Abstract
A "two-hit" model for non-alcoholic steatohepatitis (NASH) has been proposed in which steatosis constitutes the "first hit" and sensitizes the liver to potential "second hits" resulting in NASH. Oxidative stress is considered a candidate for the "second hit". N-acetylcysteine (NAC), an antioxidant, has been suggested as a dietary therapy for NASH. We examined effects of NAC in a rat total enteral nutrition (TEN) model where NASH develops as the result of overfeeding dietary polyunsaturated fat. Male Sprague-Dawley rats were fed pelleted AIN-93G diets ad libitum or were overfed a 9200 kJ˙ kg−0.75˙d−1 liquid diet containing 70% corn oil with or without 2 g˙ kg−1˙d−1 NAC intragastrically for 65 d. Hepatic steatosis was not influenced by dietary supplementation with NAC; however, the liver pathology score was significantly lower (p≤0.05) and NAC provided partial protection against alanine aminotransferase release (p≤0.05). NAC attenuated increased hepatic oxidative stress (TBARS; p≤0.05); prevented increases in cytochrome P450 2E1 apoprotein and mRNA; and tumor necrosis factor-α (TNF-α) mRNA. A decrease in titers of auto-antibodies against proteins adducted to lipid peroxidation products was observed in the NAC group relative to the 70% corn oil group (p≤0.05). NAC also decreased Picosirius red staining of collagen, a marker of fibrosis. However, markers of hepatic stellate cell activation were unaffected. Using NAC in a TEN model of NASH we have demonstrated that NAC prevents many aspects of NASH progression by decreasing development of oxidative stress and subsequent increases in TNF-α, but is unable to block development of steatosis.
Keywords: steatosis, oxidative stress, lipid peroxidation, TNF-α, fibrosis
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common of liver pathologies and is closely associated with obesity and the metabolic syndrome. There is a wide spectrum of pathologies covered by NAFLD ranging from simple reversible steatosis to nonalcoholic steatohepatitis (NASH), in which steatosis is accompanied by inflammation, necrosis, apoptosis and fibrosis. The mechanisms leading to NASH are multifactorial. In patients with NASH, there is an initial development of steatosis, plus a second insult resulting in disease progression. The “two hit” model of NASH was proposed in 1998 (1) in which alterations in lipid homeostasis associated with obesity, insulin resistance and adipokine disruption result in steatosis and constitute the “first hit”. This sensitizes the liver to potential “second hits” resulting in hepatocellular injury, inflammation and fibrosis. Oxidative stress is frequently cited as a central mechanism of hepatocellular injury in NASH, correlating with the accumulation of lipid peroxidation products, appearance of mitochondrial dysfunction (2–4) and elevation of pro-inflammatory cytokines. It is considered one of the best candidates for the “second hit” since it can explain all the recognized histological features of the disease (5–7). Glutathione (GSH) is a major endogenous anti-oxidant. N-acetylcysteine (NAC), a precursor of reduced GSH, has been in clinical use for more than 30 years as an antidote for acetaminophen overdose (8). Potential protective effects of NAC are being studied in chronic diseases characterized by decreased GSH or oxidative stress such as alcoholic and non-alcoholic steatohepatitis (9–12). Most of the beneficial effects of orally administered NAC are theorized to be a result of its ability to either reduce extracellular cystine to cysteine or to be a source of SH metabolites. As a source of SH groups, NAC can stimulate GSH synthesis, enhance glutathione-S-transferase activity, promote detoxification, and act as a scavenger of free radicals as it interacts with reactive oxygen species (ROS) (13, 14). We have developed a unique rat model in which liver pathology closely resembles clinical NASH using overfeeding of a high polyunsaturated fat diet via total enteral nutrition (TEN) (15). NASH is histologically indistinguishable from alcoholic steatohepatitis and it is proposed they may also have many similarities in the pathogenesis including oxidative stress, increased cytochrome P450 2E1 (CYP2E1) and pro-inflammatory cytokine, tumor necrosis factor-alpha (TNF-α) (1, 15, 16). We have previously shown NAC to be successful in prevention of alcohol-induced liver disease in rats fed via TEN (12). The aim of the present study was to determine the effects of NAC treatment on development of liver pathology in our TEN NASH model.
MATERIALS AND METHODS
Reagents
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Potassium chloride, potassium phosphate and potassium ferricyanide were purchased from Fisher Scientific (Hampton, NH). ECL for chemiluminescent detection in western blotting was purchased from Amersham Biosciences (Piscataway, NJ). TRI Reagent used for RNA extraction was obtained from Molecular Research Center, Inc. (Cincinnati, OH). Reagents for assessment of RNA quality using the Agilent Bioanalyzer were acquired from Agilent Technologies (Foster City, CA).
Experimental animals and diets
Male Sprague-Dawley rats (175 g) were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care approved animal facility. Animal maintenance and experimental treatments were conducted in accordance with ethical guidelines for animal research and were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. Animals were randomly assigned to diet groups. Control rats (C) had ad libitum access to water and a pelleted AIN-93G diet in which the protein source (hydrolyzed whey) matched that of the liquid diets utilized for NASH development (Table 1) (17). Rats fed by TEN received 9200 kJ˙ kg−0.75˙d−1 (which represents 17% greater energy intake than recommended by the NRC) (17, 21). TEN diets contained corn oil at a level equal to 70% of the total energy (15) with or without 2 g˙ kg−1˙d−1 NAC (high fat, HF or high fat + NAC, HF + NAC) (Table 1). TEN fed rats had an intragastric cannula surgically inserted and were allowed 7 d to recover before diet infusion as described previously (12, 15, 18–21). Vitamin and mineral content was the same in all diets (Table 1) (17, 21). All diets met energy and nutritional recommendations recommended by the NRC (17, 21). Body weights were recorded twice a week and at the beginning and end of the study. Rats were killed after 65 d and serum and livers were collected and stored at −20°C and −70°C, respectively.
Table1.
Diet Composition
| Pelleted Dieta,b | 70% Fat Dietb,c,d | |||
|---|---|---|---|---|
| Per kg | % Total kJ | Per Liter | % Total kJ | |
| Protein | 179g | 19% | 44g | 19% |
| Carbohydrate | 751g | 64% | 24.5g | 11% |
| Fat | 70g | 17% | 72.7g | 70% |
Diet made according to the AIN-93G diet formula except that corn oil replaced soy bean oil and the protein source was whey protein (New Zealand Milk Products) as described previously (17).
Whey protein was supplemented with an amino acid mix to ensure that all essential amino acids were present at levels to meet of exceed National Research Council recommended levels as described previously (17).
TEN diets formulated with vitamins and minerals added to meet or exceed National Research Council recommended levels as described previously. The caloric density was 890 kg˙l−1 and diet was fed at 9200 kJ˙kg−0.75d−1 (21).
Carbohydrate in the TEN diet was 25% maltodextrin and 75% dextrose, fat in the TEN diet was corn oil as described previously (21).
Pathological evaluation
Liver pathology was assessed by hematoxylin-eosin (H&E) and Oil Red O staining of liver sections and scored by a board-certified pathologist (L. Hennings). The pathology calculation was based on ballooning degeneration (0–2), presence or absence of serum markers of necrosis (ALT score: cut-off was 55, based on data from Charles River labs and our baseline values, <55 = 0, ≥55 = 1), the lipidosis score (based on evaluation of Oil Red O stained slides) and lobular inflammation/necrosis (0 = 0 foci, 1 = <2 foci, 2 = 2–4 foci and 3 = >4 foci) as described previously (15). Portal fibrosis was detected by Picosirius red staining of collagen (22).
Biochemical analysis
Serum alanine aminotransferase (ALT) activity levels were measured at sacrifice using the Infinity ALT liquid stable reagent (Thermo Electron Corp., Waltham, MA) according to manufacturer’s protocols. Liver microsomes were prepared by differential centrifugation and stored at −70°C until analysis. Protein concentrations of the microsomes were determined by the Bradford method using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Liver lipid peroxidation was assessed as a measure of oxidative stress as described by Ohkawa et al. (23). Western immunoblot analysis of apoprotein expression for CYP2E1 was conducted as previously described (15, 24). CYP2E1 was a gift from the laboratory of Dr. Magnus Ingelman-Sundberg (Karolinska Institute, Stockholm, Sweden) (25). Western immunoblot analysis of protein expression for malondialdehyde (MDA) was conducted as described by Polavarupu, et al. (26). Briefly, total proteins (30 µg) were loaded on SDS/PAGE and transferred to nitrocellulose membrane and incubated with 2% bovine serum albumin (BSA) in tris buffered saline (TBS)/Tween for blocking and subsequently incubated with the antibody (1 mg˙L−1) (Cosmo Bio Co., Tokyo, Japan). This procedure was followed by the addition of horseradish peroxidase-linked goat antibody to mouse IgG and detected by enhanced chemiluminescence (ECL) (27, 28). The bands were visualized by autoradiography and entire lanes quantified.
Measurement of auto-antibodies to proteins adducted with MDA and LOOH
Circulating auto-antibodies to proteins adducted with the lipid peroxidation products MDA or arachidonate hydroperoxide (LOOH) were measured as described previously by Mottaran, et al. (29).
Real-time reverse transcription-polymerase chain reaction
Total RNA was extracted from livers using TRI Reagent and cleaned using RNeasy mini columns (Qiagen, Valencia, CA). RNA quality was ascertained spectrophotometrically (ratio of A260/A280) and also by checking ratio of 28S to 18S ribosomal RNA using the RNA Nano Chip on a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Total RNA (1 µg) was reverse transcribed using the iScript Reverse Transcription kit (Biorad Laboratories, Hercules, CA) according to manufacturer’s instructions. The reverse transcribed cDNA (10 ng) was utilized for real-time PCR using the 2X SYBR green master mix and monitored on a ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). Gene-specific probes were designed using Primer Express™ Software (Applied Biosystems, Foster City, CA; Online Supporting Material, Supplemental Table 1) and the relative amounts of gene expression were quantitated using a standard curve according to manufacturer’s instructions.
Measurement of liver glutathione (GSH) concentrations
Hepatic GSH concentrations were quantified in livers from control and NAC-treated rats using a commercially available kit (703002) from Cayman Chemical Co. (Ann Arbor, MI). The kit is based on enzymic recycling of GSH using GSH reductase followed by reaction of GSH with Ellman’s reagent. The colored product is measured at 405 nm and quantified using a standard curve.
Statistical analysis
Data are expressed as means ± SEM. Densitometric quantitation of Western blot autoradiograms was performed using Quantity One software (Biorad, Hercules, CA). SigmaStat software package version 3.0 (SPSS Inc., Chicago, IL) was used to perform all statistical tests. The data were tested using Levene’s test for Equality of Variance. Pearson product moment correlation was performed using SigmaStat software. Group differences were evaluated via one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc comparisons unless the data were not normally distributed, in which case evaluation was using one way ANOVA of RANKS. P-values ≤ 0.05 were considered statistically significant.
RESULTS
Dietary supplementation with NAC partly protects against liver injury in NASH
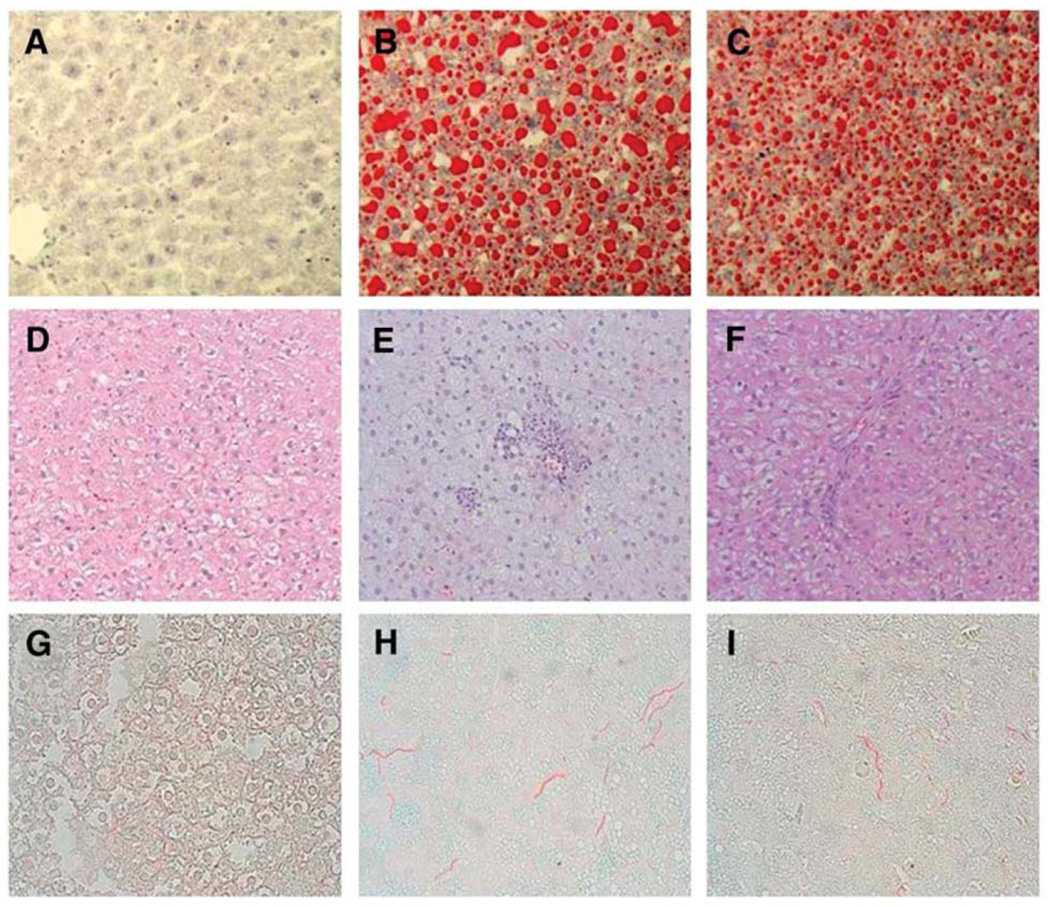
The pellet fed group C consumed a mean of 7800 kJ˙ kg−0.75˙d−1 with a growth rate of 3.83 ± 0.03 g˙d−1. In comparison, the HF TEN group infused 9200 kJ˙ kg−0.75˙d−1 had a growth rate of 6.84 ± 0.1 g˙d−1. The HF + NAC group infused the same TEN diet + NAC had a growth rate of 5.89 ± 0.08 g˙d−1. Growth rates for the HF groups were significantly higher than that of the pellet fed group (p≤0.05). Pathological examination demonstrated no histological evidence of fat accumulation or inflammation in group C (Figure 1D), but steatosis, hepatocellular ballooning, inflammation, necrosis and portal/lobular fibrosis similar to that observed in Grade 3 clinical NASH was present in the HF group (Figure 1E and Online Supporting Material, Supplemental Figure 1) consistent with previous observations for our laboratory (15). The total pathology score and pathology scores in excess of simple steatosis were reduced in the HF + NAC group (Figure 1F) compared to the HF group (p≤0.05) (Table 2). Development of NASH was accompanied by elevated serum ALT activity (p≤0.05) (Table 3) indicating necrotic injury. Addition of NAC attenuated this increase; however ALT values were still elevated relative to the C group (p≤0.05) (Table 3). Picosirius red staining demonstrated a significant increase in portal/lobular fibrosis in the HF group (Figures 1G and 1H) (p≤0.05), this was attenuated by NAC administration (p≤0.05) (Figure 1I, Table 2). Oil red O staining and biochemical analysis of triacylglycerol showed an increase in hepatic steatosis in the HF group (Figures 1A and 1B) (p≤0.05) associated with an increase in relative expression of mRNA for the FA transport associated protein CD36 from 1.0 ± 0.08 to 12.1 ± 2.7 (p≤0.05). NAC had no effect on overall level of fat accumulation in the liver or relative CD36 mRNA expression (14.1 ± 3.3) but did appear to attenuate progression of liver pathology beyond steatosis and to result in formation of smaller lipid droplets (Figure 1C, and Table 3).
Figure 1. Representative Oil Red O (A,B,C), H&E (D,E,F) and Picosirius Red (G,H,I) stained liver sections from male rats overfed high fat diets with or without NAC supplementation.
Representative Oil Red O stained liver sections: panels (A) C; (B) HF; and (C) HF + NAC (×20 magnification). Representative H&E-stained liver sections: panels (D) C; (E) HF; and (F) HF + NAC (×10 magnification). Representative Picosirius Red stained liver sections: panels (G) C; (H) HF; and (I) HF + NAC (×10 magnification).
Table 2.
Pathology scores of rats fed C, HF or HF + NAC diets for 65 d1
| Parameter | C (n=4) | HF (n=7) | HF + NAC (n=8) |
|---|---|---|---|
| Cellular Injury | 0.0 ± 0.0a | 0.9 ± 0.1b | 0.9 ± 0.1b |
| Hepatocellular Ballooning | 0.0 ± 0.0a | 2.0 ± 0.4c | 1.1 ± 0.3b |
| Lobular Inflammation | 0.6 ± 0.2a | 1.3 ± 0.2b | 1.1 ± 0.1b |
| Lipidosis (Oil Red O Staining) | 0.8 ± 0.2a | 3.5 ± 0.2b | 3.7 ± 0.2b |
| Total Score | 1.4 ± 0.3a | 8.1 ± 0.6c | 7.0 ± 0.4b |
| Total Score - Lipidosis | 0.6 ± 0.3a | 4.6 ± 0.6c | 3.3 ± 0.3b |
| Fibrosis (Picosirius Red Staining) | 3.6 ± 0.6a | 17 ± 2.8c | 7.2 ± 1.9b |
The pathology calculation was based on our previously published system for pathology scoring (15). Values are means ± SEM. Means in a row without a common letter differ, p≤0.05.
Table 3.
ALT values, serum and hepatic triacylglycerol, and MDA protein for rats fed C, HF or HF + NAC diets for 65 d1
| Parameter | C (n=4) | HF (n=7) | HF + NAC (n=8) |
|---|---|---|---|
| ALTs (U/L) | 38 ± 1.6a | 104 ± 16c | 70 ± 6.5b |
| Serum triacylglycerol (µmol/L serum) | 19.2 ± 1.9b | 12.4 ± 1.2a | 12.4 ± 2.4a |
| Liver triacylglycerol (µmol/g liver) | 11.3 ± 2.9a | 18.1 ± 3.2b | 18.1 ± 1.7b |
| MDA protein (% adjusted volume) | 8.3 ± 1.2 | 11 ± 2.9 | 5.4 ± 1.7 |
Values are means ± SEM. Means in a row without a common letter differ, p≤0.05.
Dietary NAC alleviates oxidative stress and inflammation
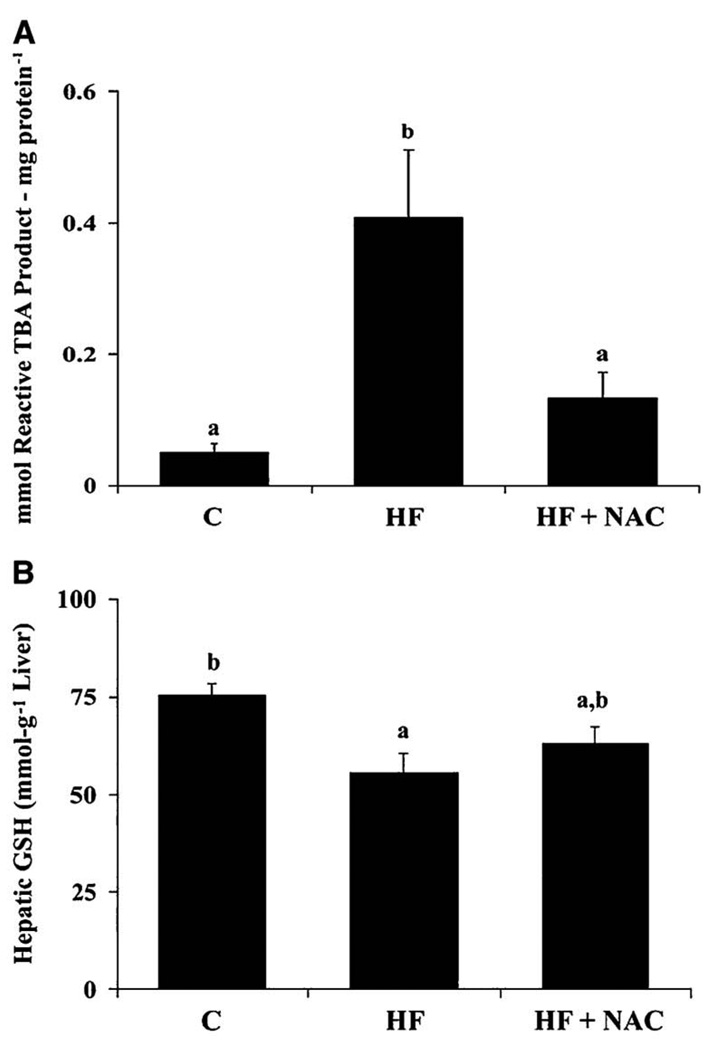
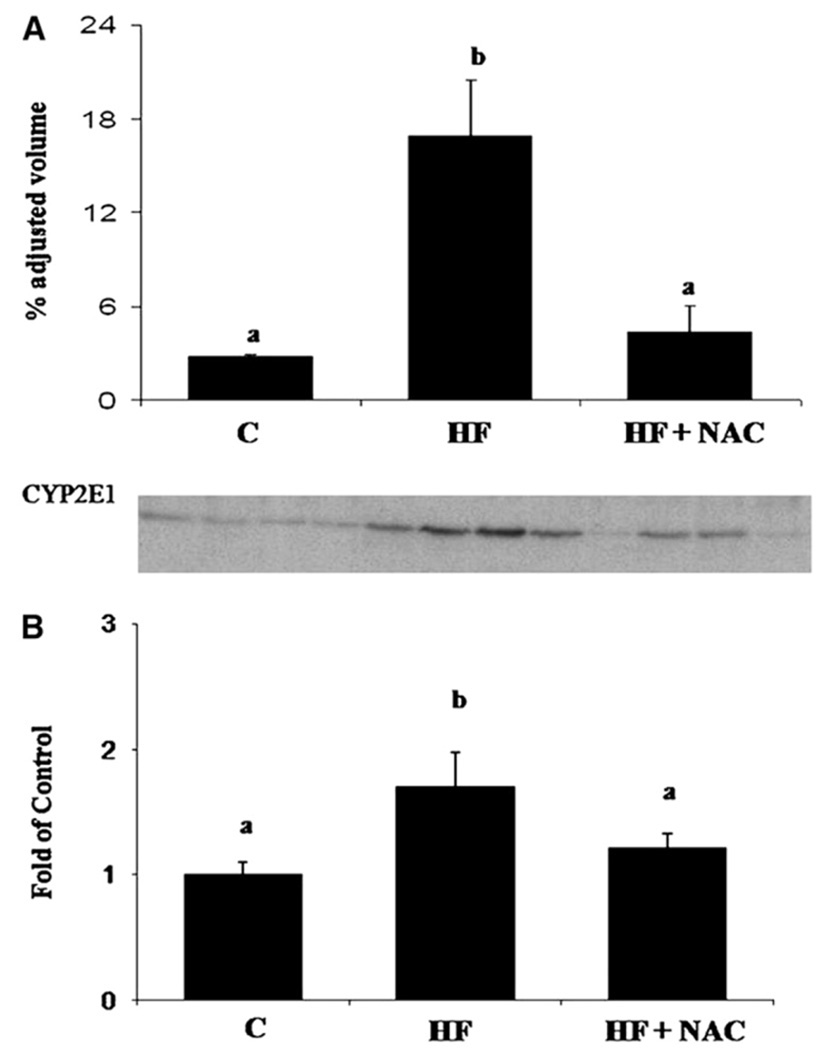
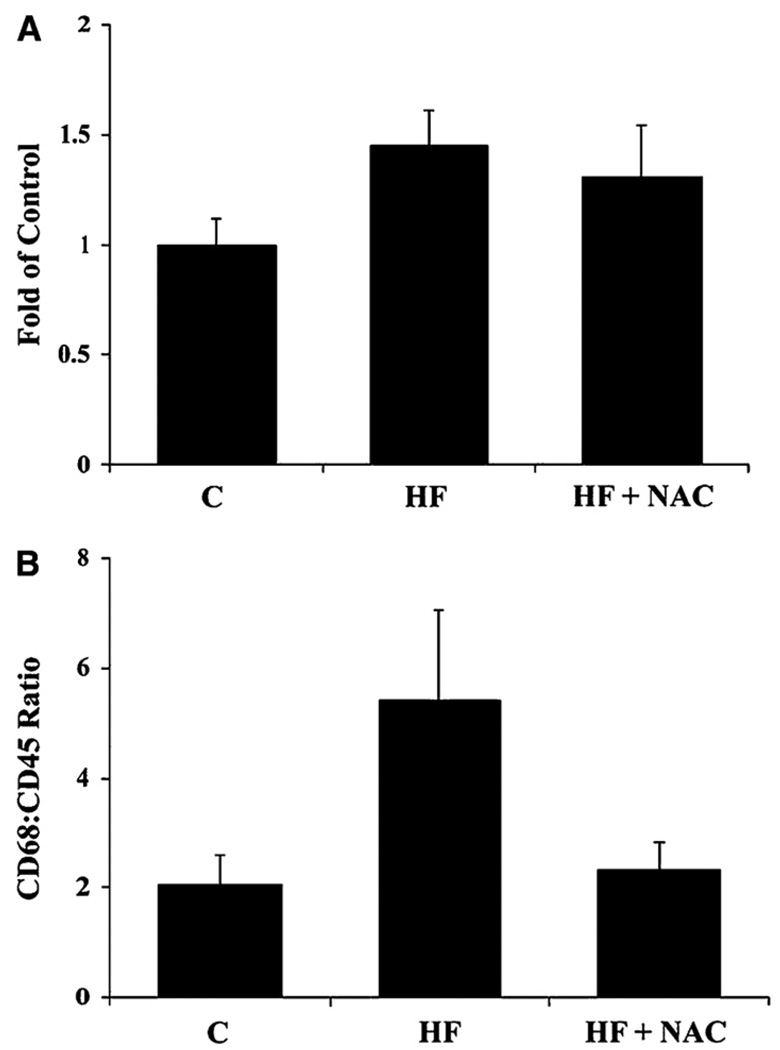
In the HF group, an increase in hepatic lipid peroxidation (TBARS; p≤0.05) was observed (Figure 2A), accompanied by decrease in hepatic GSH concentrations (p≤0.05) (Figure 2B). Although it did not reach statistical significance, as a result of inter-animal variability, an increase in mean expression of MDA-adducted proteins was also observed in Western blots from liver homogenates in the HF group (p= 0.1) (Table 3). An increase in CYP2E1 apoprotein and mRNA (p≤0.05) (Figures 3A and 3B), a source of reactive oxygen species (ROS), was also observed in the HF group as were increases in mRNA expression of the pro-inflammatory cytokine, TNF-α (p≤0.05) (Table 4). Another pro-inflammatory cytokine IL-1β mRNA was increased in the HF group (p=0.08) compared to the C group (Table 4). No increase was observed in expression of CD14 mRNA, an indicator of Kupffer cell activation (12) (Figure 4A). The ratio of CD68:CD45, an indicator of leukocyte activation and infiltration (30) tended to be greater in the HF group (p=0.077) than in the C group (Figure 4B). These indictors of oxidative stress and inflammation, except IL-1β, were attenuated by NAC supplementation (p≤0.05).
Figure 2. NAC prevents increases in hepatic TBARS (A) and partially restores hepatic GSH concentrations (B) reduced by overfeeding rats a high fat diet.
Values are means ± SEM, C (n = 4); HF (n = 7) and HF + NAC (n = 8). Means without a common letter differ, p≤0.05.
Figure 3. NAC supplementation reverses induction of rat hepatic CYP2E1 apoprotein (A) and hepatic CYP2E1 mRNA (B) expression following the overfeeding of a high fat diet.
Values are means ± SEM, C (n = 4); HF (n = 7) and HF + NAC (n = 8). Means without a common letter differ, p≤0.05. In the representative western blot each lane represents the liver microsomal protein from individual animals.
Table 4.
Relative expression of hepatic pro-inflammatory cytokines in rats fed C, HF or HF + NAC diets for 65 d1
| Gene | C (n=4) | HF (n=7) | HF + NAC (n=8) |
|---|---|---|---|
| TNF-α (fold of control) | 1.0 ± 0.40a | 13.3 ± 0.98b | 4.2 ± 1.20a |
| IL-1β (fold of control) | 1.0 ± 0.17 | 2.6 ± 0.76 | 2.5 ± 0.26 |
| IL-12 (fold of control) | 1.0 ± 0.29 | 1.0 ± 0.24 | 0.7 ± 0.32 |
Values are means ± SEM, relative expression of target gene mRNA normalized to cyclophilin A mRNA as a housekeeping gene using real time RT-PCR relative to mean value for C group = 1.0. Means in a row without a common letter differ, p≤0.05.
Figure 4. Effects of overfeeding high fat with or without NAC supplementation on rat hepatic CD14 mRNA (A) and CD68/CD45 mRNA ratio (B) an indicator of leukocyte activation and infiltration.
Values are means ± SEM, C (n = 4); HF (n = 7) and HF + NAC (n = 8). Means without a common letter differ, p≤0.05.
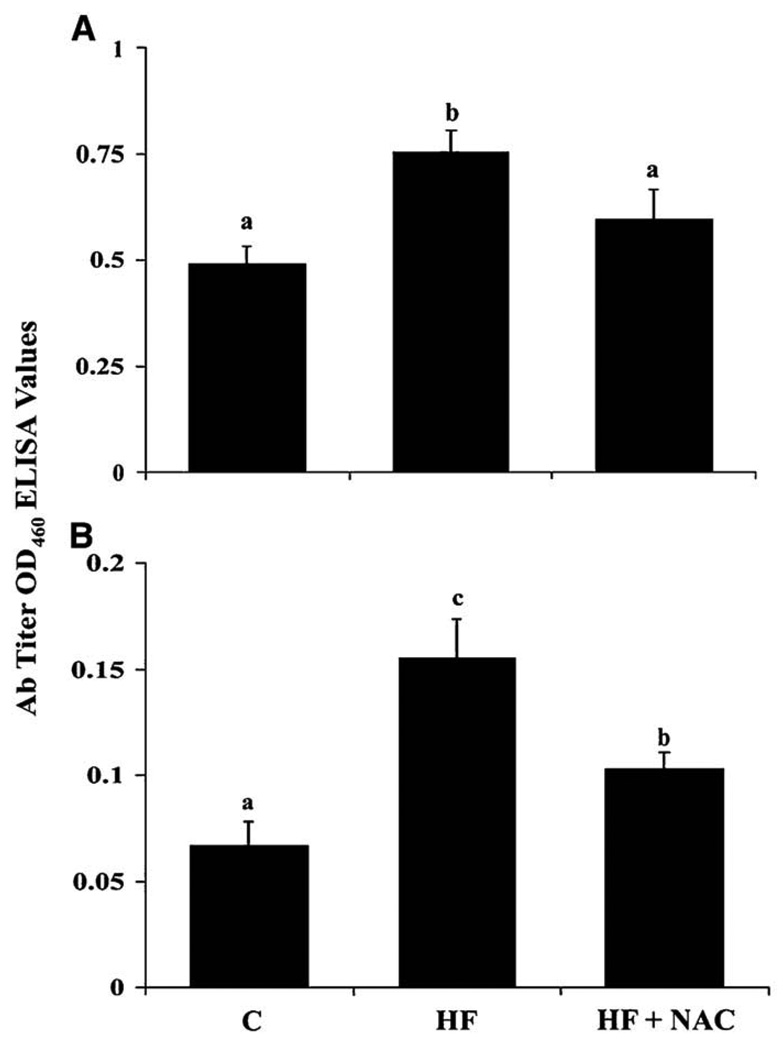
NAC supplementation reduces immune responses to protein adducts of lipid peroxidation products
Increased titers of antibodies directed against proteins adducted to the lipid peroxidation products MDA and LOOH (p≤0.05) were observed in the HF group and this was reduced by supplementation with NAC.
Effects of NAC administration on early markers of hepatic stellate cell activation and the turnover of extracellular matrix
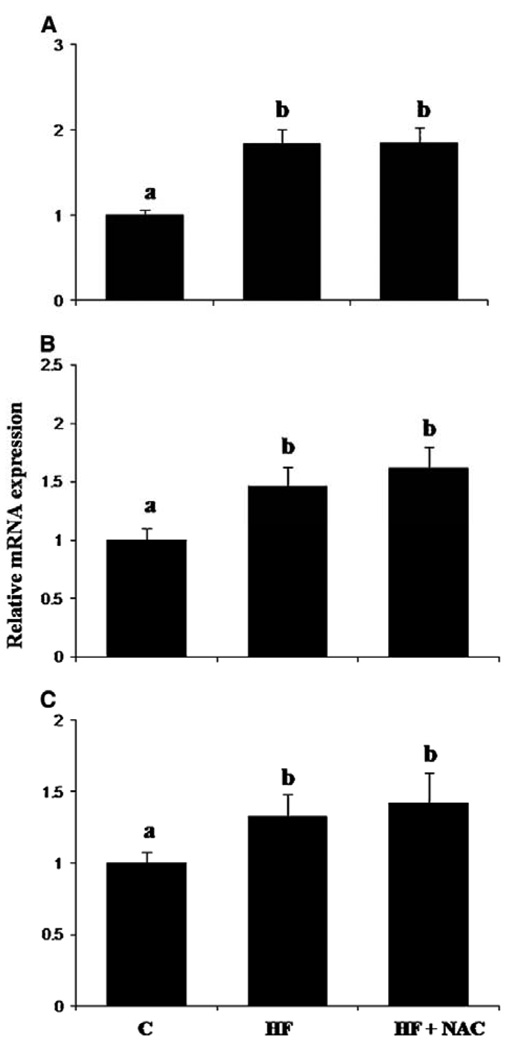
Transforming growth factor-β (TGF-β) is a cytokine linked to the activation of hepatic stellate cells (HSC) and development of fibrosis. Platelet derived growth factor receptor-β (PDGFr-β) and α-smooth muscle actin (α-SMA) are also early markers associated with HSC activation (31). Hepatic mRNA for all three were increased in the HF group compared to the C group (p≤0.05). However, elevation in expression of these mRNAs in the HF group was not affected by the addition of NAC (Figures 6A, 6B and 6C). No significant effect was observed on expression of mRNA for connective tissue growth factor (CTGF) (Table 5). Moreover, no significant effect was observed on expression of mRNA for the interstitial collagenase, MMP13 or its inhibitor, TIMP-1 (Table 5). In contrast, mRNA expression for CD44, the major cell-surface hyaluronan receptor (32), was increased in the HF group and attenuated by NAC treatment (p≤0.05) (Table 5).
Figure 6. Effects of overfeeding high fat with or without NAC supplementation on relative mRNA expression of early markers of hepatic stellate cell activation: TGF-β (A), αSMA (B) and PDGFr-β (C).
Values are means ± SEM, C (n = 4); HF (n = 7) and HF + NAC (n = 8). Means without a common letter differ, p≤0.05.
Table 5.
Relative expression of genes associated with fibrosis and modification of the extracellular matrix in rats fed C, HF or HF + NAC diets for 65 d1
| Gene | C (n=4) | HF (n=7) | HF + NAC (n=8) |
|---|---|---|---|
| CTGF (fold of control) | 1.0 ± 0.37 | 1.8 ± 0.21 | 2.1 ± 0.44 |
| MMP13 (fold of control) | 1.0 ± 0.55 | 3.6 ± 1.08 | 3.9 ± 1.3 |
| TIMP-1 (fold of control) | 1.0 ± 0.21 | 1.9 ± 0.59 | 3.9 ± 1.3 |
| CD44 (fold of control) | 1.0 ± 0.15a | 3.6 ± 0.87b | 2.2 ± 0.48a,b |
Values are means ± SEM, relative expression of target gene mRNA normalized to cyclophilin A mRNA as a housekeeping gene using real time RT-PCR relative to mean value for C group = 1.0. Means in a row without a common letter differ, p≤0.05. CD44, p≤ 0.05 by ANOVA of RANKS.
DISSCUSION
One area of general agreement between investigators studying mechanisms of NASH is that steatosis is a necessary prerequisite for any further damage. In the rat TEN NASH model, where pathology is driven by overfeeding of polyunsaturated fats, we have observed development of hepatic steatosis resulting, at least in part, from increased fatty acid uptake (15). Consistent with this, we observed a significant increase in expression of the fatty acid transport associated molecule CD36. However, neither CD36 expression nor steatosis was reversed by NAC supplementation. The lack of effect on steatosis is similar to our previous findings with NAC in a rat model of alcoholic steatohepatitis (12).
The progression from steatosis to steatohepatitis and fibrosis (“the second hit”) is complex and not well defined. Oxidative stress is believed to play an important role in the “second hit”. Peroxidation of lipids accumulated within steatotic hepatocytes has been demonstrated (5–7). Mitochondrial dysfunction and induction of hepatic CYP2E1 (33) leading to the formation of ROS (34), promote lipid peroxidation and oxidative stress. Increased staining for lipid peroxidation products in steatotic livers, with added increases in those with steatohepatitis (35) has been confirmed by immunohistochemistry. Disease progression may also involve immune responses to proteins adducted by lipid peroxidation products (36). Increases in pro-inflammatory cytokines, such as TNF-α and IL-1β, could also mediate hepatic inflammation.
Interestingly, the hepatic accumulation of lipids in patients with NASH and in the current model are associated with an increased mitochondrial β-oxidation of fatty acids (15, 37, 38), which has been suggested to be an adaptive response for limiting the accumulation of lipid. Respiratory chain complexes are reported to be poorly coupled in patients with NASH and increased mitochondrial FA oxidation may serve as a source of ROS (39). We observed hepatic lipid peroxidation which correlates with development of steatosis, oxidative stress, increased TNF-α and increased necrosis (15). Bioactive aldehydes such as MDA are chemically reactive products of lipid peroxidation that result in chemotaxis and cytotoxicity (40, 41) and the modulation of cell signaling cascades (21). Although the mechanisms whereby MDA and other aldehydes such as hydroxynonenal (HNE) elicit these effects remain to be delineated, it is likely they occur as a consequence of protein modification. As has been described in NASH patients, proteins adducted with lipid peroxidation products such as MDA and HNE and with LOOH also stimulate the host immune response which could result in an autoimmune-like disease (39). A weak correlation, r2 = 0.34, was observed in values for cellular injury and presence of auto-antibodies to LOOH-adducted proteins. The observation that NAC reduces the presence of antibodies to these protein adducts coincidental with attenuated progression of hepatic pathology suggests that immune mechanisms might be involved. Consistent with this are observations in humans showing that elevated titers of circulating IgG toward MDA and LOOH adducted proteins in a large fraction of hepatitis and/or cirrhosis patients, but only in a few subjects with simple steatosis alone (39).
Even though CD14 mRNA was not elevated in the HF group, indicating a lack of Kupffer cell activation, our data regarding CD68:CD45 ratio and TNF-α is consistent with the ability of NAC to reduce inflammation in this model. TNF-α is a cytokine proposed to play a central role in the chronic inflammatory response in NASH (42, 43). Kupffer cells are the major source of increased TNF-α secretion within the liver with infiltrating monocytes and macrophages also contributing (42, 43). TNF-α mRNA is increased in both the liver and adipose tissues in NASH patients (44). In both steatosis and steatohepatitis, soluble TNF receptor levels are elevated (45, 46), and enhanced TNF-α secretion from circulating monocytes is seen in patients with NASH compared with healthy controls (47). It has been observed that TNF receptor knockout mice have less severe steatosis than wild-type littermates (48) and a significant reduction in liver injury in steatohepatitis is seen after anti-TNF-α therapy (49–51), supporting the pathogenic role of TNF-α. In this study, an increase in the pro-inflammatory cytokine TNF-α mRNA was observed in the HF group, but was attenuated by the addition of NAC to the diet. This suggests that TNF-α elevation in NASH is the result of increased oxidative stress, perhaps secondary to an increase in CYP2E1 expression. It has recently been shown that CYP2E1 located in the mitochondrial fraction is associated with mitochondrial lipid peroxidation and damage, and is associated with TNF-α production in alcohol-induced steatohepatitis (52). NASH patients have also been shown to have up-regulation of CYP2E1, which may be one of the most important sources of ROS.
Regardless of etiology, oxidative stress plays a major role in activation of HSCs and in hepatic fibrogenesis (53). Lipid peroxidation has also been shown to stimulate collagen production in fibroblasts and HSCs (54). GSH precursors or antioxidant agents have been shown to exert protective effects against activation of HSCs (55); however, the role of oxidative stress and the beneficial effects of GSH precursors or antioxidant agents during the initial phases of liver fibrosis have not been fully investigated. The antifibrotic effects of NAC have been demonstrated in other experimental models especially in the lung (56, 57). In the current study, we observed partial protection against development of hepatic fibrosis in the NAC treated group; however, there was no reduction in TGF-β or its downstream targets α-SMA and PDGFr-β mRNA, both markers of HSC activation, compared to the HF group. In addition no significant effects were observed on MMP13 or TIMP-1. However, CD44, the major cell surface hyaluronan (HA) receptor was increased in the HF group and this was prevented by NAC. CD44 plays a role in clearing fragmented HA, which accumulates during tissue injury (32). However, HA may also play a role in promoting tissue repair as the result of interactions with Toll-like receptors (TLRs). In an acute lung injury model, over expression of high molecular mass HA resulted in protection against injury in part through TLR-mediated activation of NFκB (58). It is possible that increased HA, as the result of reduced CD44 expression in NAC treated animals, contributes to the partial protection against fibrosis observed in the current model. There is a possibility that NAC is also working downstream of TGF–β to diminish the progression of fibrosis via other signaling pathways. Dietary NAC is rapidly cleared from the body (13, 59). Assuming a constant infusion of NAC at 2000 mg˙kg−1˙d−1, steady state plasma concentrations of NAC are calculated to be ~300 µmol˙L−1. NAC has provided protection in our model, despite this relatively low predicted in vivo concentration, in comparison to in vitro studies where anti-oxidant and anti-fibrotic activities of NAC have been reported at levels in excess of 5 mmol˙L−1 (41). The observed anti-fibrotic effect may be secondary to decreases in lipid peroxidation and pro-inflammatory cytokines, such as TNF-α, both of which have been implicated in the development of fibrosis. Lipid peroxidation products can directly enhance collagen production by activated HSCs (60). Consistent with a role of lipid peroxidation in steatosis-triggered fibrosis, vitamin E supplementation in mice fed a methionine/choline-deficient diet, another animal model of NASH, inhibited fibrosis (61). The effects on CD68:CD45 ratio and TNF-α suggest that NAC’s protective effect against liver fibrosis may be associated with its ability to inhibit infiltrating macrophages. To some degree, our data parallels in vivo studies of anti-fibrotic effects of other antioxidants such as curcumin, the major polyphenolic compound in tumeric. Curcumin has been shown to inhibit the development of liver fibrosis mainly due to is anti-inflammatory activities and not by a direct anti-fibrotic effect (62).
We have shown that dietary NAC is an effective hepatic antioxidant that abolished NASH-induced lipid peroxidation, attenuated reductions in hepatic GSH, blocked NASH-associated autoimmune responses, inhibited the production of TNF-α and attenuated inflammation, leading to significant reduction in cellular damage, hepatocyte injury and fibrosis. These effects are similar to those we have previously reported for NAC treatment in a rat model of alcoholic steatohepatitis (12). To our knowledge, this is the first report that administration of NAC partially protects against progression of liver injury including fibrosis in this type of dietary model of NASH. These data suggest that NAC supplementation in conjunction with other treatments may be a valuable adjuvant therapy in NASH patients. Indeed, a clinical study has recently been published in which NASH patients treated for 12 months with 1.2 g˙d−1 NAC in conjunction with metformin demonstrated significantly improved fibrosis (63).
Supplementary Material
Figure 5. NAC supplementation reduces immune responses to protein adducts of malondialdehyde (A) and lipid hydroperoxides (B) in rats overfed a high fat diet.
Values are means ± SEM, C (n = 4); HF (n = 7) and HF + NAC (n = 8). Means without a common letter differ, p≤0.05.
ACKNOWLEDGEMENTS
We thank the following people for their technical assistance: Matt Ferguson, Jamie Badeaux, Tammy Dallari and Michele Perry.
ABBREVIATIONS USED
- α-SMA
alpha-smooth muscle actin
- ALT
alanine aminotransferase
- C
pellet-fed control
- CTGF
connective tissue growth factor
- CYP
cytochrome P450
- GSH
glutathione
- HA
hyaluronan
- HF
high fat
- HNE
hydroxynonenal
- HSC
hepatic stellate cell
- LOOH
arachidonate hydroperoxide
- MDA
malondialdehyde
- MMP13
matrix metallopeptidase 13
- NAC
N-acetylcysteine
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatoheptatitis
- PDGFr-β
platelet derived growth factor receptor-beta
- ROS
reactive oxygen species
- TEN
total enteral nutrition
- TGF-β
transfoming growth factor-beta
- TIMP-1
tissue inhibitor of metalloproteinases-1
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript that has been accepted for publication in The American Journal of Clinical Nutrition, copyright American Society for Nutrition (ASN). This manuscript may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, US Code) without permission of the copyright owner, the ASN. The final copyedited article, which is the version of record, can be found at http://www.ajcn.org/. The ASN disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
This research was supported, in part, by RO1 AA 12819 (M.J.J.R), ACNC-USDA-ARS 6251-51000-005D (T.M.B) and the NIEHS Graduate Student Training Grant (J.N.B).
Author disclosures: January N. Baumgardner, Kartik Shankar, Leah Hennings, Emanuele Albano, Thomas M. Badger, and Martin J. J. Ronis have no conflicts of interest.
A full list of real-time RT-PCR primer sequences (Supplemental Table 1) is available as Online Supporting Material with the online posting of this paper at http://jn.nutrition.org.
An enlarged, high fat group pathology figure (Supplemental Figure 1) is available as Online Supporting Material with the online posting of this paper at http://jn.nutrition.org.
Literature Cited
- 1.Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463–1466. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- 2.Albano E. Free radical mechanisms in immune reactions associated with alcoholic liver disease. Free Radic. Biol. Med. 2002;32:110–114. doi: 10.1016/s0891-5849(01)00773-0. [DOI] [PubMed] [Google Scholar]
- 3.Chawla RK, Watson WH, Eastin CE, Lee EY, Schmidt J, McClain CJ. S-adenosylmethionine deficiency and TNF-alpha in lipopolysaccharide-induced hepatic injury. Am. J. Physiol. 1998;275:G125–G129. doi: 10.1152/ajpgi.1998.275.1.G125. [DOI] [PubMed] [Google Scholar]
- 4.Osmundsen H, Bremer J, Pedersen JI. Metabolic aspects of peroxisomal beta-oxidation. Biochim. Biophys. Acta. 1991;1085:141–158. doi: 10.1016/0005-2760(91)90089-z. [DOI] [PubMed] [Google Scholar]
- 5.Mehta K, Van Thiel DH, Shah N, Mobarhan S. Nonalcoholic fatty liver disease: pathogenesis and the role of antioxidants. Nutr. Rev. 2002;60:289–293. doi: 10.1301/002966402320387224. [DOI] [PubMed] [Google Scholar]
- 6.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 7.Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria in steatohepatitis. Semin. Liver Dis. 2001;21:57–69. doi: 10.1055/s-2001-12929. [DOI] [PubMed] [Google Scholar]
- 8.Buckley NA, Whyte IM, O'Connell DL, Dawson AH. Oral or intravenous N-acetylcysteine: which is the treatment of choice for acetaminophen (paracetamol) poisoning? J. Toxicol. Clin. Toxicol. 1999;37:759–767. doi: 10.1081/clt-100102453. [DOI] [PubMed] [Google Scholar]
- 9.Gulbahar O, Karasu A, Ersoz G, Akarca US, Mosoglu A. Treatment of nonalcoholic steatohepatitis with N-acetylcysteine. Gastroenterology. 2000:118. [Google Scholar]
- 10.Gulbahar O, Karasu A, Ersoz G, Akarca US, Mosoglu A. N-Acetylcysteine in the treatment of non-alcoholic steatohepatitis. J Gastroenterology. 2003:1220–1221. doi: 10.1046/j.1440-1746.2003.03156.x. [DOI] [PubMed] [Google Scholar]
- 11.Pastor A, Collado PS, Almar M, Gonzalez-Gallego J. Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J Hepatol. 1997;27:363–370. doi: 10.1016/s0168-8278(97)80183-3. [DOI] [PubMed] [Google Scholar]
- 12.Ronis MJ, Butura A, Sampey BP, Shankar K, Prior RL, Korourian S, Albano E, Ingelman-Sundberg M, Petersen DR, et al. Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic. Biol. Med. 2005;39:619–630. doi: 10.1016/j.freeradbiomed.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vries N, De Flora S. N-acetyl-l-cysteine. J. Cell Biochem. 1993 Suppl 17F:270–277. doi: 10.1002/jcb.240531040. [DOI] [PubMed] [Google Scholar]
- 14.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quinones L, Varela N, Contreras J, Lazarte R, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. (Lond) 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 15.Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJ. A New Model For Non-Alcoholic Steatohepatitis in the Rat Utilizing Total Enteral Nutrition to Overfeed a High Polyunsaturated Fat Diet. Am. J Physiol Gastrointest. Liver Physiol. 2008;294:G27–G38. doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- 16.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 17.Ronis MJ, Rowlands JC, Hakkak R, Badger TM. Inducibility of hepatic CYP1A enzymes by 3-methylcholanthrene and isosafrole differs in male rats fed diets containing casein, soy protein isolate or whey from conception to adulthood. J. Nutr. 2001;131:1180–1188. doi: 10.1093/jn/131.4.1180. [DOI] [PubMed] [Google Scholar]
- 18.Badger TM, Ronis MJ, Lumpkin CK, Valentine CR, Shahare M, Irby D, Huang J, Mercado C, Thomas P, et al. Effects of chronic ethanol on growth hormone secretion and hepatic cytochrome P450 isozymes of the rat. J. Pharmacol. Exp. Ther. 1993;264:438–447. [PubMed] [Google Scholar]
- 19.Badger TM, Crouch J, Irby D, Hakkak R, Shahare M. Episodic excretion of ethanol during chronic intragastric ethanol infusion in the male rat: continuous vs. cyclic ethanol and nutrient infusions. J. Pharmacol. Exp. Ther. 1993;264:938–943. [PubMed] [Google Scholar]
- 20.Badger TM, Ronis MJ, Ingelman-Sundberg M, Hakkak R. Pulsatile blood alcohol and CYP2E1 induction during chronic alcohol infusions in rats. Alcohol. 1993;10:453–457. doi: 10.1016/0741-8329(93)90064-u. [DOI] [PubMed] [Google Scholar]
- 21.Ronis MJ, Korourian S, Zipperman M, Hakkak R, Badger TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr. 2004;134:904–912. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- 22.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Baumgardner JN, Shankar K, Korourian S, Badger TM, Ronis MJ. Undernutrition enhances alcohol-induced hepatocyte proliferation in the liver of rats fed via total enteral nutrition. Am. J Physiol Gastrointest. Liver Physiol. 2007;293:G355–G364. doi: 10.1152/ajpgi.00038.2007. [DOI] [PubMed] [Google Scholar]
- 25.Johansson I, Ingelman-Sundberg M. Benzene metabolism by ethanol-, acetone-, and benzene-inducible cytochrome P-450 (IIE1) in rat and rabbit liver microsomes. Cancer Res. 1988;48:5387–5390. [PubMed] [Google Scholar]
- 26.Polavarapu R, Spitz DR, Sim JE, Follansbee MH, Oberley LW, Rahemtulla A, Nanji AA. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology. 1998;27:1317–1323. doi: 10.1002/hep.510270518. [DOI] [PubMed] [Google Scholar]
- 27.Yamada S, Kumazawa S, Ishii T, Nakayama T, Itakura K, Shibata N, Kobayashi M, Sakai K, Osawa T, et al. Immunochemical detection of a lipofuscin-like fluorophore derived from malondialdehyde and lysine. J Lipid Res. 2001;42:1187–1196. [PubMed] [Google Scholar]
- 28.Uchida K, Szweda LI, Chae HZ, Stadtman ER. Immunochemical detection of 4-hydroxynonenal protein adducts in oxidized hepatocytes. Proc. Natl. Acad. Sci. USA. 1993;90:8742–8746. doi: 10.1073/pnas.90.18.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti M, Vidali M, Sartori M, Rigamonti C, et al. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic. Biol. Med. 2002;32:38–45. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- 30.O'Donohue J, Wong T, Portmann B, Williams R. Immunohistochemical differences in the portal tract and acinar infiltrates between primary biliary cirrhosis and autoimmune cholangitis. Eur J Gastroenterol Hepatol. 2002;14:1143–1150. doi: 10.1097/00042737-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Borkham-Kamphorst E, Meurer SK, Gressner AM, Weiskirchen R. Disruption of intermolecular disulfide bonds in PDGF-BB dimers by N-acetyl-L-cysteine does not prevent PDGF signaling in cultured hepatic stellate cells. Biochem. Biophys. Res. Commun. 2005;338:1711–1718. doi: 10.1016/j.bbrc.2005.10.139. [DOI] [PubMed] [Google Scholar]
- 32.Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16:693–701. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- 33.Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin. Liver Dis. 2001;21:27–41. doi: 10.1055/s-2001-12927. [DOI] [PubMed] [Google Scholar]
- 34.Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005;42:928–940. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Le TH, Caldwell SH, Redick JA, Sheppard BL, Davis CA, Arseneau KO, Iezzoni JC, Hespenheide EE, et al. The zonal distribution of megamitochondria with crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2004;39:1423–1429. doi: 10.1002/hep.20202. [DOI] [PubMed] [Google Scholar]
- 36.Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, Day CP. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54:987–993. doi: 10.1136/gut.2004.057968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miele L, Grieco A, Armuzzi A, Candelli M, Forgione A, Gasbarrini A, Gasbarrini G. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am. J Gastroenterol. 2003;98:2335–2336. doi: 10.1111/j.1572-0241.2003.07725.x. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Carreras M, Del HP, Martin MA, Rubio JC, Martin A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2005;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 39.Hensley K, Kotake Y, Sang H, Pye QN, Wallis GL, Kolker LM, Tabatabaie T, Stewart CA, Konishi Y, et al. Dietary choline restriction causes complex I dysfunction and increased H2O2 generation in liver mitochondria. Carcinogenesis. 2000;21:983–989. doi: 10.1093/carcin/21.5.983. [DOI] [PubMed] [Google Scholar]
- 40.Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197–208. doi: 10.1016/j.jhep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Zamara E, Novo E, Marra F, Gentilini A, Romanelli RG, Caligiuri A, Robino G, Tamagno E, Aragno M, et al. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60–68. doi: 10.1016/s0168-8278(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 42.Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303–306. doi: 10.1136/gut.2003.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N. Engl. J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 44.Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, Fernandez-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 45.Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, et al. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 2006;26:39–45. doi: 10.1111/j.1478-3231.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 46.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 47.Poniachik J, Csendes A, Diaz JC, Rojas J, Burdiles P, Maluenda F, Smok G, Rodrigo R, Videla LA. Increased production of IL-1alpha and TNF-alpha in lipopolysaccharide-stimulated blood from obese patients with non-alcoholic fatty liver disease. Cytokine. 2006;33:252–257. doi: 10.1016/j.cyto.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am. J Gastroenterol. 2004;99:2365–2368. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- 50.Koppe SW, Sahai A, Malladi P, Whitington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41:592–598. doi: 10.1016/j.jhep.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 51.Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 52.Lieber CS, Cao Q, DeCarli LM, Leo MA, Mak KM, Ponomarenko A, Ren C, Wang X. Role of medium-chain triglycerides in the alcohol-mediated cytochrome P450 2E1 induction of mitochondria. Alcohol Clin. Exp. Res. 2007;31:1660–1668. doi: 10.1111/j.1530-0277.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 53.Tsukamoto H. Oxidative stress, antioxidants, and alcoholic liver fibrogenesis. Alcohol. 1993;10:465–467. doi: 10.1016/0741-8329(93)90066-w. [DOI] [PubMed] [Google Scholar]
- 54.Parola M, Leonarduzzi G, Robino G, Albano E, Poli G, Dianzani MU. On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free Radic. Biol. Med. 1996;20:351–359. doi: 10.1016/0891-5849(96)02055-2. [DOI] [PubMed] [Google Scholar]
- 55.Kawada N, Seki S, Inoue M, Kuroki T. Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology. 1998;27:1265–1274. doi: 10.1002/hep.510270512. [DOI] [PubMed] [Google Scholar]
- 56.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am. J Respir. Crit Care Med. 2000;162:225–231. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- 57.Pardo A, Ruiz V, Arreola JL, Ramirez R, Cisneros-Lira J, Gaxiola M, Barrios R, Kala SV, Lieberman MW, et al. Bleomycin-induced pulmonary fibrosis is attenuated in gamma-glutamyl transpeptidase-deficient mice. Am. J Respir. Crit Care Med. 2003;167:925–932. doi: 10.1164/rccm.200209-1007OC. [DOI] [PubMed] [Google Scholar]
- 58.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 59.De CL, Ghizzi A, Costa R, Longo A, Ventresca GP, Lodola E. Pharmacokinetics and bioavailability of oral acetylcysteine in healthy volunteers. Arzneimittelforschung. 1989;39:382–386. [PubMed] [Google Scholar]
- 60.Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentilini A, Gentilini P, Dianzani MU. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem. Biophys. Res. Commun. 1993;194:1044–1050. doi: 10.1006/bbrc.1993.1927. [DOI] [PubMed] [Google Scholar]
- 61.Phung N, Farrell G, Robertson G, George G. Antioxidant therapy with vitamin E ameliorates hepatic fibrosis in MCDD-associated NASH. J Gastroenterology and Hepatology. 2001:248–251. [Google Scholar]
- 62.Bruck R, Ashkenazi M, Weiss S, Goldiner I, Shapiro H, Aeed H, Genina O, Helpern Z, Pines M. Prevention of liver cirrhosis in rats by curcumin. Liver Int. 2007;27:373–383. doi: 10.1111/j.1478-3231.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 63.de Oliveira CP, Stefano JT, de Siqueira ER, Silva LS, de Campos Mazo DF, Lima VM, Furuya CK, Mello ES, Souza FG, et al. Combination of N-acetylsysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol. Res. 2008;38:159–165. doi: 10.1111/j.1872-034X.2007.00215.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.