Abstract
The present study investigated the effect of folic acid supplementation on the Notch signaling pathway and cell proliferation in rat embryonic neural stem cells (NSCs). The NSCs were isolated from E14–16 rat brain and grown as neurospheres in serum-free suspension culture. Individual cultures were assigned to one of 3 treatment groups that differed according to the concentration of folic acid in the medium: Control (baseline folic acid concentration of 4 mg/l), low folic acid supplementation (4 mg/l above baseline, Folate-L) and high folic acid supplementation (40 mg/l above baseline, Folate-H). NSCs were identified by their expression of immunoreactive nestin and proliferating cells by incorporation of 5'bromo-2'deoxyuridine. Cell proliferation was also assessed by methyl thiazolyl tetrazolium assay. Notch signaling was analyzed by real-time PCR and western blot analyses of the expression of Notch1 and hairy and enhancer of split 5 (Hes5). Supplementation of NSCs with folic acid increased the mRNA and protein expression levels of Notch1 and Hes5. Folic acid supplementation also stimulated NSC proliferation dose-dependently. Embryonic NSCs respond to folic acid supplementation with increased Notch signaling and cell proliferation. This mechanism may mediate the effects of folic acid supplementation on neurogenesis in the embryonic nervous system.
Keywords: folic acid, neural stem cell, Notch, proliferation
Introduction
Neural stem cells (NSCs) are highly proliferative cells that can generate new NSCs and differentiated progeny such as neurons and glia [1]. Embryonic NSCs consequently play a major role in neurogenesis and gliogenesis in the development of the central nervous system (CNS). Additionally, NSCs are useful for cell replacement therapy of neurodegenerative diseases. For many clinical applications of cell replacement therapy in the CNS, the NSCs will likely need to be acquired from embryonic or fetal tissues and then expanded [2].
Nutrients, such as folate species, influence the proliferation and differentiation of NSCs. Folate deficiency or methotrexate inhibition of folate metabolism impairs cell proliferation in cultures of embryonic NSCs [3], whereas folic acid supplementation stimulates these cells to proliferate [4]. Cerebral folate deficiency is associated with developmental delay and lifelong neurological conditions [5–7]. The CNS pathologies associated with folate deficiency include malformations that develop during embryogenesis (neural tube defects) as well as neurological disorders that appear later, such as seizures, dementia and depression [8–11]. Dietary supplements containing folic acid, which can be metabolized to biologically active folate species, are therefore recommended for prevention of folate deficiency in women who are or may become pregnant. There is evidence from studies of various cell types that some effects of folate deficiency and folic acid supplementation may be mediated by altered levels of substrates (purines, thymidine, methionine and homocysteine) that change intracellular signaling pathways [3, 4, 12]. The identity of the intracellular signaling pathway through which folic acid influences the fate of embryonic NSCs is unknown. However, a possible candidate is the Notch signaling pathway, including Notch1 and its downstream effector hairy and enhancer of split 5 (Hes5), because this pathway is an important regulator of CNS growth and differentiation [13, 14].
NSCs can be isolated from rodent brains and selectively expanded in chemically defined media, giving rise to floating spheroid cell aggregates called neurospheres. In the present study, embryonic rat neurosphere cultures were used to investigate the effect of folic acid supplementation on the Notch signaling pathway and cell proliferation in rat embryonic NSCs.
Materials and Methods
Cell culture
Pregnant Sprague-Dawley rats were purchased from Beijing Medical Laboratory Animal Co. Ltd. (Beijing, China). Folic acid, Iscove’s Modified Dulbecco’s Medium (IMDM), B27 supplement, basic fibroblast growth factor, epidermal growth factor, dimethyl sulphoxide and 5'-bromo-2' deoxyuridine (BrdU) were obtained from Gibco (Carlsbad, CA). Monoclonal antibodies against BrdU, nestin, Notch1, Hes5 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All fluorescent secondary antibodies were obtained from Zhongshan Goldbridge Biotechnology (Beijing, China). Trizol and Platinum SYBR Green qPCR Supermix-UDG Kit were purchased from Invitrogen (Carlsbad, CA).
The experimental procedures were approved by the Tianjin Medical University Committee on Animal Care. E14–E16 rat brain cortices were dissected from timed-pregnant Sprague-Dawley rats and washed 3 times with culture medium (IMDM, containing 4 mg/l folic acid). The tissue was cut into small pieces and then dissociated by incubation with 0.25% parenzyme and 0.02% ethylenediaminetetraacetic acid (EDTA). This step was followed by agitation, centrifugation and resuspension of the cells in IMDM that was supplemented with 2% B27 supplement, 20 ng/ml epidermal growth factor, 20 ng/ml basic fibroblast growth factor, 2 mmol/l L-glutamine, 100 U/ml penicillin and phytomycin. The resulting cell suspension was grown at 37°C in a humidified atmosphere containing 95% air, 5% CO2. The culture medium was replaced every 2 days. Beginning on day 1 in vitro, individual cultures were assigned to one of 3 treatment groups that differed according to the concentration of folic acid in the medium: Control (baseline folic acid concentration of 4 mg/l), low folic acid supplementation (4 mg/l above baseline, Folate-L) and high folic acid supplementation (40 mg/l above baseline, Folate-H). Thus the final concentrations of folic acid were 8 mg/l and 44 mg/l in Folate-L and Folate-H, respectively.
Immunofluorescence analysis
Briefly, neurospheres on the sixth day in culture were incubated with the proliferation marker BrdU (10 µg/ml) for 24 h. Then they were stained for BrdU and the NSC marker nestin. To detect BrdU, the primary antibody was mouse anti-BrdU monoclonal antibody and the second antibody was fluorescein isothiocyanate (FITC) conjugated goat anti-mouse IgG. To detect nestin, the primary antibody was rabbit anti-nestin monoclonal antibody and the secondary antibody was tetramethylrhodamine isothiocyanate (TRITC) conjugated goat anti-rabbit IgG. Immunoreactive cells were visualized by fluorescence microscopy. The percentages of double-positive cells of all cells were calculated.
Methyl thiazolyl tetrazolium (MTT) assay
After 2 days exposure of the neurospheres to the Control, Folate-L and Folate-H treatments, cell proliferation was quantified by measuring the time-dependent increase in the abundance of viable cells. Aliquots containing 5 × 105 cells were transferred individual wells in a 96-well plate and then incubated at 37°C for the indicated periods. MTT (0.5 mg) was added to each well at 4 h before the end of the incubation period, when the MTT reaction was stopped by addition of 10% sodium dodecyl sulfate (SDS)-0.1 mol/l HCl. The cell’s formazan crystals were dissolved in dimethyl sulphoxide and then absorbance at 490 nm was measured.
Real-time PCR
Cells were harvested after 6 days of folic acid (or Control) treatment. Then quantitation of gene expression was done with the real-time PCR procedure. First-strand cDNA was synthesized from 2 µg total RNA using M-MLV reverse transcriptase according to the instructions of the manufacturer. The 25 µl of reaction volume was incubated for 60 min at 42°C, 10 min at 70°C and then held at −20°C. Real-time PCR was performed using a Platinum SYBR Green qPCR Supermix-UDG Kit. The 20 µl of PCR mixture included 10.0 µl of PCR MIX, 6.0 µl of cDNA, 0.5 µl of amplification primer I, 0.5 µl of amplification primer II and 3.0 µl of autoclaved distilled water. The reaction mixtures were incubated at 95°C for 5 min, followed by 40 amplification cycles of 95°C for 10 s and 60°C (58°C for β-actin) for 10 s and 72°C for 10 s. RT-PCR was performed using the ABI Prism 7700 sequence detector. The expression of Notch1 and Hes5 was normalized to β-actin levels. The primers employed are shown as below: β-actin: 5'GAACCCTAA GGCCAACCGTG3' and 5'AGGCATACAGGGACAACA CAGC3'; Notch1: 5'GAGGCTTGAGATGCTCCCAG3' and 5'ATTCTTACATGGTGTGCTGAGG3'; Hes5: 5'-GCACCA GCCCAACTCCAAAC-3' and 5'-TGCAGGCACCACGAG TAGCC-3'.
Western blot
The protein expression levels of Notch1, Hes5 and β-actin (loading control) were measured by western blot analysis after 6 days in culture. Cells were washed with ice-cold PBS and then lysed with radioimmunoprecipitation assay buffer. The lysates was centrifuged at 12000 rpm for 10 min at 4°C and the pellets were discarded. Protein concentrations in the supernatants were determined with a BCA protein assay kit, using bovine serum albumin as a standard. Equal amounts of protein were loaded in each well for sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and then the separated proteins were transferred to nitrocellulose membranes. The membranes were blocked in Tris-buffered saline contained Blotto solution for 2 h at room temperature. The membranes were then incubated with the primary antibodies (rabbit anti-notch1 antibody, rabbit anti-hes5 antibody, 1:1000) and β-actin (1:5000) overnight at 4°C in Tris-buffered saline containing 0.1% Tween 20 (TBS-T). Membranes were rinsed 3 times with TBS-T before being incubated with horseradish peroxidase-conjugated secondary antibody (1:30000 in TBS-T) for 50 min and detected by chemiluminescence. Quantitation of proteins was done by densitometric analysis of using NIH Image software (version 1.61).
Statistics
Numerical data are presented as mean ± standard deviation (SD) values based on n number of experiments. Differences between means were evaluated by one-way ANOVA followed by Tukey’s multiple comparison test. p<0.05 was considered statistically significant.
Results
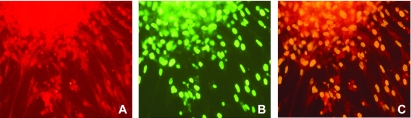
The cells cultured from embryonic brain grew in spheroids and expressed the NSC marker nestin (Fig. 1A). BrdU is a thymidine analog that is incorporated into cells during the S phase of the cell cycle in proliferating cells. In our study, many of the cells in neurospheres also incorporated BrdU, as indicated by the green staining of nuclei (Fig. 1B). Double immunofluorescence staining of cells showed colocalization of nestin-positive and BrdU-positive cells (Fig. 1C). Nestin/BrdU double-labeled cells are NSCs which possessed the abilities of proliferation.
Fig. 1.
Embryonic neurospheres contain proliferating neural stem cells (NSCs). The neurospheres were grown under control conditions in serum-free medium containing 4 mg/l folic acid. On the sixth day in culture, the neurospheres were incubated with the proliferation marker 5'bromo-2'deoxyuridine (BrdU) for 24 h. Subsequently they were stained with specific primary antibodies and fluorescent secondary antibodies to detect BrdU and the NSC marker nestin. Shown are fluorescence photomicrographs of cells expressing nestin (red fluorescence, A), BrdU (green, B), and both nestin and BrdU (yellow, C). The original magnification for fluorescence microscopy was ×200.
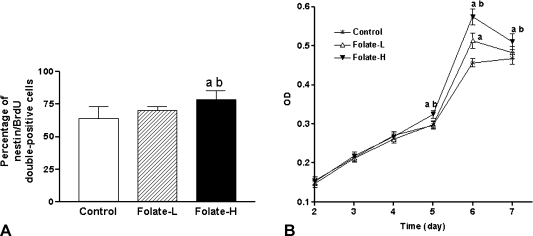
Supplementation for 6 days with 40 mg/l folic acid (Folate-H treatment) increased the percentages of cells in the neurospheres that were nestin/BrdU double-positive (Fig. 2A). This observation indicates that the additional folic acid increased rapidly the relative abundance of proliferating NSCs.
Fig. 2.
Folic acid supplementation stimulates proliferation of embryonic neural stem cells (NSCs). A: Percentage of cells that were double-positive for nestin and 5'bromo-2'deoxyuridine (BrdU), as determined by immunofluorescence analysis. B: Cell proliferation quantified by measuring the time-dependent increase in the abundance of viable cells, which were identified by methyl thiazolyl tetrazolium (MTT) assay. NSC neurospheres were incubated at 37°C, for the indicated periods, in medium containing 4 mg/l folic acid (Control), 8 mg/l folic acid (Folate-L), or 44 mg/l folic acid (Folate-H). MTT was added to each well at 4 h before the end of the incubation. Finally the resulting precipitates were dissolved in dimethyl sulphoxide and optical density (OD) was measured at 490 nm. Shown are mean ± SD values of 8 separate experiments. One-way analysis of variance and Tukey’s multiple comparison were performed to compare the mean values. ap<0.05 vs Control, bp<0.05 vs Folate-L.
The effect of folic acid supplementation on the growth of neurosphere cultures was also quantitated using the MTT assay of viable cells. As shown in Fig. 2B, the abundance of viable cells depended on time and folic acid concentration. After 5–7 days in culture, there were significantly more viable cells in cultures that were supplemented with folic acid than in those that were not (Fig. 2B). This effect was concentration-dependent because, after treatment periods of 5 days or longer, the Folate-H cultures contained more viable cells than did the Folate-L cultures (Fig. 2B). Nevertheless, the Folate-L treatment did eventually stimulate proliferation above the Control level (Fig. 2B).
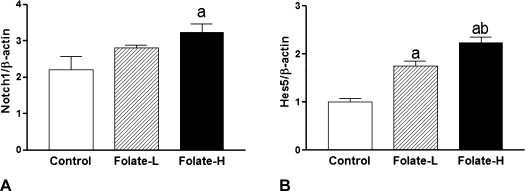
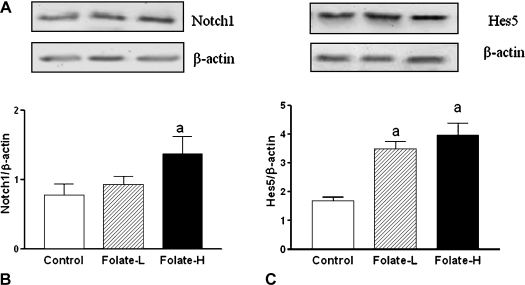
The Folate-H treatment, but not the Folate-L treatment, increased the expression of Notch1 mRNA (Fig. 3A) and protein (Fig. 4A). Both Folate-H and Folate-L stimulated Hes5 expression at the mRNA (Fig. 3B) and protein (Fig. 4B) levels. Further, the size of the effect on Hes5 mRNA expression was greater for the Folate-H treatment than for the Folate-L treatment (Fig. 3B).
Fig. 3.
Folic acid supplementation increases expression of Notch1 and Hes5 mRNAs. Neural stem cell (NSC) neurospheres were incubated for 6 days in medium containing 4 mg/l folic acid (Control), 8 mg/l folic acid (Folate-L), or 44 mg/l folic acid (Folate-H). Subsequently Notch1 and Hes5 mRNA levels were determined by real-time PCR and normalized to β-actin expression. Shown are mean ± SD values of 3 experiments. ap<0.05 vs Control, bp<0.05 vs Folate-L.
Fig. 4.
Folic acid supplementation increases expression of Notch1 and hairy and enhancer of split 5 (Hes5) proteins. Neural stem cell (NSC) neurospheres were incubated for 6 days in medium containing 4 mg/l folic acid (Control), 8 mg/l folic acid (Folate-L), or 44 mg/l folic acid (Folate-H). Subsequently Notch1 and Hes5 protein levels were determined by western blot analysis and normalized to β-actin expression. A: Representative western blots of Notch1, Hes5 and β-actin. B: Notch1: β-actin ratios. Shown are mean ± SD values of 3 experiments. ap<0.05 vs Control. C: Hes5: β-actin ratios. Shown are mean ± SD values of 3 experiments. ap<0.05 vs Control.
Discussion
The present study found that folic acid supplementation exerts proliferative actions on embryonic NSCs. Analysis of the underlying mechanism revealed that this effect of folic acid is associated with increased mRNA and protein expression of Notch1 and Hes5. Because NSCs are sources of newly generated neurons and glia in the brain, our findings suggest that folic acid may regulate neurogenesis and gliogenesis by stimulating the Notch1 signaling pathway in embryonic NSCs.
NSCs proliferate and maintain an undifferentiated state when incubated with basic fibroblast growth factor. We found that folic acid supplementation increased the percentage of nestin/BrdU double-positive cells in embryonic neurospheres grown in the presence of basic fibroblast growth factor. It can be inferred that the number of proliferative NSCs was enhanced by folic acid. Taken together, the present results indicate that folic acid stimulates the proliferation of embryonic NSCs.
There is abundant evidence that folate influences mitosis. For example, experiments with mice have shown that, compared to a normal maternal diet (2 mg folic acid/kg diet), a maternal diet that is folate-deficient (0.0 mg folic acid/kg diet) during E11–E17 decreases NSC proliferation and increases apoptosis in the E17 brain [15]. There are several ways in which folic acid supplementation may influence cell proliferation [9]. Mammalian cells cannot synthesize folate de novo, but they require folate for many metabolic functions, including the conversion of serine to glycine, the catabolism of histidine, the synthesis of thymidylate, methionine, and purine, and the DNA methylation that regulates gene expression [16].
Folic acid supplementation modulates intracellular signaling pathways that regulate mitosis and apoptosis [9]. Previously we observed that folic acid supplementation increased mRNA expression of Notch signaling molecules in neurosphere cultures of NSCs derived from neonatal rats, but proteins expression hadn’t been detected [17]. In present study, we further detected protein expression evolved in Notch signaling pathway of embryonic NSCs. The Notch signaling pathway is necessary for cell-cell communication, which involves gene regulation mechanisms that control multiple cell differentiation processes during embryonic and adult life. The Notch gene is known to suppress apoptosis and promote cell proliferation through a growth factor-mediated survival pathway. Notch proteins act as receptors in an evolutionarily conserved signaling pathway that regulates cell proliferation and differentiation. In our study, it is likely that folic acid stimulated the transcription and translation of Notch1, because folic acid supplementation increased Notch1 mRNA and protein levels in NSC neurospheres. Hes genes are the conserved targets of Notch signaling for regulating the expansion and differentiation of neural progenitors. Hes5 as a known Notch effectors, regulate the maintenance of neural stem cells and the development of the central nervous system. Previously it has been shown that Hes5 expression responds to Notch1; for example, Hes5 mRNA expression is decreased in the CNS of Notch1 deficient (Notch1–/–) mice [13]. Therefore, in the present study, elevation of Hes5 mRNA and protein levels in folic acid-supplemented NSCs may have been due to stimulation by Notch1 of Hes5 transcription and translation.
Experiments with mouse mutants have found that genetic disruptions of Notch signaling generally result in an increase in neuronal differentiation markers and a decrease in NSC markers, showing that Notch signaling maintains the NSC phenotype [18]. Further, conditional deletion of Notch1 in the telencephalon leads to decreased neuronal cell abundance in vivo and decreased neurosphere formation in vitro, most likely resulting from precocious neuronal differentiation and earlier progenitor pool depletion [19]. Other studies also found that deletion of Notch1 or Hes5 decreases neurosphere formation in suspension cultures of NSCs [20, 21]. Conversely, activation of the Notch signaling pathway inhibits differentiation of NSCs into terminal cell phenotypes [22, 23]. Thus, Notch signaling maintains the NSC pool. Hes5 is key effector in Notch signaling because this transcriptional repressor is highly expressed by NSCs and inhibits their neuronal differentiation [14, 24]. Therefore, Hes5 upregulation may maintain the capacity for self-renewal that we observed in embryonic NSCs after folic acid supplementation.
In conclusion, this study has demonstrated that embryonic NSCs respond to folic acid supplementation with increased Notch signaling and cell proliferation. These results support the hypothesis that folic acid supplementation promotes proliferation of embryonic NSCs by activating Notch signaling.
Acknowledgments
This research was supported by National Natural Science Foundation of China grants No30771797.
References
- 1.Merkle F.T., Alvarez-Buylla A. Neural stem cells in mammalian development. Curr. Opin. Cell Biol. 2006;18:704–709. doi: 10.1016/j.ceb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Sharp J., Keirstead H.S. Stem cell-based cell replacement strategies for the central nervous system. Neurosci. Lett. 2009;456:107–111. doi: 10.1016/j.neulet.2008.04.106. [DOI] [PubMed] [Google Scholar]
- 3.Kruman I.I., Mouton P.R., Emokpae R. Jr., Cutler R.G., Mattson M.P. Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuroreport. 2005;16:1055–1059. doi: 10.1097/00001756-200507130-00005. [DOI] [PubMed] [Google Scholar]
- 4.Sato K., Kanno J., Tominaga T., Matsubara Y., Kure S. De novo and salvage pathways of DNA synthesis in primary cultured neurall stem cells. Brain Res. 2006;1071:24–33. doi: 10.1016/j.brainres.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Hansen F.J., Blau N. Cerebral folate deficiency: life-changing supplementation with folinic acid. Mol. Genet. Metab. 2005;84:371–373. doi: 10.1016/j.ymgme.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Moretti P., Sahoo T., Hyland K., Bottiglieri T., Perters S., del Gaudio D., Roa B., Curry S., Zhu H., Finnell R.H., Neul J.L., Ramaekers V.T., Blau N., Bacino C.A., Miller G., Scaglia F. Cerebral folate deficiency with developmental delay, autism, and response to folinic acid. Neurology. 2005;64:1088–1090. doi: 10.1212/01.WNL.0000154641.08211.B7. [DOI] [PubMed] [Google Scholar]
- 7.Ramaekers V.T., Sequeira J.M., Artuch R., Blau N., Temudo T., Ormazabal A., Pineda M., Aracil A., Roelens F., Laccone F., Quadros E.V. Folate receptor autoantibodies and spinal fluid 5-methyltetrahydrofolate deficiency in Rett syndrome. Neuropediatrics. 2007;38:179–183. doi: 10.1055/s-2007-991148. [DOI] [PubMed] [Google Scholar]
- 8.Djukic A. Folate-responsive neurologic diseases. Pediatr. Neurol. 2007;37:387–397. doi: 10.1016/j.pediatrneurol.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Ho P.I., Ashline D., Dhitavat S., Ortiz D., Collins S.C., Shea T.B., Rogers E. Folate deprivation induces neurodegeneration: roles of oxidative stress and increased homocysteine. Neurobiol. Dis. 2003;14:32–42. doi: 10.1016/s0969-9961(03)00070-6. [DOI] [PubMed] [Google Scholar]
- 10.Clarke R. Vitamin B12, folic acid, and the prevention of dementia. N. Engl. J. Med. 2006;354:2817–2819. doi: 10.1056/NEJMe068086. [DOI] [PubMed] [Google Scholar]
- 11.Ramos M.I., Allen L.H., Mungas D.M., Jagust W.J., Haan M.N., Green R., Miller J.W. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am. J. Clin. Nutr. 2005;82:1346–1352. doi: 10.1093/ajcn/82.6.1346. [DOI] [PubMed] [Google Scholar]
- 12.Rabaneda L.G., Carrasco M., López-Toledano M.A., Murillo-Carretero M., Ruiz F.A., Estrada C., Castro C. Homocysteine inhibits proliferation of neuronal precursors in the mouse adult brain by impairing the basic fibroblast growth factor signaling cascade and reducing extracellular regulated kinase 1/2-dependent cyclin E expression. FASEB. J. 2008;22:3823–3835. doi: 10.1096/fj.08-109306. [DOI] [PubMed] [Google Scholar]
- 13.de la Pompa J.L., Wakeham A., Correia K.M., Samper E., Brown S., Aguilera R.J., Nakano T., Honjo T., Mak T.W., Rossant J., Conlon R.A. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- 14.Kageyama R., Ohtsuka T., Kobayashi T. Roles of Hes genes in neural development. Dev. Growth Differ. 2008;50 Suppl 1:S97–103. doi: 10.1111/j.1440-169X.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- 15.Craciunescu C.N., Brown E.C., Mar M.H., Albright C.D., Nadeau M.R., Zeisel S.H. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J. Nutr. 2004;134:162–166. doi: 10.1093/jn/134.1.162. [DOI] [PubMed] [Google Scholar]
- 16.Cains S., Shepherd A., Nabiuni M., Owen-Lynch P.J., Miyan J. Addressing a Folate Imbalance in Fetal Cerebrospinal Fluid Can Decrease the Incidence of Congenital Hydrocephalus. J. Neuropathol. Exp. Neurol. 2009;68:404–416. doi: 10.1097/NEN.0b013e31819e64a7. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X., Liu H., Cong G., Tian Z., Ren D., Wilson J.X., Huang G. Effects of folate on notch signaling and cell proliferation in neural stem cells of neonatal rats in vitro. J. Nutr. Sci. Vitaminol. (Tokyo) 2008;54:353–356. doi: 10.3177/jnsv.54.353. [DOI] [PubMed] [Google Scholar]
- 18.Yoon K., Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 19.Yoon K., Nery S., Rutlin M.L., Radtke F., Fishell G., Gaiano N. Fibroblast growth factor receptor signaling promotes radial glial identity and interacts with Notch1 signaling in telencephalic progenitors. J. Neurosci. 2004;24:9497–9506. doi: 10.1523/JNEUROSCI.0993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hitoshi S., Alexson T., Tropepe V., Donoviel D., Elia A.J., Nye J.S., Conlon R.A., Mak T.W., Bernstein A., van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes. Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtsuka T., Sakamoto M., Guillemot F., Kageyama R. Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. J. Biol. Chem. 2001;276:30467–30474. doi: 10.1074/jbc.M102420200. [DOI] [PubMed] [Google Scholar]
- 22.Hojo M., Ohtsuka T., Hashimoto N., Gradwohl G., Guillemot F., Kageyama R. Glial cell fate specification modulated by the bHLH gene Hes5 in mouse retina. Development. 2000;127:2515–2522. doi: 10.1242/dev.127.12.2515. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y.X., Armstrong R.C. Interaction of fibroblast growth factor 2 (FGF2) and notch signaling components in inhibition of oligodendrocyte progenitor (OP) differentiation. Neurosci. Lett. 2007;421:27–32. doi: 10.1016/j.neulet.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iso T., Kedes L., Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J. Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]