Abstract
Dietary conjugated linoleic acid (CLA) has been reported to exhibit a number of therapeutic effects in animal models and patients, such as anti-hypertensive, anti-hyperlipidemic, anti-arteriosclerotic, anti-carcinogenic, and anti-diabetic effects. However, the underlying mechanism is not well-characterized. In the present study, the effects of cis(c)9, trans(t)11-CLA on the differentiation of mouse 3T3-L1 preadipocytes into mature adipocytes were examined. Treatment with c9, t11-CLA in the presence of insulin, dexamethasone, and 3-isobutyl-1-methyl-xanthine (differentiation cocktail) significantly stimulated the accumulation of triacylglycerol. The microscopic observation of cells stained by Oil Red O demonstrated that c9, t11-CLA increases the amount and proportion of small mature adipocytes secreting adiponectin, a benign adipocytokine, when compared to the differentiation cocktail alone. Furthermore, c9, t11-CLA increased bioactive peroxisome proliferator-activated receptor γ (PPARγ) levels in a nuclear extract of 3T3-L1 cells, suggesting the enhancing effect of this fatty acid on the nuclear transmission of PPARγ, a master regulator of adipocyte differentiation, in 3T3-L1 cells. These results suggest that the therapeutic effects of c9, t11-CLA on lifestyle-related diseases are partially due to the enhanced formation of small adipocytes from preadipocytes via PPARγ stimulation.
Keywords: conjugated linoleic acid, adipocyte differentiation, 3T3-L1 cells, adiponectin, lifestyle-related diseases
Introduction
Conjugated linoleic acid (CLA) is the collective acronym for combinations of positional and geometric isomers of linoleic acid that exist naturally in dairy products and meat. It is produced in ruminant animals via the biohydrogenation of polyunsaturated fatty acids as well as during the mechanical processing of dairy products [1, 2]. CLA has been reported to exhibit a number of physiological effects in animal models and patients, such as anti-hypertensive [3], anti-hyperlipidemic [4], anti-arteriosclerotic [5], anti-carcinogenic [6], and anti-diabetic [7] effects. However, the underlying mechanism behind the effects of CLA is not fully understood.
One of the common backgrounds of hypertension, hyperlipidemia, carcinogenesis, and diabetes is thought to be obesity. Adipocyte precursor cells (i.e., preadipocytes) are present throughout life [8]. Accordingly, obesity may be partially mediated by stimulating the differentiation of preadipocytes into adipocytes or by increasing fat accumulation in the differentiated adipocytes [9]. Furthermore, adipose tissue has recently been identified as an endocrine organ that secretes various kinds of bioactive molecule called adipocytokines. Of these, tumor necrosis factor-α (TNF-α), which increases in the obese state and is expressed in enlarged adipocytes, is implicated in various metabolic disorders, whereas adiponectin, which is expressed in small adipocytes, is considered to protect against diabetes, atherosclerosis, etc. [10, 11]. Such enlarged and small adipocytes have been shown to be generated by differentiation from preadipocytes in vivo [10, 11]. Murine 3T3-L1 preadipocytes have been frequently used to study the differentiation of preadipocytes in vitro [12–14].
In the present study, we examined the effects of cis(c)9, trans(t)11-CLA, which is present at high levels in dairy products [15–17], on the differentiation of 3T3-L1 preadipocytes into adipocytes.
Materials and Methods
Materials
Mouse 3T3-L1 preadipocytes were obtained from the European Collection of Cell Cultures, Wiltshire, UK. c9, t11-CLA, and the peroxisome proliferator-activated receptor γ (PPARγ) Transcription Factor Assay kit were purchased from Cayman Chemical Co., Ann Arbor, MI. Dulbecco’s modified Eagle’s medium (DMEM), dexamethasone, 3-isobutyl-1-methyl-xanthine (IBMX), and protease inhibitor cocktail were obtained from Sigma Chemical Co., St. Louis, MO. Penicillin-streptomycin was purchased from Invitrogen, Life Technologies, Carlsbad, CA. Fetal bovine serum (FBS) was purchased from Nichirei Biosciences Inc., Tokyo, Japan. Triglyceride E-test Wako, 4% formaldehyde-phosphate buffer (pH 7.4), and Oil Red O dye were obtained from Wako Pure Chemical Industries, Limited, Osaka, Japan. GW9962 (2-chloro-5-nitrobenzanilide) was purchased from Merck Ltd., Darmstadt, Germany. All other reagents were of analytical grade.
Cell culture
3T3-L1 preadipocytes were cultured at 37°C in a humidified atmosphere of 5% CO2/95% air. The cells were maintained in growth medium containing the following: DMEM with 10% FBS and 1% penicillin-streptomycin. Differentiation was induced according to the protocol enclosed with the 3T3-L1 preadipocytes from the European Collection of Cell Cultures: differentiation of the cells was initiated 2 days after confluence for 3 days in growth medium containing 0.25 µM dexamethasone, 0.5 mM IBMX, and 1 µg/ml insulin. This was followed by 2 days in growth medium containing 1 µg/ml insulin. Thereafter, the cells were cultured in the growth medium for 2 days.
Treatment with c9, t11-CLA
c9, t11-CLA was prepared in Me2SO and added to the medium from day-3 (time of addition of dexamethasone, IBMX, and insulin) to day-9 (end point of the experiment). The Me2SO concentration was maintained up to 0.25% of the total volume, and preliminary experiments demonstrated no significant effects of 0.25% Me2SO on cell differentiation.
Oil Red O staining
Cells were fixed with 4% formaldehyde-phosphate buffer (pH 7.4) for 1 h, rinsed with water, and stained with 0.3% Oil Red O dye for 1 h. After washing again with water, cells were visually monitored by microscopic observation (10 × 10-fold or 10 × 20-fold).
Measurement of cell number and size
The 3T3-L1 cells stained with Oil Red O dye as mentioned above were used for measurement of cell number and size. The sizes of adipocytes were determined by tracing the diameters of the cells within 1,300 mm2 of the microscopic pictures using a soft ware (DP2-BSW, OLYMPUS Corporation, Tokyo, Japan).
Measurement of triacylglycerol (TG)
The cells were harvested by scraping from the culture dishes into lysis buffer [1% Triton-100, 150 mM NaCl, 4 mM EDTA, and 20 mM Tris-HCl (pH 7.4) containing protease inhibitor cocktail] and lysed completely using a horn-type sonicator. The amount of TG, an index of lipid accumulation, was quantitatively measured using a Triglyceride E-test Wako kit following normalization by protein amounts and expressed as TG contents (µg/mg protein).
Measurement of TNF-α and adiponectin
After incubation, TNF-α and adiponectin in the medium were measured using a Quantikine mouse TNF-α/TNFSF1A kit (R&D systems, Inc., Minneapolis, MN) and Mouse/rat adiponectin ELISA kit (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan), respectively.
Measurement of nuclear bioactive PPARγ
After incubation, 3T3-L1 cells were scraped, and the nuclear fraction was separated using the Nuclear Extraction kit (Marligen Biosciences Inc., Rockville, MD). Bioactive PPARγ in the nucleus was measured using the PPARγ Transcription Factor Assay kit. A specific double stranded DNA (dsDNA) sequence containing the peroxisome proliferator response element (PPRE) was immobilized onto the bottom of wells of a 96 well plate. PPARs contained in a nuclear extract, bound specifically to the PPRE. PPARγ was detected by addition of specific primary antibody directed against PPARγ. A secondary antibody conjugated to horseradish peroxidase was added to provide a sensitive colorometric readout at 450 nm.
Statistical analysis
Results are the means ± SE. The significance of differences between two groups was assessed employing the t test, and differences between multiple groups were assessed by one-way analysis of variance (ANOVA), followed by Scheffe’s multiple range test. p values less than 0.05 were considered significant.
Results and Discussion
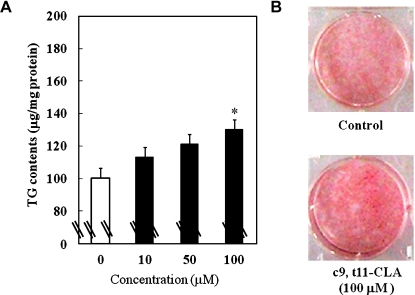
Fig. 1A shows the effect of c9, t11-CLA on the accumulation of TG, a marker of lipid accumulation, during the differentiation of 3T3-L1 preadipocytes into adipocytes. At concentrations of 10, 50, and 100 µM, c9, t11-CLA dose-dependently enhanced the TG contents (100 µM c9, t11-CLA, 1.31-fold). As shown in Fig. 1B, the cells treated with c9, t11-CLA (100 µM) were dyed red with Oil Red O more deeply than the control. These results demonstrated that c9, t11-CLA can be a stimulator of TG accumulation in adipocytes.
Fig. 1.
Alterations in TG contents (A) and Oil Red O staining (B) of 3T3-L1 adipocytes treated with c9, t11-CLA. The differentiation of 3T3-L1 preadipocytes was initiated 2 days after confluence for 3 days in growth medium containing 0.25 µM dexamethasone, 0.5 mM IBMX, and 1 µg/ml insulin. This was followed by 2 days in growth medium containing 1 µg/ml insulin. Thereafter, the cells were cultured in the growth medium for 2 days. c9, t11-CLA was added to the medium from day-3 (time of addition of dexamethasone, IBMX, and insulin) to day-9 (end point of the experiment). (A): The treated cells were lysed with lysis buffer, and the TG contents were measured using a Triglyceride E-test Wako kit. The data represent the means ± SE. of four experiments. *p<0.05 vs 0 µM. (B): The treated cells were fixed in 4% formaldehyde-phosphate buffer and stained with 0.3% Oil Red O dye. TG, triacylglycerol; IBMX, 3-isobutyl-1-methyl-xanthine.
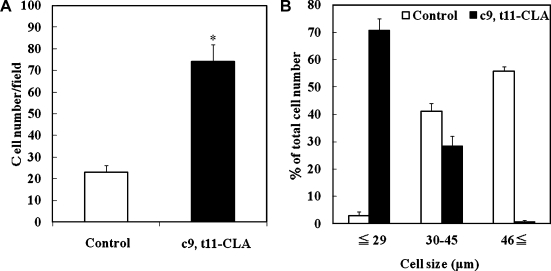
Next, 3T3-L1 adipocytes with or without c9, t11-CLA (100 µM) in the presence of a differentiation cocktail were visualized by Oil Red O staining, and the morphology of cells was observed microscopically (10 × 10-fold, 10 × 20-fold) (Fig. 2). c9, t11-CLA-treated cells contained more and smaller adipocytes with lipid filling compared to the control. Within 1,300 mm2 of the microscopic pictures, the c9, t11-CLA (100 µM)-treated group contained about 3.2-fold more Oil Red O-stained cells than the control group (Fig. 3A). About 71% of the c9, t11-CLA (100 µM)-treated cells were less than 29 µm in size, whereas about 56% of the control group comprised cells more than 46 µm in size (Fig. 3B). These results indicate that c9, t11-CLA increases the number of small adipocytes during the differentiation of 3T3-L1 cells.
Fig. 2.
Microscopic observations of Oil Red O staining of 3T3-L1 cells treated with c9, t11-CLA. The differentiation of 3T3-L1 preadipocytes was initiated 2 days after confluence for 3 days in growth medium containing 0.25 µM dexamethasone, 0.5 mM IBMX, and 1 µg/ml insulin. This was followed by 2 days in growth medium containing 1 µg/ml insulin. Thereafter, the cells were cultured in the growth medium for 2 days. c9, t11-CLA (100 µM) was added to the medium from day-3 (time of addition of dexamethasone, IBMX, and insulin) to day-9 (end point of the experiment). The treated cells were fixed in 4% formaldehyde-phosphate buffer and stained with 0.3% Oil Red O dye. IBMX, 3-isobutyl-1-methyl-xanthine.
Fig. 3.
Alterations in the cell number (A) and size (B) of c9, t11-CLA-treated 3T3-L1 adipocytes. The differentiation of 3T3-L1 preadipocytes was initiated 2 days after confluence for 3 days in growth medium containing 0.25 µM dexamethasone, 0.5 mM IBMX, and 1 µg/ml insulin. This was followed by 2 days in growth medium containing 1 µg/ml insulin. Thereafter, the cells were cultured in the growth medium for 2 days. c9, t11-CLA (100 µM) was added to the medium from day-3 (time of addition of dexamethasone, IBMX, and insulin) to day-9 (end point of the experiment). The treated cells were fixed in 4% formaldehyde-phosphate buffer and stained with 0.3% Oil Red O dye. Within 1,300 mm2 of the microscopic pictures, the total amount (A) and number of cells less than 29 µm, from 30 to 45 µm, or more than 46 µm (B) of the Oil Red O-stained cells in the presence or absence of c9, t11-CLA were assessed. The data represent the means ± SE. from four experiments. *p<0.01 vs Control. IBMX, 3-isobutyl-1-methyl-xanthine.
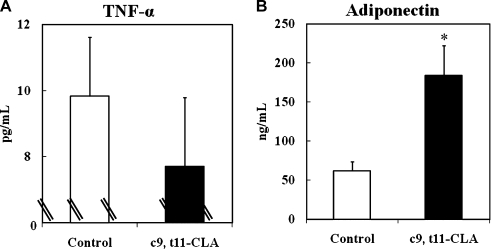
It has been reported that adipocyte size is an important determinant of adipocytokine secretion [10, 11]; an increasing adipocyte size results in a shift toward the dominance of pro-inflammatory adipocytokines like TNF-α, and a decrease in the size toward the dominance of anti-inflammatory adipocytokines like adiponectin. Fig. 4 illustrates the effects of c9, t11-CLA on the secretion of TNF-α and adiponectin from adipocytes after differentiation. c9, t11-CLA (100 µM) showed no significant effect on the secretion of TNF-α, whereas it significantly increased the secretion of adiponectin (2.7-fold), when compared to the control. Thus, it is thought that c9, t11-CLA enhances adiponectin secretion from adipocytes by increasing the emergence of small-sized cells.
Fig. 4.
Changes in the production of TNF-α (A) and adiponectin (B) in 3T3-L1 adipocytes treated with c9, t11-CLA. The differentiation of 3T3-L1 preadipocytes was initiated 2 days after confluence for 3 days in growth medium containing 0.25 µM dexamethasone, 0.5 mM IBMX, and 1 µg/ml insulin. This was followed by 2 days in growth medium containing 1 µg/ml insulin. Thereafter, the cells were cultured in the growth medium for 2 days. c9, t11-CLA (100 µM) was added to the medium from day-3 (time of addition of dexamethasone, IBMX, and insulin) to day-9 (end point of the experiment). TNF-α (A) and adiponectin (B) in the medium were measured using a Quantikine mouse TNF-α/TNFSF1A kit and Mouse/rat adiponectin ELISA kit, respectively. The data represent the means ± SE. from four experiments. *p<0.01 vs Control. IBMX, 3-isobutyl-1-methyl-xanthine; TNF-α, tumor necrosis factor-α.
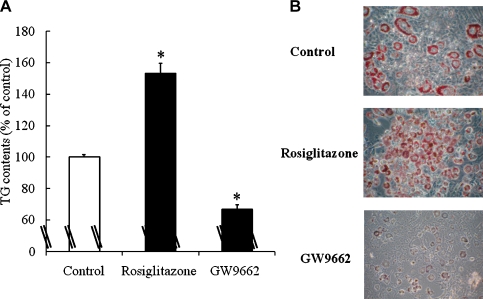
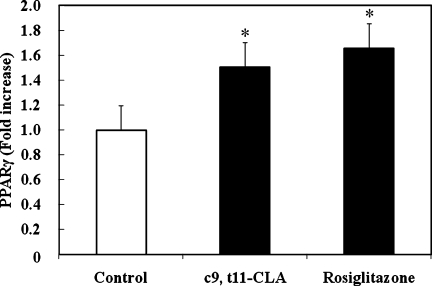
PPARγ is known as a master regulator of adipocyte differentiation [9, 10, 18, 19]. Yamauchi et al. [10] and Okuno et al. [19] have shown that thiazolidinediones, rosiglitazone and troglitazone, activate PPARγ, which is expressed primarily in adipose tissues. They suggested that the primary action of thiazolidinediones is to stimulate the accumulation of TG and the number of small adipocytes preferentially secreting adiponectin, in white adipose tissues, presumably via PPARγ. Therefore, we compared the effect of c9, t11-CLA with rosiglitazone on activating PPARγ. Rosiglitazone (1 µM) increased the TG content of 3T3-L1 cells, and GW9962 (5 µM), a PPARγ antagonist inhibited it (Fig. 4A). Rosiglitazone also increased small adipocytes when compared to the control (Fig. 4B). The addition of c9, t11-CLA (100 µM) significantly increased bioactive PPARγ in the nucleus of 3T3-L1 cells to the same extent as rosiglitazone (1 µM). These data indicate that c9, t11-CLA stimulates TG accumulation and the number of small adipocytes preferentially secreting adiponectin, in 3T3-L1 cells, through an increase in nuclear PPARγ.
To date, the effect of CLA on TG accumulation and adipocyte differentiation has been controversial. For example, Satory and Smith [20] reported that CLA isomers (41% c9, t11 isomer; 44% t10, c12 isomer; and 15% other isomers) increase adipogenesis and TG accumulation during the differentiation period of 3T3-L1 cells. In contrast, others reported that treating 3T3-L1 preadipocytes with t10, c12-CLA during the differentiation period reduced TG accumulation [21, 22]. Similarly, Choi et al. [18] reported that a mixture of c9, t11 and t10, c12-CLA attenuated differentiation marker genes such as adipocyte fatty acid-binding protein (aP2) and PPARγ in 3T3-L1 adipocytes, whereas t10, c12-CLA alone did not affect the expression levels of these genes. Under the present assay conditions, t10, c12-CLA up to 100 µM did not have any significant effect on the accumulation of TG during the differentiation of 3T3-L1 preadipocytes into adipocytes (data not shown). The difference between the results of the present study and previous observations [18, 21, 22] may be related to the way of the CLA treatment; in the experiments undertaken by Choi et al. [18], Brown et al. [21] and Evans et al. [22], CLA isomers were complex to fatty acid-free serum albumin, and added to the cultures on day 1 of the differentiation. On the other hand, in the present study, c9, t11-CLA was prepared in Me2SO and added to the medium at the same time as the previous reports. Satory and Smith [20] also used ethanol as a solvent for the CLA isomers. Thus, the direct interaction of c9, t11-CLA with 3T3-L1 cells may promote the signal transduction for the differentiation. Although a fraction of blood circulating and cell-constructed c9, t11-CLA is thought to exist in the form of the free CLA and to affect the cell functions, further studies are needed to clarify the significance of the present findings.
In conclusion, the present study is the first to show that c9, t11-CLA can be a stimulator of adiponectin secretion by forming benign small-sized adipocytes, and suggests that this effect may partially explain the anti-hypertensive, anti-hyperlipidemic, anti-arteriosclerotic, anti-carcinogenic, and anti-diabetic effects mediated by CLAs. At least, the present findings may provide new information to extend the ongoing debate as to the mechanisms through which CLAs play functional roles both physiologically and pharmacologically in animal and human bodies.
Fig. 5.
The effects of the PPARγ agonist rosiglitazone and antagonist GW9662 on the TG contents (A) and Oil Red O staining (B) of 3T3-L1 adipocytes. The differentiation of 3T3-L1 preadipocytes was initiated 2 days after confluence for 3 days in growth medium containing 0.25 µM dexamethasone, 0.5 mM IBMX, and 1 µg/ml insulin. This was followed by 2 days in growth medium containing 1 µg/ml insulin. Thereafter, the cells were cultured in the growth medium for 2 days. Rosiglitazone (1 µM) or GW9662 (5 µM) was added to the medium from day-3 (time of addition of dexamethasone, IBMX, and insulin) to day-9 (end point of the experiment). (A): The treated cells were lysed with lysis buffer, and the TG contents were measured using a Triglyceride E-test Wako kit. The data represent the means ± SE. from four experiments. *p<0.01 vs Control. (B): The treated cells were fixed in 4% formaldehyde-phosphate buffer and stained with 0.3% Oil Red O dye. PPARγ, peroxisome proliferator-activated receptor γ; IBMX, 3-isobutyl-1-methyl-xanthine; TG, triacylglycerol.
Fig. 6.
Alterations in bioactive PPARγ in the nuclear extract of 3T3-L1 cells treated with c9, t11-CLA. The differentiation of 3T3-L1 preadipocytes was initiated 2 days after confluence for 3 days in growth medium containing 0.25 µM dexamethasone, 0.5 mM IBMX, and 1 µg/ml insulin. This was followed by 2 days in growth medium containing 1 µg/ml insulin. Thereafter, the cells were cultured in the growth medium for 2 days. Rosiglitazone (1 µM) or c9, t11-CLA (100 µM) was added to the medium from day-3 (time of addition of dexamethasone, IBMX, and insulin) to day-9 (end point of the experiment). Bioactive PPARγ in the nuclear extract from treated cells was measured using the PPARγ Transcription Factor Assay kit. The data represent the means ± SE. from five experiments. *p<0.05 vs Control. PPARγ, peroxisome proliferator-activated receptor γ; IBMX, 3-isobutyl-1-methyl-xanthine.
Abbreviations
- CLA
conjugated linoleic acid
- PPARγ
peroxisome proliferator-activated receptor γ
- TG
triacylglycerol
- TNF-α
tumor necrosis factor-α
- DMEM
Dulbecco’s modified Eagle’s medium
- IBMX
3-isobutyl-1-methyl-xanthine
- FBS
fetal bovine serum
References
- 1.Kepler C.R., Tove S.B. Biohydrogenation of unsaturated fatty acids. 3. Purification and properties of a linoleate delta-12-cis, delta-11-trans-isomerase from Butyrivibrio fibrisolvens. J. Biol. Chem. 1967;242:5686–5692. [PubMed] [Google Scholar]
- 2.Gurr M.I. Isomeric fatty acids. Biochem. Soc. Trans. 1987;15:336–338. doi: 10.1042/bst0150336. [DOI] [PubMed] [Google Scholar]
- 3.Zhao W.S., Zhai J.J., Wang Y.H., Xie P.S., Yin X.J., Li L.X., Cheng K.L. Conjugated linoleic acid supplementation enhances antihypertensive effect of ramipril in Chinese patients with obesity-related hypertension. Am. J. Hypertens. 2009;22:680–686. doi: 10.1038/ajh.2009.56. [DOI] [PubMed] [Google Scholar]
- 4.Park Y., Albright K.J., Liu W., Storkson J.M., Cook M.E., Pariza M.W. Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997;32:853–858. doi: 10.1007/s11745-997-0109-x. [DOI] [PubMed] [Google Scholar]
- 5.Lee K.N., Kritchevsky D., Pariza M.W. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis. 1994;108:19–25. doi: 10.1016/0021-9150(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 6.Ha Y.L., Storkson J., Pariza M.W. Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 1990;50:1097–1101. [PubMed] [Google Scholar]
- 7.Pariza M.W. Conjugated linoleic acid may be useful in treating diabetes by controlling body fat and weight gain. Diabetes Technol. Ther. 2002;4:335–338. doi: 10.1089/152091502760098474. [DOI] [PubMed] [Google Scholar]
- 8.Ailhaud G. Some new aspects on adipose tissue development. Diabetes Metab. Rev. 1992;8:3–7. doi: 10.1002/dmr.5610080103. [DOI] [PubMed] [Google Scholar]
- 9.Takamura T., Nohara E., Nagai Y., Kobayashi K. Stage-specific effects of a thiazolidinedione on proliferation, differentiation and PPARγ mRNA expression in 3T3-L1 adipocytes. Eur. J. Pharmacol. 2001;422:23–29. doi: 10.1016/s0014-2999(01)01053-6. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi T., Kamon J., Waki H., Murakami K., Motojima K., Komeda K., Ide T., Kubota N., Terauchi Y., Tobe K., Miki H., Tsuchida A., Akanuma Y., Nagai R., Kimura S., Kadowaki T. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. J. Biol. Chem. 2001;276:41245–41254. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- 11.Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab. 2004;30:13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- 12.Green H., Kehinde O. Sublines of mouse 3T3-L1 cells that accumulate lipid. Cell. 1974;1:113–116. [Google Scholar]
- 13.Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 14.Green H., Kehinde O. An established preadipose cell line and its differentiation in culture II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 15.Pariza M.W., Ha Y.L. Newly recognized anticarcinogenic fatty acids. Basic Life Sci. 1990;52:167–170. doi: 10.1007/978-1-4615-9561-8_13. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y.C., Leudecke L.O., Schultz T.D. Effect of cheddar cheese consumption on plasma conjugated linoleic acid concentrations in men. Nutr. Res. 1994;14:373–386. [Google Scholar]
- 17.Kramer J.K., Sehat N., Dugan M.E., Mossoba M.M., Yurawecz M.P., Roach J.A., Eulitz K., Aalhus J.L., Schaefer A.L., Ku Y. Distributions of conjugated linoleic acid (CLA) isomers in tissue lipid classes of pigs fed a commercial CLA mixture determined by gas chromatography and silver ion-high-performance liquid chromatography. Lipids. 1998;33:549–558. doi: 10.1007/s11745-998-0239-1. [DOI] [PubMed] [Google Scholar]
- 18.Choi Y., Kim Y.C., Han Y.B., Park Y., Pariza M.W., Ntambi J.M. The trans-10, cis-12 isomer of conjugated linoleic acid downregulates stearoyl-CoA desaturase 1 gene expression in 3T3-L1 adipocytes. J. Nutr. 2000;130:1920–1924. doi: 10.1093/jn/130.8.1920. [DOI] [PubMed] [Google Scholar]
- 19.Okuno A., Tamemoto H., Tobe K., Ueki K., Mori Y., Iwamoto K., Umesono K., Akanuma Y., Fujiwara T., Horikoshi H., Yazaki Y., Kadowaki T. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J. Clin. Invest. 1998;101:1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satory D.L., Smith S.B. Conjugated linoleic acid inhibits proliferation but stimulates lipid filling of murine 3T3-L1 preadipocytes. J. Nutr. 1999;129:92–97. doi: 10.1093/jn/129.1.92. [DOI] [PubMed] [Google Scholar]
- 21.Brown M., Evans M., McIntosh M. Linoleic acid partially restores the triglyceride content of conjugated linoleic acid-treated cultures of 3T3-L1 preadipocytes. J. Nutr. Biochem. 2001;12:381–387. doi: 10.1016/s0955-2863(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 22.Evans M., Park Y., Pariza M., Curtis L., Kuebler B., McIntosh M. Trans-10, cis-12 conjugated linoleic acid reduces triglyceride content while differentially affecting peroxisome proliferator activated receptor γ2 and aP2 expression in 3T3-L1 preadipocytes. Lipids. 2001;36:1223–1232. doi: 10.1007/s11745-001-0836-z. [DOI] [PubMed] [Google Scholar]