenger for O2•−, and catalase, a cleaving enzyme for H2O2. Neither SOD (1 U/µl) nor catalase (1 U/µl) affected the amounts of DMPO-H and DMPO-OH (data not shown), suggesting that neither O2•− nor H2O2 is involved in the generation process of •H and •OH.
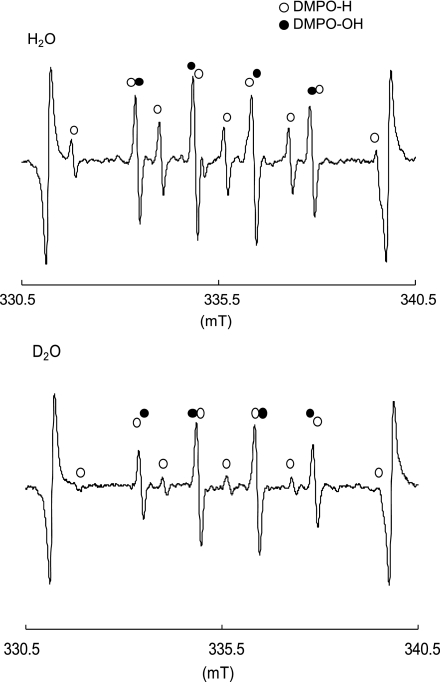
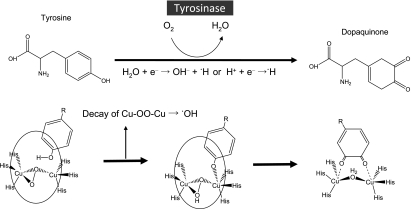
Then to further examine if H2O is a source of •H and •OH, ESR signals of DMPO-H and DMPO-OH generated by the tyrosine-tyrosinase reaction where D2O was used as a major solvent (94% D2O solution) were compared with those where H2O was used as a solvent. As shown in Fig. 2, the amount of DMPO-H was reduced by 70%, suggesting that approximately 70% of •H is derived from H2O. Thus, we assume that the electron derived from the ligand of tyrosinase reacts with H2O or H+ to form •H. That is, H2O + e− → OH− + •H or H+ + e− → •H. On the other hand, the amount of DMPO-OH was not affected by the replacement of H2O to D2O, suggesting that •OH was derived from the catalytic reaction of tyrosine to dopaquinone by tyrosinase [16]. Since catalase had no effect on the generation of two radicals as described above, Fenton-like reaction in which H2O2 reacts with transient metal copper in the structures of tyrosinase and its intermediates is least likely involved in the generation mechanism of •OH. As reported in the previous study on dinuclear copper complexes, a rather stable dicopper-peroxide intermediate in aqueous solution decays through an internal electron transfer from the ligand to produce •OH [28]. Since tyrosinase is an enzyme which contains dinuclear copper ions at the active site, [22–24], dicopper-peroxide intermediates formed during the catalytic process of tyrosine to dopaquinone possibly decay to produce •OH through an internal electron transfer from the ligand. The proposed mechanism by which •H and •OH are generated in tyrosine-tyrosinase reaction is illustrated in Fig. 3. Although tyrosine-tyrosinase reaction is a key step of melanin formation as a photoprotection, it also produces not only •H but •OH which might be a causative factor of oxidative damage of the skin. Since there are many cosmetics developed for whitening ability by inhibiting tyrosine-tyrosinase reaction, our study revealed that they also might contribute to alleviate the oxidative damage of the skin by inhibiting •OH generation via the enzyme reaction.
Fig. 2.
Deuterium oxide effect of D2O on ·H and ·OH generated by tyrosine-tyrosinase reaction (a) H2O was used as a solvent, and (b) D2O was used as a major solvent.
Fig. 3.
Schematic illustration of tyrosine-tyrosinase reaction by which tyrosine was catalyzed oxidatively to dopaquinone, and proposed mechanism of •H and •OH generation. Below shows the catalytic process of monophenol to quinone by tyrosinase, an enzyme with dinuclear copper active site [24]. Each “His” in the chemical structures of tyrosinase and its intermediates indicates histidine.
Abbreviations
- electron spin resonance
ESR
- 5,5-dimethyl-1-pyrroline-N-oxide
DMPO
- DMPO spin adduct of •OH
DMPO-OH
- DMPO spin adduct of •H
DMPO-H
- superoxide dismutase
SOD
References
- 1.Brash D.E., Ziegler A., Jonason A.S., Simon J.A., Kunala S., Leffell D.J. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J. Investig. Dermatol. Symp. Proc. 1996;1:136–142. [PubMed] [Google Scholar]
- 2.Wikonkal N.M., Brash D.E. Ultraviolet radiation signature mutations in photocarcinogenseis. J. Investig. Dermatol. Symp. Proc. 1999;4:6–10. doi: 10.1038/sj.jidsp.5640173. [DOI] [PubMed] [Google Scholar]
- 3.Mckay B.C., Stubbert L.J., Fowler C.C., Smith J.M., Cardamore R.A., Spronck J.C. Regulation of ultraviolet light-induced gene expression by gene size. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6582–6586. doi: 10.1073/pnas.0308181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agar N.S., Halliday G.M., Barnetson R.S., Ananthaswamy H.N., Wheeler M., Jones A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4954–4959. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner D.J., Doll R., Goodhead D.T., Hall E.J., Land C.E., Little J.B., Lubin J.H., Preston D.L., Preston R.J., Puskin J.S., Ron E., Sachs R.K., Samet J.M., Setlow R.B., Zaider M. Cancer risks attributable to low does of ionizing radiation: assessing what really know. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setlow R.B. Spectral regions contributing to melanoma: a personal view. J. Investig. Dermatol. Symp. Proc. 1999;4:46–49. doi: 10.1038/sj.jidsp.5640180. [DOI] [PubMed] [Google Scholar]
- 7.Moan J., Dahlback A., Setlow R.B. Epidemiological support for an hypothesis for melanoma induction indicating a role for UVA radiation. Photochem. Photobiol. 1999;70:243–247. [PubMed] [Google Scholar]
- 8.Hunt G., Kyne S., Ito S., Wakamatsu K., Todd C., Thody A. Eumelanin and pheomelanin contents of human epidermis and cultured melanocytes. Pigment Cell Res. 1995;8:202–208. doi: 10.1111/j.1600-0749.1995.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 9.Vincensi M.R., d’Ischia M., Napolitano A., Procaccini E.M., Riccio G., Monfrecola G., Santoianni P., Prota G. Pheomelanin versus eumelanin as a chemical indicator of ultraviolet sensitivity in fair-skinned subjects at high risk for melanoma: a pilot study. Melanoma Res. 1998;8:53–58. doi: 10.1097/00008390-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Kvam E., Dahle J. Pigmented melanocytes are protected against ultraviolet-A-induced membrane damage. J. Investig. Dermatol. 2003;121:564–569. doi: 10.1046/j.1523-1747.2003.12418.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki F., Okamoto H., Miyauchi-Hashimoto H., Matsumura Y., Itoh T., Tanaka K., Kunisada T., Horio T. XPA gene-deficient, SCF-transgenic mice with epidermal melanin are resistant to UV-induced carcinogenesis. J. Investig. Dermatol. 2004;123:220–228. doi: 10.1111/j.0022-202X.2004.22710.x. [DOI] [PubMed] [Google Scholar]
- 12.Wagner J.K., Parra E.J., Norton H.L., Jovel C., Shriver M.D. Skin responses to ultraviolet radiation: effects of constitutive pigmentation, sex, and ancestry. Pigment Cell Res. 2002;15:385–390. doi: 10.1034/j.1600-0749.2002.02046.x. [DOI] [PubMed] [Google Scholar]
- 13.Ortonne J.P. Photoprotective properties of skin melanin. Br. J. Dermatol. 2002;146:7–10. doi: 10.1046/j.1365-2133.146.s61.3.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi Y., Takahashi K., Zmudzka B.Z., Kornhauser A., Miller S.A., Tadokoro T., Berens W., Beer J.Z., Hearing V.J. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB. J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- 15.Tada M., Kohno M., Niwano Y. Scavenging or quenching effect of melanin on superoxide anion and singlet oxygen. J. Clin. Biochem. Nutr. 2010;46:224–228. doi: 10.3164/jcbn.09-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito S., K. Wakamatsu K. Chemistry of mixed melanogenesis—pivotal roles of dopaquinone. Photochem. Photobiol. 2008;84:582–592. doi: 10.1111/j.1751-1097.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 17.Khazaeli P., Goldoozian R., Sharififar F. An evaluation of extracts of five traditional medicinal plants from Iran on the inhibition of mushroom tyrosinase activity and scavenging of free radicals. Int. J. Cosmet. Sci. 2009;31:375–381. doi: 10.1111/j.1468-2494.2009.00503.x. [DOI] [PubMed] [Google Scholar]
- 18.Momtaz S., Mapunya B.M., Houghton P.J., Edgerly C., Hussein A., Naidoo S., Lall N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 2008;119:507–512. doi: 10.1016/j.jep.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Ng L.T., Ko H.H., Lu T.M. Potential antioxidants and tyrosinase inhibitors from synthetic polyphenolic deoxybenzoins. Bioorg. Med. Chem. 2009;17:4360–4366. doi: 10.1016/j.bmc.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Nugroho A., Choi J.K., Park J.H., Lee K.T., Cha B.C., Park H.J. Two new flavonol glycosides from Lamium amplexicaule L. and their in vitro free radical scavenging and tyrosinase inhibitory activities. Planta. Med. 2009;75:364–366. doi: 10.1055/s-0028-1112216. [DOI] [PubMed] [Google Scholar]
- 21.Wang K.H., Lin R.D., Hsu F.L., Huang Y.H., Chang H.C., Huang C.Y., Lee M.H. Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethnopharmacol. 2006;106:353–359. doi: 10.1016/j.jep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T., Shiota Y., Yoshizawa K. Quantum chemical approach to the mechanism for the biological conversion of tyrosine to dopaquinone. J. Am. Chem. Soc. 2008;130:16890–16897. doi: 10.1021/ja802618s. [DOI] [PubMed] [Google Scholar]
- 23.Chen P., Solomon E.I. O2 activation by binuclear Cu sites: noncoupled verus exchange coupled reaction mechanisms. Pros. Natl. Acad. Sci. U.S.A. 2004;101:13105–13110. doi: 10.1073/pnas.0402114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon E.I., Chen P., Metz M., Lee S.K., Palmer A.E. Oxygen binding, activation, and reduction to water by copper proteins. Angew. Chem. Int. Ed. Engl. 2001;40:4570–4590. doi: 10.1002/1521-3773(20011217)40:24<4570::aid-anie4570>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Usui N., Sinha B.K. Tyrosinase-induced free radical formation from VP-16,213: relationship to cytotoxicity. Free Radic. Res. Commun. 1990;10:287–293. doi: 10.3109/10715769009149897. [DOI] [PubMed] [Google Scholar]
- 26.Tomita Y., Hariu A., Kato C., Seiji M. Radical production during tyrosinase reaction, dopa-melanin formation, and photoirradiation of dopa-melanin. J. Investig. Dermatol. 1984;82:573–576. doi: 10.1111/1523-1747.ep12261311. [DOI] [PubMed] [Google Scholar]
- 27.Buettner G.R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987;3:259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Q., Lian Y., Thyagarajan S., Rokita S.E., Karlin K.D., Blough N.V. Hydrogen peroxide and dioxygen activation by dinuclear copper complexes in aqueous solution: hydroxyl radical production initiated by internal electron transfer. J. Am. Chem. Soc. 2008;130:6304–6305. doi: 10.1021/ja800080z. [DOI] [PubMed] [Google Scholar]